Abstract
Objectives
Severe asthma (SA) can be uncontrolled despite guideline-directed treatment. We described SA characteristics and identified factors associated with uncontrolled disease and frequent exacerbations.
Methods
Post hoc analysis of the observational IDEAL study (201722/NCT02293265) included patients with SA aged ≥12 years receiving high-dose inhaled corticosteroids plus additional controller(s) for ≥12 months. Uncontrolled SA was defined by Asthma Control Questionnaire (ACQ)-5 scores ≥1.5 or ≥1 exacerbations (prior year), and further stratified by exacerbation frequency (no/infrequent [0–1] vs frequent [≥2]; prior year); associated factors were determined using multivariate logistic regression.
Results
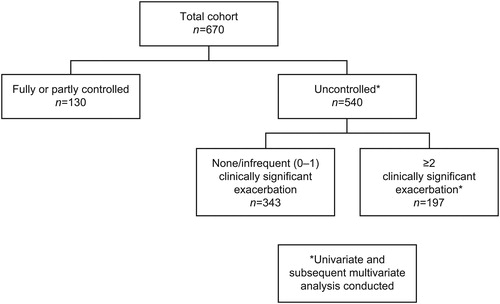
Of 670 patients with SA, 540 (81%) were uncontrolled (ACQ-5 scores ≥1.5: 80%; ≥1 exacerbations [prior year]: 71%). Uncontrolled patients had lower lung function and worse health-related quality of life (HRQoL) than controlled patients; 197/540 (37%) experienced frequent exacerbations (prior year). Worse St George’s Respiratory Questionnaire (SGRQ) total score, comorbid sinusitis, or eczema were significantly associated with uncontrolled SA; younger age, never smoker status, exacerbation requiring hospitalization (previous year), worse SGRQ symptom score, comorbid nasal polyps, COPD, or osteoporosis were significantly associated with uncontrolled SA with frequent exacerbations.
Conclusions
In IDEAL, one-fifth of patients with SA were controlled, based on symptoms. Uncontrolled, exacerbating SA was associated with specific comorbidities, frequent exacerbations, a lower lung function, and compromised HRQoL, although inference from this analysis is limited by the selective cross-sectional nature of the cohort. Nonetheless, these data highlight the need for more effective precision treatments in this population.
Introduction
Approximately 10% of patients with asthma have severe disease (Global Initiative for Asthma [GINA] Step 4 or 5), characterized by frequent exacerbations and poor symptom control, reflecting underlying chronic airways inflammation (Citation1). Severe disease presentation can occur despite optimal asthma management and present despite good adherence to treatment and correct inhalation technique (Citation2,Citation3). Asthma control represents the degree by which treatment reduces current disease manifestations, such as symptoms, and minimizes the future risk of adverse outcomes, such as exacerbations and accelerated decline in lung function (Citation4). For patients with severe asthma (SA), high-dose inhaled corticosteroids (ICS) combined with an additional controller or oral corticosteroids (OCS) are often needed to achieve asthma control (Citation5). Despite these measures, a considerable number of patients with SA continue to have uncontrolled disease (Citation3).
Although patients with uncontrolled SA account for only a small portion of the total asthma population, these patients demonstrate considerable morbidity and healthcare resource utilization (Citation6–8). Previously, patients with uncontrolled SA have been found to be older, have more comorbidities, use more controller medications, and have more frequent asthma exacerbations compared with patients with controlled SA (Citation9–13). Similarly, patients with frequent exacerbations, an indicator of uncontrolled disease, have worse health-related quality of life (HRQoL) (Citation11,Citation14).
To improve treatment outcomes for patients with uncontrolled SA, there has been an increased emphasis on identifying patient phenotypes based on biomarkers, including blood eosinophil counts and immunoglobulin (Ig) E levels (Citation15,Citation16). Two asthma phenotypes identified include severe eosinophilic asthma, characterized by eosinophilic inflammation and recurrent exacerbations, and severe allergic asthma, characterized by high serum IgE and eosinophilic inflammation (Citation5). The biomarkers associated with these phenotypes have been used to predict treatment response; therefore, the asthma characteristics associated with these phenotypes are of interest in determining the need for targeted treatment in these sub-populations.
Previously, the cross-sectional, single-visit, observational IDEAL study (Citation17) described treatment eligibility for the biologics mepolizumab (anti-interleukin [IL]-5), omalizumab (anti-IgE), and reslizumab (anti-IL-5) in patients with SA recruited from clinical practice. The objective of this analysis was to describe the characteristics of patients with SA included in the IDEAL study, and also to explore two key patient phenotypes: severe uncontrolled asthma and severe uncontrolled asthma with frequent exacerbations aiming to support future hypothesis generation.
Methods
Study design
The IDEAL study (201722; NCT02293265) was a cross-sectional, single-visit, observational, real-world study of patients with SA from specialist clinics in six countries, the USA, Canada, Australia, France, the UK, and Germany. The study was conducted in accordance with the International Conference on Harmonisation Good Clinical Practice standards and the ethical principles outlined in the Declaration of Helsinki.
Patients
Enrolled patients with SA were ≥12 years of age and had been treated with high-dose ICS plus at least one additional controller (long-acting bronchodilator antagonist [LABA], leukotriene modifier, theophylline, or continuous or near continuous OCS [ie, maintenance OCS for ≥50% of the previous year]) for ≥12 months, as defined in the American Thoracic Society/European Respiratory Society guidelines (Citation5). The maximum recommended dose of the ICS/LABA combination per local label was acceptable for fulfilling the SA criteria if a patient was on a fixed-dose combination medication. No medications were prohibited. Patients who had participated in an interventional clinical trial for asthma within the past 12 months were excluded. In short, patients were recruited from secondary care severe asthma clinics located predominantly in allergist or chest physician offices in a consecutive way, depending on their study eligibility (confirmed SA status). Further details of the IDEAL study methodology have previously been presented (Citation17).
Measures
Traits assessed in the IDEAL study included patient demographic and clinical characteristics collected at the study visit or retrieved from patients’ medical records.
The 12-month period prior to the study visit was used to derive the frequency of predefined events or to collect the most recent assessments from the patients’ medical records. Events and assessments included airway reversibility (≥12% to short-acting β2-agonist administration); lung function (forced expiratory volume in 1 s [FEV1] % predicted); asthma exacerbation history (ie, clinically significant exacerbations [requiring systemic corticosteroids and/or emergency room visit and/or hospitalization], exacerbations requiring hospitalization, and exacerbations requiring intubation); and asthma medication use. Asthma exacerbations were confirmed through a review of medical records verified by site investigator. Maintenance OCS use was defined as OCS use for at least 50% of the previous year. At the study visit, the following characteristics were assessed: patient demographics, medical history, asthma disease history, lung function (where recent data were not available), white blood cell differential count, IgE tests (performed using central laboratories), and measures of symptom control and HRQoL.
Self-administered measures of symptom control and HRQoL included the St George’s Respiratory Questionnaire (SGRQ; scores range from 0 to 100, with higher scores indicating poorer HRQoL), the Asthma Quality of Life Questionnaire (AQLQ; scores range from 0 to 7, with lower scores indicating a greater impairment), the Asthma Control Questionnaire (ACQ-5; scale ranges from 0 to 6, with higher scores indicating greater impairment), and the Work Productivity and Activity Impairment Index: General Health V2.0 (WPAI-GH; scale ranges from 0 to 100, with higher numbers indicating a greater impairment/limitation).
Study population
In this post hoc analysis, the SA population was stratified by the study definition of level of asthma control, as follows: controlled SA (had not experienced clinically significant asthma exacerbations in the prior year and had an ACQ-5 score of <1.5); uncontrolled SA (all other patients, ie, those who had experienced clinically significant asthma exacerbations in the prior year or had an ACQ-5 score of ≥1.5). Patients with uncontrolled SA were further analyzed by history of clinically significant exacerbations (no/infrequent [0–1] vs frequent [≥2] exacerbations in the prior year). An additional analysis stratified controlled versus uncontrolled patients by blood eosinophil count category (<150, ≥150–<300 or ≥300 cells/μL); patients with uncontrolled SA were further stratified by exacerbation frequency (no/infrequent [0–1] vs frequent [≥2]) in the prior year.
Statistical analysis
All demographic and clinical characteristics were analyzed descriptively and stratified by patient subgroup. For the present analysis, the two subgroups of interest included 1) patients with uncontrolled SA (vs controlled) and 2) patients with uncontrolled SA who had history of frequent (≥2) clinically significant exacerbations (≥2 events/year vs 0–1 events/year) (). Factors associated with uncontrolled SA or with uncontrolled SA with a history of frequent exacerbations were first estimated using univariate analysis, including chi-square test for categorical variables and t-test for continuous variables. All variables, except those removed due to collinearity, were included in the multivariate logistic regression model by a backward stepwise selection and the corresponding odds ratios were assigned to a 95% confidence interval. The following covariates that were removed due to collinearity included comorbid obesity (correlation with body mass index [BMI]), SGRQ domain scores (correlation with SGRQ total score) and AQLQ score (correlation with SGRQ total score). For the remaining variables, the highest observed correlation was 0.47 (between FEV1 reversibility and % predicted FEV1); there was no evidence of collinearity in the final model. Percentage overall work impairment and activity impairment were excluded from the selection process, as they were only derivable for employed patients, and history of pneumonia was also excluded due to only 2 patients responding with “Yes”. All models were also re-run with forward selection with the same result. Statistical significance for the multivariate analysis was set at p < 0.05.
Results
Characteristics of patients with SA
The total cohort consisted of 670 patients with SA, with a mean age of 50.5 years, of whom 62% were female (). The mean (standard deviation [SD]) BMI was 29.9 (7.4), and 36% of patients were former or current smokers. The mean (SD) duration of asthma was 25.8 (16.9) years, with half of patients having a family history of asthma (Table S1, supplementary material). In total, 66% of patients had a history of allergic rhinitis/hay fever, 13% sinusitis, 11% nasal polyps, and 10% aspirin sensitivity.
Table 1. Demographic characteristics and comorbidities of patients with severe asthma by asthma control.
In the previous 12 months, 57% of patients with SA had ≥1 clinically significant exacerbation, with 30%, 16%, and 8% of patients experiencing ≥2, ≥3, and ≥4 exacerbations, respectively (). In total, 9% of patients had ≥1 asthma exacerbation requiring hospitalization in the previous 12 months and 7.5% experienced asthma exacerbations requiring intubation anytime in their history. At the study visit, 14% of patients were receiving current maintenance OCS treatment and 25% were receiving omalizumab. Mean pre-bronchodilator FEV1% predicted was 68.7%, with 40% of patients having ≥12% airway reversibility. At the study visit, 63% of patients had a blood eosinophil count ≥150 cells/µL and 32% had ≥300 cells/µL, with a geometric mean of 186 cells/μL.
Table 2. Clinical characteristics and patient-reported outcomes of patients with severe asthma by asthma control.
Characteristics of patients with SA by asthma control
The majority (n = 540/670; 81%) of patients with SA were uncontrolled. Classification as uncontrolled was primarily based on ACQ-5 scores ≥1.5 (80%), followed by ≥1 exacerbations in the previous year (71%). Age and smoking status were similar across the asthma control strata and there were no notable differences in allergic and some other respiratory comorbidities (ie, allergic rhinitis/hay fever, or nasal polyps), except eczema, which was more frequent in controlled SA (n = 27/130 [21%] vs n = 58/540 [11%]) (). Patients with uncontrolled versus controlled SA were more frequently females (n = 340/540 [63%] vs. n = 75/130 [58%]), had higher mean BMIs (30.2 vs 28.5 kg/m2, respectively), and a shorter duration of asthma (25.2 vs 28.2 years, respectively; ; Table S1, supplementary material). A greater proportion of patients with uncontrolled versus controlled SA had osteoporosis (n = 62/540 [12%] vs. n = 8/130 [6%]; ) or depression (n = 87/540 [16%] vs. n = 15/130 [12%]).
Clinical characteristics and patient-reported outcomes
Mean pre-bronchodilator FEV1% predicted was lower in patients with uncontrolled versus controlled SA, whereas percent FEV1 reversibility and the proportion of patients with airway reversibility ≥12% was higher in the uncontrolled versus controlled strata (). The geometric mean for peripheral blood eosinophil count was similar in both strata (range: 185–189 cells/μL). The mean serum IgE, KU/L (geometric mean [SD log]) was lower in patients with uncontrolled versus controlled SA; omalizumab use was comparable in both strata (25% in uncontrolled; 26% in controlled).
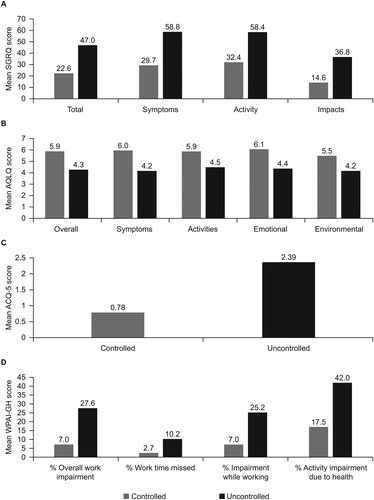
Patients with uncontrolled SA had higher SGRQ total and domain scores, indicating worse HRQoL, than patients with controlled SA (); the difference in SGRQ total scores for uncontrolled versus controlled SA was 24.4 (minimal clinically important difference [MCID]: 4 points (Citation18); ), while the difference in AQLQ total score was 1.6 (MCID: 0.5 points (Citation19); ). As would be expected based on the criteria for uncontrolled SA, mean ACQ-5 scores were higher in the uncontrolled compared with the controlled stratum, a difference of 1.6 (MCID: 0.5 points (Citation20); ). WPAI-GH scores were also higher in patients with uncontrolled versus controlled SA ().
Figure 2. Mean (a) SGRQ, (b) AQLQ, (c) ACQ-5, and (d) WPAI-GH scores in patients with severe asthma by asthma control.
ACQ, Asthma Control Questionnaire; AQLQ, Asthma Quality of Life Questionnaire; SGRQ, St George's Respiratory Questionnaire; WPAI-GH, Work Productivity and Activity Impairment Questionnaire: General Health.
Factors associated with uncontrolled SA
In the multivariate analysis, independent factors associated with uncontrolled SA were poorer HRQoL (higher SGRQ total score), and a lower frequency of comorbid sinusitis or eczema (Table S2, supplementary material). Details of the univariate analysis are included in Table S3 (supplementary material).
Characteristics of patients with uncontrolled SA by exacerbation frequency
In total, 36% (n/N = 197/540) of patients with uncontrolled SA experienced ≥2 clinically significant exacerbations in the previous 12 months (). Patients with a history of frequent (≥2) clinically significant exacerbations were younger (mean: 47.8 vs 51.9 years, respectively) and had a higher frequency of respiratory comorbidities including sinusitis, nasal polyps, or chronic obstructive pulmonary disease (COPD) than patients with no or infrequent (0–1) clinically significant exacerbations (). More patients with a history of frequent exacerbations were currently treated with OCS () and subsequently experienced higher systemic steroid-exposure–related comorbidities (eg, osteoporosis, adrenal insufficiency, and Cushing syndrome) compared with patients with no or infrequent exacerbations.
Table 3. Demographic characteristics of patients with uncontrolled severe asthma by history of clinically significant exacerbations.
Table 4. Clinical characteristics and patient-reported outcomes of patients with uncontrolled severe asthma by history of clinically significant exacerbations.
Clinical characteristics and patient-reported outcomes
A greater proportion of patients with a history of frequent (≥2) clinically significant exacerbations had <80% predicted normal FEV1 and had greater reversibility to bronchodilator challenge compared with those with no or infrequent (0–1) exacerbations (; Table S4, supplementary material). Blood eosinophil counts were similar across exacerbation strata; however, patients with frequent clinically significant exacerbations more frequently had blood neutrophil counts ≥9.0% than those with no or infrequent exacerbations. Mean SGRQ scores were higher and AQLQ scores lower (poorer HRQoL) in patients with frequent clinically significant exacerbations compared with patients with infrequent or no exacerbations ().
Factors associated with a history of frequent (≥2) clinically significant exacerbations
In the multivariate analysis, seven significant factors including a younger age (p < 0.001), being a never smoker (p = 0.006), exacerbation requiring hospitalization in the previous year (p < 0.001), higher SGRQ symptoms scores (poorer HRQoL; p = 0.026), comorbid nasal polyps (p = 0.034), current COPD (p = 0.013), and osteoporosis (p = 0.032) were associated with a history of frequent clinically significant exacerbations (Table S5, supplementary material). Details of the univariate analysis are included in Table S6 (supplementary material).
Characteristics of patients with SA by blood eosinophil counts
In the subgroup with uncontrolled SA, the proportions of patients with nasal polyps increased with increasing blood eosinophil counts (Table S7, supplementary material). Moreover, the incidence of sinusitis was also highest in patients with blood eosinophil counts ≥300 cells/μL, regardless of asthma control status (Table S7, supplementary material). The proportion of patients with airway reversibility ≥12% also increased with increasing baseline blood eosinophil count in those with uncontrolled SA (Table S8, supplementary material).
Further observations, when uncontrolled SA was stratified by blood eosinophil count and frequency of clinically significant exacerbations included trends toward decreasing FEV1 and increasing reversibility with increasing blood eosinophil count (Tables S9 and S10, supplementary material).
Discussion
This post hoc analysis of the large, observational IDEAL study of patients with SA aimed to describe key patient characteristics, and determine factors associated with uncontrolled SA and a history of frequent exacerbations in this sample of patients with uncontrolled disease. The results provide a detailed description of the population and provide insight into the factors associated with the disease subsets highlighted in the study objectives. Of the 670 patients with SA, more than 80% had uncontrolled disease despite the use of high-dose ICS plus additional controller(s) for the previous 12 months. Although the classification as uncontrolled was primarily based on ACQ-5 score (80%), the presence of clinically significant exacerbations in the previous year (71%) was highly prevalent. Uncontrolled SA was associated with worse lung function and a poorer HRQoL than controlled SA. Patients with uncontrolled SA with frequent clinically significant exacerbations in the previous year, representing more than a quarter of the study population, had more allergic comorbidities, greater OCS use and incidence of OCS-associated comorbidities, worse lung function, and a poorer HRQoL than those with no or infrequent exacerbations. These results highlight that most patients in the sample with SA lack disease control, and those who exacerbate frequently carry a substantial clinical, healthcare utilization and HRQoL/symptom burden, likely impacting their disease progression and prognosis (Citation5,Citation21–23).
Several previously published studies have indicated that uncontrolled asthma is associated with a high burden on healthcare systems, with total direct costs of uncontrolled asthma estimated to be double that of controlled asthma (Citation12). In line with previous reports, patients with uncontrolled SA in the current analysis experienced more frequent exacerbations, worse lung function, and more OCS use than patients with other levels of SA control, as would be expected based on the definition of SA control (Citation1). Additionally, a greater proportion of patients with uncontrolled versus controlled SA had osteoporosis, a comorbidity often associated with frequent OCS use (Citation24,Citation25). Indeed, it is likely that rather than being a factor associated with frequent exacerbations, the higher incidence of osteoporosis in patients who experienced frequent exacerbations was due to the chronic use of OCS, as shown in previous studies (Citation26). These factors likely contributed to the substantial HRQoL burden observed in patients with uncontrolled SA; differences in SGRQ and AQLQ scores for patients with uncontrolled versus controlled SA were approximately 6 and 3 times the MCID, respectively (Citation18,Citation20). Finally, patients with uncontrolled SA demonstrated a greater work impairment as measured by the WPAI-GH, with mean scores for work impairment, work time missed, and activity impairment 2–4-times greater than in patients with controlled disease. These results are consistent with previous studies of patients with mild to SA (10–12) and indicate a substantial disease burden for patients with SA, which worsens with decreasing asthma control.
A direct relationship between the number of exacerbations in patients with severe eosinophilic asthma and lung function decline has previously been demonstrated (Citation27). The current study supports previous observations, showing more respiratory comorbidities and worse lung function in patients with uncontrolled SA with frequent exacerbations versus those with infrequent exacerbations. Additionally, we built upon previous reports, demonstrating very poor HRQoL at the clinical visit in patients with uncontrolled SA with frequent exacerbations. SGRQ total and domain scores were 4–6 units higher in patients with uncontrolled SA with frequent exacerbations than in uncontrolled patients with no/infrequent exacerbations (MCID 4 points). By contrast, no differences in AQLQ scores were observed between these subgroups of patients suggesting that the AQLQ may not be sufficiently discriminating for assessing HRQoL in patients with SA and exacerbations. Indeed, it has been suggested that standard asthma questionnaires, such as AQLQ, may not detect symptom changes in SA as well as the SGRQ, which has been widely used in severe respiratory diseases such as COPD and has a broader focus (Citation28–34). Together, this information should be considered in the design of similar studies of severe asthma in the future.
A significant association between comorbidities and the presence of uncontrolled asthma has been demonstrated previously (Citation35). Here, we identified several factors associated with uncontrolled SA in the multivariate analysis, which aimed to provide a more realistic view of the relationship between the variables that contribute to uncontrolled asthma than the univariate analysis. These factors included two comorbidities (sinusitis and eczema), as well as higher SGRQ total score, denoting poorer HRQoL. Interestingly, eczema and sinusitis were less frequent in patients with uncontrolled asthma. This finding is in contrast to a previous review, which found a higher rate of atopic conditions such as sinusitis in patients with uncontrolled asthma, albeit in an elderly population (Citation36). Conversely, T helper 2 inflammation, which is associated with uncontrolled asthma, has been observed independent of atopic status in some SA populations (Citation37,Citation38). These previous findings support the possibility that, in this population of patients with SA, atopic conditions such as eczema and sinusitis may not have increased in prevalence with uncontrolled disease, but were associated with a lower risk of uncontrolled disease. We also identified eight factors associated with frequent (≥2) clinically significant exacerbations in the previous year in patients with uncontrolled SA. These included three comorbidities, nasal polyps, COPD, and osteoporosis, in addition to younger patient age and never smoker status. As has been demonstrated in previous analyses, the current study also found that a history of exacerbations requiring hospitalization was strongly associated with frequent exacerbation events (Citation13,Citation39), as were higher SGRQ symptom scores. Interestingly, AQLQ scores were not associated with frequent exacerbations, further suggesting the appropriateness of the SGRQ over the AQLQ in patients with SA. In terms of lung function, while pre-bronchodilator FEV1 was significantly associated with uncontrolled SA in the univariate analysis (p = 0.042), this result did not maintain significance following multivariate correction. Post-bronchodilator FEV1 may be a better factor associated with uncontrolled SA and exacerbations due to the fixed airway obstruction in patients with SA, as has been demonstrated previously (Citation40); however, post-bronchodilator FEV1 was not assessed in the current study. Notably, blood eosinophil count was not found to be a factor associated with a history of frequent clinically significant exacerbations in our analyses, despite high counts being a known risk factor for future exacerbations (Citation41). As approximately 25% of patients in each SA control stratum had omalizumab exposure in the previous 12 months prior to the study visit, this may have impacted patient blood eosinophil counts and IgE levels during this period. In addition, patients with uncontrolled SA with frequent exacerbations were more likely to have blood neutrophil counts >9% than those with no/infrequent (0–1) exacerbations, potentially indicating a mixed inflammatory phenotype which may be associated with frequent exacerbations (Citation42,Citation43).
Previous studies have estimated that around 55% of patients with SA have uncontrolled disease (Citation44,Citation45). The higher proportion of patients with uncontrolled disease shown here (>80%) is likely due to the differing definitions of uncontrolled disease used across studies. Our definition included exacerbations and ACQ score, encompassing two clinical aspects that are important to patients, whereas some studies base their definition of uncontrolled disease on just one of these elements. However, the majority of these patients were uncontrolled based on the ACQ score (80%). As only 20% of patients in the current study had controlled SA, despite treatment with high-dose ICS and additional controller(s), and approximately 25% of controlled patients receiving omalizumab, additional targeted and more effective therapeutic approaches would likely be of benefit in improving asthma control. Our analysis has presented detailed clinical characteristics of a sample of patients with SA and identified several factors that are associated with both uncontrolled SA and uncontrolled SA with frequent (≥2) exacerbations in this sample. These findings may be useful in providing targeted treatment approaches in this population.
The limitations of the IDEAL study have previously been discussed in detail by Albers and colleagues (Citation17). This observational study was a “convenience sample” rather than being based on random sampling of patients and, hence, it is possible that recruitment sites and countries may have demonstrated bias toward certain patients, potentially impacting results. Additionally, there is an unknown potential for selection bias for patients who volunteer for this type of study. This study used a cross-sectional design, which does not allow the linking of predictors with future outcomes; hence, we were limited to describing factors related to history of exacerbations or asthma control. Inclusion of traits or markers as present at study visit plus history of exacerbations allowed us to evaluate factors associated with the presence of pre-defined sub-populations or phenotypes; however, we cannot discern if these factors are drivers or consequence of these patient phenotypes. Finally, we did not collect data on socio-economic status and other social features, which could have been informative in respect to access to care or individual attitudes to health. Although all study countries were highly developed and located in the European Union, USA, or Canada, country-specific variations in exposures, socio-economic factors, and accessibility of quality care may still impact the asthma severity and/or control of the included patients. However, a relatively small sample size per country prevented us from running a meaningful country-level analysis to reflect national health system differences, therefore it should be noted that country-specific variations are not accounted for in the current study.
Conclusions/key findings
In this sample of patients from the IDEAL study, controlled SA was achieved infrequently, with approximately 80% of patients with SA having some form of uncontrolled disease. Classification of uncontrolled SA was primarily based on symptoms and clinically significant exacerbations; these patients also had poor lung function and ultimately very poor HRQoL. Comorbidities and HRQoL were found to be significantly associated with uncontrolled disease with a history of frequent (≥2) exacerbations. Identifying differences in clinical characteristics and factors associated with uncontrolled SA and frequent exacerbations can help support individualized asthma management and development of future targeted and more effective therapeutics for patients with SA.
Author’s contributions
All authors (HM, SMC, NBG, LMN, and FCA) were involved in study conception and design, data analysis and interpretation, preparation and review of the manuscript, and approved the final version to be submitted.
Funding source and role
This post hoc analysis and the original study were funded by GSK (Study 201722; NCT02293265).
Supplemental Material
Download MS Word (75.1 KB)Acknowledgements
Editorial support (in the form of writing assistance, including development of the initial draft based on author direction, assembling tables and figures, collating and incorporating authors' comments, grammatical editing, and referencing) was provided by Alex Lowe, PhD, and Elizabeth Hutchinson, PhD, CMPP, at Fishawack Indicia Ltd, UK, and was funded by GlaxoSmithKline (GSK).
Declaration of interest
SMC, NBG, and LMN are employees of GSK and own stocks/shares. HM and FCA were employees of GSK at the time of the study and submission, and own stocks/shares in GSK; HM is now employed by AstraZeneca and FCA is now employed by Avillion.
Data sharing
Anonymized individual participant data and study documents for the original IDEAL study can be requested for further research from www.clinicalstudydatarequest.com.
References
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2018. Available from: https://ginasthma.org/ [last accessed 3 December 2018].
- Wenzel SE, Busse WW. Severe asthma: lessons from the Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119(1):14–23. doi:10.1016/j.jaci.2006.10.025.
- Hekking PP, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896–902. doi:10.1016/j.jaci.2014.08.042.
- Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, Casale TB, Chanez P, Enright PL, Gibson PG, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59–99. doi:10.1164/rccm.200801-060ST.
- Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi:10.1183/09031936.00202013.
- Bahadori K, Doyle-Waters MM, Marra C, Lynd L, Alasaly K, Swiston J, FitzGerald JM. Economic burden of asthma: a systematic review. BMC Pulm Med. 2009;9(1):24. doi:10.1186/1471-2466-9-24.
- Braunstahl G-J, Deenstra M, Canvin J, Peachey G, Chen C-W, Georgiou P, Maykut R. Healthcare utilization and indirect cost of treatment associated with severe allergic asthma in a real-world setting. Clin Transl Allergy. 2013;3(suppl 1):P1. doi:10.1186/2045-7022-3-S1-P1.
- Kerkhof M, Tran TN, Soriano JB, Golam S, Gibson D, Hillyer EV, Price DB. Healthcare resource use and costs of severe, uncontrolled eosinophilic asthma in the UK general population. Thorax. 2018;73(2):116–124. doi:10.1136/thoraxjnl-2017-210531.
- Zeiger RS, Schatz M, Dalal AA, Qian L, Chen W, Ngor EW, Suruki RY, Kawatkar AA. Utilization and costs of severe uncontrolled asthma in a managed-care setting. J Allergy Clin Immunol Pract. 2016;4(1):120–129.e123. doi:10.1016/j.jaip.2015.08.003.
- Dean BB, Calimlim BM, Kindermann SL, Khandker RK, Tinkelman D. The impact of uncontrolled asthma on absenteeism and health-related quality of life. J Asthma. 2009;46(9):861–866. doi:10.3109/02770900903184237.
- Lee LK, Obi E, Paknis B, Kavati A, Chipps B. Asthma control and disease burden in patients with asthma and allergic comorbidities. J Asthma. 2018;55(2):208–219. doi:10.1080/02770903.2017.1316394.
- Pavord ID, Mathieson N, Scowcroft A, Pedersini R, Isherwood G, Price D. The impact of poor asthma control among asthma patients treated with inhaled corticosteroids plus long-acting beta2-agonists in the United Kingdom: a cross-sectional analysis. NPJ Prim Care Respir Med. 2017;27:17. doi:10.1038/s41533-017-0014-1.
- Suruki RY, Daugherty JB, Boudiaf N, Albers FC. The frequency of asthma exacerbations and healthcare utilization in patients with asthma from the UK and USA. BMC Pulm Med. 2017;17:74. doi:10.1186/s12890-017-0409-3.
- Luskin AT, Chipps BE, Rasouliyan L, Miller DP, Haselkorn T, Dorenbaum A. Impact of asthma exacerbations and asthma triggers on asthma-related quality of life in patients with severe or difficult-to-treat asthma. J Allergy Clin Immunol Pract. 2014;2(5):544–552.e541–542. doi:10.1016/j.jaip.2014.02.011.
- Wenzel S. Severe asthma: from characteristics to phenotypes to endotypes. Clin Exp Allergy. 2012;42(5):650–658. doi:10.1111/j.1365-2222.2011.03929.x.
- Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D'Agostino R, Jr., Castro M, Curran-Everett D, Fitzpatrick AM, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181(4):315–323. doi:10.1164/rccm.200906-0896OC.
- Albers FC, Mullerova H, Gunsoy NB, Shin JY, Nelsen LM, Bradford ES, Cockle SM, Suruki RY. Biologic treatment eligibility for real-world patients with severe asthma: The IDEAL study. J Asthma. 2018;55(2):152–160. doi:10.1080/02770903.2017.1322611.
- Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2(1):75–79. doi:10.1081/COPD-200050513.
- Wilson SR, Rand CS, Cabana MD, Foggs MB, Halterman JS, Olson L, Vollmer WM, Wright RJ, Taggart V. Asthma outcomes: quality of life. J Allergy Clin Immunol. 2012;129(3):S88–S123. doi:10.1016/j.jaci.2011.12.988.
- Cloutier MM, Schatz M, Castro M, Clark N, Kelly HW, Mangione-Smith R, Sheller J, Sorkness C, Stoloff S, Gergen P. Asthma outcomes: composite scores of asthma control. J Allergy Clin Immunol. 2012;129(3):S24–S33. doi:10.1016/j.jaci.2011.12.980.
- Pavord ID, Beasley R, Agusti A, Anderson GP, Bel E, Brusselle G, Cullinan P, Custovic A, Ducharme FM, Fahy JV, et al. After asthma: redefining airways diseases. Lancet. 2018;391(10118):350–400. doi:10.1016/S0140-6736(17)30879-6.
- Rennard SI, Farmer SG. Exacerbations and progression of disease in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1(2):88–92. doi:10.1513/pats.2306026.
- Stephenson JJ, Quimbo RA, Gutierrez B. Subacute lack of asthma control as a predictor of subsequent acute asthma exacerbation in a managed care population. Am J Manag Care. 2010;16(2):108–114.
- Sarnes E, Crofford L, Watson M, Dennis G, Kan H, Bass D. Incidence and US costs of corticosteroid-associated adverse events: a systematic literature review. Clin Ther. 2011;33(10):1413–1432. doi:10.1016/j.clinthera.2011.09.009.
- Sullivan PW, Ghushchyan VH, Globe G, Schatz M. Oral corticosteroid exposure and adverse effects in asthmatic patients. J Allergy Clin Immunol. 2018;141(1):110–116.e117. doi:10.1016/j.jaci.2017.04.009.
- van Staa TP, Leufkens HGM, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002;13(10):777–787. doi:10.1007/s001980200108.
- Ortega H, Yancey SW, Keene ON, Gunsoy NB, Albers FC, Howarth PH. Asthma exacerbations associated with lung function decline in patients with severe eosinophilic asthma. J Allergy Clin Immunol Pract. 2018;6(3):980–986.e981. doi:10.1016/j.jaip.2017.12.019.
- Chupp GL, Bradford ES, Albers FC, Bratton DJ, Wang-Jairaj J, Nelsen LM, Trevor JL, Magnan A, Ten Brinke A. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5(5):390–400. doi:10.1016/S2213-2600(17)30125-X.
- Filipowski M, Bozek A, Kozlowska R, Czyżewski D, Jarzab J. The influence of hospitalizations due to exacerbations or spontaneous pneumothoraxes on the quality of life, mental function and symptoms of depression and anxiety in patients with COPD or asthma. J Asthma. 2014;51(3):294–298. doi:10.3109/02770903.2013.862543.
- Jones PW. Quality of life, symptoms and pulmonary function in asthma: long-term treatment with nedocromil sodium examined in a controlled multicentre trial. Nedocromil Sodium Quality of Life Study Group. Eur Respir J. 1994;7(1):55–62.
- Kupczyk M, ten Brinke A, Sterk PJ, Bel EH, Papi A, Chanez P, Nizankowska-Mogilnicka E, Gjomarkaj M, Gaga M, Brusselle G, et al. Frequent exacerbators–a distinct phenotype of severe asthma. Clin Exp Allergy. 2014;44(2):212–221. doi:10.1111/cea.12179.
- Szende A, Svensson K, Stahl E, Meszaros A, Berta GY. Psychometric and utility-based measures of health status of asthmatic patients with different disease control level. Pharmacoecon. 2004;22(8):537–547. doi:10.2165/00019053-200422080-00005.
- Nelsen LM, Kimel M, Murray LT, Ortega H, Cockle SM, Yancey SW, Brusselle G, Albers FC, Jones PW. Qualitative evaluation of the St George's Respiratory Questionnaire in patients with severe asthma. Respir Med. 2017;126:32–38. doi:10.1016/j.rmed.2017.02.021.
- Bateman ED, Esser D, Chirila C, Fernandez M, Fowler A, Moroni-Zentgraf P, FitzGerald JM. Magnitude of effect of asthma treatments on Asthma Quality of Life Questionnaire and Asthma Control Questionnaire scores: systematic review and network meta-analysis. J Allergy Clin Immunol. 2015;136(4):914–922. doi:10.1016/j.jaci.2015.03.023.
- Hekking PP, Amelink M, Wener RR, Bouvy ML, Bel EH. Comorbidities in difficult-to-control asthma. J Allergy Clin Immunol Pract. 2018;6(1):108–113. doi:10.1016/j.jaip.2017.06.008.
- Ban GY, Trinh TH, Ye YM, Park HS. Predictors of asthma control in elderly patients. Curr Opin Allergy Clin Immunol. 2016;16(3):237–243. doi:10.1097/ACI.0000000000000273.
- Ingenito E, Bigler J, Smith D, Rowe A, Chung F, Djukanovic R, Sousa A, Adcock I, Sterk P, Nirula A. Characteristics of asthma in U-BIOPRED severe and non-severe cohorts with distinct biomarker profiles. Eur Respir J. 2015;46:PA2546. doi:10.1183/13993003.congress-2015.PA2546.
- Busse WW, Holgate ST, Wenzel SW, Klekotka P, Chon Y, Feng J, Ingenito EP, Nirula A. Biomarker profiles in asthma with high vs low airway reversibility and poor disease control. Chest. 2015;148(6):1489–1496. doi:10.1378/chest.14-2457.
- Chipps BE, Zeiger RS, Dorenbaum A, Borish L, Wenzel SE, Miller DP, Hayden ML, Bleecker ER, Simons FER, Szefler SJ, et al. Assessment of asthma control and asthma exacerbations in the epidemiology and natural history of asthma: outcomes and treatment regimens (TENOR) observational cohort. Curr Respir Care Rep. 2012;1(4):259–269. doi:10.1007/s13665-012-0025-x.
- Bateman ED, Buhl R, O'Byrne PM, Humbert M, Reddel HK, Sears MR, Jenkins C, Harrison TW, Quirce S, Peterson S, et al. Development and validation of a novel risk score for asthma exacerbations: the risk score for exacerbations. J Allergy Clin Immunol. 2015;135(6):1457–1464.e1454. doi:10.1016/j.jaci.2014.08.015.
- Zeiger RS, Schatz M, Li Q, Chen W, Khatry DB, Gossage D, Tran TN. High blood eosinophil count is a risk factor for future asthma exacerbations in adult persistent asthma. J Allergy Clin Immunol Pract. 2014;2(6):741–750. doi:10.1016/j.jaip.2014.06.005.
- Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, Wenzel SE, Peters SP, Meyers DA, Bleecker ER, et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol. 2014;133(6):1557–1563.e1555. doi:10.1016/j.jaci.2013.10.011.
- Green BJ, Wiriyachaiporn S, Grainge C, Rogers GB, Kehagia V, Lau L, Carroll MP, Bruce KD, Howarth PH. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS One. 2014;9(6):e100645. doi:10.1371/journal.pone.0100645.
- Peters SP, Jones CA, Haselkorn T, Mink DR, Valacer DJ, Weiss ST. Real-world Evaluation of Asthma Control and Treatment (REACT): findings from a national Web-based survey. J Allergy Clin Immunol. 2007;119(6):1454–1461. doi:10.1016/j.jaci.2007.03.022.
- Larsson K, Stallberg B, Lisspers K, Telg G, Johansson G, Thuresson M, Janson C. Prevalence and management of severe asthma in primary care: an observational cohort study in Sweden (PACEHR). Respir Res. 2018;19(1):12. doi:10.1186/s12931-018-0719-x.