Abstract
Objective: To describe patient characteristics, treatment patterns and healthcare utilization (HCU) of non-active users of maintenance asthma medications in the United Kingdom.
Methods: Retrospective, cohort analysis of patients with asthma, aged ≥ 6 years who were non-active users of maintenance therapy (no prescription for inhaled corticosteroids (ICS), combined ICS/long-acting beta agonists (ICS/LABA) or ‘other’ bronchodilatory therapies in last 12 months) were identified in the Clinical Practice Research Datalink (2012–2015) and followed-up for 2 years after a new prescription for an asthma maintenance medication (index date). Patient characteristics, most common maintenance treatment sequences and HCU were described.
Results: 55,293 patients were identified (ICS: 46,297, ICS/LABA: 8,367; Other: 629). Mean age was 37 years and 56% were female. During follow-up, the most common treatment sequences across groups implied intermittent use, comprising periods of maintenance therapy interspersed with maintenance-free periods. During year 1 and year 2 of follow-up, the proportion of patients prescribed OCS was 19% and 13%, prescribed ≥ 4 short-acting bronchodilators (SABD) was 24% and 19%, having ≥ 3 asthma-related primary care consultations/year was 59% and 36% and experiencing ≥ 1 exacerbation/year was 15% and 11%, respectively.
Conclusions: In previously non-active users of asthma maintenance medication subsequently commenced on maintenance therapy, intermittent use was common during the 2-year follow-up despite the potential need for regular use as evidenced by patient HCU and SABD usage patterns. This highlights the need for regular patient assessment and education on medication adherence to ensure appropriateness of prescribing to maintain asthma control.
Introduction
Asthma is one of the most common chronic, non-communicable diseases worldwide and is responsible for a substantial social and economic burden, causing lost days at work and school, and impaired quality of life through limitations in daily activities and sleep disturbances (Citation1–3). The main goals of asthma treatment are to achieve good symptom control whilst minimizing the future risk of exacerbations, fixed airflow obstruction and treatment side-effects (Citation2,Citation4–6). Successful asthma management requires a comprehensive, holistic approach in selecting the most appropriate asthma medication and inhaler for a given patient and initiating a regimen of treatment that will ensure appropriate medication-taking behaviors over time. Pharmacological therapies are only one aspect of optimal care, and treatment plans should address modifiable risk factors, ensure high-quality communication between healthcare professionals and patients, matching the patient with the most suitable inhaler, regular follow-up, and empower patient self-management through education and personalized written action plans (Citation1,Citation2,Citation4–6).
Asthma guidelines recommend a stepwise approach to pharmacological treatment, titrating changes in therapy – be it changes in dose regimen, medication or delivery device - to achieve adequate control of symptoms (Citation2,Citation4,Citation5). Inhaled corticosteroids (ICS) are considered the most effective anti-inflammatory treatment for all severities of persistent asthma, and early initiation of ICS after asthma diagnosis is associated with long-term benefits in lung function and asthma control; oral therapies such as leukotriene receptor antagonists (LTRAs) and xanthine derivatives (e.g. theophylline) are alternatives to ICS but do not have as marked an effect on inflammation (Citation2,Citation4–7).
For patients who remain poorly controlled on ICS, the addition of a long-acting beta2-agonist (LABA) is the preferred next step (Citation2,Citation8); should patients remain uncontrolled, therapy can be further intensified through increasing medication dosage, addition of another controller medication and, in refractory cases, addition of systemic therapies such as oral corticosteroids (OCS) or biologics (e.g. anti-IL5, anti-IgE) (Citation2). Ongoing evaluation of pharmacological treatment efficacy is based on the review/assessment of symptom control, risk factors, comorbidities, treatment side-effects, and patient adherence to and satisfaction with treatment (Citation1,Citation2,Citation9).
There is limited understanding of the long-term real-world maintenance treatment patterns in the broad asthma population. Most data, collected through clinical trials and observational studies, have typically evaluated outcomes associated with a specific step-up or step-down in therapy rather than describing changes in prescribed asthma therapy over time (Citation10–14). This study aims to address this knowledge gap through describing the patient characteristics, healthcare utilization (HCU) and in particular treatment patterns, of non-active users of maintenance asthma medications among the primary care population of asthmatics in the United Kingdom (UK), in a large, nationally representative, primary care dataset.
Methods
Data source and patients
This retrospective, cohort study used data extracted from the Clinical Practice Research Datalink (CPRD) (period between 1 January 2012 and 31 December 2017) (GSK study 209142). CPRD contains anonymized, longitudinal patient-level data from the electronic medical records of a representative sample of the UK primary care practices and includes data on primary care medical consultations, prescriptions, results of investigations ordered by primary care, and referrals to specialists (Citation15,Citation16).
The source population for this study was defined as individuals with a diagnosis of asthma who had not received maintenance therapy (ICS/LABA, ICS or ‘other’ indicated therapies) for a period of at least 12 months between January 1 2012 and December 31 2015 and, as such, were deemed ‘non-active’ users of maintenance therapy. Members of this ‘non-active’ population who were subsequently prescribed an asthma maintenance therapy were identified using a new user design and were the analysis population for this study; the date of this prescription was defined as the index date and the 12 months immediately prior to this index prescription as the baseline period.
Eligible patients were ≥ 6 years of age at the index date with an asthma diagnosis on or before the index date and had not been prescribed a maintenance medication for asthma in the 12 months immediately preceding their index maintenance prescription. All patients had at least 3 years of continuous data (1 year prior to and 2 years post-index date) within the study period. Patients diagnosed with one or more of chronic obstructive pulmonary disease, an interstitial lung disease, cystic fibrosis or lung cancer was excluded.
In the 2-year follow-up period from the index date, data on treatment patterns, HCU and the occurrence of asthma exacerbations (as defined below) were assessed.
The Independent Scientific Advisory Committee approved the use of CPRD data for this study (ISAC protocol 19_128R) and the protocol complied with all applicable laws regarding subject privacy. As the study involved no direct subject contact or primary collection of individual human subject data, and all data were anonymized, patient informed consent, ethics committee or IRB approval were not required.
Study variables
Patient characteristics
Patient characteristics of age, gender and ethnicity (at index date), comorbidities, history of atopy and diagnosis of exercise-induced bronchoconstriction (during baseline year), and previous asthma maintenance medication history (prior to the 1-year baseline period) were recorded.
Asthma treatment
For patients in each of the index therapy categories, prescribed asthma therapies (at the level of treatment class – e.g. ICS, ICS/LABA) over the 2-year observation period were determined. Changes in prescribed compounds were classified in terms of whether they were an augmentation (an addition of another therapy from a different class), a therapy switch (either within- or between-class) or a discontinuation of therapy. Discontinuation was defined as a gap of ≥ 61 days between the projected end date of one prescription (defined as the date of issue + days supplied) and the date of the next prescription issued to the patient (should one exist), with the date of discontinuation defined as the end date of the prescription immediately prior to the discontinuation.
Type of inhaler device prescribed was described at index, as well as during the first and second year of follow-up among those prescribed further inhalers.
Healthcare utilization (HCU)
All primary care consultations with a non-administrative healthcare professional together with those that were specifically asthma-related (i.e. an asthma Read code recorded against it) were identified. Hospitalizations, as recorded in primary care records, were identified for all-cause and asthma-related admissions. Short-acting bronchodilator (SABD) use was described as the number of SABD devices prescribed by primary care.
Asthma exacerbations were defined as an asthma-related emergency department (ED) visit/hospital admission or requirement for OCS treatment (Citation17). Additional exacerbations were required to be at least 7 days after the end of an OCS course or discharge date from ED/hospital to be considered a separate event.
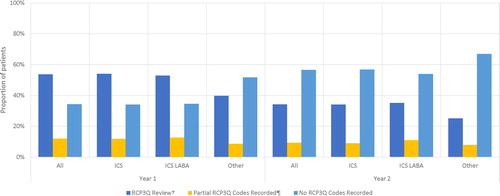
In addition to HCU assessments, the number of patients having a RCP3Q assessment was documented for year 1 and year 2 of follow-up. The RCP3Q is a component of the asthma annual review within the Quality and Outcome Framework that was added in 2012, and is a pay for performance scheme for primary care practices in the UK (Citation18,Citation19). The three questions in RCP3Q relate to the impact of asthma on sleep, symptoms and usual activities; all 3 questions should be asked on the same date to count as a valid RCP3Q assessment. Valid RCP3Q assessments were identified using the methods stipulated in V38 of the QOF Business Rules (Citation20) or recording of a single Read code denoting administration of the RCP3Q.
Statistical analysis
Descriptive data were described for all patients and by index medication class as either mean (standard deviation [SD]) or median (interquartile range [IQR]) for continuous variables and the count and proportion of patients for categorical and ordinal variables.
Prescriptions for maintenance asthma medications subsequent to the index date were used to construct treatment sequences. The five most common maintenance medication class sequences for each index maintenance therapy group (ICS/LABA, ICS, other) up to the end of the 2-year follow-up period were reported, including the mean (SD) and median (IQR) time between changes in sequences. Within index groups, the proportion of patients (%) following each of the most common treatment patterns was calculated using the total population (i.e. total number of patients across all index therapies) as the denominator in order to report the commonality of a particular treatment pattern within this cohort of patients. Where there was insufficient information to determine the duration of MDI prescription length, data were imputed using an algorithm based on modal daily dose and quantity supplied for a particular product code (Citation21). Analysis of time to first therapy change was analyzed using Kaplan-Meier estimates of the survival function. The failure event was defined as the first occurrence of one of, discontinuation, augmentation, between-class switch or within-class switch (as defined above). Censoring occurred after two years of post-index follow-up; this was the upper limit of observation time, precluding further assessment of events in those yet to experience a change in treatment.
Results
Study population
A total of 55 293 patients fulfilled the study criteria and were included in the analysis (), the majority of whom were prescribed ICS as asthma maintenance medication at the index date (n = 46 297, 84%) (). Of the 629 patients who were prescribed other medications, most were prescribed LTRA (n = 610). Whilst all patients had not received an asthma maintenance treatment in the baseline year, approximately 70% had previously been prescribed a maintenance therapy (; Table S1, supplementary material).
Table 1. Baseline demographic and clinical characteristics.
For the total population, the mean age was 37 years, 56% were female. Compared with the other groups, patients in the ICS/LABA group were older (mean age 43 years) and, consistent with this, had a higher reported incidence of cardiovascular disease, bronchitis/bronchopneumonia, and metabolic disorders ().
Asthma treatment patterns during the 2-year follow up period
Across groups, the most common treatment sequence following index therapy was discontinuation of maintenance treatment with no further prescriptions recorded up to the end of the 2-year observation period (; ). Other common treatment sequences generally showed patterns of intermittent use, comprising periods of maintenance therapy interspersed with ‘no’ therapy. The proportion of individuals following a given treatment sequence by age group and by no known previous maintenance therapy was calculated for the most common sequences in each index therapy class (see Table S2 and Table S3, respectively, supplementary material). The distribution of age and prior exposure to maintenance therapy within the most common sequences generally reflected that of the relevant index class. The time to first treatment change was later in the ICS group (; ).
Figure 2. Five most common treatment sequences by index asthma maintenance medication group.
Proportions were calculated using the total number of patients across all index therapies as the denominator (n = 55,293); durations above each arrow denote the mean time spent in a given treatment class by patients with that treatment sequence; total sequence durations may not sum to the same value due to rounding and leap years.
ICS: inhaled corticosteroid; ICS/LABA: inhaled corticosteroid/long-acting beta2-agonist
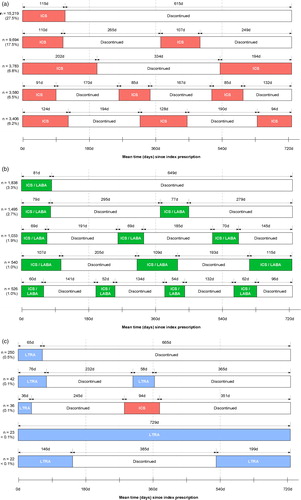
Figure 3. Survival analysis of time to first change in therapy (days) by index asthma maintenance medication group.
For description of first event, see . Other included leukotriene receptor antagonists (LTRA) and methylxanthines. ICS: inhaled corticosteroid; ICS/LABA: inhaled corticosteroid/long-acting beta2-agonist
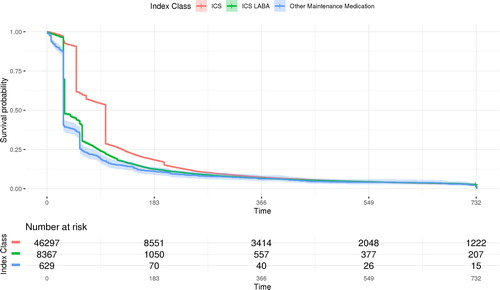
Table 2. Summary of time to first medication change and therapy change event by index treatment class.
Treatments prescribed during year 1 and year 2 post-index are summarized in and Table S4 (supplementary material). In the total population, 19% of patients in year 1 and 13% in year 2 were prescribed OCS, mostly for intermittent use, and the proportion was higher in the ICS/LABA (24% and 18%, respectively) than the ICS (18% and 12%) and Other (21% and 12%) groups. Approximately a quarter of patients were prescribed at least four SABD inhalers during the first year of follow-up ().
Figure 4. Proportion of patients prescribed ≥ 4 SABD cannisters during baseline and follow-up by index asthma maintenance medication group.
Other included leukotriene receptor antagonists (LTRA) and methylxanthines.
ICS: inhaled corticosteroid; ICS/LABA: inhaled corticosteroid/long-acting beta2-agonist
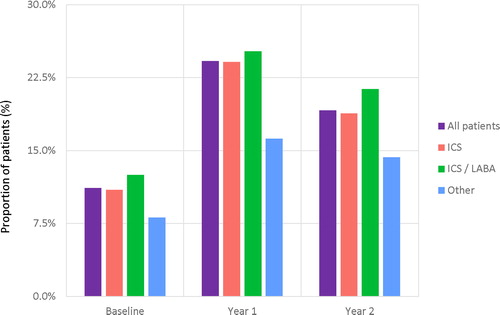
Table 3. Asthma maintenance therapies prescribed during 2-year follow-up by index asthma maintenance medication group.
During year 1 and 2 of follow up, most patients remained on the same device with respect to their maintenance therapy (Table S5, supplementary material).
Healthcare utilization
Over the 2-year follow-up period over 90% patients across groups had three or more primary care consultations per year (all-cause) (Table S6, supplementary material). Asthma-related consultations were also prevalent with 59% and 36% of patients requiring ≥ 3 visits during year 1 and year 2, respectively (Supplementary Figure S1a). During year 1, the mean (SD) exacerbation rate was 0.19 (0.5), notably higher for patients in the ICS/LABA group (0.27 (0.61) compared with the ICS (0.18 (0.48)) and Other (0.16 (0.45)) groups and a similar pattern was observed for the proportion of patients experiencing at least one exacerbation in that year (ICS: 14%; ICS/LABA: 20%; Other: 13%; Figure S1b, supplementary material). There was an attrition in exacerbations in year 2. In general hospitalizations, as recorded in primary care, were low across groups.
During year 1 post-index, 54% of patients had a valid RCPQ3 assessment i.e. were asked all three questions relating to asthma control on the same day, whilst a third of patients had no RCP3Q codes recorded (not asked any of the questions) (). In year 2, the proportion of patients with a valid assessment had decreased to 34% with over half of all patients (57%) having no RCP3Q codes recorded.
Discussion
This retrospective cohort study in non-active users of asthma maintenance therapy using a ‘new user’ study design demonstrated a pattern of intermittent use of maintenance therapies in the 2-year follow-up period. Many patients who had ≥ 3 primary care consultations for their asthma were often prescribed OCS and multiple prescriptions (≥ 4) for SABD over each 1-year period, and many had ≥ 1 exacerbations per year, indicating a potential tendency for underuse of maintenance therapies and overuse of SABD by the population studied. Although time to first treatment change occurred later in the ICS group, this was likely driven by inhaler device, as MDIs tend to contain a greater number of doses per unit in comparison to DPIs or oral medication, and they will be assumed to cover patients for a longer period of time. The relatively low proportion of patients having an annual RCP3Q assessment, may suggest that many primary care providers utilized a reactive rather than a proactive asthma management paradigm; however, elucidating the underlying reasons for this observation are not possible with the available data.
Whilst a strength of the current study was that it clearly demonstrated intermittent treatment patterns in a longitudinal design, these data could not determine how patients were taking their prescribed maintenance therapy – for example, whether they underused and stockpiled medication – only that there were periods of prescription absences. As suggested by other surveys (Citation23–25), patients may have been using their medication irregularly over an extended period of time, stopping and starting their treatment when their symptoms felt better or worse, respectively.
Our study focused on those who were being ‘stepped up’ to asthma maintenance therapy, as these patients represent a group who have objectively experienced an acute or sub-acute worsening of symptoms. This contrast with the approach taken by Gayle et al. (Citation22) who considered all asthmatics, regardless of severity, and the vast majority of individuals were at BTS/SIGN step 0 or step 1 which may have masked the treatment needs and trajectories of those with more severe asthma.
The pattern of intermittent maintenance therapy use observed in this study is consistent with findings in previous questionnaire-based surveys. In a survey of 2686 participants with asthma in Australia, of the 1601 participants using ICS or ICS/LABA inhalers, 43.2% reported using them less frequently than five days a week, and 30.5% less than weekly (Citation23). Similarly, in the pan-European REALIZE online survey, of the respondents who stated that they had a maintenance therapy inhaler (n = 3,481), over half did not use it every day as prescribed (Citation24). The latest results of the European Community Respiratory Health Survey (ECRHS III) showed that whilst there has been an increase in the use of asthma medication in the last 20 years, only 34% of subjects with persistent asthma were taking ICS on a regular basis (Citation25). This irregular use of maintenance therapies has clinical consequences. Approximately a fifth of patients in our survey had at least one asthma exacerbation requiring OCS in the first year of follow up, slightly lower than the incidence reported in the Web-based surveys (range 29%−44% in the last 12 months) (Citation22–24) but these were based on broader asthma populations in terms of their treatment and study designs. As our study solely utilized primary care data – and was not linked to Hospital Episode Statistics (HES) – there was the potential for incomplete acquisition of hospitalizations, exacerbations and emergency department visits. In our cohort (who were aged ≥ 6 years), 12.6%, 15.3% and 11.3% had ≥ 1 exacerbation during the baseline period, year 1 and year 2 respectively; accompanying crude rates (per person, per year) were 0.15, 0.19 and 0.14. It should be noted that index date is included in Year 1, which may mean that the increased rate of events during this year is attributable to the acute worsening of symptoms that prompted the change in asthma therapy. In a recent study utilizing CPRD-HES data, crude exacerbation rates of patients deemed to be of a non-atopic, non-obese phenotype were estimated at ∼0.20 and ∼0.39 for those receiving reliever and maintenance therapy respectively (Citation26). Another study estimated that 13.7% of asthma patients of all severities experience ≥ 1 exacerbation over the period of a year using the UK and US databases (8.4% and 12.5% patients with asthma had ≥ 1 exacerbation over the period of a year, respectively) (Citation17).
Having a history of even one previous exacerbation is associated with an increased risk of a subsequent exacerbation (Citation27–30). Two UK database studies in adults and adolescents with asthma showed that having one or more acute courses of OCS in the previous year was the single best predictor of having two or more subsequent exacerbations, increasing the odds by more than three-fold (Citation28,Citation29). Similar data in children showed that a past history of asthma attacks was the best predictor of future attacks (Citation30). An observational retrospective cohort study in 19 126 Canadian asthmatic patients found that, compared with non-adherent patients, those who were adherent to salmeterol/fluticasone had a significantly lower risk of exacerbations, assessed both by compliance (medication possession ratio ≥80%; risk reduction of 52%) and persistence (prescriptions of the ongoing therapy being continuously renewed without a gap of more than 30 days; risk reduction of 58%) (Citation31). These data and this study reiterate the importance of regular assessment to ensure patients are on the most appropriate therapy for them.
A substantial proportion of patients in this study were prescribed ≥ 4 SABD inhalers/year during the 2-year follow-up, also a risk factor for severe exacerbations (Citation32). An insufficient prescription of ICS and over-prescription of SABD were identified as key risk factors for asthma death in the National Review of Asthma Deaths in 2014 (Citation33). An audit of 50 UK practices identified the continuing presence of risk factors including excessive SABD and insufficient uptake on ICS prescriptions and failures to issue personal asthma action plans, highlighting an urgent need for a more proactive model of care (Citation34). Our findings are compatible with those of the Asthma UK survey which showed that 60% of 10 500 patients still do not receive basic asthma care comprising three elements: an annual asthma review, a written asthma action plan and an inhaler technique check with a healthcare professional (Citation35).
This study has some limitations. Patients who were not continuously registered at a CPRD-contributing practice would not have had their full prescription history available. Although the study aimed to investigate patients that were ‘newly’ prescribed maintenance asthma medications, only 30% had no evidence of maintenance therapy in their available history. However, the study design permitted the evaluation of a large sample of patients that were representative of asthma patients in the UK in a real-world setting. We have not attempted to compare RCP3Q score between years; it is likely that such a comparison would be subject to a range of biases. For patients who are well controlled, the assessment may take place at a routine checkup, whereas RCP3Q may be utilized as a response to an acute or subacute worsening of symptoms in those who have not attained control. Finally, investigating any associations between prescription patterns and patient outcomes was beyond the scope of this analysis. In a future study on treatment patterns, it would be interesting to superimpose patterns for maintenance, OCS and SABD therapy to determine any trends of usage in relation to each other as well as assessing what association observed treatment patterns have with scheduled and unscheduled HCU.
Conclusion
In conclusion, this study demonstrated that in previously non-active users of asthma maintenance medication subsequently commenced on maintenance therapy, intermittent use was common during the 2-year follow-up despite the potential need for regular use as evidenced by patient HCU and SABD usage patterns. Outside of a patient’s annual review, patient treatment patterns are driven by self-management and patients should be trained in the essential skills of understanding their asthma, how to maintain asthma control and medication adherence. These findings highlight the need for regular patient assessment and education to ensure patients are taking appropriate medications to maintain asthma control, to monitor their SABD usage as a potential proxy for poor symptom control, check their inhaler technique, and to empower patients to be an active partner in managing their asthma therapy.
Declaration of interest
The protocol for this research was approved by the Independent Scientific Advisory Committee (ISAC) of the Medicines and Healthcare Products Regulatory Agency (protocol number 19_128R), and the approved protocol was made available to the journal and reviewers during peer review.
At the time of study conduct, DH was an employee of GSK and held GSK shares. SBF has received an unrestricted research grant funding and personal fees from Teva Pharmaceuticals for expert contribution to advisory boards and expert presentations; and personal fees from AstraZeneca, Boehringer Ingelheim, GSK and Sanofi for expert contribution to advisory boards and expert presentations. DP has board membership with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Mylan, Mundipharma, Napp, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Teva Pharmaceuticals; consultancy agreements with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mylan, Mundipharma, Napp, Novartis, Pfizer, Teva Pharmaceuticals, Theravance; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AKL Research and Development Ltd, AstraZeneca, Boehringer Ingelheim, British Lung Foundation, Chiesi, Circassia, Mylan, Mundipharma, Napp, Novartis, Pfizer, Regeneron Pharmaceuticals, Respiratory Effectiveness Group, Sanofi Genzyme, Teva Pharmaceuticals, Theravance, UK National Health Service, Zentiva (Sanofi Generics); payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Mylan, Merck, Mundipharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Sanofi Genzyme, Teva Pharmaceuticals; payment for manuscript preparation from Mundipharma, Teva Pharmaceuticals; payment for the development of educational materials from Mundipharma, Novartis; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Circassia, Mundipharma, Napp, Novartis, Teva Pharmaceuticals; funding for patient enrollment or completion of research from Chiesi, Novartis, Teva Pharmaceuticals, Zentiva (Sanofi Generics); stock/stock options from AKL Research and Development Ltd which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 74% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); and is peer reviewer for grant committees of the Efficacy and Mechanism Evaluation program, and Health Technology Assessment.
Supplemental Material
Download Zip (260 KB)Acknowledgements
The authors are also grateful to Dr Michael Gibbs for providing a review of an early draft of this manuscript.
Availability of data and materials
Information on GSK’s data sharing commitments and requesting access can be found at: https://www.clinicalstudydatarequest.com
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. The Lancet. 2018;391(10122):783–800. doi:10.1016/S0140-6736(17)33311-1.
- Global I. for Asthma. Global Strategy for Asthma Management and Prevention; 2018 [Accessed 28 May. 2019; https://www.ginaasthma.org.
- Kant S. Socio-economic dynamics of asthma. Indian J Med Res. 2013;138(4):446–448.
- British Thoracic Society, Scottish Intercollegiate Guidelines Network. British Guideline on the Management of Asthma – A National Clinical Guideline. 2016. Available from: https://www.brit-thoracic.org.uk/standards-of-care/guidelines/btssign-british-guideline-on-the-management-of-asthma [last accessed 28 May 2019].
- National Heart, Lung, and Blood Institute. Guidelines for the Diagnosis and Management of Asthma (EPR-3. ). Bethesda: National Institutes for Health; 2007. Available from: https://www.nhlbi.nih.gov/health-topics/guidelines-for-diagnosis-management-of-asthma [accessed 28 May 2019].
- National Asthma Council Australia. Australia’s National Guidelines for Asthma Management. 2018. Available from: https://www.asthmahandbook.org.au/management/adults [accessed 28 May 2019].
- Busse WW, Pedersen S, Pauwels RA, Tan WC, Chen Y-Z, Lamm CJ, O'Byrne PM, on behalf of the START Investigators Group The Inhaled Steroid Treatment As Regular Therapy in Early Asthma (START) study 5-year follow-up: Effectiveness of early intervention with budesonide in mild persistent asthma. J Allergy Clin Immunol. 2008;121(5):1167–1174.
- Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, Pedersen SE. GOAL Investigators Group. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004;170(8):836–844. doi:10.1164/rccm.200401-033OC.
- Small M, Anderson P, Vickers A, Kay S, Fermer S. Importance of inhaler-device satisfaction in asthma treatment: real-world observations of physician-observed compliance and clinical/patient-reported outcomes. Adv Therapy. 2011;28(3):202–212. doi:10.1007/s12325-010-0108-4.
- Price DB, Colice G, Israel E, Roche N, Postma DS, Guilbert TW, van Aalderen WMC, Grigg J, Hillyer EV, Thomas V, et al. Add-on LABA in a separate inhaler as asthma step-up therapy versus increased dose of ICS or ICS/LABA combination inhaler. ERJ Open Res. 2016;2(2):00106-2015–2015. doi:10.1183/23120541.00106-2015.
- Rank MA, Branda ME, McWilliams DB, Johnson SK, Samant SA, Podjasek JC, Park MA, Volcheck GW. Outcomes of stepping down asthma medications in a guideline-based pediatric asthma management program. Ann Allergy Asthma Immunol. 2013;110(5):354–358.e2. doi:10.1016/j.anai.2013.02.012.
- Rank MA, Liesinger JT, Branda ME, Gionfriddo MR, Schatz M, Zeiger RS, Shah ND. Comparative safety and costs of stepping down asthma medications in patients with controlled asthma. J Allergy Clin Immunol. 2016;137(5):1373–1379. doi:10.1016/j.jaci.2015.08.035.
- Turner SW, Richardson K, Burden A, Thomas M, Murray C, Price D. Initial step-up treatment changes in asthmatic children already prescribed inhaled corticosteroids: a historical cohort study. NPJ Prim Care Respir Med. 2015;25:15041doi:10.1038/npjpcrm.2015.41.
- Turner S, Richardson K, Murray C, Thomas M, Hillyer EV, Burden A, Price DB. Long-acting beta-agonist in combination or separate inhaler as step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. J Allergy Clin Immunol Pract. 2017;5(1):99–106. doi:10.1016/j.jaip.2016.06.009.
- Clinical Practice Research Datalink. Available from www.cprd.com/home.
- Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, Smeeth L. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol. 2015;44(3):827–836. doi:10.1093/ije/dyv098.
- Suruki RY, Daugherty JB, Boudiaf N, Albers FC. The frequency of asthma exacerbations and healthcare utilization in patients with asthma from the UK and USA. BMC Pulm Med. 2017;17(1):74. doi:10.1186/s12890-017-0409-3.
- Pearson MG, Bucknall CE. Measuring clinical outcome in asthma: a patient focused approach. London: Royal College of Physicians; 1999. doi:10.1046/j.1365-2753.2002.00346.x.
- Pinnock H, Burton C, Campbell S, Gruffydd-Jones K, Hannon K, Hoskins G, Lester H, Price D. Clinical implications of the Royal College of Physicians three questions in routine asthma care: a real-life validation study. Prim Care Respir J. 2012; 21(3):288–294. doi:10.4104/pcrj.2012.00052.
- Quality and Outcomes Framework (QOF) business rules v 38 2017-2018 October code release; October 2018. Available from: https://digital.nhs.uk/data-and-information/data-collections-and-data-sets/data-collections/quality-and-outcomes-framework-qof/quality-and-outcome-framework-qof-business-rules/quality-and-outcomes-framework-qof-business-rules-v-38-2017-2018-october-code-release [last accessed 3 July 2019].
- Joint Formulary Committee. British National Formulary (online) London: BMJ Group and Pharmaceutical Press. Available from: https://www.medicinescomplete.com [last accessed 15 February 2019].
- Gayle A, Tebboth A, Pang M, Guelfucci F, Argoubi R, Sherman S, Mak V. 2014. Real-life prescribing of asthmatic treatments in UK general practice over time using 2014. BTS/SIGN steps. NPJ Prim Care Respir Med. 2019;29:25. doi:10.1038/s41533-019-0137-7.
- Reddel HK, Sawyer SM, Everett PW, Flood PV, Peters MJ. Asthma control in Australia: a cross-sectional web-based survey in a nationally representative population. Med J Aust. 2015;202(9):492–498. doi:10.5694/mja14.01564.
- Price D, Fletcher M, van der Molen T. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med. 2014;24:14009. doi:10.1038/npjpcrm.2014.9.
- Janson C, Accordini S, Cazzoletti L, Cerveri I, Chanoine S, Corsico A, Ferreira DS, Garcia-Aymerich J, Gislason D, Nielsen R, et al. Pharmacological treatment of asthma in a cohort of adults during a 20-year period: results from the European Community Respiratory Health Survey I, II and III. ERJ Open Res. 2019;5(1):00073-2018–2018. doi:10.1183/23120541.00073-2018.
- Nissen F, Douglas IJ, Müllerová H, Pearce N, Bloom CI, Smeeth L, Quint JK. Clinical profile of predefined asthma phenotypes in a large cohort of UK primary care patients (Clinical Practice Research Datalink). JAA. 2019;12:7–19. doi:10.2147/JAA.S182013.
- Miller MK, Lee JH, Miller DP, Wenzel SE. Recent asthma exacerbations: a key predictor of future exacerbations. Respir Med. 2007;101(3):481–489. doi:10.1016/j.rmed.2006.07.005.
- Price D, Wilson AM, Chisholm A, Rigazio A, Burden A, Thomas M, King C. Predicting frequent asthma exacerbations using blood eosinophil count and other patient data routinely available in clinical practice. J Asthma Allergy. 2016;9:1–12. doi:10.2147/JAA.S97973.
- Blakey JD, Price DB, Pizzichini E, Popov TA, Dimitrov BD, Postma DS, Josephs LK, Kaplan A, Papi A, Kerkhof M, et al. Identifying Risk of Future Asthma Attacks Using UK Medical Record Data: A Respiratory Effectiveness Group Initiative. J Allergy Clin Immunol Pract. 2017;5(4):1015–1024. doi:10.1016/j.jaip.2016.11.007.
- Turner SW, Murray C, Thomas M, Burden A, Price DB. Applying UK real-world primary care data to predict asthma attacks in 3776 well-characterised children: a retrospective cohort study. NPJ Prim Care Respir Med. 2018;28(1):28doi:10.1038/s41533-018-0095-5.
- Ismaila A, Corriveau D, Vaillancourt J, Parsons D, Stanford R, Su Z, Sampalis JS. Impact of adherence to treatment with fluticasone propionate/salmeterol in asthma patients. Curr Med Res Opin. 2014;30(7):1417–1425. doi:10.1185/03007995.2014.908827.
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention; 2019. Available from: https://www.ginaasthma.org [last accessed 3 July 2019].
- Royal College of Physicians National review of asthma deaths Why asthma still kills report and key recommendations. http://www.rcplondon.ac.uk/projects/national-review-asthma-deaths [last accessed 20 May 2019].
- Levy ML, Garnett F, Kuku A, Pertsovskaya I, McKnight E, Haughney J. A review of asthma care in 50 general practices in Bedfordshire, United Kingdom. NPJ Prim Care Respir Med. 2018;28:29. doi:10.1183/13993003.congress-2018.PA4205.
- Asthma UK. 2009. ‘The reality of asthma care in the UK’. Annual Asthma Survey 2018 Report. https://www.asthma.org.uk/support-us/campaigns/publications/survey/ [last accessed 20 May 2019].