Abstract
Objective: The prevalence of asthma among elite endurance athletes is high, but less is known about the incidence of asthma among athletes. The aim of this study was to examine the incidence rate of physician-diagnosed asthma among elite endurance athletes.
Method: An annual postal questionnaire was sent to an open prospective cohort of elite endurance athletes between 2011 and 2015. Athletes from Swedish National teams, students at universities with elite sport partnership, and pupils at Swedish National elite sport schools, competing in cross-country skiing, biathlon, ski orienteering, or orienteering were invited (n = 666). Incidence rate of physician-diagnosed asthma was calculated among those without asthma at baseline (n = 449). Risk factors for incident physician-diagnosed asthma were identified using a multivariate logistic regression analysis.
Results: Response rate was 88.7% (n = 591) at baseline. The median age of participants was 17 (range 15–36) years at inclusion. The study population included 407 (69%) skiers and 184 (31%) orienteers. The prevalence of asthma at baseline was 23.9% (n = 141). Incidence rate (95% confidence interval [CI]) of physician-diagnosed asthma was 61.2 (45.7–80.3) per 1,000 person-years. Risk factors (odds ratio [OR (95% CI)]) for incident physician-diagnosed asthma were family history of asthma (1.97 [1.04–3.68]), being a skier (3.01 [1.42–7.21]), and wheezing without having a cold (4.15 [1.81–9.26]).
Conclusion: The incidence rate of physician-diagnosed asthma is high among Swedish elite endurance athletes.
Introduction
The prevalence of physician-diagnosed asthma in a Swedish general population older than 15 years of age has been estimated to 8.3–13.3% (Citation1,Citation2). The prevalence of asthma among endurance athletes is higher but varies widely, depending on study and sport (Citation3,Citation4). In 1993, 33% out of 42 Swedish cross-country skiers were reported to have had asthma-like symptoms and bronchial hyper-reactivity (BHR), and 31% had physician-diagnosed asthma (Citation5). Almost 20 years later, the prevalence of physician-diagnosed asthma among Swedish adolescent elite skiers and orienteers has been estimated at 29% and 17%, respectively (Citation6).
The increased prevalence of asthma among endurance athletes has mostly been attributed to increased ventilation during exercise, leading to osmotic changes and epithelial damage (Citation7,Citation8). Environmental factors, such as the dry air in winter sports, also are believed to enhance the harmful effect on the airways and may increase the osmotic effect during high ventilation. In aquatic sports, chlorine compounds in swimming pools may lead to increased airway inflammation and epithelial damage due to oxidative stress (Citation9,Citation10).
The incidence rate of asthma among adults in Sweden has been estimated at 1.4–3.9 per 1,000 person-years (Citation11–13) and 1.9 per 1,000 person-years in a population 16–35 years of age (Citation11). There are a few small longitudinal observational studies (n = 3–42) on respiratory health among endurance athletes; some show increased airflow limitation (Citation14,Citation15) or airway inflammation (Citation16), and one study did not show any lung function impairment (Citation17) during the observation period. However, to the best of our knowledge, there are no published studies on the incidence of asthma among endurance athletes.
The aim of this study was to estimate the incidence rate of self-reported, physician-diagnosed asthma in a population of elite endurance athletes, consisting of skiers and orienteers. A secondary objective was to assess risk factors for incident physician-diagnosed asthma in this population.
Material and methods
Study design
This study was based on a prospective annual postal survey conducted in Sweden from 2011 to 2015 (6). The study was approved by the Regional Ethical Review Board, Umeå. Written informed consent was obtained from each participant.
Study population
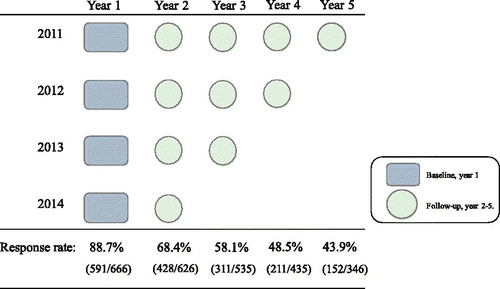
The study population was composed of Swedish elite endurance athletes competing in skiing (cross-country skiing, biathlon, or ski-orienteering) and orienteering. Athletes were eligible for inclusion if they belonged to one of the following four groups: (1) the Swedish National teams, (2) National elite upper secondary sport schools, (3) students with elite sport contracts at one of three universities, or (4) National top-80 ranking in orienteering in 2011. The study cohort was open for inclusion annually from 2011 to 2015, and each study participant received a yearly questionnaire in August–September (). In total, 666 unique athletes were invited to participate.
Questionnaire and study variables
The athletes received a postal questionnaire based on the European Community Respiratory Health Survey II (ECRHS II) questionnaire (Citation18), with additional questions about amount of training (hours/week) and main sport. One reminder was sent to non-responders.
Definitions of study variables were as follows. Physician-diagnosed asthma: “Have you ever had asthma?” and “Was it diagnosed by a doctor?” Nasal allergies: “Do you have any nasal allergies, including hay fever?” Family history of asthma: “Have any of your parents or siblings had asthma?” Shortness of breath: “Have you had an attack of shortness of breath that came following strenuous activity at any time in the last 12 months?” Wheeze without having a cold: “Have you had wheezing or whistling in your chest at any time in the last 12 months?” and “Have you had this wheezing or whistling without having a cold?” Training: “On average during the last 12 months, how many hours/week did you exercise so much that you got out of breath or became sweaty?”
Statistics
Population at risk (PAR) was defined as participants without physician-diagnosed asthma at baseline (at inclusion of the study = year 1). Incidence rate of physician-diagnosed asthma among the PAR was calculated by dividing the number of athletes that fulfilled the study criteria for physician-diagnosed asthma during follow-up, that is, number of incident cases of asthma, by the summarized time at risk in person-years. Athletes in the PAR contributed with 1 person-year for every year of follow-up without being diagnosed with asthma. An athlete diagnosed with asthma contributed with 0.5 person-years that year, since the exact date of the diagnosis was unknown. Incidence rate was expressed per 1,000 person-years. Participating athletes who neither answered the last questionnaire (year 2015), nor had incident physician-diagnosed asthma, were considered lost to follow-up. To analyze athletes lost to follow-up from the PAR, baseline characteristics of athletes in the PAR who were lost to follow-up were compared to those who completed the follow-up.
All statistical analyses were conducted using R statistical software (version 3.4.4) (Citation19). Baseline characteristics at inclusion among athletes were compared by Pearson’s χ2 for categorical variables and Student’s t-tests for continuous variables. When data was skewed, the Mann–Whitney U test was used instead of the Student’s t-test. P values <0.05 were considered significant.
Risk factors for incident physician-diagnosed asthma were identified using a generalized linear model with a binomial distribution with a logit link. The variable “sport” was grouped into skiing (cross-country skiing, biathlon, ski-orienteering) and orienteering (orienteering), based on season of competition (winter vs. summer sport). Variables included in the bivariate and multivariate logistic regression models were age, sex, family history of asthma, wheeze without having a cold, and sport (skiing vs. orienteering).
Results
The response rate was 88.7% (591/666) at baseline and decreased by year of follow-up (). The study population thus consisted of 407 skiers (279 cross-country skiers, 81 biathletes, and 47 ski orienteers) and 184 orienteers. In total, 107 athletes belonged to a national team at baseline. Median age was 17 (range 15–36) years.
At baseline, 141 (23.9%) reported physician-diagnosed asthma, and among these, 110 (18.6% of the total population) had used asthma medication during the last 12 months. Median (IQR) age at onset of asthma was 13 (11–15) years. One athlete did not answer the question regarding physician-diagnosed asthma and was excluded. Thus, the PAR consisted of 449 athletes. Respiratory symptoms, such as wheeze without having a cold and shortness of breath following exercise, were less common among athletes in the PAR compared to those with physician-diagnosed asthma at baseline, 8.2% vs. 37.4% and 5.4% vs. 30.5%, respectively (both p < 0.001). Also, nasal allergy and family history of asthma were less common in the PAR compared to those with physician-diagnosed asthma, 28.3% vs. 58.3% and 21.7% vs. 48.9% respectively (both p < 0.001) (). At baseline, 5 athletes reported less than 5 h of exercise per week, compared to 11 athletes at year 4 of follow-up.
Table 1. Basic characteristics at baseline (year 1) of the total study population, and comparison of athletes with and without physician-diagnosed asthma (asthma vs. PAR).
Incidence of physician-diagnosed asthma and risk factors
The study contributed with 849 person-years at risk, and during follow-up, 52 athletes were diagnosed with asthma. The incidence rate of physician-diagnosed asthma (95% confidence interval [CI]) was estimated at 61.2 (45.7–80.3) per 1,000 person-years. Among athletes with incident physician-diagnosed asthma, it was more common to be female (59.6% vs. 43.1%, p = 0.024), to have a family history of asthma (42.3% vs. 26.5%, p = 0.017), and to report wheeze without having a cold at baseline (23.5% vs. 6.2%, p < 0.001), compared to those who were not diagnosed with asthma during the observation period. There were no significant differences regarding nasal allergy or shortness of breath following exercise ().
Table 2. Basic characteristics of the population at risk at baseline (year 1) comparing incident cases of physician-diagnosed asthma (incident asthma) and subjects who remained without asthma diagnosis (no asthma) during follow-up.
Being a skier (compared to being an orienteer), having a family history of asthma, and reporting wheeze without having a cold in the previous 12 months were independently associated with incident physician-diagnosed asthma ().
Table 3. Risk factors at baseline for incident physician-diagnosed asthma, analyzed in bivariate and multivariate logistic regression models.
Baseline characteristics of skiers and orienteers
The orienteers were older than the skiers; median (interquartile range [IQR]) age 18 (16–22) vs. 17 (16–20), p = 0.036. Among the elite endurance athletes, the proportions of females were similar among skiers (46%) and orienteers (49%) (p = 0.577). At baseline, a higher proportion of skiers had physician-diagnosed asthma (24% vs. 13%, p < 0.001), reported wheeze without having a cold (18% vs. 8%, p = 0.002), and had more hours of training per week during the past year (median [IQR] 10 [8–13] vs. 8 [6–10], p < 0.001), compared to orienteers. Shortness of breath following exercise was reported by 12% of the skiers compared to 9% of the orienteers (p = 0.290). There were no significant differences in family history of asthma, or nasal allergy between skiers and orienteers (30% vs. 38% and 30% vs. 29%, p = 0.065 and p = 0.862), respectively.
Analysis of participants lost to follow-up
A total of 241 (53.7%) athletes of the PAR completed the follow-up, whereas the remaining 208 athletes were lost to follow-up during the study period. Although the proportion of females was higher among who completed the follow-up compared to those lost to follow-up, 50.6% vs. 38.5% (p = 0.010), the sex distribution did not differ significantly between the PAR at baseline and the group lost to follow-up (45.0 vs 38.5, p = 0.137). Furthermore, there were no significant differences regarding age, training, family history of asthma, nasal allergy, sport, wheeze without having a cold, or shortness of breath following exercise at baseline between the athletes who were lost to follow-up and those who completed the study ().
Table 4. Comparing athletes who completed follow-up and athletes who were lost to follow-up in the population at risk at baseline (year 1).
Discussion
In this large, prospective, five-year annual postal survey of Swedish elite endurance athletes, the incidence rate of self-reported, physician-diagnosed asthma during follow-up was high, it was estimated to 61 per 1,000 person-years. Family history of asthma, wheeze without having a cold, and skiing (when compared to orienteering) were independently associated with incident physician-diagnosed asthma.
In the present study, the incidence rate of physician-diagnosed asthma among elite endurance athletes was remarkably high in comparison to that of an adult general population (1.4–3.9 per 1,000 person-years) and a population of 16–35-year-olds (1.9 per 1,000 person-years) (Citation11–13). To the best of our knowledge, this study is the first large prospective study on the incidence of asthma among elite endurance athletes. However, the results are in line with previous studies in which different longitudinal aspects of asthma in athletes were investigated. In a small-scale study of Swiss elite triathletes (n = 7), bronchial reactivity increased yearly, and the athletes were postulated to develop BHR within a few years of a continued active career (Citation14). Among Finnish elite swimmers (n = 42), a five-year follow-up showed an increased eosinophilic inflammation among those athletes who were still active. In a study of Norwegian cross-country skiers, skiers older than 25 years of age had a higher frequency of BHR than younger skiers did (Citation20). Another Norwegian study showed that among cross-country skiers, the prevalence of physician-diagnosed asthma increased with increasing age, in contrast to a matched control group in which no such increase was observed (Citation21). We have recently shown that the age at onset of asthma among elite cross-country skiers is mainly during adolescence, a time that is usually characterized by an increased amount of training to pursue an elite career (Citation22). The present study shows that the incidence of asthma is also high during their career. The high incidence rate of physician-diagnosed asthma likely reflects the elite endurance athlete’s risk of developing asthma; however, it is unclear whether, and if so to what extent, increased awareness of asthma and diagnostic activity affect the results. An elite endurance athlete does have a high demand on ventilator capacity and may therefore experience more and tolerate less respiratory symptoms compared to a non-athlete; which may contribute to a lower threshold for seeking health care.
The risk factors for incident physician-diagnosed asthma were wheeze without having a cold, family history of asthma, and skiing (compared to orienteering). Wheeze is a common respiratory symptom in both the general population and an athletic population; it is a typical symptom of asthma, although not all who wheeze have asthma (Citation23–25). Family history of asthma is a recognized risk factor for asthma in the general population (Citation1,Citation26). Moreover, we recently published a study in which family history of asthma was associated with physician-diagnosed asthma among elite endurance athletes (Citation22). Skiing, compared to orienteering, was identified as a risk factor for incident physician-diagnosed asthma in our population. Both are endurance sports and thus associated with an increased risk of asthma; however, winter sports athletes may be at a greater risk because of the repeated and prolonged inhalation of cold, dry air. Others have also found that winter sport is a risk factor for the specific phenotype of sports asthma (Citation27).
In our study, asthma was defined as self-reported, physician-diagnosed asthma based on the validated ECRHS II questionnaire (Citation18,Citation28). Self-reported, physician-diagnosed asthma based on questionnaires is a common and widely used definition of asthma in epidemiological surveys. However, the lack of a standard definition of asthma in epidemiological studies has resulted in a variation of definitions of asthma that obviously limits the comparability of results between studies (Citation29). Furthermore, we defined our population at risk as athletes without physician-diagnosed asthma at baseline. However, our population at risk could still have asthma-like symptoms such as wheeze. In this setup, we cannot exclude that wheeze among athletes in the population at risk was a sign of asthma but still without a physician diagnosis. The study aim was to describe the incidence rather than relapse of asthma, therefore, all athletes with physician-diagnosed asthma were excluded at baseline, irrespective of whether they used asthma medication or not. Indeed, the definition of population at risk of incident asthma will affect the incidence rate, and this needs to be taken into account when interpreting the results (Citation12,Citation30).
In the present study, winter and summer sport athletes were pooled to obtain a larger population sample. We lacked power to perform a stratified analysis of winter and summer sports, mainly due to the limited number of participating orienteers. It should be recognized that, according to the proposed mechanisms of development of asthma in athletes, winter and summer athletes may be exposed to different amounts of airway stress due to different exposure to environmental irritants, airborne allergens, and cold air. In fact, skiers were at a higher risk of incident physician-diagnosed asthma in our study; however, previous studies show somewhat conflicting results regarding the importance of season of competition (Citation27,Citation31).
Study strengths were the high baseline response rate and prevalence of physician-diagnosed asthma. The high asthma prevalence is in line with previous studies and affirms that the study population is a representative selection of elite endurance athletes [3]. There are, however, also some limitations in our study that merit discussion. First, the diagnosis of asthma was based on questionnaire data, and we had no objective verification of asthma such as airflow variability, bronchial reversibility, or BHR. In fact, exercise-induced laryngeal obstruction (E-ILO) is an important differential diagnosis from asthma in athletes with exercise-related respiratory symptoms and has gained increased attention during the last decade. The two conditions are often similar in symptomatology and may also be prevalent at the same time (Citation23,Citation32). Second, we cannot exclude a recall bias, because the questionnaire was distributed only once a year. Third, the study lacks a control group. However, studies on incidence rate of asthma in general populations have used similar methodology allowing us to do some comparisons (Citation11–13). Fourth, there is a risk of selection bias, since athletes with asthma may be more prone to answer the questionnaire, yielding a risk for overestimation of the incidence rate. Even though the response rate was high at baseline, it declined during follow-up, which may introduce a risk of a selection bias. On the other hand, a response rate of 62% did not result in any substantial selection bias in another Swedish questionnaire study on respiratory health (Citation33). When comparing baseline data among those who were lost to follow-up with those who completed the study, there were no significant differences besides that a higher proportion of men than women were lost to follow-up; however, there was no significant difference in the sex distribution between the PAR at baseline and the group lost to follow-up. This indicates that the given results are representative for the whole study population, despite loss to follow-up during the study. Taking into account the specific study population, we consider our population to be fairly large. However, the study sample is still of limited size; consequently, the subgroup analyses should be interpreted cautiously.
Conclusion
The incidence rate of self-reported, physician-diagnosed asthma among elite endurance athletes was very high in this prospective postal questionnaire study. We believe that the high prevalence and incidence of asthma among athletes should be met with thorough diagnostic procedures within the health care. The results indicate that there is a need to evaluate primary preventive measures, especially among skiers. Future studies are also important to evaluate to what extent there is a remission of asthma among elite endurance athletes and whether it is possible to identify measures that contribute to increased remission.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of this article.
Supplemental Material
Download MS Word (1.7 MB)Acknowledgements
The authors would like to thank the coaches at the Swedish National elite sport schools for administration of the questionnaires and recruitment. This study was financially supported by the Visare Norr Fund, Northern County Councils’ Regional Federation, under grant 557141; and by the Research & Development Unit, Region Jämtland Härjedalen, under grant JLL-650771.
References
- Lotvall J, Ekerljung L, Ronmark EP, Wennergren G, Linden A, Ronmark E, Toren K, Lundback B. West Sweden Asthma Study: prevalence trends over the last 18 years argues no recent increase in asthma. Respir Res. 2009;10:94. PMC2772988. doi:10.1186/1465-9921-10-94.
- Backman H, Räisänen P, Hedman L, Stridsman C, Andersson M, Lindberg A, Lundbäck B, Rönmark E. Increased prevalence of allergic asthma from 1996 to 2006 and further to 2016-results from three population surveys. Clin Exp Allergy. 2017;47(11):1426–1435. doi:10.1111/cea.12963.
- Del Giacco SR, Firinu D, Bjermer L, Carlsen KH. Exercise and asthma: an overview. Eur Clin Respir J. 2015;2(1):27984. PMC4653278. doi:10.3402/ecrj.v2.27984.
- Carlsen KH. Exercise-induced asthma, respiratory and allergic disorders in elite athletes: epidemiology, mechanisms and diagnosis: part I of the report from the Joint Task Force of the European Respiratory Society (ERS) and the European Academy of Allergy and Clinical Immunology (EAACI) in cooperation with GA2LEN. Allergy. 2008;63(4):387–403. doi:10.1111/j.1398-9995.2008.01662.x.
- Larsson K, Ohlsen P, Larsson L, Malmberg P, Rydstrom PO, Ulriksen H. High prevalence of asthma in cross country skiers. BMJ. 1993;307(6915):1326–1329. PMC1679468. doi:10.1136/bmj.307.6915.1326.
- Norqvist J, Eriksson L, Soderstrom L, Lindberg A, Stenfors N. Self-reported physician-diagnosed asthma among Swedish adolescent, adult and former elite endurance athletes. J Asthma. 2015;52(10):1046–1053. doi:10.3109/02770903.2015.1038389.
- Anderson SD, Daviskas E. The mechanism of exercise-induced asthma is. J Allergy Clin Immunol. 2000;106(3):453–459. doi:10.1067/mai.2000.109822.
- Kippelen P, Anderson SD. Pathogenesis of exercise-induced bronchoconstriction. Immunol Allergy Clin North Am. 2013;33(3):299–312. vii doi:10.1016/j.iac.2013.02.002.
- Couto M, Kurowski M, Moreira A, Bullens DMA, Carlsen KH, Delgado L, Kowalski ML, Seys SF. Mechanisms of exercise-induced bronchoconstriction in athletes: Current perspectives and future challenges. Allergy. 2018;73(1):8–16. doi:10.1111/all.13224.
- Bougault V, Turmel J, St-Laurent J, Bertrand M, Boulet LP. Asthma, airway inflammation and epithelial damage in swimmers and cold-air athletes. Eur Respir J. 2009;33(4):740–746. doi:10.1183/09031936.00117708.
- Toren K, Ekerljung L, Kim JL, Hillstrom J, Wennergren G, Ronmark E, Lotvall J, Lundback B. Adult-onset asthma in west Sweden--incidence, sex differences and impact of occupational exposures. Respir Med. 2011;105(11):1622–1628. doi:10.1016/j.rmed.2011.06.003.
- Ekerljung L, Ronmark E, Larsson K, Sundblad BM, Bjerg A, Ahlstedt S, Dahlen SE, Lundback B. No further increase of incidence of asthma: incidence, remission and relapse of adult asthma in Sweden. Respir Med. 2008;102(12):1730–1736. doi:10.1016/j.rmed.2008.07.011.
- Toren K. A prospective study of asthma incidence and its predictors: the RHINE study. Eur Respir J. 2004;24(6):942–946. doi:10.1183/09031936.04.00044804.
- Knopfli BH, Luke-Zeitoun M, von Duvillard SP, Burki A, Bachlechner C, Keller H, Bacharach DW. High incidence of exercise-induced bronchoconstriction in triathletes of the Swiss national team. Br J Sports Med. 2007;41(8):486–491; discussion 491. PMC2465447. doi:10.1136/bjsm.2006.030569.
- Verges S, Flore P, Blanchi MP, Wuyam B. A 10-year follow-up study of pulmonary function in symptomatic elite cross-country skiers – athletes and bronchial dysfunctions. Scand J Med Sci Sports. 2004;14(6):381–387. doi:10.1111/j.1600-0838.2004.00383.x.
- Helenius I, Rytila P, Sarna S, Lumme A, Helenius M, Remes V, Haahtela T. Effect of continuing or finishing high-level sports on airway inflammation, bronchial hyperresponsiveness, and asthma: a 5-year prospective follow-up study of 42 highly trained swimmers. J Allergy Clin Immunol. 2002;109(6):962–968. doi:10.1067/mai.2002.124769.
- Kippelen P, Caillaud C, Robert E, Connes P, Godard P, Prefaut C. Effect of endurance training on lung function: a one year study. Br J Sports Med. 2005;39(9):617–621. PMC1725299. doi:10.1136/bjsm.2004.014464.
- European Community Respiratory Health Survey, IISC, The European Community Respiratory Health Survey II. Eur Respir J. 2002;20(5):1071–1079.
- Team, RC, R. A language and environment for statistical computing. Vienna, Australia: R Foundation for Statistical Computing, 2018. Available from: https://www.R-project.org/.
- Stensrud T, Mykland KV, Gabrielsen K, Carlsen KH. Bronchial hyperresponsiveness in skiers: field test versus methacholine provocation? Med Sci Sports Exerc. 2007;39(10):1681–1686. doi:10.1249/mss.0b013e31813738ac.
- Heir T, Oseid S. Self-reported asthma and exercise-induced asthma symptoms in high-level competetive cross-country skiers. Scand J Med Sci Sports. 2007;4(2):128–133. doi:10.1111/j.1600-0838.1994.tb00415.x.
- Eriksson LM, Irewall T, Lindberg A, Stenfors N. Prevalence, age at onset, and risk factors of self-reported asthma among Swedish adolescent elite cross-country skiers. Scand J Med Sci Sports. 2018;28(1):180–186. doi:10.1111/sms.12879.
- Johansson H, Norlander K, Alving K, Hedenstrom H, Janson C, Malinovschi A, Nordang L, Emtner M. Exercise test using dry air in random adolescents: temporal profile and predictors of bronchoconstriction. Respirology. 2016;21(2):289–296. doi:10.1111/resp.12682.
- Nystad W, Harris J, Borgen JS. Asthma and wheezing among Norwegian elite athletes. Med Sci Sports Exerc. 2000;32(2):266–270. doi:10.1097/00005768-200002000-00003.
- Weiss P, Rundell KW. Imitators of exercise-induced bronchoconstriction. Allergy Asthma Clin Immunol. 2009;5(1):7doi:10.1186/1710-1492-5-7. PMC2794850.
- Backman H, Hedman L, Jansson SA, Lindberg A, Lundback B, Ronmark E. Prevalence trends in respiratory symptoms and asthma in relation to smoking - two cross-sectional studies ten years apart among adults in northern Sweden. World Allergy Organ J. 2014;7:1–1. PMC3929247. doi:10.1186/1939-4551-7-1.
- Couto M, Stang J, Horta L, Stensrud T, Severo M, Mowinckel P, Silva D, Delgado L, Moreira A, Carlsen K-H. Two distinct phenotypes of asthma in elite athletes identified by latent class analysis. J Asthma. 2015;52(9):897–904. doi:10.3109/02770903.2015.1067321.
- Pekkanen J, Sunyer J, Anto JM, Burney P, S European Community Respiratory Health, Operational definitions of asthma in studies on its aetiology. Eur Respir J. 2005;26(1):28–35. doi:10.1183/09031936.05.00120104.
- Sa-Sousa A, Jacinto T, Azevedo LF, Morais-Almeida M, Robalo-Cordeiro C, Bugalho-Almeida A, Bousquet J, Fonseca JA. Operational definitions of asthma in recent epidemiological studies are inconsistent. Clin Transl Allergy. 2014;4:24. PMC4136946. doi:10.1186/2045-7022-4-24.
- Lundback B, Ronmark E, Jonsson E, Larsson K, Sandstrom T. Incidence of physician-diagnosed asthma in adults – a real incidence or a result of increased awareness? Report from The Obstructive Lung Disease in Northern Sweden Studies. Respir Med. 2001;95(8):685–692. doi:10.1053/rmed.2001.1126.
- Selge C, Thomas S, Nowak D, Radon K, Wolfarth B. Asthma prevalence in German Olympic athletes: A comparison of winter and summer sport disciplines. Respir Med. 2016;118:15–21. doi:10.1016/j.rmed.2016.07.008.
- Hanks CD, Parsons J, Benninger C, Kaeding C, Best TM, Phillips G, Mastronarde JG. Etiology of dyspnea in elite and recreational athletes. Phys Sportsmed. 2012;40(2):28–33. doi:10.3810/psm.2012.05.1962.
- Ronmark EP, Ekerljung L, Lotvall J, Toren K, Ronmark E, Lundback B. Large scale questionnaire survey on respiratory health in Sweden: effects of late- and non-response. Respir Med. 2009;103(12):1807–1815. doi:10.1016/j.rmed.2009.07.014.