Abstract
Objective: Asthma is frequently associated with chronic rhinosinusitis with nasal polyps (CRSwNP). Although endoscopic sinus surgery (ESS) improves asthma control in CRSwNP patients with asthma, the mechanism that underlies the response to surgical treatment is still unclear. We evaluated the relevance of changes in asthma control and changes in airway/systemic inflammation in eosinophilic CRSwNP patients with not well controlled asthma who underwent ESS.
Methods: We prospectively assessed changes in the asthma control questionnaire (ACQ) score, blood eosinophil counts (B-Eos), forced expiratory volume in 1 s (FEV1), and fraction of exhaled nitric oxide (FeNO) levels at 1-week before and 8 and 52 weeks after ESS.
Results: Twenty-five subjects were analyzed. The ACQ score, B-Eos, and FeNO decreased, and FEV1 increased significantly after ESS. In the period from baseline to 52 weeks after ESS, changes in ACQ were significantly correlated with the changes in blood eosinophil counts (r = 0.58, p<.01) and FeNO (r = 0.45, p<.05). Ten subjects (40%) showed consistently improved asthma control at 52-weeks after ESS. In the remaining subjects, although the ACQ score temporarily improved at 8-weeks after ESS, but eventually deteriorated at 52-weeks. Higher levels of total immunoglobulin E were associated with long-term improved asthma control after ESS.
Conclusions: In eosinophilic CRSwNP patients with asthma, sinus surgery impacts asthma control through the suppression of airway/systemic type 2 inflammation. The present study reinforced the common pathophysiology of type 2 inflammation between the upper and lower airways.
Introduction
Asthma is frequently associated with chronic rhinosinusitis (CRS) and a correlation between the two diseases has been confirmed (Citation1–3). CRS is a heterogenous disease and classified into CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP) according to the presence or absence of nasal polyps. CRSwNP, which is a refractory type of CRS, is more likely to be associated with asthma than those with CRSsNP. Approximately 20–60% of CRSwNP patients have comorbid asthma (Citation4–6), suggesting the presence of a correlation between CRSwNP and asthma.
Although several types of mechanisms between asthma and CRSwNP are considered, one of the underlying mechanisms between the two diseases is presumed to be a systemic type 2 inflammation. CRS with eosinophilic type and asthma share a common pathophysiology of eosinophil infiltration into the upper and lower airways through blood circulation of type 2 cytokine, such as interleukin (IL)-4, IL-5, and IL-13 (Citation7–9). Although recent articles and a meta-analysis described that endoscopic sinus surgery (ESS), which is an endoscopic surgical procedure to remove nasal polyps and clear the blockage of sinuses (Citation10), improved asthma control and reduced the frequency of asthma exacerbations in patients with CRS (Citation11–18), the mechanism is not well understood.
We hypothesized that changes in the asthma control evaluated by Asthma control questionnaires (ACQ) of the CRSwNP patients with asthma who underwent ESS were linked to changes in airway/systemic inflammation. In this prospective, longitudinal observational study, we evaluated the relevance of changes in asthma control and changes in type 2-mediated airway/systemic inflammation in eosinophilic CRSwNP patients with asthma who underwent ESS. Moreover, we made a comparison of characteristics of subjects with and without improvement in asthma control.
Methods
Study subjects
All the subjects were recruited from January 2008 to July 2013 at Wakayama Medical University Hospital. Although Asian CRSwNP patients are classified by eosinophilic type, neutrophilic type, and noneosinophilic nonneutrophilic type (Citation19), to focus on assessment of type 2 inflammation, only CRSwNP patients with eosinophilic type based on the pathological assessment were eligible for the present study. Eligibility criteria were as follows: (i) Subjects over 20 years old; (ii) satisfied the standard criteria for asthma (Citation20); (iii) diagnosis of CRSwNP based on the definition in the European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS) 2012 (Citation21) with nasal symptoms; (iv) infiltration of eosinophils into nasal polyps (more than 70 eosinophils per a microscopic field), and (v) not well controlled asthma (ACQ score >0.75). Exclusion criteria were as follows: (i) a clinical diagnosis of respiratory diseases other than asthma; (ii) a clinical diagnosis of eosinophilic lung disease such as eosinophilic granulomatosis with polyangiitis and allergic bronchopulmonary mycosis; (iii) not diagnosed with CRSwNP pathologically; (iv) current smoking, and/or necessity for alternation of asthma controller during follow up period; (v) necessity for administration of systemic corticosteroids within 2 weeks before the observational visit; and (vi) individuals judged unsuitable (i.e. poor treatment adherence). This study was approved by the ethics committee of Wakayama Medical University (institutional review board #526) and all patients gave written informed consent. The study was prospectively registered with the University Hospital Medical Information Network (UMIN) in Japan (protocol ID 000006299).
Study design
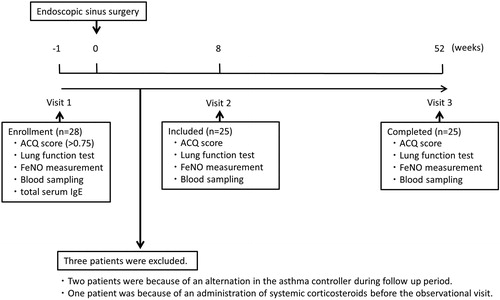
This was a prospective 53-week single-center, observational study (visits 1–3) (). Asthma patients with CRSwNP were enrolled at 1 week before ESS as the treatment for mitigation of nasal symptoms (i.e. nasal obstruction, olfactory impairment) and then attended for visits at 8 and 52 weeks after ESS. Various parameters were assessed at the visits.
Figure 1. Study design. At 1 week before ESS (Visit 1), ACQ score, lung function test, FeNO, blood sampling and total serum IgE were measured in 28 patients. Three patients were excluded. At 8 weeks after ESS (Visit 2) and 52 weeks after ESS (Visit 3), ACQ score, lung function test, FeNO and blood sampling were measured in the included 25 patients.
Evaluation of asthma control
The ACQ score, which is a validated tool for measurement of asthma control, was used for the assessment of asthma control in the present study (Citation22,Citation23). ACQ score < 0.75 was previously considered to indicate well controlled asthma (Citation24) and ACQ > 0.75 was thereby defined as not well controlled asthma in the present study. An ACQ score change of at least 0.50 has been previously defined as the minimal important difference (Citation25). Subjects were divided into two groups based on the change in ACQ score. We defined that those with a decrease in the ACQ score ≥ 0.5 at 52 weeks after ESS were the improved group and a decrease in the ACQ score < 0.5 were the unimproved group.
Blood sampling
Complete blood cell counts, differential counts of leukocytes, total serum immunoglobulin E (IgE) levels, and specific IgE for common inhaled allergens including house dust, mite, anthoxanthum odoratum, chamomile, ragweed, mugwort, aspergillus, candida, dog, and cat were examined using the ImmunoCAP system (Pharmacia Diagnostics, Uppsala, Sweden). Blood eosinophil counts 300/µL or more were considered as elevated according to the previous reports (Citation26,Citation27). Positive specific IgE (>0.7 UA/mL) to at least one allergen was assumed to confirm the presence of atopy.
Pulmonary function and exhaled nitric oxide measurement
Vital capacity (VC), forced vital capacity (FVC), and forced expiratory volume in 1 s (FEV1) were measured using a dry-rolling seal spirometer (CHESTAC-8800; Chest Co., Tokyo, Japan). The predictive values were estimated by the prediction formula of the Japanese Respiratory Society. The exhaled nitric oxide fraction (FeNO) was measured by a widely used electrochemical nitric oxide analyzer (NIOX MINO; Aerocrine, Solna, Sweden) as previously described (Citation28). FeNO 35 ppb or more was considered as elevated according to the previous reports (Citation26,Citation29).
Statistical analyses
For comparison between baseline characteristics and the characteristics after ESS, Wilcoxon signed rank sum test was used. The Spearman’s rank-order correlation coefficient analysis was performed to validate the correlations between change in ACQ score, change in blood eosinophil counts and in FeNO. For a comparison of subjects with and without improvement in asthma control, Mann-Whitney U-test was used. Repeated-measures analysis of variance (ANOVA) was used to compare variables between the three visits (at 1 week before ESS, 8 and 52 weeks after ESS). p<.05 was considered statistically significant.
All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan; http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmedEN.html; Kanda, 2012), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria, version 2.13.0) (Citation30).
Results
Subject characteristics at baseline and 52 weeks after ESS
A total of 28 patients were recruited for the study, and three patients were excluded; 2 were because of an alteration in the asthma controller during follow up period, and one was because of the administration of systemic corticosteroids within 2 weeks before the observational visit. Finally, 25 CRSwNP patients associated with not well controlled asthma (ACQ score> 0.75) were enrolled in our analysis. ESS was performed with conventional methods and the intervention was identical in all subjects. All the subjects received inhaled corticosteroids plus long-acting beta-agonist therapy. The characteristics of the baseline and 1 year after ESS are shown in . About one half of the patients were female and aged 53.8 ± 12.6 years and all subjects were atopic. The specific IgE against Staphylococcus aureus enterotoxin and a history of aspirin exacerbated respiratory disease (AERD) were not evaluated. Peripheral blood eosinophil counts and FeNO at baseline were elevated. ACQ score significantly decreased 52 weeks after ESS (p<.001). Blood eosinophil counts and FeNO significantly decreased 52 weeks after ESS (p<.0001). FEV1 and FEV1 percentage of predicted value significantly increased after ESS (p=.007 and p=.009).
Table 1. Characteristics of baseline and 1-year after sinus surgery.
Change in ACQ score and type 2 biomarkers at 8 and 52 weeks after ESS
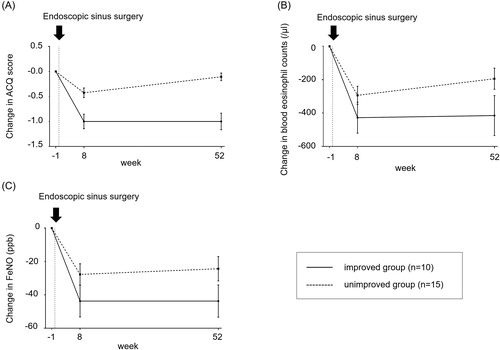
Ten (40%) patients were in the improved group, and the ACQ score was consistently improved at 8 and 52 weeks after ESS. Fifteen (60%) patients were in the unimproved group (). In the unimproved group, although the ACQ score temporarily improved at 8-weeks after ESS, it eventually deteriorated at 52-weeks after ESS. In the period from 8-weeks to 52-weeks after ESS, their type 2-inflammation biomarkers, such as blood eosinophil counts and FeNO changed in parallel with their ACQ score (). Repeated-measures ANOVA revealed significant changes in ACQ score (p<.01), blood eosinophil counts (p<.001), FeNO (p<.005), and percent predicted of FVC (p<.01) at 1 week before ESS, 8 and 52 weeks after ESS.
Figure 2. A–C: The short/long-term mean (standard deviation) changes in ACQ, blood eosinophil counts and FeNO from 1-week before endoscopic sinus surgery. Ten subjects (40%) were in the improved group, while 60% (n = 15) of the patients were in the unimproved group. The ACQ score temporally improved in the short-term even in the ACQ score unimproved group. Transitions in type 2 inflammation biomarkers from 8 weeks to 52 weeks after ESS were concordant with those in the ACQ score in the unimproved group.
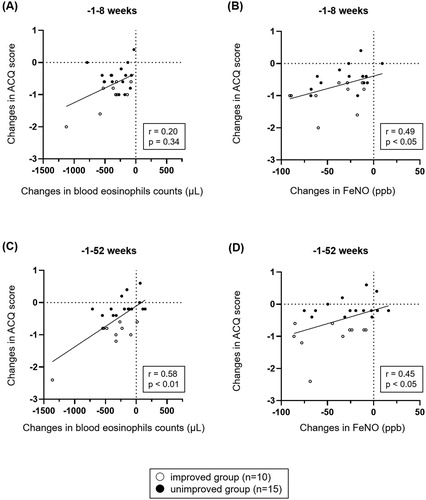
The short/long-term relevance of changes in ACQ and changes in type 2 airway/systemic inflammation
In the short-term after ESS, changes in ACQ score were not correlated with the changes in blood eosinophil counts (r = 0.20, p = .34, ), but significantly correlated with the changes in FeNO (r = 0.49, p<.05, ). In the long-term after ESS, changes in ACQ score were significantly correlated with the changes in blood eosinophil counts (r = 0.58, p<.01, ) and FeNO (r = 0.45, p<.05, ).
A comparison of characteristics of subjects with and without improvement in asthma control
In , the improved group showed significantly higher total IgE levels compared with the unimproved group (p<.027). No significant differences were seen in the patients’ characteristics at baseline, such as age, BMI, pulmonary function, ACQ score, peripheral blood eosinophil counts, and FeNO except for the total IgE level. Based on twice the reference value of serum IgE levels, patients divided into higher IgE group (baseline total IgE ≥ 601 IU/mL) and lower IgE group. In short/long-term after ESS, ACQ score in the higher IgE group decreased more than in the lower IgE group (). Compared the lower IgE group with the higher IgE group, except for baseline ACQ score and FEV1 none of the characteristics were significant. Moreover, no significant correlations were observed between changes in ACQ score after ESS and ACQ and FEV1 values at baseline (data not shown).
Figure 4. A and B: Changes in ACQ score from baseline to 8 weeks (A) and from baseline to 52 weeks (B) in subjects with baseline total IgE <601 IU/mL and ≥601 IU/mL after ESS. The data are presented as median (interquartile range). For the comparison, Mann-Whitney U-test was used. ACQ score in subjects with baseline total IgE ≥ 601 IU/mL were more improved than those with baseline total IgE < 601 IU/mL.
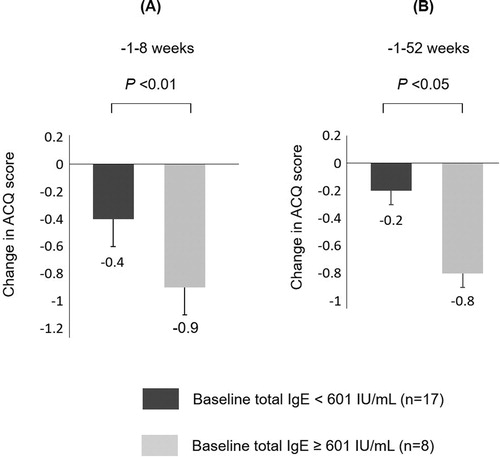
Table 2. A comparison of characteristics of subjects with and without improvement in asthma control.
Discussion
We verified our hypothesis that changes in asthma control were related to short/long-term changes in airway/systemic type 2 inflammation in eosinophilic CRSwNP patients with asthma who underwent ESS. To our knowledge, this is the first study demonstrating the short/long-term impact of sinus surgery on airway/systemic type 2 inflammation in asthma patients with eosinophilic CRSwNP. Recently, Jerschow et al. reported the short-term study that the type 2 inflammation, such as FeNO and blood eosinophil counts, were decreased after the sinus surgery in patients with AERD (Citation31). Because our study evaluated not only short-term but also evaluated long-term impact of ESS on type 2 inflammation, the present study reinforced the pathophysiology regarding the association and management of airway/systemic type 2 inflammation between the upper and lower airways.
Our study demonstrated strong correlation between asthma control and type 2 airway/systemic inflammation in eosinophilic CRSwNP patients with asthma after ESS. Recently, cluster analysis of nasal polyps confirmed that CRSwNP patients predominantly showed a type 2 molecular phenotype cytokines (Citation32), which led to hypothesize that type 2 cytokines from nasal polyp adversely affect asthma control in asthma patients with CRSwNP. In fact, one of the underlying pathophysiology between asthma and CRSwNP was elucidated to be a systemic type 2 inflammation (Citation7–9). Recently, the sinus surgery reduced serum periostin, a surrogate for type 2 inflammation, in asthma with eosinophilic CRSwNP (Citation33). Consequently, in the present study, improvement in both asthma control and type 2 airway/systemic inflammation after ESS is presumed to be due to reduction in type 2 cytokine after surgical removal of nasal tissues. We previously reported that nasal corticosteroids significantly improved ACQ score in asthma patients with allergic rhinitis (Citation34). Moreover, the study included correlations between the changes in FeNO and ACQ after nasal corticosteroid treatment (Citation34). In accordance with these findings, another previous report demonstrated that the severity of allergic rhinitis was correlated with asthma control (Citation35). Our results are in line with these previous studies. Considering these findings, surgical treatment for eosinophilic CRSwNP may have a potential to improve asthma control through suppression of type 2 inflammation. Interestingly, the ACQ score temporally improved after ESS in the short-term even in the unimproved group, although it cannot be denied that this might be due to temporal avoidance of exposures to a specific allergen in temporally improved subjects. However, subjects with these short-term improvements finally showed deteriorated asthma control in association with increased blood eosinophil counts and FeNO. In the temporally improved subjects, transitions in type 2 inflammation biomarkers (i.e. blood eosinophil counts and FeNO) from 8 weeks to 52 weeks after ESS were concordant with those in the ACQ score, suggesting the usefulness of monitoring of type 2 inflammation biomarkers for the prediction of asthma worsening after ESS.
Baseline total IgE level was associated with long-term improved asthma control after ESS. Our study found that the ACQ score showed greater improvements in the higher IgE group (baseline total IgE ≥ 601 IU/mL) than in the lower IgE group (baseline total IgE < 601 IU/mL). It cannot be denied that there was more room for improvement of ACQ score in the higher IgE group, as the higher IgE group showed significantly worse ACQ score and FEV1 at baseline compared with the lower IgE group. However, no correlations were observed between changes in ACQ score after ESS and ACQ and FEV1 values at baseline. Generally, the total IgE levels are recognized as an indicator of type 2 inflammation, while lower baseline total IgE level was associated with worse asthma control by ESS in our study. Remarkably, even in three patients with total serum IgE levels greater than 1500 IU/mL, which cannot be neutralized by anti-IgE antibody treatment, responded to the ESS surgery. This cannot be explained only by allergic response with T-helper cell type 2 cells. One plausible explanation might be that nonallergic eosinophilic airway inflammation, which originates from innate immunity of more refractory immune mechanism than allergic response, was dominant in the lower IgE group in our study. Nonallergic eosinophilic airway inflammation asthma, which is defined by the absence of serum IgE against specific antigens, has recently been considered more important in allergic diseases (Citation36). Nonallergic eosinophilic airway inflammation asthma involves group 2 innate lymphoid cells (ILC2), and ILC2 was elevated in chronic rhinosinusitis with nasal polyps (Citation37). Interestingly, some recent studies have reported that ILC2s in response to IL-33 in vitro produced high levels of IL-5 and IL-13 and low levels of IL-4, which induces IgE production from B cells (Citation38,Citation39).
There are several limitations to our study. First, the sample size was small and further studies in larger samples might be required to strengthen our result. Second, many details of the underlying cytokine network in the upper and lower airway remain unknown, and we did not measure the serum and sputum concentrations of type 2 cytokines such as IL-4, IL-5, and IL-13. Third, we measured serum IgE only at baseline, and our study did not measure serum IgE in the same period of the year. Therefore, it cannot be denied that seasonal variation of serum IgE affected our results. Further immunological and pathological studies are needed to clarify the underlying pathophysiology of CRSwNP and asthma in detail. Forth, our study had no control groups of CRSwNP subjects without asthma, since asthma patients with CRSwNP were enrolled for treatment to improve nasal, and this was a purely observational study. However, it was difficult to compare CRSwNP patients with and without asthma, because CRSwNP is frequently associated with asthma. Finally, our study did not evaluate the number of asthma exacerbations and the CRS clinical parameters such as the 22-item Sino-Nasal Outcome Test (SNOT-22) and Lund-Mackay score and did not evaluate whether nasal polyps relapsed after ESS. Management of severe asthma with sinusitis is an unresolved issue and further studies are needed in this field.
Conclusions
Sinus surgery impacts asthma control through suppression of airway/systemic type 2 inflammation in eosinophilic CRSwNP with asthma. The present study reinforced the pathophysiology regarding the association and management of airway/systemic type 2 inflammation between the upper and lower airways. Further study is required to confirm the predictive factors for long-term improved asthma control after ESS and to establish optimal treatments for CRSwNP patients with asthma.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgement
The authors thank Mr. Brent Bell for reading the manuscript.
References
- ten Brinke A, Grootendorst DC, Schmidt JT, De Bruine FT, van Buchem MA, Sterk PJ, Rabe KF, Bel EH. Chronic sinusitis in severe asthma is related to sputum eosinophilia. J Allergy Clin Immunol 2002;109(4):621–626. doi:10.1067/mai.2002.122458.
- Philpott CM, Erskine S, Hopkins C, Kumar N, Anari S, Kara N, Sunkaraneni S, Ray J, Clark A, Wilson A, et al. Prevalence of asthma, aspirin sensitivity and allergy in chronic rhinosinusitis: data from the UK National Chronic Rhinosinusitis Epidemiology Study. Respir Res 2018;19(1):129. doi:10.1186/s12931-018-0823-y.
- Langdon C, Mullol J. Nasal polyps in patients with asthma: prevalence, impact, and management challenges. J Asthma Allergy 2016;9:45–53. doi:10.2147/JAA.S86251.
- Promsopa C, Kansara S, Citardi MJ, Fakhri S, Porter P, Luong A. Prevalence of confirmed asthma varies in chronic rhinosinusitis subtypes. Int Forum Allergy Rhinol 2016;6(4):373–377. doi:10.1002/alr.21674.
- Klossek JM, Neukirch F, Pribil C, Jankowski R, Serrano E, Chanal I, El Hasnaoui A. Prevalence of nasal polyposis in France: a cross-sectional, case-control study. Allergy 2005;60(2):233–237. doi:10.1111/j.1398-9995.2005.00688.x.
- Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, Van Cauwenberge P, Bachert C. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol 2008;122(5):961–968. doi:10.1016/j.jaci.2008.07.008.
- Beeh KM, Beier J, Kornmann O, Meier C, Taeumer T, Buhl R. A single nasal allergen challenge increases induced sputum inflammatory markers in non-asthmatic subjects with seasonal allergic rhinitis: correlation with plasma interleukin-5. Clin Exp Allergy 2003;33(4):475–482. doi:10.1046/j.1365-2222.2003.01632.x.
- Braunstahl GJ, Fokkens WJ, Overbeek SE, KleinJan A, Hoogsteden HC, Prins JB. Mucosal and systemic inflammatory changes in allergic rhinitis and asthma: a comparison between upper and lower airways. Clin Exp Allergy 2003;33(5):579–587. doi:10.1046/j.1365-2222.2003.01652.x.
- Braunstahl G-J, Kleinjan A, Overbeek SE, Prins J-B, Hoogsteden HC, Fokkens WJ. Segmental bronchial provocation induces nasal inflammation in allergic rhinitis patients. Am J Respir Crit Care Med 2000;161(6):2051–2057. doi:10.1164/ajrccm.161.6.9906121.
- Kennedy DW. Functional endoscopic sinus surgery. Tech Arch Otolaryngol 1985;111(10):643–649. doi:10.1001/archotol.1985.00800120037003.
- DeMarcantonio MA, Han JK. Nasal polyps: pathogenesis and treatment implications. Otolaryngol Clin North Am. 2011;44(3):685–695. ix. PMID:21621054. doi:10.1016/j.otc.2011.03.005.
- Ebbens FA, Toppila-Salmi SK, Renkonen JA, Renkonen RL, Mullol J, van Drunen CM, Fokkens WJ. Endothelial L-selectin ligand expression in nasal polyps. Allergy 2010;65(1):95–102. doi:10.1111/j.1398-9995.2009.01986.x.
- Ehnhage A, Olsson P, Kolbeck KG, Skedinger M, Dahlen B, Alenius M, Stjarne P, for the NAF2S2 Study Group. Functional endoscopic sinus surgery improved asthma symptoms as well as PEFR and olfaction in patients with nasal polyposis. Allergy 2009;64(5):762–769. doi:10.1111/j.1398-9995.2008.01870.x.
- Lavigne F, Nguyen CT, Cameron L, Hamid Q, Renzi PM. Prognosis and prediction of response to surgery in allergic patients with chronic sinusitis. J Allergy Clin Immunol 2000;105(4):746–751. doi:10.1067/mai.2000.105218.
- Scangas GA, Remenschneider AK, Su BM, Shrime MG, Metson R. The impact of asthma on the cost effectiveness of surgery for chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol 2017;7(11):1035–1044. doi:10.1002/alr.22013.
- Schlosser RJ, Smith TL, Mace J, Soler ZM. Asthma quality of life and control after sinus surgery in patients with chronic rhinosinusitis. Allergy 2017;72(3):483–491. doi:10.1111/all.13048.
- van Drunen CM, Reinartz S, Wigman J, Fokkens WJ. Inflammation in chronic rhinosinusitis and nasal polyposis. Immunol Allergy Clin North Am 2009;29(4):621–629. doi:10.1016/j.iac.2009.07.003.
- Vashishta R, Soler ZM, Nguyen SA, Schlosser RJ. A systematic review and meta-analysis of asthma outcomes following endoscopic sinus surgery for chronic rhinosinusitis. Int Forum Allergy Rhinol 2013;3(10):788–794. doi:10.1002/alr.21182.
- Ikeda K, Shiozawa A, Ono N, Kusunoki T, Hirotsu M, Homma H, Saitoh T, Murata J. Subclassification of chronic rhinosinusitis with nasal polyp based on eosinophil and neutrophil. Laryngoscope 2013;123(11):E1–E9. doi:10.1002/lary.24154.
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention. Updated 2019. Available from: https://ginasthma.org/gina-reports/ [last accessed 15 January 2020].
- Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhin 2012;50(1):1–12. doi:10.4193/Rhino50E2.
- Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J 1999;14(4):902–907. doi:10.1034/j.1399-3003.1999.14d29.x.
- Reddel HK, Taylor DR, Bateman ED, Boulet L-P, Boushey HA, Busse WW, Casale TB, Chanez P, Enright PL, Gibson PG, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med 2009;180(1):59–99. doi:10.1164/rccm.200801-060ST.
- Juniper EF, Bousquet J, Abetz L, Bateman ED, Committee G. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med 2006;100(4):616–621. doi:10.1016/j.rmed.2005.08.012.
- Juniper EF, Svensson K, Mork AC, Stahl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med 2005;99(5):553–558. doi:10.1016/j.rmed.2004.10.008.
- Matsunaga K, Yanagisawa S, Hirano T, Ichikawa T, Koarai A, Akamatsu K, Sugiura H, Minakata Y, Matsunaga K, Kawayama T, et al. Associated demographics of persistent exhaled nitric oxide elevation in treated asthmatics. Clin Exp Allergy 2012;42(5):775–781. doi:10.1111/j.1365-2222.2011.03945.x.
- Sin DD, Miravitlles M, Mannino DM, Soriano JB, Price D, Celli BR, Leung JM, Nakano Y, Park HY, Wark PA, et al. What is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur Respir J 2016;48(3):664–673. doi:10.1183/13993003.00436-2016.
- Matsunaga K, Hirano T, Kawayama T, Tsuburai T, Nagase H, Aizawa H, Akiyama K, Ohta K, Ichinose M. Reference ranges for exhaled nitric oxide fraction in healthy Japanese adult population. Allergol Int 2010;59(4):363–367. doi:10.2332/allergolint.10-OA-0197.
- Yanagisawa S, Ichinose M. Definition and diagnosis of asthma-COPD overlap (ACO). Allergol Int 2018;67(2):172–178. doi:10.1016/j.alit.2018.01.002.
- Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl 2013;48(3):452–458. doi:10.1038/bmt.2012.244.
- Jerschow E, Edin ML, Chi Y, Hurst B, Abuzeid WM, Akbar NA, Gibber M, Fried MP, Han W, Pelletier T, et al. Sinus surgery is associated with a decrease in aspirin-induced reaction severity in patients with aspirin exacerbated respiratory disease. J Allergy Clin Immunol Pract 2019;7(5):1580–1588. doi:10.1016/j.jaip.2018.12.014.
- Tomassen P, Vandeplas G, Van Zele T, Cardell LO, Arebro J, Olze H, Forster-Ruhrmann U, Kowalski ML, Olszewska-Ziaber A, Holtappels G, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol 2016;137(5):1449–1456. doi:10.1016/j.jaci.2015.12.1324.
- Ohta N, Suzuki Y, Ikeda H, Noguchi N, Kakuta R, Suzuki T, Ikeda R, Yamazaki M, Saito Y, Kusano Y, et al. Efficacy of endoscopic sinus surgery for eosinophilic chronic rhinosinusitis with asthma. Allergol Int 2020;69(1):144–145. doi:10.1016/j.alit.2019.08.004.
- Oka A, Matsunaga K, Kamei T, Sakamoto Y, Hirano T, Hayata A, Akamatsu K, Kikuchi T, Hiramatsu M, Ichikawa T, et al. Ongoing allergic rhinitis impairs asthma control by enhancing the lower airway inflammation. J Allergy Clin Immunol Pract 2014;2(2):172–178. doi:10.1016/j.jaip.2013.09.018.
- Oka A, Hirano T, Yamaji Y, Ito K, Oishi K, Edakuni N, Kawano R, Matsunaga K. Determinants of incomplete asthma control in patients with allergic rhinitis and asthma. J Allergy Clin Immunol Pract 2017;5(1):160–164. doi:10.1016/j.jaip.2016.08.002.
- Kubo M. Innate and adaptive type 2 immunity in lung allergic inflammation. Immunol Rev 2017;278(1):162–172. doi:10.1111/imr.12557.
- Ho J, Bailey M, Zaunders J, Mrad N, Sacks R, Sewell W, Harvey RJ. Group 2 innate lymphoid cells (ILC2s) are increased in chronic rhinosinusitis with nasal polyps or eosinophilia. Clin Exp Allergy 2015;45(2):394–403. doi:10.1111/cea.12462.
- Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 2010;463(7280):540–544. doi:10.1038/nature08636.
- Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 2010;464(7293):1367–1370. doi:10.1038/nature08900.