Abstract
Objective
Oral corticosteroids (OCS) are frequently used as relievers for acute asthma and controllers for severe asthma. However, the relief offered by OCS is counterbalanced by adverse effects. We aimed to describe how patients perceive OCS treatment benefits and risks, and how this could affect their adherence to the treatment.
Methods
Patients aged ≥18 years with asthma registered with Carenity, an online patient community, were invited to respond to a questionnaire containing 35 closed and 3 open questions to assess their asthma and perceptions of OCS.
Results
268/300 respondents were receiving or had received OCS for asthma (58 for long-term use and 107 for short-term use). The mean age at diagnosis was 21.3 years. 66% had uncontrolled asthma (GINA control score 3 or 4). Although 42% perceived OCS to be efficacious, 46% mentioned adverse effects. Respondents were mostly satisfied with OCS (median = 7.0/10), particularly for efficacy (median = 8.0/10). Respondents reported having strategies to avoid OCS, mainly because of adverse effects. 26% of respondents had previously reduced or stopped OCS; this proportion was 22% for short-term OCS users and 36% for long-term users. 15% of the respondents not receiving long-term OCS would take the treatment without doing anything else if long-term OCS were prescribed; 42% would seek an alternative treatment.
Conclusions
OCS for asthma is perceived efficient but associated with adverse effects. Patients seek alternative treatment.
Keywords:
Introduction
Asthma is estimated to affect 334 million people worldwide and usually presents with chronic airway inflammation resulting in nonspecific hyperactivity (Citation1–4). Cortisone has been the cornerstone of treatment for inflammatory diseases, including asthma, since the 1950s (Citation5–7). In particular, oral corticosteroids (OCS) are very widely used in asthma which represents by far the commonest reason to prescribe OCS (Citation8,Citation9).
Depending on disease activity and severity, asthma treatment can include inhaled or oral corticosteroid treatment (or both) (Citation10). In particular, OCS can be used as relievers for acute asthma and as controllers in patients with severe asthma (Citation10). OCS are also prescribed to some patients for self-administration at the patient’s discretion in the event of asthma exacerbation in order to initiate treatment before a medical visit, also known as rescue treatment (Citation10–12). However, OCS-related adverse events, such as those affecting the cardiovascular, gastrointestinal, and musculoskeletal systems, as well as infections, are common and such events can be fatal. These OCS-related side-effects have been reported to be more frequent in patients with severe asthma who are under daily maintenance treatment than in those with severe asthma who have frequent rescue courses and in those with mild/moderate asthma (Citation3,Citation13,Citation14). Indeed, cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry reported a prevalence of OCS-related comorbidities of 34% for high blood pressure, 16% for osteoporosis, 10% for type 2 diabetes, 10% for cardiovascular disease, 9% for cataract, 4% for glaucoma (Citation3,Citation15–17). Despite the risk of such complications, about 30% to 45% of patients with severe asthma receive oral corticosteroids (OCS) as a maintenance treatment (Citation18,Citation19).
Although adherence to treatment dosing schedules is essential for treatment efficacy, it has been reported that 45% to 70% adult patient do not take their asthma treatment as prescribed, even in a clinical trial setting or in a self-treatment, partly due to the fear of treatment-related adverse effects (Citation20–27). In a cross-sectional, questionnaire-based survey of symptom, treatment concerns and adherence, including both patients with asthma and physicians who treat asthma, Cooper et al. (Citation28) reported a disparity in perceptions of OCS-related side-effects between patients and clinicians, these perceptions being worse among patients. They also noted poor adherence to OCS as a consequence of negative patient perceptions of OCS.
Online social networks for health have been developed in recent years, which are potentially a rich source of information on the perceptions of OCS by patients and on patient’s attitudes to a proposed prescription of OCS. In an online cross-sectional survey (604 patients, only 8.9% with a lung disease), Costello et al. (Citation29) reported that the three side effects of OCS that were of most importance to participants were weight gain, followed by insomnia and moon face. However, to date, no study using online social networks has interviewed patients with asthma about their experience with short-term or long-term OCS treatments.
The primary objective of the present study was to describe perceptions of OCS treatment by adult patients with asthma belonging to an online patient community who had used or were using OCS on a short-term or long-term basis for their asthma. The secondary objective was to assess the attitudes (adherence, information seeking, etc.) and expectations of patients if their physician was to prescribe them long-term OCS.
Methods
Study design
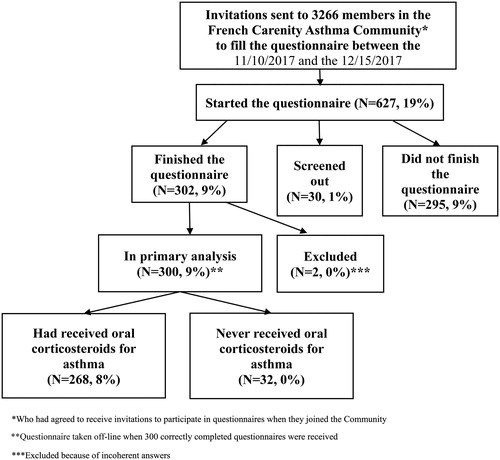
CARENITY (www.carenity.com) is an international online patient community devoted to people with chronic diseases that has existed since 2011. Patients who register on Carenity can integrate a patient group specific for their illness. They can obtain information about their illness and treatment, share their experience with other patients and contribute to medical research in various therapeutic areas, by generating real-world patient insights through online surveys. Patients registered in the asthma community on the Carenity platform and living in France were invited by email to participate in an online patient satisfaction survey to assess their perceptions of OCS treatment for their asthma. Respondents agreed to participate between 11/10/2017 and 12/15/2017. 302 patients responded to the questionnaire during this period ().
Eligibility criteria
Patients aged 18 years and older, registered in the Carenity online asthma community, living in France, had self-reported asthma and were aware of OCS treatment for asthma were eligible to respond to the survey. The results presented in this manuscript are restricted to respondents who said they had received OCS treatment for their asthma.
Survey questionnaire
The online questionnaire was developed by Carenity, with the participation of two of the authors who are pulmonologists (Arnaud Bourdin, Dany Jaffuel), Caroline Fabry and Elsa Darnal from AstraZeneca and a patient with asthma registered on the Carenity community who was receiving continuous OCS treatment. The questionnaire contained 35 closed questions and 3 open questions organized around three themes: demographic and disease characteristics (23 closed questions and 1 open question); perception of OCS treatment (6 closed questions and 2 open questions); and experience with OCS treatment (6 closed questions) (Supplementary material). Certain items were rated using a scale from 0 to 10. Verbatim answers to open-ended questions were grouped into themes.
Statistical analyses
Descriptive univariate and multivariate analyses were performed, as appropriate for the type of data. For univariate analyses, between-group differences were tested using Student’s t-test or the Wilcoxon test (when n < 30 and the population was not normally distributed) for continuous data and the Chi-square test for categorical data. The 95% confidence intervals (CIs) of the mean were calculated assuming a standard normal distribution of the standard deviation. Excel 2013® was used to perform the descriptive analyses. RStudio® (v3.5.0) was used to perform statistical analyses. P values were calculated with a confidence interval of 95%. A p values <0.05 was considered to be statistically significant.
Results
From 11/10/2017 to 12/15/2017, 300/302 patients completed correctly the online questionnaire out of 3266 members of the Carenity Asthma Community who were invited to participate (). The average questionnaire completion time was 18 min. For the analyses reported here, 32 respondents were excluded as they finally declared having never received OCS for their asthma.
Description of respondents and their OCS treatment
Patient characteristics
The overall mean age of the 268 included respondents was 49.9 years and 82% (n = 219) were women. Their geographical distribution covered the whole country. The mean age at diagnosis was 21.3 years and 57% (n = 152) of the respondents had been diagnosed before the age of 20. More than 63% (n = 170) of the respondents had been diagnosed with asthma more than 20 years earlier.
GINA control scores
The overall median GINA control score was 2.8; 66% (n = 177) of respondents had uncontrolled asthma (GINA control score of 3 or 4) and 9% (n = 23) had well-controlled asthma (GINA control score of 0). A summary of the symptoms reported by the respondents is presented in .
Unscheduled consultations and hospitalization in previous year
In the previous year, 59% (n = 158) of the respondents had reported at least one unscheduled medical visit for asthma and 27% (n = 72) had been hospitalized for >24 h. One hundred and twenty-four of the 158 respondents (78%) who had an unscheduled visit for asthma and 62 of the 72 respondents (86%) who were hospitalized for >24 h had uncontrolled asthma.
Medical care
Asthma care was managed by a hospital pulmonologist for 38% (n = 102) of respondents, by a community-based pulmonologist for 27% (n = 73) and by a general practitioner for 33% (n = 88). The mean number of visits per year was six (median: 3); on average 71% (n = 191) of the respondents visited their physician less than five times a year and 10% (n = 26) consulted at least eleven times a year.
Respondents with uncontrolled asthma were more frequently managed by a hospital pulmonologist (76/102; 75%) compared to a community-based pulmonologist (42/73; 58%; p = 0.03) or to a general practitioner (56/88; 64%; p = 0.14). The difference between those followed by a non-hospital pulmonologist and a general practitioner was statistically non-significant (p = 0.53). There was also a tendency for respondents who consulted their physician for asthma more often to have uncontrolled asthma (p = 0.16): 39/51 (76%) for those consulting 5–10 a year and 24/26 (92%) for those consulting ≥11 times.
During the previous year, 63% (n = 168) had received a fixed combination of inhaled corticosteroids (ICS) and long-acting beta-agonist (LABA), 49% (n = 132) were receiving ICS alone and 10% (n = 27) had been injected biological therapies.
OCS treatment
Overall, 22% (n = 58) were requiring long-term OCS (i.e.≥6/12 months), while 40% (n = 107) required repeated short-term OCS courses (i.e.≥2 courses of >3 days) () over the previous year. Among them, 60% (64/107) were given between 2 and 4 courses, 26/107 (24%) received 5 or 6 and 17/107 (16%) received ≥7 courses of OCS. During the previous 12 months, 16% (n = 43) had received OCS for ≤3 days. Respondents followed by a hospital-based pulmonologist were more likely to be prescribed a long-term OCS treatment (). Respondents (46/58 (79%)) who were receiving long-term OCS treatment had even more uncontrolled asthma when compared to 30/60 (50%) who did not require any OCS treatment for their asthma in the previous 12 months ().
Table 1. Oral corticosteroid treatment in the last 12 months as a function of coordinating physician, level of asthma control (GINA control score) and hospitalization or unscheduled consultation for asthma.
A total of 61/150 (41%) respondents had a reserve OCS prescription. Respondents followed by a hospital-based pulmonologist tend to have more frequently a reserve OCS prescription (27/56 (48%)) compared with those followed by a community-based pulmonologist (15/42 (36%)) or a general practitioner (17/49 (35%)) (p = 0.29).
Overall 71% (n = 190) of respondents were also given antibiotics concomitant to the OCS treatment; 22% (n = 58) reported this co-prescription to be systematic, 28% (n = 76) occasional and 21% (n = 56) rare. This was moreover true for respondents managed by a hospital or non-hospital-based pulmonologist (77/102 (76%) and 57/73 (78%), vs. 54/88 (61%) for those managed by their general practitioner).
Respondents’ perception of OCS treatment
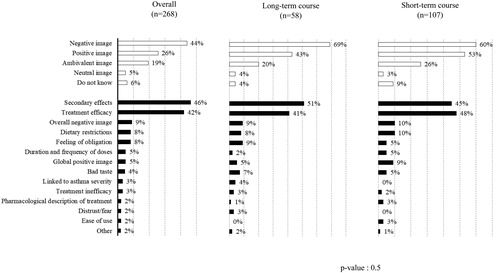
In response to the following open question “Could you describe in a few words the image you have of cortisone tablets (corticosteroids)?” (in French “Pourriez-vous décrire en quelques mots l’image que vous avez de la cortisone en comprimés (corticoïdes)?“) , 44% (n = 118) reported a negative image of OCS compared with 26% (n = 69) who said they had a positive image (). Overall, 42% (n = 113) of the respondents perceived OCS treatment to be efficacious, although 46% (n = 124) spontaneously associated OCS treatment with adverse effects. Interestingly, more respondents on short-term courses had a negative image of OCS treatment (53/107; 50%) compared to those on long-term courses (20/58; 34%).
Figure 2. Summary of responses to open question about respondents’ image of oral corticosteroid treatment. The question asked was: “Could you describe in a few words the image you have of cortisone tablets (corticosteroids)?” (in French “Pourriez-vous décrire en quelques mots l’image que vous avez de la cortisone en comprimés (corticoïdes)? “).
When respondents were spontaneously asked about the potential inconveniences of OCS treatment, adverse effects were cited as the main inconvenience (77%) followed by the need to adapt their lifestyle (n = 26, 10%) and factors related to its mechanism of action, for example interactions with other drugs, duration of action, and possible dependency (n = 24, 9%).
When respondents were asked to grade the inconveniences imposed by OCS treatments on a scale from 0 to 10, modification of diet was ranked as the most bothersome adjustment (median = 7/10, with 38% (n = 102) giving a rating between 8 and 10). This was followed by the requirement to take it daily (median = 5/10, n = 75, 28%), then by the need to attend more doctor visits (median = 5/10, n = 59, 22%). The need to adapt other treatments, such as insulin for diabetes or pills to control blood pressure but also contraceptives and vaccinations came after. Respondents requiring long-term OCS gave higher median scores (the higher the worst) for the need to observe the treatment daily and to adapt other treatments ().
Table 2. Perceptions of bothersome adjustments associated with oral corticosteroid treatment as a function of type of oral corticosteroid treatment (median scores out of 10 (range), with 10 being the most bothersome).
Patients’ experience with OCS treatment
Efficacy
Quite unexpectedly, respondents expressed globally to be satisfied with their OCS treatments (median general satisfaction score: 7.0 on a scale from 0 to 10), particularly with their efficacy (median general satisfaction score: 8.0). Respondents receiving long-term or short-term courses reported nearly similar levels of efficacy of OCS (median scores of 8.0 and 9.0, respectively).
Adverse effects
Respondents reported many adverse effects due to OCS (). The most frequently reported adverse effects were sleep disturbance (n = 226, 84%), fatigue (n = 220, 82%) and behavioral changes (n = 211, 79%). Worries about increased risks of stroke or infarction (n = 111, 41%) and uncontrolled diabetes (n = 102, 38%) were the least frequently reported adverse effects. Respondents exposed to short-term bursts frequently reported adverse effects, but at a lower frequency than respondents on long-term treatment (). Fatigue, skin disorders, loss of muscular strength, high blood pressure, bone fragility/osteoporosis and vision concerns were more frequently reported by long-term OCS users.
Table 3. Summary of oral corticosteroid-related adverse effects reported by respondents who had received oral corticosteroid treatment for asthma in the previous 12 months (n (%)).
When respondents experienced adverse effects, they were asked to grade the impact of each adverse effect on their well-being on a scale from 0 (no impact) to 10 (very important impact). This was found to be unaffected by the duration of OCS treatment (). Additionally, respondents reported that they were relatively dissatisfied with the information they had received on OCS-related long-term adverse effects and their management (median score for both: 4/10).
Table 4. Assessment of the impact of oral corticosteroid-related adverse effects on well-being (0 = no impact; 10= very significant impact).
Inconveniences associated with OCS treatment
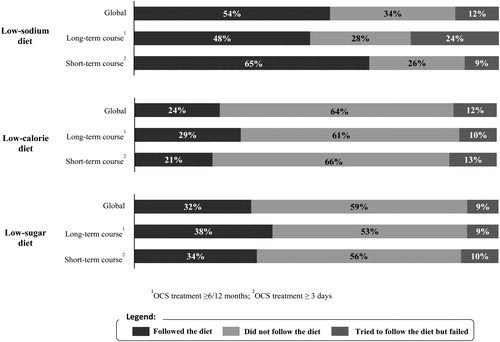
Overall, 54% (n = 114) said they followed a low-sodium diet when they were receiving OCS treatment as 32% (n = 86) followed a low-sugar diet and 24% (n = 63) a low-calorie diet (). 12% (n = 32) of respondents reported being unsuccessful at adopting a low-sodium or low-calorie diet compared to 9% (n = 23) for low-sugar diet. Respondents receiving long-term therapy failed to follow a low-sodium diet (14/58; 24%) more frequently (p = 0.02) than respondents on short-term therapy (10/107; 9%).
Adherence to prescribed OCS treatment
Only 37% (n = 99) of the respondents declared that they had completely respected their latest OCS prescription with respect to both dose and duration (), with 85% (n = 229) of the respondents saying they had respected the duration of their latest OCS prescription. Most of the respondents who had not respected the duration had reduced it. Poor adherence to the treatment duration was unaffected by the level of asthma control according to the GINA control scores.
Moreover, 44% (n = 117) of the respondents reported their willingness to reduce the dose and 34% (n = 90) had wanted to stop OCS treatment, even though 19% (n = 52) said they did actually reduce the dose and 15% (n = 41) that they did stop their OCS treatment without consulting their doctor or against their doctor’s advice.
Fear of long-term adverse effects was the main reason for wanting to reduce OCS treatment (70/133, 53%), followed by the severity of the adverse effects (66/133, 50%) and their asthma being controlled (57/133, 43%). Among the respondents who wanted to stop OCS treatment, only 9/102 (9%) wanted to stop because of lack of efficacy. The main reasons for stopping were the severity of adverse effects (57/102, 56%), fear of long-term adverse effects (50/102, 49%), and current asthma control (32/102, 31%).
Respondents’ responses and actions when facing a prescription of oral corticosteroid
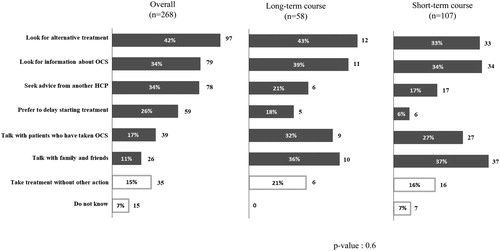
Almost half the respondents not requiring long-term OCS said they would have a very negative reaction if their physician were to prescribe them long-term OCS. Only 15% (35/230) said they would take the treatment without doing anything else, whereas 42% (97/230) acknowledged they would try to find an alternative treatment, and 34% (78/230) would seek advice from another healthcare professional or look for additional information on OCS treatment (). The mean number of actions that would be undertaken was 1.7, and 68 respondents (30%) said they would undertake ≥3 of the proposed actions.
Discussion
It is known that the OCS treatment is associated with an increased risk of adverse effects and that patients’ perceptions of OCS treatment can differ, depending on their experience (Citation29–31). To our knowledge, this is one of the first studies to provide real-world perspectives on how patients with asthma perceive the benefits and risks of their OCS treatment and how this perception could affect their adherence to the treatment. Given the inclusion criteria, the studied population likely fits with the one eligible to biologics as these patients report frequent if not permanent OCS exposure and poor levels of asthma control.
The results from this survey of volunteer members of an online asthma community, who were receiving or had received OCS treatment for their asthma, showed that although more than 40% of respondents perceived OCS treatment to be efficacious, numerous adverse effects were reported, such as sleep disturbances (84%), fatigue (82%) and behavioral changes (79%). Short-term (such as weight gain or fatigue) and long-term (such as cardiovascular risks or osteoporosis) adverse effects associated with OCS treatment have been described in several studies (Citation32–34).
Patients requiring long-term OCS treatment reported more adverse effects, in terms of both the type of adverse effects experienced and the impact on their well-being. Nevertheless, more patients receiving repeated short-term courses reported a negative image of OCS treatment. This would suggest that patients on long-term treatment are more ready to cope with adverse effects because of their perception of OCS efficacy on the severity of their asthma. Potentially, these patients may have neglected the connection between some of their comorbidities and the OCS (such as diabetes or increased risks of stroke/infarction).
Differences between patients’ and doctors’ perceptions of the significance of these various adverse effects have been reported (Citation28,Citation35). In our study, patients were not satisfied with the information on adverse effects provided by their doctor. It has been shown that clinicians underestimate the impact of some of the effects of OCS, such as mood and sleep disturbances, on patients’ well-being, whereas patients seem to be more concerned about these effects and in particular want to be better informed on their impact and their management (Citation35). Shared-decision making is advocated by all patient organizations and the European Lung Foundation.
In addition to adverse effects, numerous inconveniences associated with OCS treatment were reported in our study, such as the need to observe the OCS treatment daily, having to adapt their medical management and having to change their diet. 12% of respondents reported failures to follow a low-sodium or low-calorie diet, compared with 9% for a low-sugar diet. Respondents receiving long-term OCS treatment failed to follow a low-sodium diet more frequently than patients receiving repeated bursts.
It has been suggested that corticosteroid-sparing alternatives should be considered to avoid poor adherence to OCS and potential adverse effects (Citation17,Citation31). IL5-targeting drugs such as mepolizumab and more recently benralizumab and dupilumab evidenced their impressive OCS-sparing effects (Citation36) when given to severe asthma patients who were requiring repeated bursts (demonstrated in trials: SIROCCO, CALIMA, MENSA, Liberty asthma QUEST) (Citation37–40) or maintenance OCS treatments (demonstrated in the trials: SIRIUS, ZONDA, Liberty asthma VENTURE) (Citation41–43). Insufficient or inaccurate awareness of OCS harmfulness especially on the long term may limit access to these biologics. Noteworthy, this awareness should also be raised at managing physicians’ level to prevent them “stocking” patients with OCS. When considering the well-established risks of maintenance OCS regimens, now clearly acknowledged in GINA, we considered the present results as another argument supporting the need for referral to asthma expert centers. We acknowledged that we missed the opportunity to ask whether these patients were tested for eligibility to biologics (i.e.skin prick tests, blood eosinophil count, FeNO). It has to be noted that biological therapies are high cost treatments and are not the only alternatives to OCS treatments (ICS-LABA for instance). In our study, respondents reported having developed strategies such as reducing the dose or duration of treatment. Overall, 26% of respondents had previously reduced or stopped their OCS therapy; this was also reported by 22% and 36% of those receiving short-term and long-term OCS, respectively. 42% of the respondents not receiving long-term OCS said they would seek an alternative if they were prescribed long-term OCS. However, prior to initiating an alternative treatment, it is essential to evaluate adherence to treatment (Citation44). Targeted communication about long-term adverse effects might also be useful to encourage switching from OCS to biological therapies.
We acknowledge some limitations inherent to the design of our survey. For example, we surveyed patients with asthma via Internet which allowed us to obtain real-world data on their perception of and concerns about their OCS treatment and its impact on their well-being. Belonging to a patient community likely reflects the need to self-assess and compare the level of the disease. Poorly controlled, highly symptomatic and heavily treated patients may then be overrepresented and our findings should be extrapolated only cautiously. However, the majority of people use the Internet and a large proportion use it for health-related research. In 2018, 75% of French people said they used the Internet every day, and 46% of them used the Internet to look for health-related information (Citation45). Moreover, it has been shown that Carenity communities, when compared with the SNIIRAM database (an exhaustive massive claim database available in France where all individual refunding acts are listed but without any disease-related information), reflect the main characteristics of online users willing to share their experience with a disease, with an over-representation of female patients aged from 25 to 54 (Citation46). Furthermore, as we used a non-probabilistic sampling approach, we may have a selection bias with an over-representation of patients willing to participate. The study sample was relatively representative of the target population in terms of age, gender, geographical area and type of care received. However, the number of participants is relatively small, and patients are unlikely to be representative of all patients with asthma in France.
As with any study relying on self-report of disease status, there is also a risk of incorrect asthma diagnoses. It is recognized that the diagnosis of asthma can be difficult due to the large number of phenotypes with different triggers that have been identified (Citation47). However, the respondents’ mean age at diagnosis (21.3 years, 57% of them were diagnosed before the age of 20 years) and the date of diagnosis (asthma diagnoses had been made more than 28 years prior for 46% of the respondents) lead us to think that most of the asthma diagnoses were correct.
Conclusions
This study provides original information on patients’ perception of OCS treatment for asthma, showing that OCS-related complications are a major concern for them, and these can lead them to delay treatment and even encourage them to look for alternative treatments.
Sources of funding
This work was supported by AstraZeneca.
Supplemental Material
Download PDF (869.8 KB)Acknowledgements
The authors would like to thank the patients from the Carenity Asthma Community who participated in this survey. Caroline Fabry and Elsa Darnal from AstraZeneca contributed to the development of the survey and provided funds for operational support. They would also like to acknowledge medical writing and editorial services on an earlier version from Margaret Haugh, MediCom Consult, Villeurbanne, France.
Declaration of interest
Ophélie Wilczynski and Emilie Pain report commercial sponsorship from AstraZeneca relating to the conduct of the study. Arnaud Bourdin and Dany Jaffuel report commercial sponsorship from Carenity relating to the conduct of the study. Arnaud Bourdin reports grants, personal fees and non-financial support from AstraZeneca and Boehringer Ingelheim, personal fees and non-financial support from Chiesi, GlaxoSmithKline, Novartis and Teva, non-financial support and other support (investigator) from Cephalon outside the submitted work. Dany Jaffuel reports personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi and Philips, personal fees and non-financial support from GlaxoSmithKline, LÖWENSTEIN Médical and SEFAM, non-financial support from ResMed outside the submitted work. Caroline Fabry and Elsa Darnal are employees of AstraZeneca.
References
- Aaron SD, Vandemheen KL, FitzGerald JM, Ainslie M, Gupta S, Lemière C, Field SK, McIvor RA, Hernandez P, Mayers I, Mulpuru S. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317(3):269–279. doi:10.1001/jama.2016.19627.
- Al Efraij K, Johnson KM, Wiebe D, Sadatsafavi M, FitzGerald JM. A systematic review of the adverse events and economic impact associated with oral corticosteroids in asthma. J Asthma. 2019;56(12):1334–1346.
- Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, Ortega HG, Pavord ID. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–1197. doi:10.1056/NEJMoa1403291.
- Benard-Laribiere A, Pariente A, Pambrun E, Begaud B, Fardet L, Noize P. Prevalence and prescription patterns of oral glucocorticoids in adults: a retrospective cross-sectional and cohort analysis in France. BMJ Open. 2017;7(7):e015905. doi:10.1136/bmjopen-2017-015905.
- Berthon BS, MacDonald-Wicks LK, Wood LG. A systematic review of the effect of oral glucocorticoids on energy intake, appetite, and body weight in humans. Nutr Res. 2014;34(3):179–190. doi:10.1016/j.nutres.2013.12.006.
- Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, Sproule S, Gilmartin G, Aurivillius M, Werkstrom V, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2115–2127. doi:10.1016/S0140-6736(16)31324-1.
- Bleecker ER, Menzies-Gow AN, Price DB, Bourdin A, Sweet S, Martin AL, Alacqua M, Tran TN. Systematic literature review of systemic corticosteroid use for asthma management. Am J Respir Crit Care Med. 2020;201(3):276–293. [Epub ahead of print]. doi:10.1164/rccm.201904-0903SO.
- Bourdin A, Fabry-Vendrand C, Ostinelli J, Ait-Yahia M, Darnal E, Bouee S, Laurendeau C, Bureau I, Gourmelen J, Chouaid C. The burden of severe asthma in France: a case-control study using a medical claims database. J Allergy Clin Immunol Pract. 2019;7(5):1477–1487. doi:10.1016/j.jaip.2018.12.029.
- Bourdin A, Molinari N, Vachier I, Pahus L, Suehs C, Chanez P. Mortality: a neglected outcome in OCS-treated severe asthma. Eur Respir J. 2017;50(5):1701486. doi:10.1183/13993003.01486-2017.
- Bousquet J, Chanez P, Lacoste JY, Barneon G, Ghavanian N, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323(15):1033–1039. doi:10.1056/NEJM199010113231505.
- Busse WW, Maspero JF, Rabe KF, Papi A, Wenzel SE, Ford LB, Pavord ID, Zhang B, Staudinger H, Pirozzi G, et al. Liberty Asthma QUEST: phase 3 randomized, double-blind, placebo-controlled, parallel-group study to evaluate dupilumab efficacy/safety in patients with uncontrolled, moderate-to-severe asthma. Adv Ther. 2018;35(5):737–748. doi:10.1007/s12325-018-0702-4.
- Chapman KR, Verbeek PR, White JG, Rebuck AS. Effect of a short course of prednisone in the prevention of early relapse after the emergency room treatment of acute asthma. N Engl J Med. 1991;324(12):788–794. doi:10.1056/NEJM199103213241202.
- Chung LP, Upham JW, Bardin PG, Hew M. Rational oral corticosteroid use in adult severe asthma: A narrative review. Respirology. 2020;25(2):161–172. doi:10.1111/resp.13730.
- Cooper V, Metcalf L, Versnel J, Upton J, Walker S, Horne R. Patient-reported side effects, concerns and adherence to corticosteroid treatment for asthma, and comparison with physician estimates of side-effect prevalence: a UK-wide, cross-sectional study. NPJ Prim Care Respir Med. 2015;25:15026.
- Costello R, Patel R, Humphreys J, McBeth J, Dixon WG. Patient perceptions of glucocorticoid side effects: a cross-sectional survey of users in an online health community. BMJ Open. 2017;7(4):e014603. doi:10.1136/bmjopen-2016-014603.
- Ellis A. The pathological anatomy of bronchial asthma. Am J Med Sci. 1908;136:407–429.
- Eurostat. Internet use by individuals 2019. [Last accessed: 30 January 2019]. https://ec.europa.eu/eurostat/search?p_auth=RJlCSCPp&p_p_id=estatsearchportlet_WAR_estatsearchportlet&p_p_lifecycle=1&p_p_state=maximized&p_p_mode=view&_estatsearchportlet_WAR_estatsearchportlet_action=search&text=Internet+use+by+individuals.
- FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, Ferguson GT, Busse WW, Barker P, Sproule S, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–2141. doi:10.1016/S0140-6736(16)31322-8.
- Gamble J, Stevenson M, McClean E, Heaney LG. The prevalence of nonadherence in difficult asthma. Am J Respir Crit Care Med. 2009;180(9):817–822. doi:10.1164/rccm.200902-0166OC.
- Global Asthma Network (GAN) Steering Group. The Global Asthma Report 2014 [Last accessed: 30 January 2019]. https://www.globalasthmareport.org/2014/about/executive.php.
- Global Initiative for Asthma. GINA report, global strategy for asthma management and prevention 2018 [Last accessed: 30 January 2019]. https://ginasthma.org/gina-reports/.
- Hamilton D, Lehman H. Asthma phenotypes as a guide for current and future biologic therapies. Clin Rev Allergy Immunol. 2019. doi:10.1007/s12016-019-08760-x.
- Hench PS, Kendall EC, et al. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone; compound E) and of pituitary adrenocorticotropic hormone on rheumatoid arthritis. Proc Staff Meet Mayo Clin. 1949;24:181–197.
- Lee J, Tay TR, Radhakrishna N, Hore-Lacy F, Mackay A, Hoy R, Dabscheck E, O'Hehir R, Hew M. Nonadherence in the era of severe asthma biologics and thermoplasty. Eur Respir J. 2018;51(4):1701836. doi:10.1183/13993003.01836-2017.
- Lefebvre P, Duh MS, Lafeuille MH, Gozalo L, Desai U, Robitaille MN, Albers F, Yancey S, Ortega H, Forshag M, et al. Acute and chronic systemic corticosteroid-related complications in patients with severe asthma. J Allergy Clin Immunol. 2015;136(6):1488–1495. doi:10.1016/j.jaci.2015.07.046.
- Manser R, Reid D, Abramson M. Corticosteroids for acute severe asthma in hospitalised patients. Cochrane Database Syst Rev. 2000;Cd001740.
- Milgrom H, Bender B, Ackerson L, Bowry P, Smith B, Rand C. Noncompliance and treatment failure in children with asthma. J Allergy Clin Immunol. 1996;98(6):1051–1057. doi:10.1016/S0091-6749(96)80190-4.
- Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, Calhoun WJ, Castro M, Chung KF, Clark MP, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119(2):405–413. doi:10.1016/j.jaci.2006.11.639.
- Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, Barker P, Sproule S, Ponnarambil S, Goldman M. Oral glucocorticoid–sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376(25):2448–2458. doi:10.1056/NEJMoa1703501.
- Nobelprize.org. The Nobel Prize in Physiology or Medicine 1950. Nobel Media AB; 2014 [Last accessed: 28 June 2018]. https://www.nobelprize.org/nobel_prizes/medicine/laureates/1950/.
- Ortega HG, Yancey SW, Mayer B, Gunsoy NB, Keene ON, Bleecker ER, Brightling CE, Pavord ID. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med. 2016;4(7):549–556. doi:10.1016/S2213-2600(16)30031-5.
- Perdoncini-Roux A, Blanchon T, Hanslik T, Lasserre A, Turbelin C, Dorleans Y, Cabane J, Fardet L. General practitioners’ perception of the impact of corticosteroid-induced adverse events. Rev Epidemiol Sante Publique. 2009;57(2):93–97. doi:10.1016/j.respe.2008.12.009.
- Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, Zhu H, Hamilton JD, Swanson BN, Khan A, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378(26):2475–2485. doi:10.1056/NEJMoa1804093.
- Raïs S, Radoszycki L, Dourgnon P, Rochaix L, Chekroun M. Accurate representation of patients’ opinions for decisionmaking: are online health communities good candidates? 2017. [Last accessed: 30 January 2019]. https://www.carenity.co.uk/static/doc/corporate/poster-ISPOR2017-Accurate-Representation-of-patients-opinions-for-decision-making.pdf.
- Rand C, Bilderback A, Schiller K, Edelman JM, Hustad CM, Zeiger RS. Adherence with montelukast or fluticasone in a long-term clinical trial: results from the mild asthma montelukast versus inhaled corticosteroid trial. J Allergy Clin Immunol. 2007;119(4):916–923. doi:10.1016/j.jaci.2006.12.664.
- Randolph TG, Rollins JP. The effect of cortisone on bronchial asthma. J Allergy. 1950;21(4):288–295. doi:10.1016/0021-8707(50)90060-8.
- Rowe BH, Vethanayagam D. The role of inhaled corticosteroids in the management of acute asthma. Eur Respir J. 2007;30(6):1035–1037. doi:10.1183/09031936.00119907.
- Shaw DE, Sousa AR, Fowler SJ, Fleming LJ, Roberts G, Corfield J, Pandis I, Bansal AT, Bel EH, Auffray C, et al. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur Respir J. 2015;46(5):1308–1321. doi:10.1183/13993003.00779-2015.
- Sherman J, Patel P, Hutson A, Chesrown S, Hendeles L. Adherence to oral montelukast and inhaled fluticasone in children with persistent asthma. Pharmacotherapy. 2001;21(12):1464–1467. doi:10.1592/phco.21.20.1464.34485.
- Sullivan PW, Ghushchyan VH, Globe G, Schatz M. Oral corticosteroid exposure and adverse effects in asthmatic patients. J Allergy Clin Immunol. 2018;141(1):110–116. doi:10.1016/j.jaci.2017.04.009.
- Sweeney J, Patterson CC, Menzies-Gow A, Niven RM, Mansur AH, Bucknall C, Chaudhuri R, Price D, Brightling CE, Heaney LG. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax. 2016;71(4):339–346. doi:10.1136/thoraxjnl-2015-207630.
- van der Palen J, Klein JJ, Zielhuis GA, van Herwaarden CL, Seydel ER. Behavioural effect of self-treatment guidelines in a self-management program for adults with asthma. Patient Educ Couns. 2001;43(2):161–169. doi:10.1016/S0738-3991(00)00155-5.
- Volmer T, Effenberger T, Trautner C, Buhl R. Consequences of long-term OCS therapy and its side effects in severe asthma in adults - a focused review of the impact data in the literature. Eur Respir J. 2018;52(4):1800703. doi:10.1183/13993003.00703-2018.
- Voorham J, Xu X, Price DB, Golam S, Davis J, Zhi Jie Ling J, Kerkhof M, Ow M, Tran TN. Healthcare resource utilization and costs associated with incremental systemic corticosteroid exposure in asthma. Allergy. 2019;74(2):273–283. doi:10.1111/all.13556.
- Vuillermin PJ, South M, Carlin JB, Biscan MI, Brennan SL, Robertson CF. Parent-initiated oral corticosteroid therapy for acute asthma: a survey of current practice. J Paediatr Child Health. 2007;43(6):443–445. doi:10.1111/j.1440-1754.2007.01108.x.
- Waljee AK, Rogers MA, Lin P, Singal AG, Stein JD, Marks RM, Ayanian JZ, Nallamothu BK. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ. 2017;357:j1415.
- Wraight JM, Cowan JO, Flannery EM, Town GI, Taylor DR. Adherence to asthma self-management plans with inhaled corticosteroid and oral prednisone: a descriptive analysis. Respirology. 2002;7(2):133–139. doi:10.1046/j.1440-1843.2002.00374.x.
Appendix
Table 1. Summary of symptoms used to calculate GINA control scores.
Appendix
Table 2. Reported modifications (dose reduction or treatment discontinuation) to the respondent’s last OCS treatment.