Abstract
Objective
This study aims to describe the eligibility for biologic therapies for severe asthma (SA) in a cohort of patients attending the Program for Control of Asthma (ProAR) in Bahia, Brazil.
Methods
Data from SA patients (≥18 years old) attending the ProAR, that were included in a case-control study conducted from 2013 to 2015, were used to reassess patients according to a modified ERS/ATS 2014 SA criteria. Patients were then classified according to the eligibility for SA biological therapy based on current prescription labels.
Results
From 544 patients in the cohort, 531 (97.6%) were included and 172 (32.4%) were identified as SA patients according to the ERS/ATS 2014 modified criteria. Of these 172 patients, 69 (40.1%) were ineligible for any of the biologicals approved for asthma (omalizumab, mepolizumab, reslizumab and benralizumab), 60 (34.9%) patients were eligible for one of the biological therapies, and 10 (5.8%) patients were eligible for all biological therapies.
Conclusions
More than half of patients with SA were eligible for biologic therapy in our study, but none of them received this form of treatment. Almost half of them were not eligible to any of the approved biologics, however. The variability and overlap in patients’ eligibility highlight the importance of evaluating each patient individually for a more personalized treatment approach. While there is a need to increase access for some of those eligible that may really need a biologic treatment, continuous efforts are required to develop alternatives to those who are not eligible.
Introduction
Despite many advances, the disease burden from severe asthma (SA) remains high (Citation1). Classical epidemiological studies showed great variability in prevalence of asthma among countries, suggesting geographical and environmental influence in the occurrence of the disease (Citation2,Citation3). It is increasingly recognized that distinct immune inflammatory mechanisms (endotypes) and clinical phenotypes contribute to the heterogeneity seen in SA (Citation4,Citation5).
It is estimated that up to 3.6% of people with asthma have SA, requiring high-doses of standard treatments ([inhaled corticosteroids (ICS) and long-acting bronchodilator] and other controllers such as ICS monotherapy or oral corticosteroids (Citation6)), but the disease can remain ‘uncontrolled’ despite this therapy in a significant proportion of these patients worldwide (Citation7). Patients with uncontrolled SA suffer from frequent exacerbations, which can often require emergency department visits and hospitalizations, contributing to work and school absenteeism, use of multiple drugs to control the disease, higher healthcare resource utilization and higher mortality risk (Citation1,Citation7).
To better understand and treat the extensive heterogeneity of asthma, many expert groups have proposed distinctive classifications of SA (Citation7–11), with the goal of advancing research and treatment paradigms toward precision medicine. In recent years, much research has led to the identification of SA phenotypes wherein specific biological pathways and mechanisms (endotypes) are being identified to explain the observable properties of the phenotypes, and improving the future prospects for these difficult-to-treat patients (Citation12–14).
In this scenario, biologics have emerged as promising personalized medicines in the treatment of SA. Humanized monoclonal antibodies against immunoglobulin E (IgE) to treat severe allergic asthma, anti-interleukin-5 (IL-5) and anti-IL5 receptor (IL-5R) for severe eosinophilic asthma, have recently entered the clinic as approved therapies (Citation12–14). The addition of a biologic therapy in appropriate patients diagnosed with SA can lead to more effective control of the underlying inflammation and improve some asthma-related outcomes, such as number of exacerbations, quality of life and lung function (Citation12–15). However as new biologicals come forward it is important to identify the characteristics of SA patients who would most benefit from each therapy to achieve better outcomes, whilst allocating limited healthcare resources most efficiently.
In Brazil, the Program for Control of Asthma in Bahia (acronym ProAR) is a program whose main objective is to provide comprehensive medical care to SA patients, in Salvador, Bahia (Citation16). ProAR started in 2003 as an assistive, teaching and research program offering medical care, free medication, psychological assistance, and asthma education (Citation16). The patients followed in ProAR constitute a cohort of SA patients (Citation17), providing a unique opportunity of evaluating the characteristics of SA patients in Brazil, especially in patients who have optimal treatment available (Citation17). The objective of this study was to reassess SA patients followed-up by ProAR to estimate the frequency of eligibility and overlaps for treatment with biologics in Brazilian SA patients and to describe the clinical and sociodemographic characteristics of these patients.
Methods
Study design and study population
This is an analysis of the database of the ProAR Cohort (Citation17), which comprises 544 adult patients (≥18 years old) with SA according to previous definitions of the Education Prevention Program – Practical guide for the diagnosis and management of asthma/NHLBI-NIH (1997) (Citation18), and that were included in a case-control study conducted from 2013–2015 (Citation19). Patients with chronic conditions such as COPD, structural changes of the lungs and pregnancy were excluded. Details and results of the case-control study have been published previously (Citation19).
In the present study, patients were reclassified using modified criteria of the ERS/ATS Severe Asthma Task Force 2014 (7) as this was the definition used for consideration for treatment with SA biologicals. The ERS/ATS criteria use a history of ICS or oral steroids in the previous 12 months to classify SA. We were limited in having 6 months’ medical history available, therefore we describe our analysis according to modified ERS/ATS criteria. Medical records about prescribed asthma medications 6 months prior to starting the case-control study were used to reclassify the patients. Sociodemographic and clinical characteristics, inflammatory markers and the asthma control (Asthma Control Questionnaire (ACQ-6) and GINA asthma symptoms control questionnaire (2012)) collected on the previous conducted case-control study were reassessed in order to characterize the SA ProAR Cohort, as described below.
Eligibility criteria were defined according the label indication for anti-IgE (omalizumab), anti-IL5 (mepolizumab, reslizumab) and anti-IL5R (benralizumab) based on the number of exacerbations in the last 12 months, levels of IgE and eosinophils as summarized in .
Table 1. Eligibility criteria used to classify patients for each biologic treatment.
Consent to use baseline data and medical records were obtained from the SA cohort patients; 531 of the 544 patients were included in the present analysis. The study protocol was approved by applicable institutional review boards/independent ethics committees (approval number: 1.646.061) and was conducted in accordance with the Declaration of Helsinki. All data collected were anonymized.
Variables
The variables included in the analysis were age (years), sex, nutritional status, ethnicity (self-reported as black, white, mixed, indigenous or Asiatic), educational level (self-reported as illiterate, elementary school, high school or college), monthly family income per capita, age at asthma onset, comorbidities (self-reported), blood eosinophils (cells/μl), immunoglobulin E (IgE) (IU/mL), C-reactive protein (mg/L), oral corticosteroids (OCS) (number of cycles), number of emergency room (ER) visit and hospitalization due to asthma in the last year. Nutritional status was calculated using the following body mass index (BMI) cutoff points: “underweight” <18.5 kg/m2; “eutrophic” 18.5 to 24.9 kg/m2; “overweight” 25 to 29.9 kg/m2; “obesity I” ≥30 to 34.9 kg/m2; “obesity II” ≥35 to 39.9 kg/m2; and “obesity III” ≥40 kg/m2.
Blood eosinophils were evaluated as continuous and as categorical variables, considering four cutoffs: < 150 cells/µL; ≥ 150 − 199 cells/µL; ≥ 200 − 299 cells/µL; ≥ 300 − 399 cells/µL and ≥ 400 cells/µL. Immunoglobulin E (IgE) was measured by chemiluminescence. IgE values <160 IU/mL were considered as normal for this study’s age group. The number of exacerbations was defined as the self-reported number of OCS cycles, ER visits due to asthma or hospitalizations due to asthma in the last year. Cross-sectional data were used for Asthma Control Questionnaire-6 (ACQ-6) (Citation20) and GINA assessment of asthma symptom control questionnaire (Citation1). “Controlled” and “partially controlled” were grouped (Citation21). Monthly family income was categorized as quintiles, expressed in Brazilian monetary currency (BRL).
Data analysis
Data from the SA patients’ medical records on prescribed asthma medications 6 months prior to ProAR baseline visit was used to reclassify asthma severity according to the modified ERS/ATS 2014 guidelines criteria (Citation7), where SA was defined as treatment with high doses of inhaled corticosteroid (ICS), i.e. ≥1000 mcg of fluticasone or equivalent, plus the use of an additional controller. Patients using ICS plus the highest ICS/LABA (long-acting beta2-agonist) dose available were considered as using ICS in high doses. The modification adopted in the ERS/ATS 2014 (4) criteria for this study was the time required for use of high doses inhaled corticosteroids, from 12 months to a shorter period of 6 months. In the case of missing information on doses during the 6-month period, it was assumed that the dose had not changed since the previous prescription. Sociodemographic and clinical characteristics were described for the SA patients identified through the ERS/ATS 2014 modified definition as a post-hoc analysis. The ERS/ATS 2014 criteria was used as it uses higher ICS doses in its definition than the GINA 2017 criteria, and thus it may better identify SA patients at maximum bronchodilation. Results are descriptive and no statistical hypothesis tests were applied. Continuous variables were expressed as mean and standard deviation, and categorical ones as absolute and relative frequencies. All analyses were performed using SPSS version 24.
Results
In the ProAR database, 531 of the 544 patients consented to the use of their data in the analysis. Of these 531 patients, 172 (32.4%) were classified as SA by the ERS/ATS 2014 modified criteria. The group was predominantly female (84.3%) with a mean (standard deviation (SD)) age of 53 (12) years. Most of the patients defined their ethnicity as “mixed”, had attended elementary school or high school, had a low family income and were overweight or obese (). With respect to the clinical characteristics observed among these 172 patients with modified ERS/ATS 2014 SA, all patients reported having at least one chronic disease in addition to asthma, and approximately 70% reported having ≥3 comorbid conditions. The most common reported comorbidities were rhinitis (96.5%) and gastroesophageal reflux disease (GERD) (79.1%) ().
Table 2. Demographic and clinical characteristics of patients with severe asthma (SA) from the Brazilian ProAR Cohort according to the ERS/ATS 2014 definition modified.
In terms of inflammatory biomarkers, the mean (SD) number of blood eosinophils was 310 (317) cells/µL and 64.1% of the modified ERS/ATS 2014 SA patients presented levels ≥150 cells/µL. High levels of total IgE (≥ 160 IU/mL) were observed in 66.1% of the patients and 63.4% were atopic. Mean (SD) C-reactive protein was 6.47 (6.66) mg/L ().
Table 3. Description of inflammatory markers in severe asthma from the Brazilian ProAR Cohort patients according to ERS/ATS 2014 definition modified.
A high proportion of patients had poor asthma control; 55.8% of the patients had uncontrolled asthma according to ACQ-6 scores and 36.0% were classified as “uncontrolled” according to the GINA questionnaire. Approximately 78% of the SA patients had at least one exacerbation in the last year, 15% reported three exacerbations and 22% had five or more exacerbations reflecting the poor asthma control in this population ().
Table 4. Assessment of asthma control and number of self-reported exacerbations in severe asthma (SA) from the Brazilian ProAR Cohort patients according to ERS/ATS 2014 definition modified.
The second controller most frequently prescribed was LABA (associated with ICS in the same device) and 84.3% of the patients reported using at least two different inhalers (one with ICS alone and another with ICS/LABA) as a way to increase the dose of ICS without going beyond the dose limit of the LABA. Although high ICS doses plus a second controller had been prescribed to all patients in the last 6 months, approximately 14% of the patients admitted not following the recommendation of the attending physician and used the ICS in lower doses than prescribed, suggesting under treatment and non-adherence ().
Table 5. Patient reported use of inhaler combinations from the Brazilian ProAR Cohort severe asthma patients according to ERS/ATS 2014 definition modified.
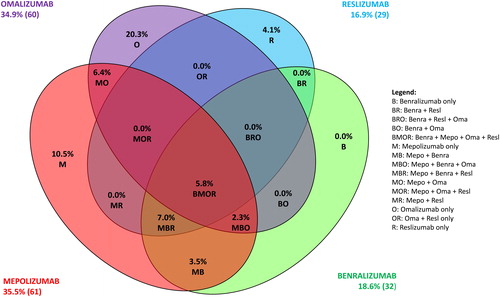
The frequency of patients eligible for the different biological therapies are shown in ; 69 of the 172 SA patients (40.1%) were not eligible to any biologicals approved in Brazil For the remaining patients, 60 (34.9%) were eligible for omalizumab, 61 (35.5%) were eligible for mepolizumab, 32 (18.6%) for benralizumab and 29 (16.9%) for reslizumab. Only 10 (5.8%) patients were eligible for all 4 therapies, and importantly 60 (34.9%) were only eligible for only one of the therapies alone. For patients who were only eligible for a single biological therapy, 35 (20.3%) patients were eligible for omalizumab alone, 7 (4.1%) for reslizumab alone and 18 (10.5%) for mepolizumab alone. 6.4% were eligible for both mepolizumab and omalizumab, 3.5% were eligible for both mepolizumab and benralizumab, 7% were eligible for mepolizumab, benralizumab and reslizumab, 2.3% were eligible for mepolizumab, benralizumab and omalizumab.
Discussion
This study found that many patients with SA in Brazil were eligible for approved biologics, and that in most of the eligible patients, there was more than one biologic treatment option. Our study also gave insights into SA patients in Brazil, based on the ProAR Cohort, contributing to the knowledge about SA in Brazil.
In the current study, patients with SA as per the modified ERS/ATS 2014 criteria were predominantly female, overweight/obese, in the 5th decade of life, with mean age of onset of symptoms at 15 years, confirming an older population with long-lasting asthma. Our cohort is therefore comparable to other SA cohorts (Citation22–24), where the mean age of patients with SA was also over 50 years. A recent cross-sectional review of the ProAR cohort compared all subjects using different classification criteria, and reclassified patients according to ATS 2000 severe asthma criteria (Citation11), criteria proposed to WHO in 2010 and ERS/ATS criteria 2014. Using rigorously the criteria proposed by ERS/ATS 2014 for SA, patients were required to be followed up for ≥ 1 year using high dose of inhaled corticosteroids combined with other controller medications to be considered as such. Only 88 subjects were identified by this criterion (Citation25), as many did not have ≥ 6 months of regular treatment. The novel GINA strategy for management of SA proposes 3 to 6 months of treatment in a reference center before considering biologics for the treatment. In the present study we decided to modify the ERS/ATS 2014 criteria to make it compatible to GINA proposal for considering the use of biologics for SA. There were no significant differences in baseline characteristics of the 88 patients who met the unmodified criteria in comparison to the 82 who met the modified criteria, and hence we had decided to include the entire 172 patients since the results should not be impacted. In terms of modification of the ERS/ATS criteria, the only modification made was that the duration of medication recorded was 6 months instead of 12; as such this does not require a validation dataset since this duration of medication follow-up is used for classification of severe asthma in other international asthma guidelines such as GINA.
Almost 60% patients classified as having SA are eligible for biological therapies, suggesting that personalized therapy with appropriate biologics may further enhance asthma control and disease outcomes in Brazil. A higher portion of patients with SA was eligible for biologics than in the recent IDEAL study assessing eligibility of biologics in European, North American and Australian populations where 24% − 35% of patients were eligible for therapies (Citation26). This difference is likely driven by the differences in IgE and eosinophil levels between the two studies. The mean number of blood eosinophils observed in this cohort (310 cells/uL) was much higher than that observed in the IDEAL study (186 cells/uL). The mean number of eosinophils of 310 cells/µL and levels of total IgE above the reference value (160 IU/mL) in most patients (66.1%) suggest an eosinophilic and atopic inflammatory profile in a large proportion this group. We further investigated these results looking at the records of a systematic parasitic stool test performed in all subjects, which was negative in 76% of patients, and the mean IgE levels were still elevated in the group with a negative stool test.
Our study showed that the eligibility for the different biologics can be quite varied, even for biologics potentially targeting the same mechanism, e.g.IL5. Of the 103 patients eligible for biologics, only 10 patients were eligible for all 4 therapies that we examined. As expected, a large portion of those patients eligible for anti-IgE therapy was only eligible for omalizumab (35 out of 60 patients) and 42% of patients eligible for omalizumab were also eligible for mepolizumab but fewer patients who were eligible for omalizumab were also eligible for reslizumab, which is comparable to the IDEAL study. Our study adds information to the previous literature since the IDEAL study did not assess eligibility for benralizumab. Within the anti-IL5 therapies, due to the difference in the eosinophil cutoffs, it was interesting to note that all patients eligible for benralizumab were also eligible for another biologic, whereas this was not the case for mepolizumab or reslizumab, although the latter is not currently marketed in Brazil. The lack of overlap in eligibility suggests that careful consideration in the prescription of biologics is required and that patients and physicians would further benefit from different choices within the healthcare system. Conversely, the overlap between eligibility for different therapies seen in a subgroup of patients would suggest that some patients may benefit from one biologic even where they do not respond to another, which has been the case with biologic therapies in other diseases such as Rheumatoid arthritis (Citation27).
One key finding of our study was the poor level of asthma control among SA patients despite use of high doses of ICS plus a second controller. People with SA in the international U-BIOPRED cohort also had poorly controlled asthma, showing a poor response to current therapy and higher burden of disease, in which therapeutic alternatives are urgently needed (Citation22). A high frequency of patients with SA who do not respond satisfactorily to nonspecific treatment with ICS plus other controllers has also been reported in previous studies (Citation28–30).
Many of the patients in ProAR recognized as having uncontrolled asthma may not truly have SA, as there may be modifiable factors worsening the disease (Citation31). One of these factors is the presence of other chronic morbidities in addition to asthma (Citation32,Citation33), of which there was a high incidence among the individuals with ERS/ATS 2014 SA, corroborating the findings of Hekking (Citation34).
Uncontrolled asthma predisposes to asthma exacerbations, which is an important morbidity indicator and can be life-threatening for asthma patients. Eligibility for most biologics is only considered when patients have either experienced 1 or 2 exacerbations in the previous 12 months. This study showed a higher proportion of patient (78%) that had at least one asthma exacerbation in the previous year. Indeed, the literature also points to a high number of SA patients experiencing exacerbations in other cohorts, with 41% to 83% of SA patients reporting at least one exacerbation in the last year in these studies (Citation22–24,Citation28,Citation32). Although the frequency reported varied among studies, probably because of methodological differences and definition of exacerbations, all studies report that these patients are experiencing a high frequency of exacerbations, highlighting what is still an unmet need for these patients.
Other factors that may influence asthma control are adherence to treatment and an adequate inhaler technique, which are crucial to deliver the right amount of drug to the lungs. Previous studies have described an association between the use of multiple inhalers or devices and non-consented switches of inhalers, with higher rates of non-adherence (Citation35,Citation36). Most of the ERS/ATS 2014 SA patients (>80%) in our study used at least two different inhalers (one device for the ICS and another for the ICS plus LABA), bringing complexity to the therapeutic approach, potentially affecting treatment adherence and, ultimately, asthma control.
Our study has some limitations. Most of these data, including exacerbations, were self-reported by the patient and thus may be subject to recall bias. However, the higher prevalence of some comorbidities (e.g. hypertension and GERD) and higher frequency of poorer asthma control observed in patients classified by ERS/ATS 2014 were consistent with patients that require higher doses of ICS. We did not specifically measure patient adherence in this study and cannot exclude noncompliance as a confounder in disease classification. Also, there is no gold standard for the assessment of asthma severity (Citation37,Citation38). We did not evaluate dupilumab, the anti-IL4/13 receptor monoclonal antibody, as it was not an approved therapy and therefore had no label at the time of conducting our analyses. Despite these limitations, this study provides meaningful data to understand the clinical features of Brazilian SA patients according to an alternative definition, and that still do not have their main treatment needs met, considering currently available medicines.
Conclusions
This real-world study provides a unique opportunity to explore the characteristics of SA and eligibility for biologics in Brazil. The study confirms a large proportion of subjects with SA are eligible for add-on biological therapies, but none of them had access to this form of treatment in our cohort. Almost half of them were not eligible to any of the approved biologics, however. Eligibility for biologics needs to be carefully considered as some patients may only be eligible for one type of anti-IL5 therapy or for anti-IgE therapy exclusively. Conversely, overlap between biological therapy eligibility would suggest where patients do not respond to one therapy, they may benefit from trial of another therapy although further research needs to be carried out in this regard. Overall, this emphasizes the need for a more personalized approach, targeting better disease outcomes and improved quality of life for the patient. While there is a need to increase access for some of those eligible that may really need a biologic treatment, continuous efforts are required to develop alternatives to those who are not eligible to approved options.
Acknowledgements
The authors wish to acknowledge Sofia Castro, GSK intern, Ronan Valladares, GSK complementary employee. Editorial support in the form of copyediting was provided by Kate Hollingworth of Continuous Improvement Ltd and was funded by GlaxoSmithKline (GSK).
Declaration of interest
LMM, AAC, and ALM received grants from GSK for conducting this study. KPV, FMS, LTMS, MLK and CDT are employees of GSK. EL, DM, CRS and JR are employees of GSK and hold shares. GAA is a GSK complementary employee. GPP, EP and VB do not have conflicts of interest to declare. AAC declares: consultancy, advisory board attendance, and speaker’s bureau attendance for AstraZeneca, Ciraccia, Novartis, and Boehringer Ingelheim; consultancy and speaker’s bureau attendance for Eurofarma and Chiesi; advisory board attendance for Roche and Sanofi; compensation received as a trial investigator for Sanofi and MSD; grants received from National Institutes of Health, National Institute for Health Research, and National Research Council of Brazil (CNPq); positions held as Full Professor of Medicine (officially retired but working under a voluntary agreement) at Federal University of Bahia, Executive Director of ProAR Foundation, member of the Board of Directors of GINA, and member of the Planning Group of WHO/GARD.
Data availability
To request access to patient-level data and documents for this study, please submit an enquiry via www.clinicalstudydatarequest.com.
Additional information
Funding
References
- GINA. Global Initiave for Asthma. Global Strategy for Asthma Management and Prevention 2018. [accessed Sept 2019]. www.ginasthma.org.
- Pearce N, Ait-Khaled N, Beasley R, Mallol J, Keil U, Mitchell E, Robertson C, and the ISAAC Phase Three Study Group. Worldwide trends in the prevalence of asthma symptoms: phase III of the International Study of Asthma and Allergies in Childhood (ISAAC. Thorax. 2007;62(9):758–766. doi:10.1136/thx.2006.070169.
- Sunyer J, Anto JM, Tobias A, Burney P. Generational increase of self-reported first attack of asthma in fifteen industrialized countries. European Community Respiratory Health Study (ECRHS). Eur Respir J. 1999;14:885–891. doi:10.1034/j.1399-3003.1999.14d26.x.
- Deliu M, Yavuz TS, Sperrin M, Belgrave D, Sahiner UM, Sackesen C, Kalayci O, Custovic A. Features of asthma which provide meaningful insights for understanding the disease heterogeneity. Clin Exp Allergy. 2018;48(1):39–47. doi:10.1111/cea.13014.
- Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D'Agostino R, Castro M, Curran-Everett D, Fitzpatrick AM, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181(4):315–323. doi:10.1164/rccm.200906-0896OC.
- Hekking PP, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896–902. doi:10.1016/j.jaci.2014.08.042.
- Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Resp J. 2014;43(2):343–373. doi:10.1183/09031936.00202013.
- Bousquet J, Mantzouranis E, Cruz AA, Ait-Khaled N, Baena-Cagnani CE, Bleecker ER, Brightling CE, Burney P, Bush A, Busse WW, et al. Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization Consultation on Severe Asthma. J Allergy Clin Immunol. 2010;126(5):926–938. doi:10.1016/j.jaci.2010.07.019.
- Chung KF, Godard P, Adelroth E, Ayres J, Barnes N, Barnes P, Bel E, Burney P, Chanez P, Connett G, et al. Difficult/therapy-resistant asthma: the need for an integrated approach to define clinical phenotypes, evaluate risk factors, understand pathophysiology and find novel therapies. ERS Task Force on Difficult/Therapy-Resistant Asthma. Eur Respir J. 1999;13:1198–1208. doi:10.1034/j.1399-3003.1999.13e43.x.
- Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, Casale TB, Chanez P, Enright PL, Gibson PG, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59–99. doi:10.1164/rccm.200801-060ST.
- Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. American Thoracic Society. Am J Respir Crit Care Med. 2000;162:2341–2351. doi:10.1164/ajrccm.162.6.ats9-00.
- Godar M, Blanchetot C, de Haard H, Lambrecht BN, Brusselle G. Personalized medicine with biologics for severe type 2 asthma: current status and future prospects. MAbs. 2018;10(1):34–45. doi:10.1080/19420862.2017.1392425.
- Viswanathan RK, Busse WW. Biologic therapy and asthma. Semin Respir Crit Care Med. 2018;39(01):100–114. doi:10.1055/s-0037-1606218.
- Pepper AN, Renz H, Casale TB, Garn H. Biologic therapy and novel molecular targets of severe asthma. J Allergy Clin Immunol Pract. 2017;5(4):909–916. doi:10.1016/j.jaip.2017.04.038.
- Lougheed MD. Choosing wisely in the era of biologics for asthma. Ann Allergy Asthma Immunol. 2018;120(4):345–346. doi:10.1016/j.anai.2017.11.013.
- Ponte E, Franco RA, Souza-Machado A, Souza-Machado C, Cruz ÁA. Impacto de um programa para o controle da asma grave na utilização de recursos do Sistema Único de Saúde. J Bras Pneumol. 2007;33(1):15–19. doi:10.1590/S1806-37132007000100006.
- Ponte EV, Almeida PCA, Biao-Lima V, Figueiredo CA, Riley JH, Myles D, Bansal AT, Souza-Machado A, Group PS. Cross-sectional analysis of adults with previously untreated severe asthma (SA) followed up aftertreatment: A Brazilian severe asthma cohort (ProAR). Am J Respir Crit Care Med. 2018;197:A1382. doi:10.1164/ajrccm-conference.2018.197.1_MeetingAbstracts.A1382.
- National Institutes of Health NH, Lung, and Blood Institute. Practical guide for the diagnosis and management of asthma. National Asthma Education Prevention Program. 1997. [last accessed Sept 2019]. https://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln_archive.pdf.
- Lima-Matos A, Ponte EV, de Jesus JPV, Almeida PCA, Lima VB, Kwon N, Riley J, de Mello LM, Cruz ÁA. Eosinophilic asthma, according to a blood eosinophil criterion, is associated with disease severity and lack of control among underprivileged urban Brazilians. Respir Med. 2018;145:95–100. doi:. doi:10.1016/j.rmed.2018.10.025.
- Juniper EF, Svensson K, Mork AC, Stahl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med. 2005;99(5):553–558. doi:10.1016/j.rmed.2004.10.008.
- Koshak EA. Classification of asthma according to revised 2006 GINA: evolution from severity to control. Ann Thorac Med. 2007;2(2):45–46. doi:10.4103/1817-1737.32228.
- Shaw DE, Sousa AR, Fowler SJ, Fleming LJ, Roberts G, Corfield J, Pandis I, Bansal AT, Bel EH, Auffray C, et al. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur Respir J. 2015;46(5):1308–1321. doi:10.1183/13993003.50779-2015.
- Heffler E, Blasi F, Latorre M, Menzella F, Paggiaro P, Pelaia G, Senna G, Canonica GW, Barbuto S, Bradicich M, et al. The Severe Asthma Network in Italy: Findings and Perspectives. J Allergy Clin Immunol Pract. 2018;7(5):1462–1468. doi:https://doi.org/ doi:10.1016/j.jaip.2018.10.016.
- Kim MH, Kim SH, Park SY, Ban GY, Kim JH, Jung JW, Moon JY, Song WJ, Kwon HS, Kwon JW, et al. Characteristics of adult severe refractory asthma in korea analyzed from the severe asthma registry. Allergy Asthma Immunol Res. 2019;11(1):43–54. doi:10.4168/aair.2019.11.1.43.
- Alves AM, Marques de Mello L, Lima Matos AS, Cruz AA. Severe asthma: Comparison of different classifications of severity and control. Respir Med. 2019;156:1–7. doi:10.1016/j.rmed.2019.07.015.
- Albers FC, Mullerova H, Gunsoy NB, Shin JY, Nelsen LM, Bradford ES, Cockle SM, Suruki RY. Biologic treatment eligibility for real-world patients with severe asthma: The IDEAL study. J Asthma. 2018;55(2):152–160. doi:10.1080/02770903.2017.1322611.
- Hyrich KL, Lunt M, Watson KD, Symmons DP, Silman AJ, British Society for Rheumatology Biologics Register. Outcomes after switching from one anti-tumor necrosis factor alpha agent to a second anti-tumor necrosis factor alpha agent in patients with rheumatoid arthritis: results from a large UK national cohort study. Arthritis Rheum. 2007;56(1):13–20. doi:10.1002/art.22331.
- Chipps BE, Haselkorn T, Paknis B, Ortiz B, Bleecker ER, Kianifard F, Foreman AJ, Szefler SJ, Zeiger RS. More than a decade follow-up in patients with severe or difficult-to-treat asthma: The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) II. J Allergy Clin Immunol. 2018;141(5):1590–1597. e1599. doi:10.1016/j.jaci.2017.07.014.
- Jang AS. Steroid response in refractory asthmatics. Korean J Intern Med. 2012;27(2):143–148. doi:10.3904/kjim.2012.27.2.143.
- Athanazio R, Carvalho-Pinto R, Fernandes FL, Rached S, Rabe K, Cukier A, Stelmach R. Can severe asthmatic patients achieve asthma control? A systematic approach in patients with difficult to control asthma followed in a specialized clinic. BMC Pulm Med. 2016;16(1):153. doi:10.1186/s12890-016-0314-1.
- Tay TR, Hew M. Comorbid “treatable traits” in difficult asthma: Current evidence and clinical evaluation. Allergy. 2018;73(7):1369–82. doi:10.1111/all.13370.
- Chung KF. Diagnosis and Management of Severe Asthma. Semin Respir Crit Care Med. 2018;39(1):91–99. doi:10.1055/s-0037-1607391.
- Marques de Mello L, Cruz ÁA. A proposed scheme to cope with comorbidities in asthma. Pulm Pharmacol Ther. 2018;52:41–51. doi:. doi:10.1016/j.pupt.2018.08.005.
- Hekking PP, Amelink M, Wener RR, Bouvy ML, Bel EH. Comorbidities in difficult-to-control asthma. J Allergy Clin Immunol Pract. 2018;6(1):108–113. doi:10.1016/j.jaip.2017.06.008.
- Mäkelä MJ, Backer V, Hedegaard M, Larsson K. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med. 2013;107(10):1481–1490. doi:10.1016/j.rmed.2013.04.005.
- Björnsdóttir US, Gizurarson S, Sabale U. Potential negative consequences of non-consented switch of inhaled medications and devices in asthma patients. Int J Clin Pract. 2013;67(9):904–910. doi:10.1111/ijcp.12202.
- Colice GL. Categorizing asthma severity: an overview of national guidelines; 2004. [last accessed Sept 2019]. https://www.ncbi.nlm.nih.gov/pubmed/15931352.
- Lommatzsch M, Virchow JC. Severe asthma: definition, diagnosis and treatment. Dtsch Arztebl Int. 2014;111:847–855. doi:10.3238/arztebl.2014.0847.