Abstract
Objective
Daily inhaled corticosteroid (ICS) and long-acting beta-2-agonist (LABA) combinations comprising either regular maintenance therapy with ICS/LABA plus as-needed short-acting beta-2-agonist (SABA) or ICS-formoterol combinations used as maintenance and reliever therapy (MART) are recommended for moderate asthma. This analysis compares the direct costs of twice-daily fluticasone propionate/salmeterol (FP/salm) and budesonide/formoterol MART in three Southeast Asian countries.
Methods
A literature review identified three randomized trials in patients with asthma (≥ 12 years) comparing regular twice-daily FP/salm with as-needed SABA versus MART in moderate asthma: AHEAD (NCT00242775/17 countries/2309 patients), COMPASS (AstraZeneca study SD-039-0735/16 countries/3335 patients), and COSMOS (AstraZeneca study SD-039-0691/16 countries/2143 patients). Economic analyses, conducted from a healthcare sector perspective (medication costs + healthcare utilization costs), applied unit costs from countries where healthcare costs are publicly available: Indonesia, Thailand and Vietnam. Results are expressed in British pound sterling (GBP/patient/year).
Results
Annual exacerbation rates were low and differences between treatment strategies were small (range, FP/salm: 0.31–0.38, MART: 0.24–0.25) although statistically significant in favor of MART. Total average (minimum-maximum) direct costs (in GBP/patient/year) across the three studies were £187 (£137–£284), £158 (£125–£190), and £151 (£141–£164) for those who used FP/salm, and £242 (£217–£267), £284 (£237–£340) and £266 (£224–£315) for MART in Indonesia, Thailand and Vietnam, respectively. On average, total direct costs/patient/year with FP/salm were 22.8%, 44.6% and 43.0% lower than with MART for Indonesia, Thailand and Vietnam, respectively.
Conclusions
In the three countries evaluated, total treatment costs with regular twice-daily FP/salm were consistently lower than with budesonide/formoterol MART due to lower direct healthcare costs.
Introduction
The severity of a patient’s asthma is based on the level of treatment required to control symptoms and to minimize the risk of exacerbations and, in this context, moderate asthma is defined as asthma that is well controlled with low dose daily inhaled corticosteroid (ICS) and long-acting beta-2-agonist (LABA) combinations comprising either regular maintenance therapy with ICS/LABA plus as-needed short-acting beta-2-agonist (SABA) as reliever therapy or ICS-formoterol combinations used as both maintenance and reliever therapy (MART) (Citation1). Approximately 10–30% of patients with asthma are reported to experience exacerbations (Citation2–4). Although poor asthma control and risk of exacerbation are related, they are not exactly concordant (Citation5) and it is therefore important to assess both separately when considering treatment options.
In clinical practice, choice of treatment is based on evidence of efficacy and safety, cost, and individual patient factors (Citation1). The efficacy and safety of regular maintenance therapy with the ICS/LABA combination fluticasone propionate/salmeterol (FP/salm) has been well-established in patients with moderate to severe asthma (Citation6–15). MART treatment regimen with the combination of budesonide and formoterol has well-described efficacy in reducing the risk of severe exacerbations in studies of patients with moderate to severe asthma and a history of exacerbations (Citation16,Citation17).
In Southeast Asia, chronic respiratory disease is a leading cause of morbidity and mortality, imposing a huge economic burden (Citation18). The cost of healthcare, including access to cost-effective medications, is an important aspect of tackling this burden (Citation18). Previous studies comparing the costs of the FP/salm and MART regimens have mainly focused on high-income countries in Europe (Citation19–22), Canada (Citation23) and Australia (Citation21). To date, cost comparison data have been reported rarely for middle- or lower middle-income countries. A cost comparison in Thailand based on one head-to-head study reported slightly lower drug costs with the FP/salm compared with the MART regimen and similar overall direct costs (Citation24).
The aim of this analysis was to compare the direct costs of FP/salm and MART in three Southeast Asian countries (Indonesia, Thailand and Vietnam) based on published data from three RCTs where the two treatments have been compared head-to-head (Citation25–27). These countries were selected since the cost data for medications and health resources are publicly available. They may also be considered to be a representative sample of Southeast Asia in terms of population diversity, development and access to healthcare and medicines.
Methods
Clinical trials
A literature review was performed using Pubmed to identify RCTs comparing regular twice-daily FP/salm plus as needed SABA (hereafter referred to as FP/salm) versus budesonide/formoterol MART (hereafter referred to as MART) in adults and adolescents aged ≥12 years with asthma. Pubmed search terms included, “salmeterol/fluticasone”; “sal/flu”; “maintenance and reliever therapy”; “MART”; “randomized controlled trials”; “RCTs”. Studies that recorded resource utilization data prospectively were identified, namely the AHEAD (NCT00242775 (Citation25), COMPASS study (AstraZeneca study code SD-039-0735) (Citation26) and COSMOS (AstraZeneca study code SD-039-0691) (Citation27) trials. A summary of the study designs for each trial is shown in . AHEAD and COMPASS were double-blind, 6-month studies and COSMOS was an open-label 12-month study. The patient populations were similar across studies (mean baseline percent of predicted FEV1 71% to 73%). For this analysis, only data from the relevant FP/salm and MART treatment arms have been included.
Table 1. Study design of the three clinical studies.
Resource utilization data
During all studies, the following resource use data were collected via patient notebooks or event logs: hospitalizations (intensive care and general ward), emergency room (ER) visits, and other healthcare visits including general practitioner (GP), specialist, physiotherapist and nurse (Citation25–27). Scheduled study visits were excluded from the cost analyses. Medication use was either recorded in daily diaries and checked by the investigators at study visits (AHEAD and COMPASS) (Citation25,Citation26), or retrospective patient-reported medication use during the preceding 2 weeks recorded at clinic visits (COSMOS) (Citation27). The dosing regimen for FP/salm and MART differed for the three studies in terms of medication inhalations per day as shown in . In addition, dose titrations were permitted in COSMOS but not in AHEAD or COMPASS.
Data concerning resource use were pooled across participating countries for each study and, for comparative purposes, data from the 6-month studies were extrapolated to 12 months - as reported previously (Citation22).
Economic analysis
Unit costs of branded medications and healthcare services in individual countries were obtained from publicly available sources (Citation24,Citation28–39) (). Where available, costs were based on the licensed dose (i.e. the dose approved in each country) and 60-dose (dry powder inhaler) or 120-dose (metered dose inhaler) packs, and were expressed as per dose/puff costs. In Thailand, terbutaline cost was not available so SABA costs were based on cost of salbutamol. In Vietnam, FP/salm 100/50 (i.e. dry powder device) cost was not available, and per unit cost was assumed to be twice that of the FP/salm 125/25 (i.e. metered dose inhaler) unit cost, since these are clinically equivalent. Where the cost of health care visits for ‘other health care personnel’ (physiotherapist, occupational therapist or similar professional) was unavailable in the specific country, this was priced as the average cost of a GP visit and a visit to a physiotherapist.
Table 2. Country specific unit costs (2019, conversion to British pound sterling (GBP)).
Unit costs in local currency were converted to British pound sterling (GBP) using the 2019 average exchange rate (Citation28). An expert in each country provided external validity of individual costings. Total direct costs (including medication and non-medication costs) per patient per year were calculated for each type of healthcare resource by multiplying the frequency of use over one year by unit costs for each country.
Several univariate sensitivity analyses were performed to test the robustness of the results in this analysis. The first sensitivity analysis, examined the impact on results of changing the unit cost of hospitalizations. In the main analyses, the differentiated unit cost at an intensive care unit and general ward was used. In the first sensitivity analysis, the average cost of ICU and general ward was used for hospitalization costs. In the second sensitivity analysis, the unit cost for a visit to a specialist was set equal to the unit cost for a GP visit. This was done to reflect clinical practice, where patients most often visit their GP with respect to their asthma. Finally, in the last two analyses, the unit costs for hospitalizations, health care visits and home visits were increased or decreased by 10%.
A sensitivity analysis was also performed by varying the health care unit costs by 10% or 20% to give estimated boundaries for the total costs.
Results
Efficacy
In the three comparator studies between FP/salm and MART selected because they could provide health economic data for this analysis, the primary endpoint, time to first severe exacerbation, was significantly prolonged in patients using MART compared with FP/salm in the COSMOS and COMPASS studies (Citation26,Citation27) with no significant difference between treatments shown in the AHEAD study (Citation25). The yearly exacerbation rate ranged from 0.24 to 0.25 for the MART and 0.31–0.38 for FP/salm treatment. Both treatments resulted in similar improvements in asthma control scores, asthma symptoms and lung function.
Resource utilization data
Overall, healthcare resource use was relatively low in all three studies (). The most common healthcare use in all studies were visits to GPs and specialists and there were no notable differences between treatments in the frequency of these visits.
Table 3. Average resource use per patient per year by treatment option in each study.
Economic analysis
Average total direct costs per patient per year were consistently lower for those who used FP/salm compared with MART, with one exception (Indonesia/COMPASS study) (; ). Total average (minimum-maximum) direct costs (in GBP/patient/year) across the three studies were £187 (£137–£284), £158 (£125–£190), and £151 (£141–£164) for those who used FP/salm, and £242 (£217–£267), £284 (£237–£340) and £266 (£224–£315) for MART in Indonesia, Thailand and Vietnam, respectively. On average, total costs per-patient per-year were 22.8%, 44.6% and 43.0% lower with FP/salm than MART for Indonesia, Thailand and Vietnam, respectively ().
Figure 1. Average total direct costs by treatment option in each study in (a) Indonesia, (b) Thailand and (c) Vietnam.
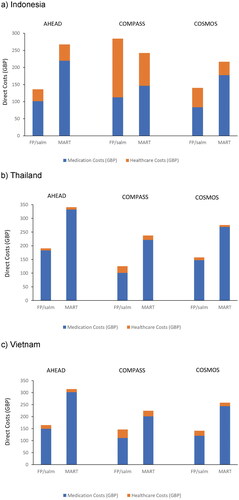
Figure 2. Average total direct costs by treatment option across all studies in Indonesia, Thailand and Vietnam.
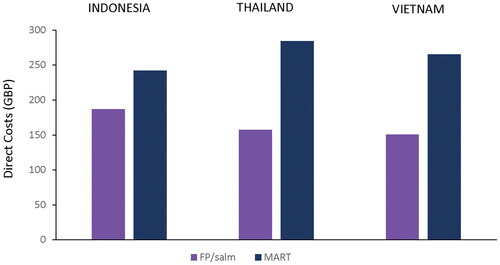
Table 4. Average total direct costs by treatment option in each study by country.
These differences were largely driven by the medication costs of each treatment regimen as in all countries the cost of MART treatment was higher than FP/salm treatment, whereas healthcare costs were generally higher with FP/salm versus MART treatment (). The exceptions to this were that healthcare costs in Indonesia and Thailand in the AHEAD study were slightly lower with FP/salm treatment versus MART.
The robustness of the presented analyses was tested by varying several parameters which could have impacted the unit costs of either hospitalizations or other healthcare visits. In each of the four sensitivity analyses conducted, the results of the analysis and conclusions remained unchanged.
Discussion
The results of this analysis showed that, overall, regular twice-daily FP/salm was associated with lower direct healthcare costs compared with MART, despite the slightly higher exacerbation rates, and these findings were largely consistent across the three studies and the three countries evaluated. In general, healthcare resource use was low in all three studies, in keeping with the relatively low reported rates of severe exacerbations and accounting for the observation that differences in total direct costs were driven by the higher costs of MART than FP/salm treatment regimens.
Asthma-related costs impose a high burden on individual countries and costs differ between countries depending on several factors such as a country’s gross domestic product (GDP), type of healthcare system and public health resources (Citation40). Studies reporting the economic costs of the FP/salm and MART regimens in high-income countries, have shown less marked differences between treatments in terms of total direct costs, and no trend on one direction across studies, probably related to their different healthcare systems and drug pricing criteria (Citation19–23). A previous analysis of cost data in Thailand based on the COMPASS study reported slightly lower drug costs with the FP/salm versus MART regimen and similar overall direct costs (Citation24). Payers in Southeast Asian countries define cost thresholds or economic evaluation criteria relevant and specific to their healthcare system. In Indonesia, there is a single-payer, universal healthcare program called the National Health Insurance System (Jaminan Kesehatan Nasional – JKN) which provides cover for the majority of the population (Citation41). In Thailand, universal healthcare is provided and reimbursed through three government schemes, and direct medical costs and specifically drug costs are an important consideration when prescribing medication. Vietnam has a tiered health system and per patient visit cost thresholds are applicable within all medical care institutions other than the largest, regional central hospitals. In order to fall within thresholds, physicians may reduce the time between patient visits to reduce medication costs.
Our goal in this analysis was to reflect clinical practice, drug usage and costing; therefore, the real-world price of treatments in each country were used in our calculations. The difference in drug acquisition cost was a key driver of the overall cost presented per regime in this study, and these data will provide important information to decision makers. Both physicians and payers have to weigh up the overall evidence based on clinical data (efficacy, safety) and costs. In the three countries evaluated in this analysis, local asthma guidelines recommend both regular ICS/LABA and MART as preferred treatment options for moderate/severe asthma (Citation42–44). In a recent survey of physicians in Asia, in addition to treatment guidelines and physician’s personal experience, patient affordability of treatment was identified as an important factor influencing the choice of treatment by physicians for patients with asthma and co-existent rhinitis (Citation45). Although hospitalizations due to asthma pose a significant cost, medications are the major contributor to overall costs of maintenance-treatment asthma management (Citation1,Citation46), since there are many patients with asthma but only a relative minority experience hospital admission (Citation4,Citation46). The data from our analysis showed that costs of medication was the most important driver of direct costs.
In the three studies that could be included in this analysis, differences in yearly exacerbation rates were very small but were significantly in favor of the MART strategy (Citation25–27). Even in these studies where MART was associated with lower exacerbation rates, direct healthcare costs were lower with FP/salm. Across a range of clinical studies, superior benefit of MART is not consistently demonstrated (Citation15,Citation16,Citation47–51). The cost advantage of FP/salm reported in this analysis, may therefore also apply more widely, dependent on local medication and treatment costs. In future research, inclusion of a budget impact analysis of different treatment regimens for asthma with country specific epidemiological data, or factoring asthma control into healthcare cost computations would be of added interest.
A main limitation of this analysis is that we could not include indirect costs associated with asthma, such as absenteeism, because of difficulties in obtaining consistent estimates of unit costs in the countries included in this analysis; instead we focused on direct costs associated with healthcare resource use and medication costs. Even though the countries included in this analysis were similar in terms of populations, development and access to healthcare, the costing data with these three countries in Southeast Asia may not be representative more extensively. However, our analysis prepares the way for future such research. While the majority of unit costs data were obtained from publicly available, verifiable resources, we were required to make a limited number of assumptions about individual unit costings where a particular drug or drug formulation was not available in a particular country. However, these were rational assumptions based on clinically equivalent alternatives, and were validated with a local expert in the relevant country, as is a standard practice for these types of healthcare cost studies. A further criticism may be the lack of inclusion of recent clinical trials; all of those included here were undertaken over a decade ago, although the approach in clinical practice and treatment guideline recommendations for moderate asthma are largely the same (Citation1,Citation51). Another limitation is that resource use data were based on clinical trials only, using data collected in notebooks or event logs, although this is commonly used in cost-effectiveness analyses and reported to have high internal validity (Citation22). The same resource use data have been used and reported in several other cost analyses publications based on these studies (Citation19–24). With respect to external validity, the three countries included in this analysis participated in the AHEAD study, Thailand and Vietnam participated in the COMPASS study, and Vietnam participated in the COSMOS study. The external validity of these data was also provided by advice taken from experts in each country. While the use of clinical trials data was helpful at standardizing data collection across multiple geographies, further data collection should be done to measure the real-world impact of different treatment approaches in asthma.
Conclusion
This study showed that, overall, treatment with regular twice-daily FP/salm is associated with lower direct healthcare costs than budesonide/formoterol MART. This difference was consistent across the three countries evaluated. These data provide important information for both physicians and payers with respect to treatment decisions for patients with moderate asthma in Indonesia, Thailand and Vietnam.
Acknowledgements
The authors would like to thank Wenny Yang (GSK, Indonesia), Torsak Bunupuradah (GSK, Thailand) and Hoang Chan Huynh (GSK, Vietnam) for providing local data and helpful comments on the manuscript, and Aman Kapil Butta (GSK, Singapore) for reviewing this manuscript. Editorial support was provided by Kate Hollingworth of Continuous Improvement Ltd.
Declaration of interest
BA, PWJ, AI and SA are employees of GSK and hold GSK stocks/shares. WB has received personal fees from AstraZeneca Thailand, Boehringer Ingelheim and Thai Otsuka. FY and LTTL report no conflicts of interest.
Availability of data and materials
Information on GSK’s data sharing commitments and requesting access can be found at: https://www.clinicalstudydatarequest.com.
References
- Global Initiative for Asthma. Global strategy for asthma management and prevention; 2020. Available from: https//www.ginaasthma.org [last accessed 15 July 2020].
- Dougherty RH, Fahy JV. Acute exacerbations of asthma: epidemiology, biology and the exacerbation-prone phenotype. Clin Exp Allergy. 2009;39(2):193–202. doi:https://doi.org/10.1111/j.1365-2222.2008.03157.x.
- Woodcock A, Vestbo J, Bakerly ND, New J, Gibson JM, McCorkindale S, Jones R, Collier S, Lay-Flurrie J, Frith L, et al. Effectiveness of fluticasone furoate plus vilanterol on asthma control in clinical practice: an open-label, parallel group, randomised controlled trial. Lancet. 2017;390(10109):2247–2255. doi:https://doi.org/10.1016/S0140-6736(17)32397-8.
- Gibbons DC, Aggarwal B, Fairburn-Beech J, Hinds D, Fletcher M, Bosnic-Anticevich S, Price D. Treatment patterns among non-active users of maintenance asthma medication in the United Kingdom: a retrospective cohort study in the Clinical Practice Research Datalink. J Asthma. 2020:1–12. doi:https://doi.org/10.1080/02770903.2020.1728767.
- Blakey JD, Woolnough K, Fellows J, Walker S, Thomas M, Pavord ID. Assessing the risk of attack in the management of asthma: a review and proposal for revision of the current control-centred paradigm. Prim Care Respir J. 2013;22(3):344–352. doi:https://doi.org/10.4104/pcrj.2013.00063.
- Jenkins C, Woolcock AJ, Saarelainen P, Lundback B, James MH. Salmeterol/fluticasone propionate combination therapy 50/250 microg twice daily is more effective than budesonide 800 microg twice daily in treating moderate to severe asthma. Respir Med. 2000;94(7):715–723. doi:https://doi.org/10.1053/rmed.2000.0875.
- Juniper EF, Jenkins C, Price MJ, James MH. Impact of inhaled salmeterol/fluticasone propionate combination product versus budesonide on the health-related quality of life of patients with asthma. Am J Respir Med. 2002;1(6):435–440. doi:https://doi.org/10.1007/BF03257170.
- Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJH, Pauwels RA, Pedersen SE. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004;170(8):836–844. doi:https://doi.org/10.1164/rccm.200401-033OC.
- Dahl R, Chuchalin A, Gor D, Yoxall S, Sharma R. EXCEL: a randomised trial comparing salmeterol/fluticasone propionate and formoterol/budesonide combinations in adults with persistent asthma. Respir Med. 2006;100(7):1152–1162. doi:https://doi.org/10.1016/j.rmed.2006.03.001.
- Woodcock A, Bagdonas A, Boonsawat W, Gibbs M, Bousquet J, Bateman E. Improvement in asthma endpoints when aiming for total control: salmeterol/fluticasone propionate versus fluticasone propionate alone. Prim Care Respir J. 2007;16(3):155–161. doi:https://doi.org/10.3132/pcrj.2007.00043.
- Bateman ED, Jacques L, Goldfrad C, Atienza T, Mihaescu T, Duggan M. Asthma control can be maintained when fluticasone propionate/salmeterol in a single inhaler is stepped down. J Allergy Clin Immunol. 2006;117(3):563–570. doi:https://doi.org/10.1016/j.jaci.2005.11.036.
- Bateman ED, Bousquet J, Keech ML, Busse WW, Clark TJ, Pedersen SE. The correlation between asthma control and health status: the GOAL study. Eur Respir J. 2007;29(1):56–62. doi:https://doi.org/10.1183/09031936.00128505.
- Bateman E, Bousquet J, Busse W, Clark T, Gul N, Gibbs M, Pedersen S. Stability of asthma control with regular treatment: an analysis of the Gaining Optimal Asthma controL (GOAL) study. Allergy. 2008;63(7):932–938. doi:https://doi.org/10.1111/j.1398-9995.2008.01724.x.
- Tabri NA, Supriyadi M, Yunus F, Wiyono WH. The efficacy of combination of inhalation salmeterol and fluticasone compare with budesonide inhalation to control moderate persistent asthma by the use of Asthma Control Test as evaluation tool. J Respir Indones. 2010;30(3):152–158.
- Stempel DA, Raphiou IH, Kral KM, Yeakey AM, Emmett AH, Prazma CM, Buaron KS, Pascoe SJ. Serious asthma events with fluticasone plus salmeterol versus fluticasone alone. N Engl J Med. 2016;374(19):1822–1830. doi:https://doi.org/10.1056/NEJMoa1511049.
- Cates CJ, Karner C. Combination formoterol and budesonide as maintenance and reliever therapy versus current best practice (including inhaled steroid maintenance), for chronic asthma in adults and children. Cochrane Database Syst Rev. 2013;(4):CD007313. doi:https://doi.org/10.1002/14651858.CD007313.pub3.
- Kew KM, Karner C, Mindus SM, Ferrara G. Combination formoterol and budesonide as maintenance and reliever therapy versus combination inhaler maintenance for chronic asthma in adults and children. Cochrane Database Syst Rev. 2013;(12):CD009019. doi:https://doi.org/10.1002/14651858.CD009019.pub2.
- Castillo-Carandang NT, Buenaventura RD, Chia YC, Do Van D, Lee C, Duong NL, Ng CH, Robles YR, Santoso A, Sigua HS, et al. Moving towards optimized noncommunicable disease management in the ASEAN Region: recommendations from a review and multidisciplinary expert panel. Risk Manag Healthc Policy. 2020;13:803–819. doi:https://doi.org/10.2147/RMHP.S256165.
- Johansson G, Andreasson EB, Larsson PE, Vogelmeier CF. Cost effectiveness of budesonide/formoterol for maintenance and reliever therapy versus salmeterol/fluticasone plus salbutamol in the treatment of asthma. Pharmacoeconomics. 2006;24(7):695–708. doi:https://doi.org/10.2165/00019053-200624070-00008.
- Tamminen K, Laine J, Soini E, Martikainen J, Kankaanranta H. Cost-effectiveness analysis of budesonide/formoterol maintenance and reliever therapy versus fixed combination treatments for asthma in Finland. Curr Med Res Opin. 2008;24(12):3453–3461. doi:https://doi.org/10.1185/03007990802567566.
- Price D, Wirén A, Kuna P. Cost-effectiveness of budesonide/formoterol for maintenance and reliever asthma therapy. Allergy. 2007;62(10):1189–1198. doi:https://doi.org/10.1111/j.1398-9995.2007.01466.x.
- Wickstrøm J, Dam N, Malmberg I, Hansen BB, Lange P. Cost-effectiveness of budesonide/formoterol for maintenance and reliever asthma therapy in Denmark-cost-effectiveness analysis based on five randomised controlled trials . Clin Respir J. 2009;3(3):169–180. doi:https://doi.org/10.1111/j.1752-699X.2009.00134.x.
- Miller E, Sears MR, McIvor A, Liovas A. Canadian economic evaluation of budesonide-formoterol as maintenance and reliever treatment in patients with moderate to severe asthma. Can Respir J. 2007;14(5):269–275. doi:https://doi.org/10.1155/2007/560819.
- Boonsawat W. Cost-effectiveness of budesonide/formoterol maintenance and rescue therapy in Thailand. Asian Biomed. 2010;4(4):571–578. doi:https://doi.org/10.2478/abm-2010-0072.
- Bousquet J, Boulet LP, Peters MJ, Magnussen H, Quiralte J, Martinez-Aguilar NE, Carlsheimer A. Budesonide/formoterol for maintenance and relief in uncontrolled asthma vs. high-dose salmeterol/fluticasone. Respir Med. 2007;101(12):2437–2446. doi:https://doi.org/10.1016/j.rmed.2007.07.014.
- Kuna P, Peters MJ, Manjra AI, Jorup C, Naya IP, Martínez-Jimenez NE, Buhl R. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. Int J Clin Pract. 2007;61(5):725–736. doi:https://doi.org/10.1111/j.1742-1241.2007.01338.x.
- Vogelmeier C, D’Urzo A, Pauwels R, Merino JM, Jaspal M, Boutet S, Naya I, Price D. Budesonide/formoterol maintenance and reliever therapy: an effective asthma treatment option?Eur Respir J. 2005;26(5):819–828. doi:https://doi.org/10.1183/09031936.05.00028305.
- UNCTADstat annual currency exchange rates; 2019. Available from: https://unctadstat.unctad.org/wds/TableViewer/tableView.aspx?ReportId=117[last accessed 6 July 2020].
- Peraturan Menterl Kesehatan Republik Indonesia Nomor 64 Tahun; 2016. Available from: http://hukor.kemkes.go.id/uploads/produk_hukum/PMK_No._64_ttg_Standar_Tarif_Pelayanan_Kesehatan_Dalam_Penyelenggaraan_Program_Jaminan_Kesehatan_.pdf [last accessed 15 July 2020].
- Tarif RSUP Persahabatan 2017. Available from: https://rsuppersahabatan.co.id/tarif [last accessed 15 July 2020].
- eCatalog obat 2018 v5.0. Available from: https://e-katalog.lkpp.go.id/ [last accessed 15 July 2020].
- MIMS Indonesia; 2020. Available from: https://www.mims.com/indonesia/drug/info/seretide [last accessed 6 August 2020].
- Puranitee P, Kamchaisatian W, Manuyakorn W, Vilaiyuk S, Laecha O, Pattanaprateep O, Benjaponpitak S. Direct medical cost of Thai pediatric asthma management: a pilot study. Asian Pac J Allergy Immunol. 2015;33:296–300. doi:https://doi.org/10.12932/AP0494.33.4.2015.
- Health Intervention and Technology Assessment Program (HITAP), Ministry of Public Health, Thailand; 2010. Available from: http://costingmenu.hitap.net/ [last accessed 15 July 2020].
- National drug information, Thailand, version dated 9 December 2019. Available from: http://ndi.fda.moph.go.th/drug_value/index/public/ [last accessed 15 July 2020].
- Hospital price list (current version in 2019). Ho Chi Minh City, Vietnam: University Medical Center.
- Lai CKW, Kim Y-Y, Kuo S-H, Spencer M, Williams AE. Cost of asthma in the Asia-Pacific region. Eur Respir Rev. 2006;15(98):10–16. doi:https://doi.org/10.1183/09059180.06.00009802.
- Brakema E, Nguyen Thanh L, Kasteleyn M, van der Kleij R, Hasenack B, Nguyn Nhat Q, Nguyen Lam V, Nguyen Nhu V, Diep Tuan T, Postma M, et al. Fresh air: health economic burden of asthma & COPD in Vietnam; 2020. Available from:https://old.theipcrg.org/download/attachments/38535324/Evelyn%20Brakema%20-%20FRESH%20AIR%20Health%20Economic%20Burden%20of%20Asthma%20and%20COPD%20in%20Vietnam.pdf?version=1&modificationDate=1510911834000&api=v2 [last accessed 15 July 2020].
- Daily Item Price List Report; 17 June 2020. Published by Zuellig Pharma Internal system.
- Nunes C, Pereira AM, Morais-Almeida M. Asthma costs and social impact. Asthma Res Pract. 2017;3:1. doi:https://doi.org/10.1186/s40733-016-0029-3.
- Agustina R, Dartanto T, Sitompul R, Susiloretni KA, Suparmi MKM, Achadi EL, Taher A, Wirawan F, Sungkar S, Sudarmono P, et al. Universal health coverage in Indonesia: concept, progress, and challenges. Lancet. 2019;393:75–102. doi:https://doi.org/10.1016/S0140-6736(18)31647-7.
- Perhimpunan Dokter Paru Indonesia. Penatalaksanaan Asma Stabil. Dalam: Asma. Pedoman diagnosis dan penatalaksanaan di Indonesia. Jakarta: Balai Penerbit FKUI; 2019. p. 38. (Guidelines for management of stable asthma in Indonesia; Jakarta: FKUI Publisher Center; 2019. p. 38).
- Guidelines for diagnosis and treatment of asthma in Thailand for adults; 2019. Available from: http://www.tac.or.th/index.php/en/download/category/1-guidelines [last accessed 10 August 2020].
- Guideline of clinical professional documentation in diagnosis and treatment of adults and children >12 years of age, asthma; 2020. Available from: https://kcb.vn/vanban/quyet-dinh-so-1851-qd-byt-ngay-24-thang-4-nam-2020-cua-bo-y-te-ve-viec-ban-hanh-tai-lieu-chuyen-mon-huong-dan-chan-doan-va-dieu-tri-hen-phe-quan-nguoi-lon-va-tre-em-tu-12-tuoi-tro [last accessed 7 August 2020].
- Aggarwal B, Shantakumar S, Hinds D, Mulgirigama A. Asia-Pacific Survey of Physicians on Asthma and Allergic Rhinitis (ASPAIR): physician beliefs and practices about diagnosis, assessment, and treatment of coexistent disease. J Asthma Allergy. 2018;11:293–307. doi:https://doi.org/10.2147/JAA.S180657.
- Bahadori K, Doyle-Waters MM, Marra C, Lynd L, Alasaly K, Swiston J, FitzGerald JM. Economic burden of asthma: a systematic review. BMC Pulm Med. 2009;9:24. doi:https://doi.org/10.1186/1471-2466-9-24.
- Chapman KR, Barnes NC, Greening AP, Jones PW, Pedersen S. Single maintenance and reliever therapy (SMART) of asthma: a critical appraisal. Thorax. 2010;65(8):747–752. doi:https://doi.org/10.1136/thx.2009.128504.
- Louis R, Joos G, Michils A, Vandenhoven G. A comparison of budesonide/formoterol maintenance and reliever therapy vs. conventional best practice in asthma management. Int J Clin Pract. 2009;63(10):1479–1488. doi:https://doi.org/10.1111/j.1742-1241.2009.02185.x.
- Sears MR, Boulet LP, Laviolette M, Fitzgerald JM, Bai TR, Kaplan A, Smiljanic-Georgijev N, Lee JS-M. Budesonide/formoterol maintenance and reliever therapy: impact on airway inflammation in asthma. Eur Respir J. 2008;31(5):982–989. doi:https://doi.org/10.1183/09031936.00104007.
- Demoly P, Louis R, Søes-Petersen U, Naya I, Carlsheimer A, Worth H, Almeida J, Sears MR. Budesonide/formoterol maintenance and reliever therapy versus conventional best practice. Respir Med. 2009;103(11):1623–1632. doi:https://doi.org/10.1016/j.rmed.2009.07.018.
- Rogliani P, Ritondo BL, Ora J, Cazzola M, Calzetta L. SMART and as-needed therapies in mild to severe asthma: a network meta-analysis. Eur Respir J. 2020;56(3):2000625. doi:https://doi.org/10.1183/13993003.00625-2020.
