Abstract
Objective
Pharmacological treatment plays a key role in the management of asthma, but medication adherence is generally low. Our aim was to assess factors associated with dispensing patterns of, and adherence to, asthma medication in young adults with asthma.
Methods
The study included young adults (age 22–24 years) from the Swedish population-based birth cohort BAMSE (n = 3,064) with linkage to register data on dispensed asthma medications and recorded diagnosis. Dispensing information was collected in January 2014–June 2019 (the study period) to cover the period of questionnaire data. Adherence to asthma medication was defined as refilling a prescription within 18 months.
Results
In total, 234 individuals (7.6%) had asthma (doctor’s diagnosis of asthma in combination with respiratory symptoms) and had been dispensed at least one prescription of asthma medication during the study period. Among them, 77% were dispensed a controller medication. The mean number of prescriptions dispensed per individual was higher in males than females (11.0 vs. 7.2; p < 0.01). The proportion of asthmatics with only a short-acting β2-agonist (SABA) dispensed was 22%, of which 33% were classified as having uncontrolled asthma. Adherence to controller medication was 60% and higher among those with an asthma diagnosis from specialized care than those diagnosed in primary care (RR 1.32 95% CI 1.03–1.69). Sex, socioeconomic status, and non-allergic comorbidity did not affect adherence.
Conclusion
Young adults with asthma had few prescriptions of asthma medication dispensed, indicating sub-optimal treatment. A considerable proportion was dispensed only SABA. Furthermore, adherence to controller medication was relatively low.
Keywords:
Introduction
Pharmacological treatment is key to asthma management (Citation1). Asthma medications are used for symptom relief and control and to minimize future risks. Controller medications containing inhaled corticosteroids (ICS) should be introduced (as needed or regularly) swiftly after an asthma diagnosis (Citation1–3). Overuse of short-acting β2-agonists (SABA) is associated with higher risk of exacerbation and death, also in patients with mild asthma (Citation2–6).
Generally, asthma medication adherence is low in all ages (Citation7,Citation8). A recent meta-analysis calculated the pooled prevalence of ICS adherence to 28% in young adults (aged 15–30 years) (Citation7). The World Health Organization defines adherence as “the extent to which a person’s behavior corresponds with agreed recommendations from healthcare provider” (Citation9). Medication adherence is suggested to be affected by several factors. The meta-analysis stated that personality, illness perceptions, and treatment beliefs were all identified as important predictors of ICS adherence in young adults (Citation7). A qualitative study found three key factors for improving controller medication adherence: patient knowledge about their medication, information adapted to patient needs, and a patient-centered approach in healthcare (Citation10). Also, sex is known to be associated with asthma medication dispensing patterns and controller medication adherence in children (Citation11,Citation12).
It is known that adolescents and young adults with asthma need support in disease management, beyond what is offered today (Citation13). They also need information and training to confidently self-manage their asthma during the transition into adult care (Citation13). The transition process is complex and encompasses two parts: education of the adolescents and transfer of healthcare (Citation13). One important issue is treatment adherence, known to be poor in this age group (Citation7). Simplifying medication regimens and implementing medication reminders are recommended to increase adherence (Citation13).
Healthcare utilization has been reported to be low among young adults with asthma (Citation14,Citation15). In a recent Swedish study of this group, only 37% had at least one healthcare visit within four years of their 18th birthday (Citation14). In an Australian study, 59% of adults with asthma (mean age 39 years) did not have a visit to a general practitioner within the preceding year (Citation15).
More research on why adherence to pharmacological treatment is low is needed, especially in young adults. Asthma medication adherence is known to be multifactorial, but studies on socioeconomic factors and comorbidity are scarce (Citation7,Citation10). A previous study from our group showed that adherence, measured as refilling controller medication within 18 months, was lower in girls than boys aged 0–17 years (Citation11). Although overuse of SABA has been highlighted recently (Citation1,Citation3,Citation6), it remains uncertain if SABA is used alone or combined with controller medication, especially among young adults.
Therefore, the aims of this study were to assess factors associated with dispensing patterns of, and adherence to, asthma medication in young adults and to assess SABA treatment among young adults with asthma in a population-based cohort.
Methods
Study design and population
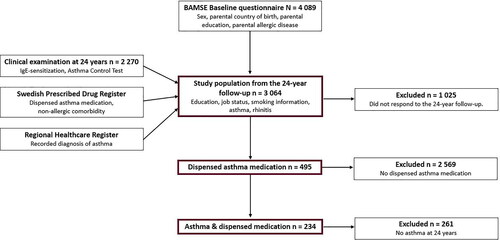
This study was based on data from the ongoing population-based birth cohort BAMSE (Barn/Children, Asthma, Milieu, Stockholm, Epidemiology) (Citation16). The BAMSE cohort encompasses 4,089 participants born in Stockholm, Sweden, in 1994–1996 (Citation16). The participants have been followed through questionnaires and clinical examinations from birth to age 24 years (Citation17,Citation18). All individuals answering the 24-year follow-up questionnaire were included in this study (n = 3,064, response rate 75% of original cohort; ). The young adults answered the questionnaire in November 2016–May 2019. The clinical examination at 24 years included blood sampling for analysis of IgE antibodies and filling out the Asthma Control Test (ACT) form.
Data sources and measurements
The questionnaire data were linked to data on dispensed medications from the Swedish Prescribed Drug Register (SPDR) (Citation19,Citation20). Dispensing information was collected in January 2014–June 2019 to cover the entire data collection period for the 24-year follow-up (the study period). SPDR includes patient-level data of all prescriptions dispensed at Swedish pharmacies, for the entire population. Data on recorded asthma diagnosis were obtained from the administrative healthcare databases in Region Stockholm (Citation11,Citation21–23). These databases contain information on both main and contributing diagnoses from inpatient care, specialized ambulatory care, and primary care. The measurements used in this study were defined based on three types of data sources: registers, questionnaires data, and clinical examination ().
Register data
Dispensed asthma medications were identified from SPDR by Anatomical Therapeutic Chemical (ATC) code: SABA (R03AC02, R03AC03); ICS (R03BA); Leukotriene receptor antagonist, LTRA (R03DC); Long-acting β2-agonist, LABA (R03AC12, R03AC13); fixed combination of ICS and LABA (R03AK). Controller medication was defined as ICS, LTRA, or fixed combination. At least one SABA, ICS, LTRA, LABA, or fixed combination was denoted “any asthma medication.”
Non-allergic comorbidity was identified from SPDR using ATC code: Drugs used in diabetics (A10), Cardiovascular system (C), or Psychoanaleptics and anxiolytics (N06, N05), with at least one dispensation during the study period.
Information on doctor’s diagnosis of asthma was obtained from the administrative healthcare databases and defined as any recorded (main or contributing) diagnosis of ICD-10 J45.
Asthma medication adherence was measured as refilling a controller medication prescription (ICS, LTRA, or fixed combination) within 18 months (Citation11,Citation24,Citation25). This definition was used to take into consideration recommendations of using controller medication only when having symptoms (Citation1,Citation25). Individuals with a first dispensing date after January 2018 were excluded if they did not refill a prescription, as their follow-up periods were under 18 months.
Questionnaire data
Parental ethnicity was defined as foreign if at least one parent was born outside Scandinavia.
Higher parental education was defined as having at least one parent with a university or college degree at baseline.
Higher education at 24 years was defined as a university or college degree or equivalent.
Employment was defined as having a job at the 24-year follow-up.
Daily smoking was defined based on response to the 24-year questionnaire (yes, daily/occasional).
Parental allergic disease was defined as having at least one parent with a doctor’s diagnosis of asthma and asthma medication and/or diagnosis of hay fever in combination with allergy to furred pets and/or pollen at baseline.
Asthma onset was defined using three phenotypes: early-onset transient, fulfilling the definition of asthma at 1, 2, or 4 years but not later; adolescent-onset, fulfilling the definition of asthma at 12, 16 or 24 years but not earlier; persistent asthma, fulfilling the definition of asthma at 1, 2, 4 or 8 years AND at 12, 16 or 24 years.
Asthma at 24 years was defined as having a doctor’s diagnosis of asthma in combination with respiratory symptoms within the last year.
Uncontrolled asthma was defined based on the modified GINA criteria (Citation1) as at least one of the following in the last 4 weeks: daytime wheeze ≥ 3 times/week; any night-time awakening; activity limitation due to asthma; and use of a symptom reliever ≥3 times/week.
Rhinitis at 24 years was defined as symptoms from eyes or nose after exposure to furred animals or pollen (without having the flu) within the last year.
Clinical examination
IgE sensitization at 24 years was defined as having a positive Phadiatop test (≥0.35 kU/l) for inhalant allergens (cat, dog, horse, house dust mite, timothy grass, birch, mugwort, or mould) and/or a positive fx5 test (≥0.35 kU/l) for food allergens (wheat, egg, cow’s milk, soya, peanut, or fish) (Citation17).
ACT score ≥20 points was used to define controlled asthma (Citation1).
Statistical analysis
Descriptive statistics including frequencies and proportions were used to describe the study population. The Z-test was used for test of means and the proportion test for test of number of prescriptions and individuals. The mean numbers of prescriptions and of defined daily doses (DDDs) were calculated per individual. DDD is a volume measure defined as the assumed average maintenance dose per day for a drug used for its main indication in adults (Citation26).
Logistic regression was used to estimate the odds ratios (ORs) with 95% confidence intervals (CIs) for the associations between factors identified from the literature and asthma medication dispensation with adjustment for possible confounders. The included exposure variables were sex (male/female), parent born outside Scandinavia (yes/no), parental allergic disease (yes/no), higher parental education at baseline (yes/no), employment at 24 years (yes/no), daily smoking at 24 years (yes/no), asthma onset (early-onset transient/adolescent-onset/persistent), rhinitis at 24 years (yes/no), and non-allergic comorbidity (yes/no). The model was adjusted for sex, parental allergic disease, daily smoking at 24 years, higher parental education, having a job at 24 years, non-allergic comorbidity, and asthma at 24 years. The factors were included based on significant associations in the unadjusted models.
A log-binomial regression model was used to estimate the relative risk (RR) with 95% CI for the associations between the exposure variables and adherence among participants with asthma and at least one dispensed controller medication. The model included the factors stated above, plus recorded asthma diagnosis (yes/no) and recorded diagnosis from specialized care (yes/no). The model was adjusted for sex, parental allergic disease, rhinitis at 24 years, and non-allergic comorbidity. The factors included were selected based on their association with asthma medication. All statistical analyses were performed using STATA Statistical Software (release 16.0; College Station, TX, USA).
The study was approved by the Regional Ethics Committee, Karolinska Institutet, Stockholm, Sweden and participants gave written informed consent.
Results
Among the 3,064 participants in the study population, 47% were male and 11% fulfilled the study definition of asthma at 24 years (). In total, 495 individuals were dispensed at least one asthma medication during the study period (234 fulfilled the criteria for asthma; 261 did not). Among those with dispensed asthma medication, the proportion of males were lower compared to the entire population (39% vs. 47%; p < 0.01), while the proportion of rhinitis was higher (52% vs. 31%; p < 0.01). Similar patterns were seen among those who were dispensed asthma medication and had asthma (). In contrast, there was no difference in prevalence of socioeconomic factors.
Table 1. Background characteristics in the study population based on the 24-year follow-up questionnaire, among all individuals with dispensed asthma medication and among individuals with asthma (i.e., doctor’s diagnosis of asthma and symptoms) and dispensed asthma medication.
Factors associated with asthma medication dispensation
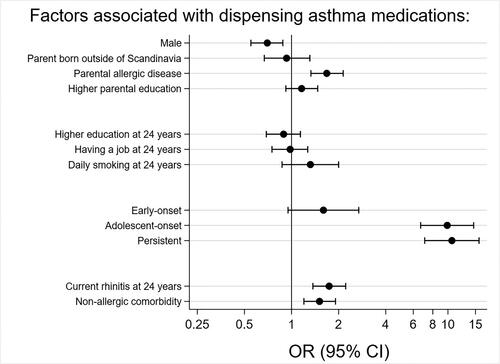
In a multivariable analysis, male sex was inversely associated with asthma medication dispensation (adj.OR 0.70, 95% CI 0.55–0.88). Parental allergic disease (1.68, 1.33–2.14), asthma onset (adolescent-onset 9.89, 6.69–14.6, persistent asthma 10.6, 7.10–15.8), rhinitis at 24 years (1.74, 1.37–2.22), and non-allergic comorbidity (1.51, 1.20–1.91) were all associated with increased odds of asthma medication dispensation (). None of the socioeconomic factors were associated with asthma medication dispensation.
Figure 2. Factors associated with dispensing asthma medication in the study population from the 24-year follow-up questionnaire (n = 3,064). Odds ratios (ORs) adjusted for sex, parental allergic disease, daily smoking at 24 years, higher parental education at baseline, having a job at 24 years, non-allergic comorbidity, and asthma at 24 years.
Asthma medication dispensing patterns among young adults with asthma
Among the 495 individuals with dispensed asthma medication during the study period of 5.5 years, 234 (7.6% of the entire population) were defined as having asthma at 24 years. Among them, 77% were dispensed at least one controller medication (ICS, LTRA, or fixed combination; ), while 86% were dispensed at least one reliever medication (SABA). The proportion of dispensed reliever medication was higher for females than males (89% vs. 81%; p < 0.01). The mean number of controller medication dispensations per individual was 5.9, corresponding to one per year on average. Males were dispensed more prescriptions per individual than females (11.0 vs. 7.2; p < 0.01 for any asthma medication). In addition, the volume of asthma medications (measured as mean DDDs/individual) was higher for males than females (1,313 vs. 791; p < 0.01). The corresponding figures for controller medications were 985 for males and 660 for females (p < 0.01).
Table 2. Dispensing patterns of asthma medications during the study period January 2014–June 2019 among young adults fulfilling the study definition of asthma (n = 234).
Among the 234 individuals with dispensed asthma medication and asthma, 22% (n = 51) were dispensed only SABA. Of these, a lower proportion was male than in the group dispensed controller medication (22% vs. 41%; p = 0.01; ). Moreover, the proportion of individuals with adolescent-onset asthma was higher among those dispensed only SABA (47% vs. 33%, p < 0.01). Among individuals dispensed only SABA, 33 were classified as having controlled asthma (ACT score ≥20 points), comparable to the proportion among individuals with controller medication (65% vs. 63%; p = 0.46). The proportions of IgE-sensitized individuals at 24 years were also similar (57% for only SABA vs. 60% for controller medication, p = 0.57). The proportion of individuals with exacerbations, i.e., dispensed oral corticosteroid or self-reported acute medical care/hospitalization due to respiratory symptoms, was lower among those dispensed only SABA, albeit not statistically significantly (29% vs. 43%; p = 0.07).
Table 3. Characterization of young adults with asthma dispensed only SABA and young adults with asthma and dispensed controller medication (n = 234).
Asthma medication adherence
Of the 181 participants with asthma at 24 years and at least one dispensed controller medication, 168 were included in the adherence model (13 excluded due to follow-up <18 months). Of them, 100 (60%) were classified as adherent after 18 months (67% in males, 55% in females, p = 0.11; ). Sex, socioeconomic factors, asthma onset, rhinitis, and non-allergic comorbidity were not associated with adherence. However, having a higher education at 24 years was non-statistically inversely associated with asthma medication dispensation (RR 0.76, 95% CI 0.57–1.01).
Table 4. Adherence, i.e., refilled a prescription of controller medication within 18 months, and background characteristics among individuals fulfilling the study definition of asthma at 24 years (n = 168).
The proportion of adherent young adults was higher among those with a recorded diagnosis of asthma from healthcare registers than those without, but not statistically significantly (64% vs. 49%; p = 0.07). Having a recorded diagnosis of asthma from specialized care was associated with higher adherence than having a diagnosis from primary care (68% vs. 55%; p = 0.18). The corresponding adjusted relative risk for adherence after 18 months among young adults with an asthma diagnosis recorded in specialized care was 1.32 (95% CI 1.03–1.69; ).
Discussion
In this population-based cohort study of young adults, questionnaire data were linked to information on clinical examinations and healthcare registers in order to assess factors associated with dispensing patterns of, and adherence to, asthma medication in young adults with asthma. We found that sex, parental allergic disease, asthma onset, rhinitis, and non-allergic comorbidity were associated with asthma medication dispensation. In general, individuals with asthma had sub-optimal treatment with small amounts and few prescriptions of asthma medications dispensed. One in five with asthma was dispensed only SABA. Males were dispensed more prescriptions and DDDs per individual than females, and the proportion dispensed only SABA was higher among females. Controller medication adherence (i.e., refilling a prescription within 18 months) was relatively low and not related to sex, socioeconomic factors, or non-allergic comorbidity. Having a recorded diagnosis of asthma increased adherence, especially when the diagnosis was recorded in specialized healthcare. However, two in five with a doctor’s diagnosis of asthma had not refilled a prescription of controller medication within 18 months. Three in ten diagnosed in specialized care and half in primary care were non-adherent.
We found that, on average, only one prescription of controller medication was dispensed per year. In the Swedish reimbursement system, a prescription for medication for a chronic disease is normally refilled after 3 months. A Norwegian study of ICS dispensing patterns among children (0–5 years) found similar results: one prescription of ICS per year was most common in all age groups (Citation12). Boys were in general dispensed more medications than girls; the proportion with dispensed ICS was higher in boys than girls and the number of prescriptions was larger (Citation12). In a previous study from our group, the number of prescriptions and the volume dispensed (DDDs/child) were higher in boys than girls aged 0–17 years (Citation11).
In this study, a larger proportion of females than males were dispensed SABA overall. In an Israelian study using questionnaires and data on dispensed medication, the mean number of SABA prescriptions/individual in the preceding year was calculated to 3.4 (mean age 37.7 years) (Citation27). The number of SABA prescriptions was greater in uncontrolled than controlled asthma. Furthermore, the proportion of males was higher in the controlled group. In our study, one in five young adults with asthma were dispensed only SABA. Overuse of SABA is associated with increased risk of exacerbation (Citation6). In our study, one in three young adults with asthma and dispensed only SABA was classified as having uncontrolled disease, and one in three had experienced an exacerbation in the last year. This number was somewhat lower compared to individuals with controller medication, indication that young adults with SABA only may have a milder asthma. However, both groups are having uncontrolled asthma to the same extent. Furthermore, the proportion of young adults with more than 12 episodes of wheeze in the last 12 months (one measure of severe asthma) did not differ between those with only SABA and among those with controller medication. In a recent review, the authors concluded that SABA should not be used as sole therapy (Citation1,Citation28).
In our study, controller medication adherence was 60% after 18 months, in line with an American study showing ICS adherence below 50% (Citation8). In a recent study using electronic medication data, ICS adherence among adults with asthma and chronic obstructive pulmonary disease was calculated to 53.7% (Citation29). In our study, sex, level of education, employment, and asthma phenotype did not change the adherence. In contrary, having the asthma diagnosis confirmed in specialized care resulted in higher adherence than confirmation in primary care. However, a considerable proportion of young adults in both categories had not refilled a prescription of controller medication within 18 months. To our knowledge, no other study has evaluated this. In a recent study of our prospective birth cohort, we found that young adults with asthma had few consultations with healthcare, before and after transition (Citation14). In addition, a Swedish qualitative study of young adults with asthma confirmed that they feel lost in the transition process (Citation30). The infrequent consultations and sense of being lost may explain the relatively low asthma medication adherence.
Strengths and limitations
This study used data from a large population-based birth cohort with high response rate (Citation17). A strength was the combination of data from questionnaires, clinical examination, and national and regional healthcare registers which together constitute novel data on young adults with asthma. The SPDR data cover the entire population of Sweden (coverage > 99%) (Citation19). All dispensed medications are included, regardless of reimbursement status. Also, Örtqvist et al. have shown that dispensing data from SPDR is a good proxy for having asthma (Citation31). The regional healthcare register includes full information on recorded diagnoses which is important when studying young adults’ diagnoses after transition within the healthcare system. In Sweden and many other countries, young adults with asthma are treated mainly in primary care (Citation1). However, it is difficult to define asthma regardless of data source.
Longitudinal analyses of dispensing data are considered the gold standard when measuring medication adherence (Citation32); still, it should be emphasized that dispensation does not equate to actual medication use. Also, young adults classified as being non-adherent may have mild asthma with the recommendation to use controller medication only when having symptoms. The GINA guidelines recommending use controller medication combined with a reliever on an as-needed basis (Citation1) were published at the end of the study period. Therefore, it is unlikely that they affected the results of this study.
In conclusion, young adults with asthma had few prescriptions of asthma medication dispensed, indicating sub-optimal treatment. A considerable proportion were dispensed only reliever medication. Controller medication adherence was rather low and was not associated with sex, socioeconomic status, or non-allergic comorbidity.
Acknowledgments
We thank the children and parents participating in the BAMSE cohort and all staff involved in the study through the years.
Declaration of interest
EM has received advisory board reimbursement from AstraZeneca, Chiesi, Novartis and Sanofi outside the submitted work. No other author reported any conflict of interest.
Additional information
Funding
References
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2020 [accessed 2020 Jun]. https://ginasthma.org/wp-content/uploads/2020/06/GINA-2020-report_20_06_04-1-wms.pdf
- Reddel HK, Busse WW, Pedersen S, Tan WC, Chen YZ, Jorup C, Lythgoe D, O’Byrne PM. Should recommendations about starting inhaled corticosteroid treatment for mild asthma be based on symptom frequency: a post-hoc efficacy analysis of the START study. Lancet. 2017;389(10065):157–166.
- Reddel HK, Ampon RD, Sawyer SM, Peters MJ. Risks associated with managing asthma without a preventer: urgent healthcare, poor asthma control and over-the-counter reliever use in a cross-sectional population survey. BMJ Open. 2017;7(9):e016688.
- O’Byrne PM, FitzGerald JM, Bateman ED, Barnes PJ, Zhong N, Keen C, Jorup C, Lamarca R, Ivanov S, Reddel HK. Inhaled combined budesonide-formoterol as needed in mild asthma. N Engl J Med. 2018;378(20):1865–1876.
- Bateman ED, Reddel HK, O’Byrne PM, Barnes PJ, Zhong N, Keen C, Jorup C, Lamarca R, Siwek-Posluszna A, FitzGerald JM. As-needed budesonide-formoterol versus maintenance budesonide in mild asthma. N Engl J Med. 2018;378(20):1877–1887.
- Nwaru BI, Ekström M, Hasvold P, Wiklund F, Telg G, Janson C. Overuse of short-acting β. Eur Respir J. 2020;55(4):1901872.
- Murphy J, McSharry J, Hynes L, Matthews S, Van Rhoon L, Molloy GJ. Prevalence and predictors of adherence to inhaled corticosteroids in young adults (15-30 years) with asthma: a systematic review and meta-analysis. J Asthma. 2020;58(5):683–705.
- Desai M, Oppenheimer JJ. Medication adherence in the asthmatic child and adolescent. Curr Allergy Asthma Rep. 2011;11(6):454–464.
- World Health Organization. Adherence to long-term therapies: evidens for action. Geneva (Switzerland): WGO; 2003 [accessed 2020 Jun]. https://www.who.int/chp/knowledge/publications/adherence_full_report.pdf.
- Peláez S, Lamontagne AJ, Collin J, Gauthier A, Grad RM, Blais L, Lavoie KL, Bacon SL, Ernst P, Guay H, et al. Patients’ perspective of barriers and facilitators to taking long-term controller medication for asthma: a novel taxonomy. BMC Pulm Med. 2015;15:42.
- Dahlén E, Komen J, Jonsson EW, Almqvist C, Kull I, Wettermark B. Eliminated patient fee and changes in dispensing patterns of asthma medication in children—an interrupted time series analysis. Basic Clin Pharmacol Toxicol. 2019;125(4):360–369.
- Øymar K, Mikalsen IB, Furu K, Nystad W, Karlstad Ø. Prescription patterns of inhaled corticosteroids for preschool children—a Norwegian register study. Pediatr Allergy Immunol. 2015;26(7):655–661.
- Roberts G, Vazquez-Ortiz M, Knibb R, Khaleva E, Alviani C, Angier E, Blumchen K, Comberiati P, Duca B, DunnGalvin A, et al. EAACI guideline on the effective transition of adolescents and young adults with allergy and asthma. Allergy. 2020;75(11):2734–2752.
- Ödling M, Andersson N, Hallberg J, Almqvist C, Janson C, Bergström A, Melén E, Kull I. A gap between asthma guidelines and management for adolescents and young adults. J Allergy Clin Immunol Pract. 2020;8(9):3056–3065.e2.
- Wang L, Palmer AJ, Otahal P, Cocker F, Sanderson K. Multimorbidity and health care service utilization in the Australian workforce: findings from the National Health Survey. J Occup Environ Med. 2017;59(8):795–802.
- Wickman M, Kull I, Pershagen G, Nordvall SL. The BAMSE project: presentation of a prospective longitudinal birth cohort study. Pediatr Allergy Immunol. 2002;13(s15):11–13.
- Melén E, Bergström A, Kull I, Almqvist C, Andersson N, Asarnoj A, Borres MP, Georgellis A, Pershagen G, Westman M, et al. Male sex is strongly associated with IgE-sensitization to airborne but not food allergens: results up to age 24 years from the BAMSE birth cohort. Clin Transl Allergy. 2020;10:15.
- Wang G, Hallberg J, Um Bergström P, Janson C, Pershagen G, Gruzieva O, van Hage M, Georgelis A, Bergström A, Kull I, et al. Assessment of chronic bronchitis and risk factors in young adults: results from BAMSE. Eur Respir J. 2020;57(3):2002120.
- Wettermark B, Hammar N, Fored CM, MichaelFored C, Leimanis A, Olausson PO, Persson I, Sundström A, Westerholm B, Rosén M. The new Swedish Prescribed Drug Register–opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16(7):726–735.
- Wallerstedt SM, Wettermark B, Hoffmann M. The first decade with the Swedish Prescribed Drug Register—a systematic review of the output in the scientific literature. Basic Clin Pharmacol Toxicol. 2016;119(5):464–469.
- Eriksson I, Cars T, Piehl F, Malmström RE, Wettermark B, von Euler M. Persistence with dimethyl fumarate in relapsing-remitting multiple sclerosis: a population-based cohort study. Eur J Clin Pharmacol. 2018;74(2):219–226.
- Forslund T, Wettermark B, Hjemdahl P. Comparison of treatment persistence with different oral anticoagulants in patients with atrial fibrillation. Eur J Clin Pharmacol. 2016;72(3):329–338.
- Zarrinkoub R, Wettermark B, Wändell P, Mejhert M, Szulkin R, Ljunggren G, Kahan T. The epidemiology of heart failure, based on data for 2.1 million inhabitants in Sweden. Eur J Heart Fail. 2013;15(9):995–1002.
- Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, Wong PK. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47.
- Dahlén E, Ekberg S, Lundholm C, Jonsson EW, Kull I, Wettermark B, Almqvist C. Sibship and dispensing patterns of asthma medication in young children-a population-based study. Pharmacoepidemiol Drug Saf. 2019;28(8):1109–1116.
- WHO Collaborating Center for Drug Statistics Methodology, Oslo. Guidelines for ATC classification and DDD assignment; 2021 [accessed 2021 Jan]. https://www.whocc.no/filearchive/publications/2021_guidelines_web.pdf
- Shlomi D, Katz I, Segel MJ, Oberman B, Peled N. Determination of asthma control using administrative data regarding short-acting beta-agonist inhaler purchase. J Asthma. 2018;55(5):571–577.
- O’Byrne PM, Reddel HK, Beasley R. The management of mild asthma. Eur Respir J. 2020:2003051.
- Kaye L, Theye B, Smeenk I, Gondalia R, Barrett MA, Stempel DA. Changes in medication adherence among patients with asthma and COPD during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020;8(7):2384–2385.
- Ödling M, Jonsson M, Janson C, Melén E, Bergström A, Kull I. Lost in the transition from pediatric to adult healthcare? Experiences of young adults with severe asthma. J Asthma. 2019;57(10):1119–1127.
- Örtqvist AK, Lundholm C, Wettermark B, Ludvigsson JF, Ye W, Almqvist C. Validation of asthma and eczema in population-based Swedish drug and patient registers. Pharmacoepidemiol Drug Saf. 2013;22(8):850–860.
- Vrijens B, Dima AL, Van Ganse E, van Boven JF, Eakin MN, Foster JM, de Bruin M, Chisholm A, Price D. What we mean when we talk about adherence in respiratory medicine. J Allergy Clin Immunol Pract. 2016;4(5):802–812.