Abstract
Objective
Asthma is frequently accompanied by dysfunctional breathing of which hyperventilation has been recognized as a subtype. The prevalence of hyperventilation in stable asthma has been scantily studied using blood gas analysis. Hence, a reliable estimate of its prevalence is lacking. It is unknown whether the Nijmegen Questionnaire (NQ) is a useful screening tool for hyperventilation in asthma. Therefore, the primary aim of this study was to determine the prevalence of hyperventilation in a large sample of patients with asthma in a stable state of disease. Secondary aims were to compare the clinical characteristics between patients with and without hyperventilation, and, to examine the concurrent validity of the NQ to detect hypocapnia in patients with asthma.
Methods
A real-world, observational, multicenter study was conducted. Capillary blood gas analysis was performed in adults with a confirmed diagnosis of stable asthma. A subset of patients completed the NQ.
Results
A blood gas analysis was obtained in 1006 patients. In 17% of the patients an acute hyperventilation was found, and in another 23% a chronic hyperventilation was uncovered. Patients with a chronic hyperventilation blood gas were more often female, were younger and had a better spirometric outcomes. The NQ appeared not to correlate with PCO2.
Conclusion
Hyperventilation is common in patients with stable asthma. Chronic hyperventilation is more often found in females of younger age and with the best spirometric outcomes compared to patients without hyperventilation. The NQ is not a suitable screening tool for the presence of hyperventilation in stable asthmatics.
Introduction
Asthma is defined according to the Global Initiative for Asthma (GINA) as a heterogeneous disease, usually characterized by reversible airflow obstruction and chronic airway inflammation. It is defined with a history of respiratory symptoms such as wheeze, shortness of breath, chest tightness and cough, that varies over time and in intensity, together with variable expiratory airflow limitation (Citation1). Although precise figures on the prevalence of asthma are difficult to obtain because of significant under- and overdiagnosis (Citation2), it is estimated that asthma is affecting more than 300 million people worldwide (Citation3).
Pharmacological treatment with inhaled corticosteroids is the cornerstone of the clinical management in asthmatic patients. It aims at reduction of the impact of asthma on daily life and reducing the risk of future adverse events such as exacerbations (Citation1). However, despite the presence of effective pharmacotherapy for patients with asthma, a recent pan European observational ‘real-life’ study revealed that asthma control measured with the Asthma Control Questionnaire (ACQ) was suboptimal in 56% of the more than 8000 included patients (Citation4). Also in the Netherlands, there is still a significant number of patients in whom disease control is not (27%) or only partially achieved (36%), even when medication is increased in accordance with guidelines (Citation5). These undesirable outcomes may result from inappropriate pharmacological asthma management from clinician and/or patient perspective (Citation6), but can also be attributed to unrecognized concomitant conditions which are highly prevalent in patients with asthma (Citation7).
A respiratory condition frequently co-occurring with asthma is dysfunctional breathing (DB) (Citation8,Citation9). DB is defined as chronic or recurrent changes in breathing pattern resulting in respiratory and non-respiratory symptoms in the absence of, or alongside respiratory disease (Citation10). DB is usually diagnosed with the Nijmegen Questionnaire (NQ) (Citation11), although originally not developed for this purpose (Citation12). Several subtypes of DB are distinguished of which hyperventilation is one (Citation13). Recognition of hyperventilation, that is, breathing beyond the metabolic needs resulting in hypocapnia, is relevant in asthma because of the potential role of hypocapnia in the pathophysiology of bronchoconstriction (Citation14). The gold standard for diagnosing hyperventilation is blood gas analysis and assessment of the presence of respiratory alkalosis, whether or not metabolically compensated (Citation15). Unfortunately, blood gas analysis has been scantily applied to patients with asthma in a stable state of disease, that is outside the remits of an acute exacerbation, hence a reliable estimate of its prevalence is lacking (Citation16). Whether the NQ could be used as a screening tool for the presence of hyperventilation in patients with asthma is unknown because it has never been validated against the gold standard, which is blood gas analysis in this population. Therefore, the aims of the present study were: 1) to determine the prevalence of a blood gas analysis confirmed hyperventilation in a large sample of patients with asthma in a stable state of disease; 2) to compare the clinical characteristics between patients with and without hyperventilation, and, 3) to examine the concurrent validity of the NQ to detect hypocapnia in patients with asthma.
Methods
Study design
A real-world, observational, multicenter study was conducted. Data were collected in stable patients referred for an elective, outpatient consultation with a pulmonologist to one of the following hospitals: Amphia Hospital (Breda, the Netherlands), Franciscus Gasthuis & Vlietland Hospital (Rotterdam, the Netherlands) or Radboudumc (Nijmegen, the Netherlands). By stable is meant outside the remits of an acute exacerbation. These patients participated in a standardized, yet comprehensive, diagnostic care pathway which was developed and implemented specifically for patients with chronic airways disease (Citation17). The Committee on Human Research in the Nijmegen-Arnhem region approved the study. Due to the observational nature of the study and the provision of usual care, written informed consent could be waived (reference number 2018–4357). Subsequently, the local Research Ethics Committees of Radboudumc (reference number 2018–4357), Amphia Hospital (reference number 2019–0221) and Franciscus Vlietland (reference number 2018–108/T110) permitted the conductance of this study in their institutions. Preliminary results have been presented at the 2018 European Respiratory Society annual congress (Citation18).
Participants
All adult patients (≥ 18 year) with a diagnosis of asthma confirmed by the pulmonologist according to the GINA guidelines (Citation1), who were consecutively referred between April 2013 and December 2017, were deemed eligible for participation. Patients were excluded if no capillary blood gas analysis was obtained, or if they had had a self-reported acute exacerbation within the last 6 weeks.
Assessments
Blood gas analysis
A capillary blood gas analysis was performed according to guidelines to determine the presence of hyperventilation (Citation19). Capillary samples accurately reflects arterial PCO2 (Citation20), and bicarbonate concentration (HCO3–) (Citation21). For this purpose, blood samples were obtained from either earlobe or fingertip under resting conditions and at least one hour after use of a bronchodilator. HCO3– was measured not calculated in all three biochemicals laboratories.
Three subtypes of asthmatic patients were distinguished according to internationally accepted blood gas interpretation rules: 1) patients with an acute hyperventilation blood gas (PCO2 < 4.7 kPa/35 mm Hg and bicarbonate ≥ 23 mmol/L), 2) patients with a chronic hyperventilation blood gas (PCO2 < 4.7 kPa/35 mm Hg and HCO3– < 23 mmol/L), and, 3) patients without a hyperventilation blood gas (Citation22,Citation23).
Patient characteristics
The following patient characteristics were assessed: sex (female/male), age (in years), body mass index (BMI, in kg/m2), pulmonary function (spirometry and flow-volume curve, using the Global Lung Initiative (GLI) equations) (Citation24), self-reported smoking status (current/former-never and number of packyears), and the number of asthma exacerbations in the past 12 months. Medication use was recorded and classified according to GINA treatment steps (Citation1).
Asthma control and disease specific quality of life
Asthma control was measured with the Asthma Control Questionnaire (ACQ) and quality of life with the Asthma Quality of Life Questionnaire (AQLQ) (Citation25,Citation26). Both questionnaires were in Dutch. Based on the scores of the ACQ, patients were classified as having controlled (score < 0.75 points), partially controlled (score 0.75–1.5 points) or uncontrolled asthma (score >1.5 points).
Dysfunctional breathing
Patients referred to the Radboudumc were requested to complete the NQ to assess the presence of dysfunctional breathing which was defined as a score ≥23 points on the NQ (Citation11).
Statistical analysis
Descriptive statistics were used to summarize the data as means (standard deviations) or frequencies (proportions), as appropriate. A One-way-Anova was applied with a Bonferroni post hoc test to test for any difference in continuous variables among the three subtypes of patients according to their blood gas interpretation, that is, normal, acute hyperventilation, or chronic hyperventilation. Categorical variables were analyzed with the Chi-square test and post hoc converting Z-values into probability values. Because PCO2 values might be lower in females, especially of younger age, PCO2 values in females < 40 year of age were also compared to a reference value 4.9 kPa, applying a One-Sample T-test (Citation27). Pearson’s correlation coefficients were calculated between PCO2 values and NQ scores and between ACQ and AQLQ and NQ scores to examine the relationship between these variables. All data were treated confidentially and in line with The code of Ethics of the World Medical Association (Declaration of Helsinki) (Citation28). Statistical analyses were conducted using SPSS Version 27 (IBM Corp., Armonk, NY, USA). Significance levels were set to P < 0.05.
Results
In the study period a total of 1118 patients with a confirmed diagnosis of asthma had been referred to the three hospitals participating in this study. Thirteen patients were excluded because of an acute exacerbation < 6 weeks. A valid blood gas analysis could be obtained in 1006 patients. A total of 99 patients (9%) were excluded due to a lack of blood gas results. This was either the result of failing to take a blood sample or failing its analysis. No patients refused to take the test. Patient characteristics are shown in . Patients were on average 47 ± 15 years of age (range 18–88) and were mostly women (62%). Post-bronchodilator spirometry (FVC% pred and FEV1%pred) was on average slightly decreased compared to the GLI reference values. The majority of patients (60%) had uncontrolled asthma, 25% was partially controlled, and only 15% had controlled asthma. Patient characteristics showed no differences between the three participating sites.
Table 1. Patient characteristics.
Prevalence of hyperventilation
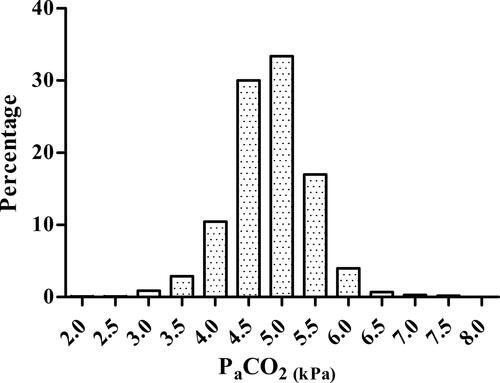
The PCO2 values of these 1006 patients were normally distributed with a mean value of 4.8 ± 0.60 kPa/36 ± 4.5 mmHg (). Of this study group, 172 patients (17%) had an acute hyperventilation blood gas, 229 patients (23%) a blood gas indicating the presence of chronic hyperventilation, and 605 (60%) had a normal blood gas. In the patient characteristics of each subgroup are listed. Patients with a chronic hyperventilation blood gas were significantly more often female (P < 0.001), were younger (P < 0.001) and had a better spirometric outcomes (P < 0.001) compared to patients with a normal blood gas. Patients with an acute hyperventilation blood gas differed only in FEV1/FVC from patients with a normal blood gas. Except for the pH, PCO2 and HCO3–, all other clinical characteristics were comparable between the two hyperventilation groups. Also asthma control, asthma-specific quality of life and GINA treatment steps did not differ between subgroups. The mean PCO2 value of a subgroup of females < 40 years (4.5 ± 0.5 kPa) differed statistically significantly from a reference value of 4.9 kPa (t (193) = −9,15, P < 0.001).
Concurrent validity of NQ for the presence of hyperventilation in patients with asthma
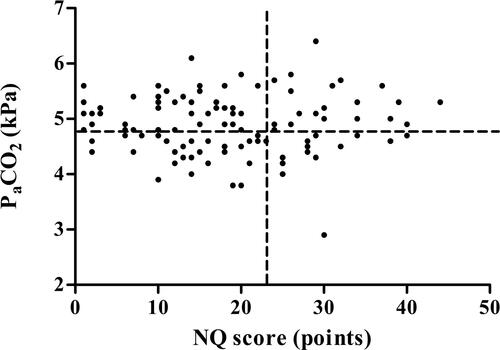
Valid NQ scores were obtained in 164 patients, namely from 101 patients (62%) without hyperventilation, from 30 patients (18%) with acute hyperventilation and from 33 patients (20%) with chronic hyperventilation. NQ scores did not differ between subgroups nor did the proportions of patients with a positive NQ (score ≥ 23 points). A scatterplot visualized the absence of a relationship between PCO2 values and NQ scores (), which was confirmed by a non-significant Pearson correlation coefficient (r = 0.05; P = 0.52). By contrast, significant correlation coefficients were found between NQ scores and ACQ (r = 0.50; P < 0.001) and NQ scores and AQLQ (r = –0.63; P < 0.001).
Figure 2. Scatterplot of Nijmegen Questionnaire scores (N = 164) and PCO2 values. The vertical dashed line represents the threshold for a positive score (≥23 points) on the Nijmegen Questionnaire. The horizontal dashed line delineates the border of the presence of hypocapnia (PCO2 <4.7 kPa/35 mm Hg).
Discussion
In this study, for the first time large-scale blood gas analysis was performed in patients with stable asthma, that is, without acute exacerbation. A remarkably large proportion of patients, namely 40%, appeared to have a hyperventilation blood gas. A metabolic compensated respiratory alkalosis was found in 23% of the patients, indicating the presence of a longer lasting hyperventilation. An uncompensated respiratory alkalosis was observed in another 17% of the patients, representing an acute hyperventilation. Patients with a chronic hyperventilation were younger, more often female and had the best spirometric outcomes compared to patients without hyperventilation. The NQ turned out not to be a valid tool for use as a screening instrument for hyperventilation in patients with asthma.
While blood gas disturbances have been demonstrated in patients with an acute asthma exacerbation, with respiratory alkalosis present predominantly in patients with mild airway obstruction (Citation29), few studies evaluated its presence in a stable state of disease, that is, outside the remits of an acute exacerbation (Citation16). The prevalence of hypocapnia in 40% of the patients with asthma in a stable state of disease in the current study is higher compared to the few earlier studies addressing this issue. In the study by Osborne et al. 7 out of 23 patients (30%) had hypocapnia (Citation30), whereas in the study by Tai et al. hypocapnia was present in 14 out of the 64 patients (22%) (Citation31). The higher prevalence found in the present study is not readily explained due to the lack of comparability of study populations. Osborne included younger patients of on average 26 years of age with normal spirometry values but did not examine asthma control or quality of life (Citation30). The paper by Tai et al. lacks any detail about the study population (Citation31). The large sample taken from a real world population and over a wide range of disease severity favors in our view the validity of the outcomes of the present study. Further supporting the validity of the findings is the significantly lower PCO2 value found in young women when compared with a downwardly adjusted reference value of 4.9 kPa for this subgroup. The current study was conducted in patients with asthma referred for an elective, outpatient consultation with a pulmonologist. This might limit the generalizability of the findings for instance to patients treated in primary care. It should be noted however, that disease severity of pulmonary patients treated in the different echelons of the healthcare system show considerable overlap and these differences might be lesser than expected (Citation32). The cross-sectional study design we employed, precludes obviously any conclusions regarding changes over time of the presence of hyperventilation, and this requires additional research.
Clinical relevance of the presence of hyperventilation in patients with asthma
There is compelling evidence that the presence of hypocapnia during an asthma attack may perpetuate bronchospasm and culminate in a vicious cycle of progressive hypocapnia and increasing bronchospasm (Citation33). In addition, in a review of existing literature, Bruton and Holgate, nicely summarized the body of knowledge, also building a strong case for a bronchconstrictive effect of hypocapnia and a bronchodilatory effect of hypercapnia in individuals with asthma in stable state of disease (Citation34). The present study shows that hyperventilation is a prevalent trait in asthma patients referred for an outpatient consultation to a pulmonologist. The frequent occurrence of hypocapnia in combination with the evidence on its possible role in the development, aggravating and/or maintenance of bronchoconstriction in patients with asthma as summarized above (Citation33,Citation34), might explain why some patients do not or only limited respond to escalations in prescribed medications, even with powerful next generation biologicals (Citation35). The absence of poorer asthma control and quality of life in the current study in the hyperventilating patients does not in our view detract from its potential clinical relevance.
This view is supported by the observation that the subgroup with chronic hyperventilation differed significantly from the other groups owing to a relatively large proportion of women of a younger than average age, and with the best spirometric outcomes. It could be argued that in this specific group the individual burden of asthma could be more attributed to the symptom amplifying effect of over breathing and the resulting hypocapnia rather than the severity of the physiological impairment. Such a reasoning would be in line with observations of discordant interplay between objective physiological, inflammatory markers and asthma control (Citation36). Future studies are warranted to investigate this assumption. Intervention studies could be relevant for this that investigate a link between a reduction in overbreathing and better asthma control. Another issue arising from this study is why in some patients acute or chronic over breathing resulting in hypocapnia was present and why not in some others? The capillary blood sample procedure itself likely did not affect PCO2, for a recent study showed the absence of effect of an arterial puncture on PETCO2 (Citation37). Knowledge about the trigger(s) for excessive breathing is relevant from the perspective of the choice of appropriate intervention(s). It should be noticed that the present study was designed to examine the prevalence of hyperventilation in patients with asthma and to assess whom it afflicts, not to unveil a possible working mechanism. Such a study would require extensive documentation of the physiological, inflammatory as well as psychological profiles of patients. Anxiety disorders (i.e. generalized anxiety disorder, panic disorder) are common psychological comorbidities in asthma (Citation38), and indeed have been linked with hyperventilation in these patients (Citation39).
NQ as screening tool for hyperventilation in asthma
Another novel result of the present study is the absence of association between NQ scores and PCO2 measurements. These two features apparently represent independent traits of patients with asthma. Given the significant correlation between the NQ scores and ACQ and AQLQ, a high score on the NQ expresses poor asthma control and impaired quality of life rather than the presence of hyperventilation (Citation40). This finding is not surprising considering that only five of the sixteen questions of the NQ refer to central nervous system symptoms relating to hypocapnia (Citation13). The remaining eleven question relate to respiratory symptoms closely mirroring asthma symptoms. Recently, Denton et al. also concluded that DB in patients with asthma mirrors worse asthma status (Citation41). Because of this result, we do not recommend using the NQ as a screening tool for the presence of hyperventilation in people with asthma. Our findings contrast the results from a study by Grammamtopulou and coworkers (Citation42). They found that applying a cutoff value of > 17 points on the NQ could identify asthma patients with the presence of hyperventilation (Citation39). A possible explanation for this difference is that while we used the original 16-item version of the NQ, Grammatopoulou et al. used a version reduced to 11 items via principal component analysis. This might have resulted in a higher discriminatory power due to the relative increase of items more related to central nervous system symptoms relating to hypocapnia.
To detect dysfunctional breathing in patients with asthma, the recently proposed Brompton Breathing Pattern Assessment Tool (BPAT) seems promising. Instead of relying on symptoms perceptions it scores features of breathing which are more central to the definition of dysfunctional breathing (Citation43). Due to the noninvasive nature of the BPAT it is easier to use in everyday clinical practice than blood gas analysis, and future research should examine whether this tool is valid to detect the presence of hyperventilation in patients with asthma.
Therapeutic options for asthma patients with hyperventilation
Breathing-control and relaxation exercises seems an obvious intervention for hyperventilation in patients with asthma. Indeed these exercises have been advocated as adjunct treatment option for asthmatic patients with persisting symptoms (Citation1). Nevertheless, a 2013 Cochrane systematic review concluded that no conclusive evidence supports or refutes the efficacy of breathing exercises in the treatment of adults patients with asthma (Citation44). It should be noted however, that studies on the effects of breathing exercises included into this review applied broad entry criteria and for instance did not use the presence of confirmed hyperventilation or objectively determined breathing pattern alterations as patient selection criterion. Hypocapnia was also never used as outcome measure. Therefore, we suggest that future investigations use the presence of these features for patient selection and as outcome measure in studies on breathing exercises. Breathing exercises specifically targeting increasing PCO2 could be a good intervention choice in cases of hyperventilation since this intervention has been shown to result in a higher PCO2 level which was associated with better asthma control (Citation45). In addition, voice bubbling therapy for uncontrolled asthma patients with chronic hyperventilation and a positive screening for vocal cord dysfunction, resulted in a higher PCO2 level, suggesting that such an approach may also be a useful means to effectively reduce over breathing in asthmatic patients (Citation46).
Conclusions
In conclusion, this large scale study confirms that hyperventilation is a common feature in patients with stable asthma. Chronic hyperventilation is more often found in females of younger age and with the best spirometric outcomes compared to asthma patients without hyperventilation. The NQ is not a suitable screening tool for the presence of hyperventilation in patients with stable asthma. Capillary blood gas analysis should be a mandatory component of a comprehensive analysis of health status in these patients.
| Abbreviations | ||
| ACQ | = | asthma control questionnaire |
| AQLQ | = | asthma quality of life questionnaire |
| BMI | = | Body mass index |
| DB | = | dysfunctional breathing |
| FEV1 | = | forced expiratory volume in one second |
| FVC | = | forced vital capacity |
| GINA | = | Global Initiative for Asthma |
| GLI | = | Global Lung Initiative |
| HCO3 – | = | bicarbonate |
| NQ | = | Nijmegen Questionnaire |
| PCO2 | = | arterial carbon dioxide tension |
Acknowledgment
Adjan Witte, MD and Noortje Koolen, MsC are thanked for their valuable contributions during the data collection for this study.
Declaration of interest
All authors hereby declare that they have no conflicts of interest including funding sources that supported their work and any commercial associations. These include consultant arrangements, speakers’ bureau participation, stock or other equity ownership, patent licensing arrangements, support such as financial or materials grants for research, employment, or expert witness testimony. There were also no financial relationships for themselves and their immediate family/significant others.
References
- Global Initiative for Asthma. Global strategy for asthma management and prevention. 2020. Available from: https://www.ginaasthma.org/ [last accessed January 2021].
- Aaron SD, Boulet LP, Reddel HK, Gershon AS. Underdiagnosis and overdiagnosis of asthma. Am J Respir Crit Care Med. 2018;198(8):1012–1020. doi:https://doi.org/10.1164/rccm.201804-0682CI.
- Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259. doi:https://doi.org/10.1016/S0140-6736(17)32154-2.
- Braido F, Brusselle G, Guastalla D, Ingrassia E, Nicolini G, Price D, Roche N, Soriano JB, Worth H, LIAISON Study Group. Determinants and impact of suboptimal asthma control in Europe: The International Cross-Sectional and Longitudinal Assessment on Asthma Control (LIAISON) study. Respir Res. 2016;17(1):51. doi:https://doi.org/10.1186/s12931-016-0374-z.
- van der Meer V, van Stel HF, Bakker MJ, Roldaan AC, Assendelft WJJ, Sterk PJ, Rabe KF, Sont JK, the SMASHING (Self-Management of Asthma Supported by Hospitals, ICT, Nurses and General practitioners) Study Group. Weekly self-monitoring and treatment adjustment benefit patients with partly controlled and uncontrolled asthma: an analysis of the SMASHING study. Respir Res. 2010;11(1):74. doi:https://doi.org/10.1186/1465-9921-11-74.
- Thomas M. Why aren’t we doing better in asthma: time for personalised medicine?NPJ Prim Care Respir Med. 2015;25:15004. doi:https://doi.org/10.1038/npjpcrm.2015.4.
- Porsbjerg C, Menzies-Gow A. Co-morbidities in severe asthma: clinical impact and management. Respirology. 2017;22(4):651–661. doi:https://doi.org/10.1111/resp.13026.
- Veidal S, Jeppegaard M, Sverrild A, Backer V, Porsbjerg C. The impact of dysfunctional breathing on the assessment of asthma control. Respir Med. 2017;123:42–47. doi:https://doi.org/10.1016/j.rmed.2016.12.008.
- Connett GJ, Thomas M. Dysfunctional breathing in children and adults with asthma. Front Pediatr. 2018;6:406. doi:https://doi.org/10.3389/fped.2018.00406.
- Vidotto LS, Carvalho CRF, Harvey A, Jones M. Dysfunctional breathing: what do we know?J Bras Pneumol. 2019;45(1):e20170347. doi:https://doi.org/10.1590/1806-3713/e20170347.
- van Dixhoorn J, Duivenvoorden HJ. Efficacy of Nijmegen Questionnaire in recognition of the hyperventilation syndrome. J Psychosom Res. 1985;29(2):199–206. doi:https://doi.org/10.1016/0022-3999(85)90042-X.
- van Dixhoorn J, Folgering H. The Nijmegen Questionnaire and dysfunctional breathing. ERJ Open Res. 2015;1(1):00001-2015. doi:https://doi.org/10.1183/23120541.00001-2015.
- Boulding R, Stacey R, Niven R, Fowler SJ. Dysfunctional breathing: a review of the literature and proposal for classification. Eur Respir Rev. 2016;25(141):287–294. doi:https://doi.org/10.1183/16000617.0088-2015.
- Vasileiadis I, Alevrakis E, Ampelioti S, Vagionas D, Rovina N, Koutsoukou A. Acid-base disturbances in patients with asthma: a literature review and comments on their pathophysiology. J Clin Med. 2019;8(4):563. doi:https://doi.org/10.3390/jcm8040563.
- Gardner WN. The pathophysiology of hyperventilation disorders. Chest. 1996;109(2):516–534. doi:https://doi.org/10.1378/chest.109.2.516.
- van’t Hul AJ, Deenstra DD, Djamin RS, Antons JC, van Helvoort HA. Hypocapnia correction as a working mechanism for breathing retraining in asthma. Lancet Respir Med. 2018;6(4):e14. doi:https://doi.org/10.1016/S2213-2600(18)30071-7.
- van den Akker EFMM, van’t Hul AJ, Chavannes NH, Braunstahl G-J, van Bruggen A, Rutten-van Mölken MPMH, In’t Veen JCCM. Development of an integral assessment approach of health status in patients with obstructive airway diseases: the CORONA study. Int J Chron Obstruct Pulmon Dis. 2015;10:2413–2422. doi:https://doi.org/10.2147/COPD.S90680.
- Deenstra D, van Helvoort HAC, Koolen EH, Djamin RS, Antons JC, van’t Hul AJ. Chronic hyperventilation in asthma. Eur Resp J. 2018;52(PA3985):PA3985. doi:https://doi.org/10.1183/13993003.congress-2018.PA3985.
- Davis MD, Walsh BK, Sittig SE, Restrepo RD. AARC clinical practice guideline: blood gas analysis and hemoximetry: 2013. Respir Care. 2013;58(10):1694–1703. doi:https://doi.org/10.4187/respcare.02786.
- Zavorsky GS, Cao J, Mayo NE, Gabbay R, Murias JM. Arterial versus capillary blood gases: a meta-analysis. Respir Physiol Neurobiol. 2007;155(3):268–279. doi:https://doi.org/10.1016/j.resp.2006.07.002.
- Heidari K, Hatamabadi H, Ansarian N, Alavi-Moghaddam M, Amini A, Safari S, Darbandsar Mazandarani P, Vafaee A. Correlation between capillary and arterial blood gas parameters in an ED. Am J Emerg Med. 2013;31(2):326–329. doi:https://doi.org/10.1016/j.ajem.2012.08.025.
- Sood P, Paul G, Puri S. Interpretation of arterial blood gas. Indian J Crit Care Med. 2010;14(2):57–64. doi:https://doi.org/10.4103/0972-5229.68215.
- Cowley NJ, Owen A, Bion JF. Interpreting arterial blood gas results. BMJ. 2013;346:f16. doi:https://doi.org/10.1136/bmj.f16.
- Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MSM, Zheng J, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi:https://doi.org/10.1183/09031936.00080312.
- Juniper EF, Guyatt GH, Ferrie PJ, Griffith LE. Measuring quality of life in asthma. Am Rev Respir Dis. 1993;147(4):832–838. doi:https://doi.org/10.1164/ajrccm/147.4.832.
- Juniper EF, O′Byrne PM, Guyatt Gh, Ferrie Pj, King Dr. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902–907. doi:https://doi.org/10.1034/j.1399-3003.1999.14d29.x.
- Klaestrup E, Trydal T, Pedersen JF, Larsen JM, Lundbye-Christensen S, Kristensen SR. Reference intervals and age and gender dependency for arterial blood gases and electrolytes in adults. Clin Chem Lab Med. 2011;49(9):1495–1500. doi:https://doi.org/10.1515/CCLM.2011.603.
- World Medical Association. WMA international code of medical ethics amended by the 57th WMA General Assembly, Pilanesberg, South Africa, October 2006. Available from: https://www.wma.net/policies-post/wma-international-code-of-medical-ethics/ [last accessed January 2021].
- Raimondi GA, Gonzalez S, Zaltsman J, Menga G, Adrogue HJ. Acid-base patterns in acute severe asthma. J Asthma. 2013;50(10):1062–1068. doi:https://doi.org/10.3109/02770903.2013.834506.
- Osborne CA, O’Connor BJ, Lewis A, Kanabar V, Gardner WN. Hyperventilation and asymptomatic chronic asthma. Thorax. 2000;55(12):1016–1022. doi:https://doi.org/10.1136/thorax.55.12.1016.
- Tai E, Read J. Blood-gas tensions in bronchial asthma. Lancet. 1967;1(7491):644–646. doi:https://doi.org/10.1016/S0140-6736(67)92541-X.
- de Klein MM, Peters JB, van’t Hul AJ, Akkermans RP, In’t Veen JC, Vercoulen JH, Bischoff EW, Schermer TR. Comparing health status between patients with COPD in primary, secondary and tertiary care. NPJ Prim Care Respir Med. 2020;30(1):39. doi:https://doi.org/10.1038/s41533-020-00196-7.
- Laffey JG, Kavanagh BP. Hypocapnia. N Engl J Med. 2002;347(1):43–53. doi:https://doi.org/10.1056/NEJMra012457.
- Bruton A, Holgate ST. Hypocapnia and asthma: a mechanism for breathing retraining?Chest. 2005;127(5):1808–1811. doi:https://doi.org/10.1378/chest.127.5.1808.
- McCracken JL, Tripple JW, Calhoun WJ. Biologic therapy in the management of asthma. Curr Opin Allergy Clin Immunol. 2016;16(4):375–382. doi:https://doi.org/10.1097/ACI.0000000000000284.
- Stahl E. Correlation between objective measures of airway calibre and clinical symptoms in asthma: a systematic review of clinical studies. Respir Med. 2000;94(8):735–741.
- Sahni AS, Gonzalez H, Tulaimat A. Effect of arterial puncture on ventilation. Heart Lung. 2017;46(3):149–152. doi:https://doi.org/10.1016/j.hrtlng.2017.01.011.
- Cooley C, Park Y, Ajilore O, Leow A, Nyenhuis SM. Impact of interventions targeting anxiety and depression in adults with asthma. J Asthma. 2020:1–24. doi:https://doi.org/10.1080/02770903.2020.1847927.
- Meuret AE, Ritz T. Hyperventilation in panic disorder and asthma: empirical evidence and clinical strategies. Int J Psychophysiol. 2010;78(1):68–79. doi:https://doi.org/10.1016/j.ijpsycho.2010.05.006.
- Warburton CJ, Jack S. Can you diagnose hyperventilation?Chron Respir Dis. 2006;3(3):113–115. doi:https://doi.org/10.1191/1479972306cd116ed.
- Denton E, Bondarenko J, Tay TR, Lee J, Radhakrishna N, Hore-Lacy F, Martin C, Hoy R, O’Hehir R, Dabscheck E, et al. Factors associated with dysfunctional breathing in patients with difficult to treat asthma. J Allergy Clin Immunol Pract. 2019;7(5):1471–1476. doi:https://doi.org/10.1016/j.jaip.2018.11.037.
- Grammatopoulou EP, Skordilis EK, Georgoudis G, Haniotou A, Evangelodimou A, Fildissis G, Katsoulas T, Kalagiakos P. Hyperventilation in asthma: a validation study of the Nijmegen Questionnaire–NQ. J Asthma. 2014;51(8):839–846. doi:https://doi.org/10.3109/02770903.2014.922190.
- Todd S, Walsted ES, Grillo L, Livingston R, Menzies-Gow A, Hull JH. Novel assessment tool to detect breathing pattern disorder in patients with refractory asthma. Respirology. 2018;23(3):284–290. doi:https://doi.org/10.1111/resp.13173.
- Freitas DA, Holloway EA, Bruno SS, Chaves GS, Fregonezi GA, Mendonca KP. Breathing exercises for adults with asthma. Cochrane Database Syst Rev. 2013;(10):CD001277.
- Ritz T, Rosenfield D, Steele AM, Millard MW, Meuret AE. Controlling Asthma by Training of Capnometry-Assisted Hypoventilation (CATCH) vs slow breathing: a randomized controlled trial. Chest. 2014;146(5):1237–1247. doi:https://doi.org/10.1378/chest.14-0665.
- Eindhoven SC, Turk Y, van der Veer T, et al. Voice bubbling therapy for vocal cord dysfunction in difficult-to-treat asthma - a pilot study. J Asthma. 2020:1–6. doi:https://doi.org/10.1080/02770903.2020.1837156.