Abstract
Objective
Treatment with fluticasone furoate/vilanterol (FF/VI), an inhaled corticosteroid/long-acting β2-agonist therapy, reduces the risk of severe asthma exacerbations and improves lung function and symptom control in patients with asthma. However, real-world data remain limited among asthma patients in the United States (US).
Methods
This retrospective cohort study propensity score (PS) matched adult asthma patients initiating once-daily FF/VI 100/25 mcg with patients initiating twice-daily budesonide/formoterol (B/F) 160/4.5 mcg using a US claims database (January 1, 2015–December 31, 2018). Asthma control was measured by the mean number of short-acting β2-agonist (SABA) canisters dispensed per patient-year (PPY) during follow-up. Time to first, and rates of, overall and severe asthma exacerbations were also measured.
Results
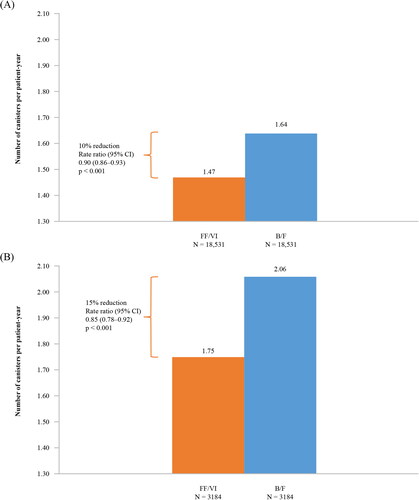
After PS matching, 18,531 patients receiving FF/VI were matched to 18,531 patients receiving B/F. Mean SABA canisters dispensed PPY was significantly lower for FF/VI users compared with B/F users (FF/VI: 1.47, B/F: 1.64; p < 0.001). FF/VI use resulted in 13% significantly lower risk of having an overall asthma-related exacerbation and 22% lower risk of a severe exacerbation versus B/F use (overall exacerbation hazard ratio [HR] [95% confidence interval (CI)]: 0.87 [0.82–0.92], p < 0.001; severe exacerbation HR [95% CI]: 0.78 [0.63–0.97], p = 0.027). Asthma-related exacerbation rates per 100 patient-days were also significantly lower for the FF/VI group compared with the B/F group (overall: 0.0475 vs. 0.0558, p < 0.001; severe: 0.0026 vs. 0.0033, p = 0.020).
Conclusions
In real-world practice, initiation of once-daily FF/VI 100/25 mcg in adults with asthma was associated with lower use of SABA and fewer asthma-related exacerbations, which may indicate better asthma control, when compared with use of twice-daily B/F 160/4.5 mcg.
Introduction
Treatment recommendations for asthma define long-term goals as the control of symptoms and prevention of exacerbations (Citation1,Citation2). Good control of asthma can be achieved in most patients (Citation3) through the assessment of triggering factors, medication adherence, and establishing the appropriate level of treatment for each patient (Citation2). Despite these strategies, some patients with asthma continue to experience persistent symptoms and exacerbations (Citation2,Citation4,Citation5). Poorly controlled asthma is associated with increased use of rescue medication, accelerated pulmonary function loss, increased risk of exacerbations, healthcare resource utilization (HRU), and healthcare costs (Citation2–9). From a patient perspective, poor asthma control is associated with significant patient and caregiver burden and may translate into functional limitations, reduced quality of life (QoL), and loss of productivity (Citation2,Citation4,Citation5,Citation10).
Asthma symptoms and airflow limitation associated with poor control can fluctuate; however, they can represent a negative and impactful experience for the patient and can be life threatening (Citation2,Citation5). Although there is currently no global consensus on the definition, exacerbations are widely defined as an acute change in the patient’s usual asthma status that requires an urgent change in the patient’s treatment regimen (Citation2,Citation10); they are characterized by a progressive increase in symptoms, including breathlessness, chest tightness, or wheezing, and an acute decline in lung function (Citation2). In the appropriate patient populations, a reduction in exacerbations is increasingly recognized as a useful measure of treatment efficacy (Citation11).
The Global Initiative for Asthma (GINA) and the National Asthma Education and Prevention Program (NAEPP) recommend a stepwise approach to achieve sufficient control of asthma, including reduction in the risk of exacerbations. If control is not achieved, an increase in controller therapy dose or the introduction of additional maintenance therapies are recommended (Citation2,Citation12). Control may also be improved by correcting for poor inhaler technique and/or treatment adherence, identifying comorbidities that may contribute to poor asthma control, and patient education (Citation2,Citation12,Citation13). To control symptoms and minimize future risk, GINA recommends low-dose inhaled corticosteroids (ICS)/formoterol (a long-acting β2-agonist [LABA]) as both as-needed reliever therapy and controller therapy, with dosing increased as severity progresses (Steps 1–4), and referral for phenotypic assessment and additional long-acting muscarinic agonist therapy at Step 5 (2). NAEPP recommends as-needed short-acting β2-agonist (SABA) for intermittent asthma (Step 1) (Citation13). For persistent asthma, NAEPP recommends daily low-dose ICS and as-needed SABA at Step 2, daily and as-needed ICS/formoterol at Step 3 (low-dose) and Step 4 (medium-dose), daily medium-high dose ICS/LABA + LAMA and as-needed SABA at Step 5, and daily high-dose ICS/LABA + oral systemic corticosteroids + as-needed SABA at Step 6. Recommendations by GINA represent a global strategy; therefore, professional judgment, local and national guidelines, and currently licensed drug doses should be taken into account as some GINA recommendations may be ‘off-label’ in some geographical locations (Citation2).
Fluticasone furoate/vilanterol (FF/VI) is an ICS/LABA combination therapy licensed for the once-daily treatment of asthma in adults and adolescents aged ≥12 years in Europe (Citation14) and in adults aged ≥18 years in the United States (US) (Citation15). FF/VI is recommended by GINA as an alternative controller therapy for Step 3 (2). Once-daily use of FF/VI 100/25 mcg has been shown to reduce the risk of severe exacerbations (defined by the European Respiratory Society/American Thoracic Society [ERS/ATS] Task Force as “events that require systemic corticosteroids for ≥3 days and/or hospitalization/emergency room visit for asthma requiring systemic corticosteroids” (Citation10)) and significantly improve lung function compared with FF alone (Citation16). Another study demonstrated that use of FF/VI 100/25 mcg is associated with significant improvement in symptom control, compared with usual care (other ICS ± LABA) (Citation17). Furthermore, data from a UK-based real-world study have demonstrated that FF/VI 100/25 mcg improves asthma control and reduces the risk of exacerbations, in addition to improving quality of life, compared with usual care (other ICS/LABA) (Citation18).
However, there are limited real-world data from the US among patients with asthma receiving FF/VI 100/25 mcg versus other ICS/LABAs, particularly those providing total daily ICS doses falling under GINA’s low-dose categories. As such, this retrospective study compared asthma symptom control, including use of rescue medication (SABA) and asthma-related exacerbations, among adult patients with asthma who received once-daily FF/VI 100/25 mcg versus patients who received twice-daily budesonide/formoterol (B/F) 160/4.5 mcg, a widely used ICS/LABA that is recommended by GINA from Steps 1–4 and fulfills the low-dose category (B: 200–400 mcg per day), using a large, multi-payer US claims database.
Methods
Study design
This was a retrospective matched cohort study using medical and pharmacy claims data from the IQVIA PharMetrics® Plus Database of adult patients with asthma initiating FF/VI 100/25 mcg or B/F 160/4.5 mcg between January 1, 2015 and December 31, 2018 (). The IQVIA PharMetrics® Plus database contains fully adjudicated medical and pharmacy claims data for more than 190 million unique enrollees since 2006. Data were de-identified and compliant with the Health Insurance Portability and Accountability Act.
Figure 1. Study design.
Abbreviations: B/F, budesonide/formoterol fumarate; FF/VI, fluticasone furoate/vilanterol.
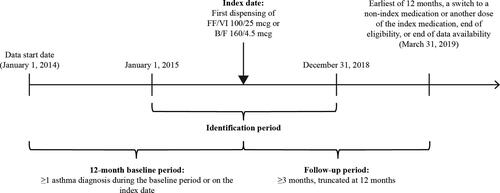
The index date was defined as the date on which FF/VI 100/25 mcg or B/F 160/4.5 mcg was first dispensed and patients were assigned to one of the two mutually exclusive cohorts based on their index medication. The baseline period was defined as the 12-month period preceding the index date and was used to assess patients’ baseline characteristics. The identification period was defined as the time frame from January 1, 2015 to December 31, 2018, over which patients initiating therapy with FF/VI 100/25 mcg or B/F 160/4.5 mcg were identified. The follow-up period was defined as the day immediately following the index date up to the earliest of 12 months, a switch to another ICS/LABA or a different dose of the index medication, health plan disenrollment, or end of data availability (March 31, 2019), and was used to evaluate study endpoints.
Study participants
Patients were included in the study if they were ≥18 years of age on the index date and had: ≥1 pharmacy claim for FF/VI 100/25 mcg or B/F 160/4.5 mcg between January 1, 2015 and December 31, 2018; ≥12 months of continuous enrollment with medical and pharmacy coverage prior to the index date (baseline period); ≥3 months of continuous enrollment with medical and pharmacy coverage following the index date (follow-up); and ≥1 medical claim with a primary or secondary diagnosis of asthma (as defined by the International Classification of Diseases [ICD], Ninth or Tenth Revision (Citation19)) during the baseline period or on the index date. Patients were excluded from the study if they had: ≥1 pharmacy claim for any fixed-dose ICS/LABA, including the index ICS/LABA, during the 12-month baseline period; ≥1 pharmacy claim for more than one type of fixed-dose ICS/LABA (i.e. both FF/VI 100/25 mcg and B/F 160/4.5 mcg) on the index date; or ≥1 medical claim with a primary or secondary diagnosis for chronic obstructive pulmonary disease, acute respiratory failure, or cystic fibrosis during the baseline period or on the index date.
Primary endpoint
The primary endpoint was asthma control, as measured by the mean number of SABA canisters dispensed per patient-year (PPY). This was calculated by dividing the total number of SABA canisters dispensed during the follow-up period (excluding any dispensed on the index date) by the total patient-years of follow-up. Canister equivalents (CEs) were assigned to each dispensed SABA product to account for metered-dose inhaler (MDI) and nebulized SABA use; one CE was defined as 200 metered actuations of albuterol or levalbuterol, or 100 3-ml ampules of nebulized SABA.
Secondary endpoints
Secondary endpoints included time to first, and rates of, overall or severe asthma-related exacerbation. Asthma-related exacerbations were evaluated during the follow-up period, excluding any on the index date. Overall asthma-related exacerbations were defined as a moderate or severe exacerbation, where:
A moderate exacerbation was defined as an asthma-related emergency department (ED) or outpatient visit with a systemic/oral corticosteroid dispensing within ±5 days of the visit
A severe exacerbation was defined as an asthma-related hospitalization or asthma-related ED visit resulting in a hospitalization within +1 day.
If two or more exacerbations occurred within 14 days of each other, these were considered as one exacerbation and classified according to the highest severity. Rates of overall and severe asthma-related exacerbations were reported per-patient per-year (PPPY) and per 100 patient-days during the follow-up period for up to 12 months, with testing for statistical significance performed on asthma-related exacerbations per 100 patient-days only.
Statistical analysis
Patients initiating FF/VI 100/25 mcg were matched 1:1 with patients initiating B/F 160/4.5 mcg using multivariable propensity score (PS) matching adjusting for the following covariates: age, gender, region, insurance, prescriber physician specialty, year and quarter of index date, Quan-Charlson comorbidity index (CCI), asthma-related exacerbations at baseline and on the index date, asthma medication ratio (AMR), respiratory medications at baseline, SABA use on the index date, all-cause and asthma-related baseline HRU, all-cause and asthma-related baseline costs, and asthma-related and baseline comorbidities (those with a prevalence ≥5%).
Patient characteristics were evaluated during the baseline period and compared between the FF/VI 100/25 mcg and B/F 160/4.5 mcg cohorts. Descriptive statistics including mean, standard deviation (SD), and median values for continuous variables, and relative frequencies and proportions for categorical variables were reported for the unmatched and matched cohorts. Differences in characteristics between cohorts were assessed using standardized differences; those with <10% difference were not considered to be statistically relevant.
For the primary objective, rates of SABA canisters dispensed PPY were compared between matched cohorts with rate ratios (RR) estimated using Poisson regressions with 95% confidence intervals (CIs) and p values generated from non-parametric bootstrap procedures. For the secondary objectives, Kaplan-Meier (KM) analysis was used to estimate the time to first overall and severe asthma-related exacerbation. Hazard ratios (HRs) from a Cox proportional hazards regression with robust standard errors were used to compare time to first overall and severe asthma-related exacerbation between matched cohorts. Rates of overall and severe asthma-related exacerbations were also calculated and reported PPPY as well as per 100 patient-days of follow-up. Asthma-related exacerbation rates per 100 patient-days of follow-up were compared between matched cohorts with RRs from a Poisson regression with bootstrapped 95% CIs and p values.
All outcomes were also evaluated in a subgroup of patients with ≥1 asthma-related exacerbation during the 12-month baseline period. Among this subgroup, patients treated with FF/VI 100/25 mcg were re-matched 1:1 with patients treated with B/F 160/4.5 mcg using multivariable PS matching to adjust for differences in baseline characteristics. Variables used in the PS calculation of the subgroup analysis were the same as those used in the main study population.
Results
Study population and baseline characteristics
A total of 306,633 patients met the study eligibility criteria (), of whom 70,655 received FF/VI 100/25 mcg and 235,978 received B/F 160/4.5 mcg. After the application of study inclusion and exclusion criteria, 18,531 patients were included in the FF/VI 100/25 mcg cohort and 51,537 were included in the B/F 160/4.5 mcg cohort ( displays unmatched baseline characteristics for patients in the two treatment cohorts). Following PS matching, all 18,531 patients in the FF/VI 100/25 mcg cohort were matched to 18,531 patients in the B/F 160/4.5 mcg cohort.
Figure 2. Inclusion and exclusion of patients.
aPatients initiating treatment with both FF/VI 100/25 mcg and B/F 160/4.5 mcg on the index date were classified as being in the B/F 160/4.5 mcg cohort and subsequently excluded (N = 301).
bContinuous eligibility was defined as continuous health plan enrollment with medical and pharmacy coverage.
cIndex date was defined as the date of the first claim for FF/VI 100/25 mcg or B/F 160/4.5 mcg during the patient identification period (January 1, 2015 – December 31, 2018).
dBaseline period was defined as 12 months of continuous enrollment with medical and pharmacy coverage prior to the index date.
eFollow-up period was defined from the index date + 1 day until the earliest of 12 months, a switch to a non-index medication or another dose of the index medication, health plan disenrollment, or end of data availability (March 31, 2019).
fDiagnosis of asthma was based on ICD-9-CM codes 493.xx; ICD-10-CM codes J45.3x, J45.4x, J45.5x, J45.9x.
gCOPD diagnosis was based on ICD-9-CM codes 491.xx, 492.x, 496; ICD-10-CM codes J41.x, J42, J43.x, J44.x.
hAcute respiratory failure diagnosis was based on ICD-9-CM code 518.81; ICD-10 CM codes J96.0, J96.2.
iCystic fibrosis diagnosis was based on ICD-9-CM code 277.0; ICD-10-CM codes E84.0-E84.9.
Abbreviations: B/F, budesonide/formoterol fumarate; COPD, chronic obstructive pulmonary disease; FF/VI, fluticasone furoate/vilanterol; ICD-9-CM, International Classification of Disease, Ninth Revision, Clinical Modification; ICD-10-CM, International Classification of Disease, Tenth Revision, Clinical Modifications; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist.
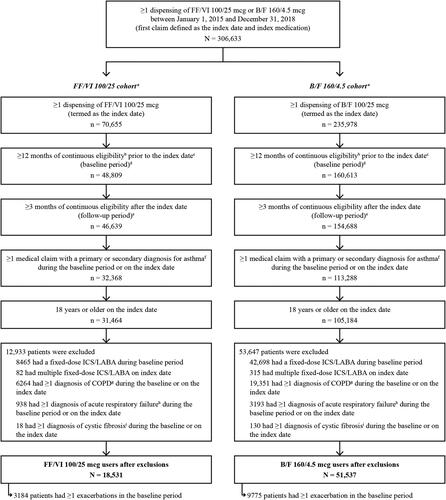
Table 1. Post-propensity score matching baseline demographics for the main analysis.
Baseline characteristics were well balanced between matched cohorts as evidenced by standardized differences of ≤10% (). Mean (SD) age was 46.7 (13.6) and 46.8 (13.5) years for the FF/VI 100/25 mcg and B/F 160/4.5 mcg cohorts, respectively. Females comprised 63.4% of patients in the FF/VI 100/25 mcg cohort and 63.8% of patients in the B/F 160/4.5 mcg cohort. A total of 17.2% and 17.5% of patients receiving FF/VI 100/25 mcg and B/F 160/4.5 mcg, respectively, experienced at least 1 exacerbation during the baseline period. In both cohorts, a higher proportion of patients had primary care compared with allergist and pulmonologist care (48.2% vs. 29.4% in the FF/VI 100/25 mcg cohort and 49.9% vs. 26.9% in the B/F 160/4.5 mcg cohort) as the prescribing physician specialty on the index date. The mean (SD) number of asthma-related ED visits during the baseline period was 0.05 (0.31) and 0.06 (0.29) in the FF/VI 100/25 mcg and B/F 160/4.5 mcg cohorts, respectively. In both matched cohorts, 0.9% of patients had ≥1 asthma-related hospitalization during the baseline period. The mean (SD) duration of follow-up was 314 (84) days in the FF/VI 100/25 mcg cohort and 315 (83) days in the B/F 160/4.5 mcg cohort.
Asthma control, as measured by mean number of SABA canisters
Patients who initiated FF/VI 100/25 mcg used significantly fewer canisters during the follow-up period compared with patients initiated on B/F 160/4.5 mcg (1.47 vs. 1.64 PPY; RR [95% CI] = 0.90 [0.86–0.93], p < 0.001) ().
Time to first overall or severe asthma-related exacerbation
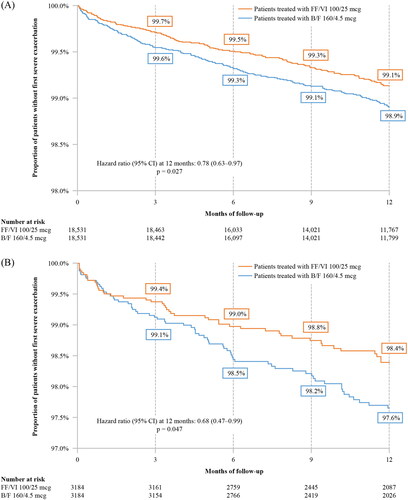
Time to first overall (moderate or severe) or severe asthma-related exacerbation, as defined within the methods, was significantly longer in the FF/VI 100/25 mcg cohort relative to the B/F 160/4.5 mcg cohort at 3, 6, 9, and 12 months of follow-up (p < 0.05 at all time points). More specifically, the KM rate of FF/VI 100/25 mcg patients without an overall exacerbation at 12 months was 86.5% compared to 84.7% in the B/F 160/4.5 mcg cohort, representing a 13% lower risk of overall asthma-related exacerbations over 12 months of follow-up for patients in the FF/VI 100/25 mcg cohort (HR [95% CI] = 0.87 [0.82–0.92], p < 0.001) ().
Figure 4. Time to first overall asthma-related exacerbation (A) in the main analysis and (B) in patients with ≥1 prior exacerbation.
Abbreviations: B/F, budesonide/formoterol; CI, confidence interval; FF/VI, fluticasone furoate/vilanterol.
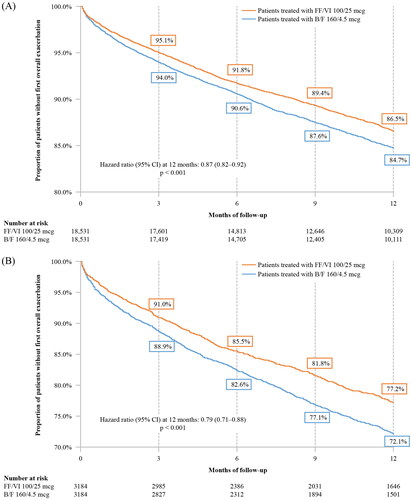
KM rates of patients without a severe asthma-related exacerbation at 12 months were 99.1% and 98.9% for the FF/VI 100/25 mcg and B/F 160/4.5 mcg cohorts, respectively, representing a 22% lower risk of severe asthma-related exacerbations over 12 months of follow-up for patients in the FF/VI 100/25 mcg cohort (HR [95% CI] = 0.78 [0.63–0.97], p = 0.027) ().
Rate of overall and severe asthma-related exacerbations
The rates of overall and severe asthma-related exacerbations were low in both cohorts. However, initiation of FF/VI 100/25 mcg was associated with a significantly lower rate of overall asthma-related exacerbations per 100 patient-days compared with initiation of B/F 160/4.5 mcg (0.0475 vs. 0.0558, RR [95% CI] = 0.85 [0.81–0.91], p < 0.001) (). Similarly, initiation of FF/VI 100/25 mcg was associated with a significantly lower rate of severe asthma-related exacerbations per 100 patient-days compared with initiation of B/F 160/4.5 mcg (0.0026 vs. 0.0033, RR [95% CI] = 0.78 [0.62–0.96], p = 0.020) (). Initiation with FF/VI 100/25 mcg was also associated with a lower rate of overall (0.1796 vs. 0.2081) and severe (0.0097 vs. 0.0131) asthma-related exacerbations PPPY compared with initiation of B/F 160/4.5 mcg ().
Table 2. Overall and severe asthma-related exacerbations during follow-up in the main analysis (N = 37,062).
Subgroup analysis (≥1 exacerbation during the baseline period)
Of the 18,531 patients in the FF/VI 100/25 mcg cohort, 3,184 had ≥1 exacerbation during the baseline period and were included in the subgroup analysis. Of the 51,537 patients in the B/F 160/4.5 mcg cohort, 9,775 had ≥1 exacerbation during the baseline period and were included in the subgroup analysis. All 3,184 patients in the FF/VI 100/25 mcg cohort who had ≥1 exacerbation during the baseline period were matched to 3,184 patients in the B/F 160/4.5 mcg cohort who had ≥1 exacerbation during the baseline period.
All baseline characteristics of the PS-matched patients with a prior history of exacerbations were well balanced between the FF/VI 100/25 mcg and B/F 160/4.5 mcg cohorts; however, these baseline characteristics tended to differ from those of the main study population ( and ). In the subgroup with a history of prior exacerbations, slightly more patients received at least one dispensing of ICS, oral corticosteroid, or SABA during the baseline period than those in the main study population. Moreover, compared with the main study population, patients with a prior history of exacerbations had a higher mean number of asthma-related ED visits (FF/VI 100/25 mcg: 0.26 vs. 0.05; B/F 160/4.5 mcg: 0.27 vs. 0.06), a greater proportion of asthma-related hospitalizations (5.3% vs. 0.9% in the FF/VI 100/25 mcg cohort; 5.2% vs. 0.9% in the B/F 160/4.5 mcg cohort), and higher asthma-related mean total healthcare costs during the baseline period ($2,575 vs. $1,003 in the FF/VI 100/25 mcg cohort; $2,370 vs. $993 in the B/F 160/4.5 mcg cohort). For patients included in the subgroup, most had exacerbations as defined by oral corticosteroid use during the baseline period compared with exacerbations as defined by hospitalizations (∼95% vs. ∼5%).
Similar to the results of the main study population, patients with ≥1 prior exacerbation (overall or severe) who were initiated on FF/VI 100/25 mcg used significantly fewer SABA canisters during the follow-up period (1.75 vs. 2.06 PPY; RR [95% CI] = 0.85 [0.78–0.92], p < 0.001; ), had significantly longer time to first overall (moderate or severe) or severe asthma-related exacerbation (p < 0.05), and had a 21% lower risk of overall asthma-related exacerbations (HR [95% CI] = 0.79 [0.71–0.88], p < 0.001; ) and a 32% lower risk of severe asthma-related exacerbations at 12 months post-index (HR [95% CI] = 0.68 [0.47–0.99], p = 0.047; ) than patients in the B/F 160/4.5 mcg cohort. Initiation with FF/VI 100/25 mcg was associated with a significantly lower rate of overall (RR [95% CI] = 0.80 [0.72–0.90]; p < 0.001) and severe (RR [95% CI] = 0.62 [0.42–0.98]; p = 0.028) asthma-related exacerbations per 100 patient-days compared with initiation of B/F 160/4.5 mcg (Supplementary Table 3). PPPY, initiation with FF/VI 100/25 mcg was associated with a lower rate of overall (0.3361 vs 0.4103) and severe (0.0189 vs 0.0332) asthma-related exacerbations compared with initiation of B/F 160/4.5 mcg (Supplementary Table 3).
Discussion
In this retrospective analysis of medical and pharmacy claims data from a large US healthcare claims database, the use of once-daily FF/VI 100/25 mcg was associated with a significant reduction in the use of SABA canisters and in overall and severe asthma-related exacerbations compared with patients using twice-daily B/F 160/4.5 mcg, potentially reflecting improved asthma control. Similar results were seen in the subgroup of patients with a prior history of exacerbations (i.e. ≥1 asthma-related exacerbation during the baseline period). FF/VI 100/25 mcg has been previously shown in a clinical study to reduce asthma-related exacerbations when compared with FF 100 mcg alone (Citation16); the data from this real-world study provide further evidence as to the effectiveness of FF/VI 100/25 mcg in reducing exacerbations compared with B/F 160/4.5 mcg.
Asthma control encompasses current clinical state, symptoms and functional limitations, and future risk of adverse outcomes including exacerbations, pulmonary function loss, poor QoL, and mortality (Citation3,Citation4,Citation10). Future health risk is also associated with significant direct costs such as increased HRU, and indirect costs including impaired work productivity (Citation4). Achieving and maintaining asthma control is therefore an important treatment goal (Citation2). In this study, SABA use served as a proxy for asthma symptom control. Although current GINA recommendations differ, SABA was recommended by both GINA (Citation20) and the US National Heart, Lung, and Blood Institute asthma guidelines (Citation12) as the preferred reliever during the period of observation for this study, and due to delays between the publication of recommendations and uptake in clinical practice (Citation21), patients may still have used SABA when poorly controlled. However, current GINA recommendations state that overuse of SABA is associated with detrimental outcomes, including poor symptom control, exacerbations, and a high risk of mortality (Citation2). SABA does not address the underlying pathology that is related to a worsening of asthma symptoms (Citation22) and regular use may be associated with worse outcomes and lower lung function than daily ICS used from diagnosis (Citation2). Due to safety concerns, GINA recommends reducing and eliminating the need for SABA reliever therapy as an important goal of asthma management and a measure of a treatment’s success (Citation2). In this study, it is promising that participants who received FF/VI 100/25 mcg used SABA 10% less often than those who received B/F 160/4.5 mcg. The magnitude of difference was more pronounced (15% reduction) in the subset of participants who had experienced ≥1 prior exacerbation during the baseline period. These results are consistent with those reported by Jacques et al., where participants who received FF/VI 100/25 mcg as part of a real-world study received significantly fewer prescriptions for salbutamol inhalers compared with those prescribed fluticasone propionate/salmeterol (FP/SAL) (Citation18).
While SABA use alone may not capture as many aspects of symptom control as patient-reported outcome (PRO) instruments, evaluating SABA use in claims database studies offers an objective measure of symptom control that is not subject to recall bias, which is a limitation of PROs. However, information in claims databases on SABA use is limited to the number of canisters dispensed rather than actual use. Despite this, the reduction in use of SABA and risk of asthma-related exacerbations associated with initiation of FF/VI 100/25 mcg compared with initiation of B/F 160/4.5 mcg in this study is an important finding. Minimizing future risk of asthma-related mortality and exacerbations is a key long-term treatment goal recommended by GINA (Citation2). Briggs et al. have demonstrated that asthma exacerbations have a significant impact on the patient, with symptoms, SABA use, and frequency of nighttime awakenings increasing up to 14 days before an exacerbation and only returning to baseline up to 28 days after an exacerbation (Citation23). By reducing the frequency or prolonging the time to exacerbation, patient QoL is improved, with fewer symptoms and less reliever medication required (Citation23). In addition, fewer exacerbations are associated with a reduction in emergency HRU, thereby reducing direct costs (Citation1). Future long-term studies could investigate if the improved asthma control and reduced risk of exacerbations associated with initiation of FF/VI 100/25 mcg compared with initiation of B/F 160/4.5 mcg could improve long-term patient QoL and HRU outcomes.
Although treatment adherence was not directly measured, the results of this study could be at least partially attributed to the simplified (once-a-day) dosing of FF/VI 100/25 mcg (Citation24), compared with twice-daily dosing of B/F 160/4.5 mcg. A significant improvement in treatment adherence and persistence has been previously reported in a study of similar design by Averell et al. in which initiation of once-daily FF/VI 100/25 mcg was associated with significantly greater adherence and treatment persistence than twice-daily B/F 160/4.5 mcg or FP/SAL 250/50 mcg (Citation25). Furthermore, a study by Stanford et al. reported greater adherence in adults with asthma initiating once-daily FF/VI 100/25 mcg compared with those initiating twice-daily B/F 160/4.5 mcg. Using the same definitions for overall and severe asthma-related exacerbations as described in this study, the authors also reported a comparable risk of overall exacerbations (HR [95% CI] = 0.989 [0.85–1.15], p = 0.884) and a 31% reduction in risk of severe exacerbations (HR [95% CI] = 0.69 [0.48–0.99], p = 0.045) in those initiating FF/VI to adults with asthma initiating B/F 160/4.5 mcg (Citation26). Poor treatment adherence is associated with a greater risk of uncontrolled asthma (Citation4), with improved adherence having a positive impact on symptoms, as described by Parimi et al. in a retrospective, new-user, active comparator database study (Citation24). A previous retrospective longitudinal analysis using US-based medical and pharmacy claims data on 12,907 patients with asthma reported that a 25% improvement in medication adherence was associated with a 10% reduction in the odds of receiving SABA, and a 10% reduction in the odds of requiring an asthma-related ED visit or hospitalization (Citation27). Moreover, a systematic literature review in patients with asthma has demonstrated that a 25% improvement in adherence was associated with a 10% reduction in the risk of severe exacerbations (as reported by four US-based high-quality studies in adults included in the analysis) (Citation28). While improved adherence to a simplified, once-daily treatment regimen may have led to improved asthma control in our study, further research is needed to better understand the underlying determinants of the results reported.
This study has several limitations that primarily reflect the use of claims-based data in observational research studies. The analysis used medical and pharmacy claims and therefore, measures of asthma control and exacerbations were indirect. Data on specific clinical measures, including asthma symptoms and pulmonary function tests, and select patient characteristics, such as tobacco use, were not collected. The database mainly covers commercially insured patients; therefore, the results may be most applicable to patients in the US with continuous commercial enrollment. Nonetheless, the study data represent a large sample of asthma patients across diverse geographic regions in the US and as such, have the potential for broader generalization. A further limitation is that the presence of a dispensed medication does not indicate that the medication was consumed or taken as prescribed. Moreover, medications not recorded in the claims data, such as over-the-counter drugs, samples, and inpatient medications were not captured. It should also be recognized that administrative claims data may be vulnerable to coding inaccuracies; for example, the presence of a diagnosis code may not indicate the presence of disease as it may be inaccurately coded, or was included as a rule-out criterion rather than actual disease, leading to potential misclassification bias. Finally, this was a non-randomized study and residual confounding not addressed by PS matching could still be present. Therefore, any outcome attributable to treatment is by association only. Despite these limitations, claims data remain a valuable source of real-world data in the US.
Conclusions
In real-world practice, initiation of once-daily FF/VI 100/25 mcg in adults with asthma was associated with significantly lower use of SABA and fewer asthma-related exacerbations, which may indicate better asthma control, when compared with use of twice-daily B/F 160/4.5 mcg.
Declarations
Ethics approval and consent to participate
This study complied with all applicable laws regarding subject privacy. No direct subject contact or primary collection of individual human subject data occurred. Study results were in tabular form and aggregate analyses omitted subject identification, therefore informed consent, ethics committee, or Institutional Review Board approval were not required.
Funding statement
The study was funded by GlaxoSmithKline (GSKIDHO-19–19563). Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Rosie Robson MSc., of Ashfield MedComms, an Ashfield Health company, and was funded by GlaxoSmithKline plc.
Trademarks and copyright
Trademarks are owned by or licensed to their respective owners (IQVIA [PharMetrics® Plus]).
Author contributions
CMA, FL, GG, MSD, RL, and DJS were involved in study conception/design; FL, GG, MSD, and MM were involved in data acquisition; CMA, FL, GG, MSD, RL, MM, and DJS were involved in data analysis and/or interpretation. All authors were involved in writing/critical review of draft versions of this manuscript and all approved the final version to be submitted for publication.
Author disclosures
CMA and DJS are GlaxoSmithKline plc. employees and hold shares in GlaxoSmithKline plc. RL was an employee at the time of the study and hold shares in GlaxoSmithKline plc. FL, GG, MM, and MSD are employees of Analysis Group Inc., a consulting company that has received research funds from GlaxoSmithKline plc. to conduct the current study.
HO-19-19563_Primary_manuscript_Supplementary_Table_3.docx
Download MS Word (15.9 KB)HO-19-19563_Primary_manuscript_Supplementary_Table_2.docx
Download MS Word (21.4 KB)HO-19-19563_Primary_manuscript_Supplementary_Table_1.docx
Download MS Word (21.3 KB)References
- Fuhlbrigge A, Peden D, Apter AJ, Boushey HA, Camargo CA Jr., Gern J, Heymann PW, Martinez FD, Mauger D, Teague WG, et al. Asthma outcomes: exacerbations. J Allergy Clin Immunol. 2012;129(3 Suppl):S34–S48. doi:https://doi.org/10.1016/j.jaci.2011.12.983.
- Global Initiative for Asthma [GINA]. Global strategy for asthma management and prevention (2021 report). 2021. Available from: https://ginasthma.org/gina-reports/.
- Papaioannou AI, Kostikas K, Zervas E, Kolilekas L, Papiris S, Gaga M. Control of asthma in real life: still a valuable goal? Eur Respir Rev. 2015;24(136):361–369. doi:https://doi.org/10.1183/16000617.00001615.
- Pavord ID, Mathieson N, Scowcroft A, Pedersini R, Isherwood G, Price D. The impact of poor asthma control among asthma patients treated with inhaled corticosteroids plus long-acting beta2-agonists in the United Kingdom: a cross-sectional analysis. NPJ Prim Care Respir Med. 2017;27(1):17. doi:https://doi.org/10.1038/s41533-017-0014-1.
- FitzGerald JM, Hamelmann E, Kerstjens HAM, Buhl R. Asthma exacerbations and worsenings in patients aged 1-75 years with add-on tiotropium treatment. NPJ Prim Care Respir Med. 2020;30(1):38. doi:https://doi.org/10.1038/s41533-020-00193-w.
- Peters SP, Jones CA, Haselkorn T, Mink DR, Valacer DJ, Weiss ST. Real-world Evaluation of Asthma Control and Treatment (REACT): findings from a national Web-based survey. J Allergy Clin Immunol. 2007;119(6):1454–1461. doi:https://doi.org/10.1016/j.jaci.2007.03.022.
- Oppenheimer J, Slade DJ, Hahn BA, Zografos L, Gilsenan A, Richardson D, McSorley D, Lima R, Molfino NA, Averell CM. Real-world evidence: patient views on asthma in respiratory specialist clinics in America. Ann Allergy Asthma Immunol. 2021;126(4):385–393.e2. doi:https://doi.org/10.1016/j.anai.2020.12.015.
- Sullivan PW, Ghushchyan VH, Campbell JD, Globe G, Bender B, Magid DJ. Measuring the cost of poor asthma control and exacerbations. J Asthma. 2017;54(1):24–31. doi:https://doi.org/10.1080/02770903.2016.1194430.
- Mungan D, Aydin O, Mahboub B, Albader M, Tarraf H, Doble A, Lahlou A, Tariq L, Aziz F, El Hasnaoui A. Burden of disease associated with asthma among the adult general population of five Middle Eastern countries: Results of the SNAPSHOT program. Respir Med. 2018;139:55–64. doi:https://doi.org/10.1016/j.rmed.2018.03.030.
- Reddel HK, Taylor DR, Bateman ED, Boulet L-P, Boushey HA, Busse WW, Casale TB, Chanez P, Enright PL, Gibson PG, American Thoracic Society/European Respiratory Society Task Force on Asthma Control and Exacerbations, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59–99. doi:https://doi.org/10.1164/rccm.200801-060ST.
- Virchow JC, Backer V, de Blay F, Kuna P, Ljørring C, Prieto JL, Villesen HH. Defining moderate asthma exacerbations in clinical trials based on ATS/ERS joint statement. Respir Med. 2015;109(5):547–556. doi:https://doi.org/10.1016/j.rmed.2015.01.012.
- National Asthma Education Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–S138. doi:https://doi.org/10.1016/j.jaci.2007.09.043.
- Cloutier MM, Baptist AP, Blake KV, Brooks EG, Bryant-Stephens T, DiMango E, Dixon AE, Elward KS, Hartert T, Krishnan JA, Expert Panel Working Group of the National Heart, Lung, and Blood Institute (NHLBI) and National Asthma Education and Prevention Program Coordinating Committee (NEPPCC), et al. 2020 Focused updates to the asthma management guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol. 2020;146(6):1217–1270. doi:https://doi.org/10.1016/j.jaci.2020.10.003.
- GlaxoSmithKline (Ireland) Limited. Summary of product characteristics. Relvar Ellipta 92 micrograms/22 micrograms inhalation powder, pre-dispensed. 2021.
- GlaxoSmithKline. Highlights of prescribing information. BREO ELLIPTA (fluticasone furoate and vilanterol inhalation powder), for oral inhalation use. 2019.
- Bateman ED, O’Byrne PM, Busse WW, Lötvall J, Bleecker ER, Andersen L, Jacques L, Frith L, Lim J, Woodcock A. Once-daily fluticasone furoate (FF)/vilanterol reduces risk of severe exacerbations in asthma versus FF alone. Thorax. 2014;69(4):312–319. doi:https://doi.org/10.1136/thoraxjnl-2013-203600.
- Woodcock A, Vestbo J, Bakerly ND, New J, Gibson JM, McCorkindale S, Jones R, Collier S, Lay-Flurrie J, Frith L, et al. Effectiveness of fluticasone furoate plus vilanterol on asthma control in clinical practice: an open-label, parallel group, randomised controlled trial. The Lancet. 2017;390(10109):2247–2255. doi:https://doi.org/10.1016/S0140-6736(17)32397-8.
- Jacques L, Bakerly ND, New JP, Svedsater H, Lay-Flurrie J, Leather DA. Effectiveness of fluticasone furoate/vilanterol versus fluticasone propionate/salmeterol on asthma control in the Salford Lung Study. J Asthma. 2019;56(7):748–757. doi:https://doi.org/10.1080/02770903.2018.1490751.
- World Health Organization. ICD-10: international statistical classification of diseases and related health problems: tenth revision. World Health Organization. 2004.
- Global Initiative for Asthma [GINA]. Global strategy for asthma management and prevention (2018 report). 2018. Available from: https://ginasthma.org/gina-reports/.
- Fischer F, Lange K, Klose K, Greiner W, Kraemer A. Barriers and strategies in guideline implementation-a scoping review. Healthcare (Basel). 2016;4(3):36. doi:https://doi.org/10.3390/healthcare4030036.
- Nwaru BI, Ekstrom M, Hasvold P, Wiklund F, Telg G, Janson C. Overuse of short-acting beta2-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J. 2020;55(4):1901872. doi:https://doi.org/10.1183/13993003.01872-2019.
- Briggs A, Nasser S, Hammerby E, Buchs S, Virchow JC. The impact of moderate and severe asthma exacerbations on quality of life: a post hoc analysis of randomised controlled trial data. J Patient Rep Outcomes. 2021;5(1):6. doi:https://doi.org/10.1186/s41687-020-00274-x.
- Parimi M, Svedsater H, Ann Q, Gokhale M, Gray CM, Hinds D, Nixon M, Boxall N. Persistence and adherence to ICS/LABA drugs in UK patients with asthma: a retrospective new-user cohort study. Adv Ther. 2020;37(6):2916–2931. doi:https://doi.org/10.1007/s12325-020-01344-8.
- Averell CM, Stanford RH, Laliberté F, Wu JW, Germain G, Duh MS. Medication adherence in patients with asthma using once-daily versus twice-daily ICS/LABAs. J Asthma. 2021;58(1):102–111. doi:https://doi.org/10.1080/02770903.2019.1663429.
- Stanford RH, Averell C, Parker ED, Blauer-Peterson C, Reinsch TK, Buikema AR. Assessment of adherence and asthma medication ratio for a once-daily and twice-daily inhaled corticosteroid/long-acting beta-agonist for asthma. J Allergy Clin Immunol Pract. 2019;7(5):1488–1496 e7. doi:https://doi.org/10.1016/j.jaip.2018.12.021.
- Delea TE, Stanford RH, Hagiwara M, Stempel DA. Association between adherence with fixed dose combination fluticasone propionate/salmeterol on asthma outcomes and costs. Curr Med Res Opin. 2008;24(12):3435–3442. doi:https://doi.org/10.1185/03007990802557344.
- Engelkes M, Janssens HM, de Jongste JC, Sturkenboom MC, Verhamme KM. Medication adherence and the risk of severe asthma exacerbations: a systematic review. Eur Respir J. 2015;45(2):396–407. doi:https://doi.org/10.1183/09031936.00075614.