Abstract
Objective
The aim of our study is to provide a novel strategy to administer treatment at the first signs of severe air pollution and before patients experience symptoms for preventing airway damage.
Methods
This single-center, prospective, randomized and standard treatment parallel control clinical trial recruited adult asthma patients. The patients were randomized into either the rescue intervention strategy (RIS) group or control group. The rescue intervention strategy for the RIS group included budesonide/formoterol plus the original treatment until the severe pollution ended. The control group was maintained on the original treatment. The follow-up observation period was 1 year.
Results
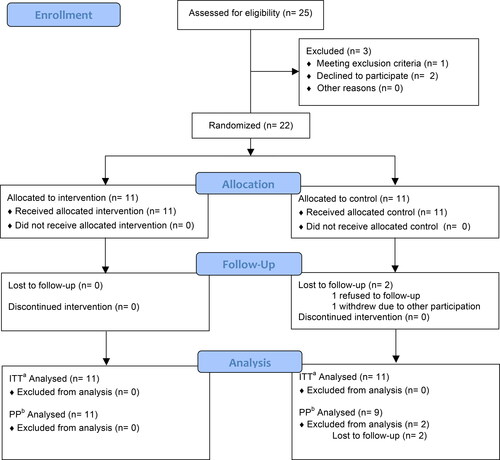
Overall, 22 participants were enrolled and 20 completed the follow-up (11 in the RIS group and 9 in the control group). Two participants dropped out of the trial for personal reasons before the first follow-up. In the intention-to-treat analysis, the frequency of asthma exacerbations per year was significantly lower in the RIS group than in the control group (RIS vs. control, 0.55 vs. 2.67; risk rate [RR] [95% confidence interval {CI}], 0.21 [0.08-0.50]; p = 0.001). The mean number of unplanned outpatient visits per person per year was also lower in the RIS group than in the control group (RIS vs. control, 0.18 vs. 1.11; RR [95% CI], 0.16 [0.04-0.75]; p = 0.019).
Conclusion
A novel strategy to administer treatment at the first signs of severe air pollution and before patients experience symptoms may decrease the risk of asthma exacerbations and negative outcomes under severe air pollution conditions.
Trial registration
ChiCTR, ChiCTR1900026757. http://www.chictr.org.cn.
Introduction
Air pollution and its serious consequences, particularly in China and other developing countries, have drawn increased attention. Air pollution can cause critical public health problems. A retrospective study of 80 515 deaths in Beijing during 2004-2008 revealed that the reduction in life expectancy was associated with increased air pollution. More specifically, an interquartile range increase in particulate matter (PM) with an aerodynamic diameter <2.5 μm (PM2.5), PM10, SO2, and NO2 was associated with 15.8, 15.8, 16.2, and 15.1 years of life lost, respectively (Citation1).
Asthma is a common chronic airway inflammatory disease, and more than 45 million adults in China have asthma (Citation2). Asthma exacerbation accelerates disease progression and increases the incidence of hospitalizations and deaths. Furthermore, air pollution can cause asthma exacerbations (Citation3). Unfortunately, few treatment strategies have been recommended to reduce severe air pollution-related asthma exacerbations. Inhaled corticosteroids (ICSs)/long-acting β-agonists (LABAs) with single maintenance and relief therapy (SMART) significantly reduce asthma exacerbations (Citation4). The SMART strategy might relieve the airway inflammation by a symptom-driven additional use of ICSs/formoterol and then prevent the onset of asthma exacerbations (Citation5). Our previous HEART study found that air pollution led to a delayed inflammatory burst in lung (Citation6), which provide a critical interventional time window to prevent the formation of inflammatory response. Once the respiratory symptoms occur, it means that the airway injury and inflammation induced by air pollution have already developed.
Therefore, we speculate that the symptom-driven SMART strategy might be insufficient to prevent the air pollution related asthma exacerbations. We hypothesize that the rescue intervention strategy of budesonide/formoterol plus original treatments under severe pollution may reduce the risk of asthma exacerbations, which is measured by the frequency of asthma exacerbations per year. The aim of our study is to provide a novel strategy to administer treatment at the first signs of severe air pollution and before patients experience symptoms for preventing airway damage.
Methods
Trial design and participants
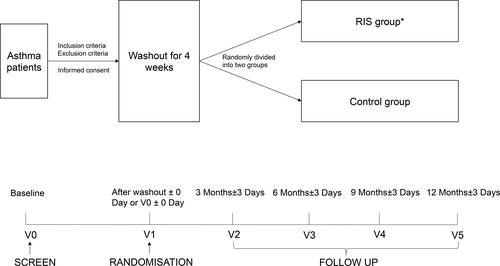
This was a single-center, prospective, randomized and standard treatment parallel control clinical trial. The study design flowchart is shown in , and the study protocol has been previously published (Citation7).
Figure 1. The study design flowchart (*RIS group, rescue intervention strategy group: when the air quality index (AQI) is more than or equal to 200 at the monitoring point near the patient’s location, the RIS group will accept Budesonide/Formoterol (160 μg/4.5 μg/dose, 1 dose/time, b.i.d) plus the original treatment until the end of severe pollution (AQI < 200). Control group: maintain the original treatment.).
Participants were recruited from Peking University First Hospital, and informed consent was obtained at screening. Basic data, including sex, age, education, income, type of medical insurance, workplace/home addresses, the air pollution monitoring station for the study (defined as the nearest air pollution monitoring station from the workplace for employees or from home for nonemployees), medical/surgical history, suspected allergen contact history (such as pets), therapeutic scheme for asthma, and any exacerbations experienced within the past 3 months, were collected at the baseline visit (V0). Physical examinations (including those for height, weight, body mass index, heart rate, and blood pressure) were also performed. We used Chinese versions of the asthma assessment scales, such as the Mini Asthma Quality of Life Questionnaire (miniAQLQ), the Asthma Control Questionnaire (ACQ) 5, the ACQ7, and Asthma Control Test (ACT), which have shown good validity (Citation8). Lung function testing (bronchodilator reversibility test) and fraction of exhaled nitric oxide (FeNO) measurements were also performed.
Several interventions, such as no use of inhaled medication, the use of short-acting β-agonists (SABAs) on demand, or the use budesonide/formoterol (160 μg/4.5 μg/dose, 1-2 dose/time, b.i.d.), were acceptable as the original treatment in our study. Participants receiving other treatments entered a washout period ( and ). Thereafter, they were randomly divided into two groups: the rescue intervention strategy (RIS) group and the control group. Exacerbation situations in the past 3 months, physical examinations, asthma assessment scales, lung function tests (bronchodilator reversibility tests), and FeNO measurements were repeated on the randomization day (V1).
Table 1. The therapeutic replacement scheme for the washout period.
Participants visited Peking University First Hospital every 3 months and were followed up for 1 year (V2-V5). At each visit, exacerbations in the past 3 months, physical examinations, asthma assessment scales, and lung function tests (bronchodilator reversibility tests) were repeated. FeNO measurements were repeated only at the final visit (V5).
Inclusion criteria
The inclusion criteria were as follows: (1) age between 18 and 80 years (male or female individuals); (2) patients with asthma at the non-exacerbation stage according to Global Initiative for Asthma 2017 guidelines; (3) smoking cessation for ≥6 months or no smoking history; (4) no restrictions in performing daily activities; (5) a resident of Beijing (there should be an air pollution monitoring station within 5 km an employee’s workplace and or within 5 km of their home for nonemployees); (6) a smartphone available at their disposal; (7) willing to provide written informed consent; and (8) willing to follow the research program.
Exclusion criteria
The exclusion criteria were as follows: (1) diagnosis of another chronic respiratory disease, such as chronic obstructive pulmonary disease, lung cancer, tuberculosis, bronchiectasis, and diffuse lung disease (interstitial pneumonia, occupational lung disease, sarcoidosis, etc.); (2) a history of lobectomy, lung transplantation, or pleural disease; (3) severe underlying disease (including severe psychiatric disorders, developmental delay, nervous system disease, other malignant tumor, chronic liver disease, heart failure, autoimmune disease, and chronic kidney disease); (4) life expectancy of <3 years; (5) no participation in outdoor activities; (6) expecting to move out of Beijing within 2 years; (7) planning to decorate home or workplace during the research period; (8) alcohol or substance abuse; (9) allergy history or other contraindication against the medicine used in this trial; (10) participating in other clinical trials; (11) poor compliance; (12) unwilling to provide written informed consent; (13) diagnosis of osteoporosis or diabetes owing to the risk of adverse effects related to using budesonide/formoterol; or (14) cigarette smoking ≥10 pack-years.
Randomization and grouping
Block randomization was used to generate random codes. The random codes were designed in a 1:1 ratio (RIS group or control group) using the SAS 9.2 software package (SAS Institute, Cary, NC). A researcher not participating in this study generated the random codes. A participant was assigned to the RIS or control group according to the randomization result. Only the participant and researchers responsible for grouping and intervention were informed of the randomization result.
Intervention
After randomization and grouping, all participants were asked to become friends with the intervention clinician on WeChat, a popular social app provided by Tencent Company, China. Communications between participants and the intervention clinician were mainly conducted through the WeChat app. The data in this app were saved and backed up.
The real-time air quality index (AQI) was collected from the Beijing Air Pollution app (provided by the Beijing Municipal Environmental Monitoring Center, China). The intervention clinician sent the real-time AQI from the air pollution monitoring station for the study to each participant via WeChat between 9 AM and 10 AM every day. When the AQI was ≥200, the intervention clinician would ask participants in the RIS group to take budesonide/formoterol (160 μg/4.5 μg/dose, 1 dose/time, b.i.d.) plus the original treatment until the severe pollution ended (AQI <200). At the same time, the control group was asked to focus on protective strategies (avoid outdoor activities, for example) and maintain their original treatments. Those in the intervention group were also given information on protective strategies. The follow-up observation period was 1 year.
Follow-up and data collection
Participants were asked to visit the intervention clinician when they visited the hospital to receive their medication for asthma or because of respiratory symptoms. The intervention clinician would assign a clinician from our study to provide medical services to the participants. Medical records were completed and saved in the Medical Record System of Peking University First Hospital. If the participant visited another hospital for an emergency, medical records from the hospital would be photographed or scanned by the intervention clinician to preserve the data.
Participants were asked to visit Peking University First Hospital every 3 months for 1 year (V2-V5). At each visit, exacerbations in the past 3 months, physical examinations, asthma assessment scales, and lung function tests (bronchodilator reversibility tests) were repeated. Exacerbation situations included moderate exacerbations (defined as the use of relief therapy for more than 2 days) and severe exacerbations (defined as the occurrence of unplanned outpatient visits, emergency visits, and hospitalizations). The exacerbation situation was verified using medical records from Peking University First Hospital and other hospitals. All visits (V0-V5) and data were recorded in case report forms.
To reduce the risk of severe acute respiratory syndrome coronavirus 2 infection, follow-up visits were changed to online visits by WeChat during the pandemic period. For each online visit, data regarding any exacerbations experienced during the previous 3 months were still collected, and the asthma assessment scales were still used. However, data regarding physical examinations, lung function tests (bronchodilator reversibility tests), and ACQ7 and FeNO measurements were not acquired. These modifications to the protocol were approved by the Peking University First Hospital Institutional Review Board.
Clinicians and blinding method
All researchers participating in this clinical study received systematic training before patient enrollment. Throughout the study, the researchers responsible for the interventions and randomization and grouping were separated from the other researchers. As a result, those responsible for providing medical services and measuring asthma assessment scales were blinded to the study grouping. Data analysts were also blinded by having the data labeled as “group A” or “group B” when data analysis was performed.
Outcomes
The primary outcome was the frequency of asthma exacerbations per year, defined as the mean number of asthma exacerbations per person per year at the end of the 1-year follow-up period. Asthma exacerbation situations included moderate exacerbations (defined as the use of relief therapy for more than 2 days) and severe exacerbations (defined as the occurrence of unplanned outpatient visits, emergency visits, and hospitalizations).
The secondary outcomes included the mean number of unplanned outpatient visits, emergency visits, hospitalizations, medical costs, and mortality caused by asthma exacerbations per person per year at the end of the 1-year follow-up period.
Safety and adverse events
Adverse events are any untoward medical occurrence that may present during treatment but which does not necessarily have a causal relationship with this treatment. Data regarding adverse events were systematically collected via spontaneous self-reporting. All adverse events were carefully monitored, managed, and tracked in a timely manner until they were properly resolved, stabilized, or returned to normal. The occurrence of adverse events was recorded from the beginning to the end of the study. Severe adverse events were analyzed every 3 months during the study. If there was a definite benefit (P < 0.01) or an obvious disadvantage (P ≤ 0.05), the study would be stopped after discussion with the center and the approval of the ethics committee.
Sample size
According to a previous study, asthma-related outpatient visits were increased by 0.65% and emergency visits by 0.49% in Beijing for every 10 μg/m3 PM2.5 increase (Citation9). Furthermore, the incidence of asthma exacerbations increased by 3-6% for every 10-μg/m3 PM10 increase (Citation10). The Beijing Environmental Statement published in 2016 by the Beijing Environmental Protection Agency showed that the monthly mean concentration of PM2.5 increased from approximately 70 μg/m3 to 150 μg/m3, and the mean concentration of PM10 increased from 52 μg/m3 to 150 μg/m3 (Citation11). This meant that the risk of asthma exacerbations increased by at least 30% as air pollution changed. Assuming an exacerbation frequency rate ratio of 0.85 in the RIS group compared to the control group, a total of 60 subjects (30 in each group) were required to detect a 75% reduction in air pollution-related exacerbation at 90% power with a two-sided significance level of 0.05. We aimed to recruit a total of 72 subjects considering a dropout rate of 20%.
Statistical analyses
Statistical analysis was conducted using SPSS 14.0 software (International Business Machines Corp., New Your, USA). All statistical analyses were performed by the two-sided test. P values <0.05 were considered statistically significant (unless otherwise specified). Numeric variables are presented as the mean (standard deviation [SD]) or median (minimum, maximum range), and categorical variables are presented as the number of cases (percentage). Baseline characteristics were summarized using the equilibrium test. Numeric variables were analyzed using the independent sample t-test or Wilcoxon rank sum test. Categorical variables were analyzed using chi-square test, continuity-corrected chi-square test, or Fisher’s exact test. Primary and secondary outcomes (including the frequency of asthma exacerbations per year, the mean number of unplanned outpatient visits, emergency visits, hospitalizations, and mortality caused by asthma exacerbations per person per year) were compared between the two groups by the Poisson regression model for calculating the 95% confidence intervals (CIs) and the risk ratios (RRs). The mean value of medical costs caused by asthma exacerbations per person per year and other data at the end of the follow-up were compared between the two groups by the generalized linear mixed model for calculating the 95% confidence intervals (CIs) and the mean differences. We had no imputation plans for missing data. The population of the intention-to-treat (ITT) analysis was defined as participants who completed randomization. The population of the per-protocol (PP) analysis was defined as participants who strictly observed the intervention protocol and completed the follow-up. The population of the safe set (SS) analysis was defined as participants who completed randomization. We had no plans to conduct a modified ITT analysis.
Results
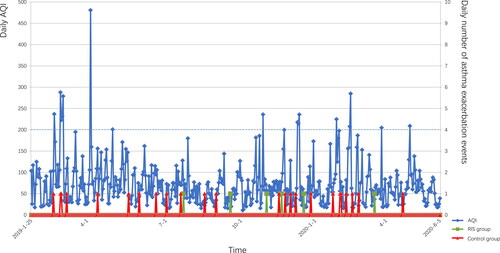
Twenty-five participants were assessed for eligibility from January 2019 to June 2019. The recruitment period was limited by the time period of funding support. One participant met the exclusion criteria of cigarette smoking >10 pack-years and two others refused to sign the informed consent. Finally, 22 participants were enrolled and randomly divided into either the RIS or control group. This population was enrolled in the ITT and SS analyses. Two participants dropped out of this trial before the first follow-up visit (V1): one refused follow-up for a personal reason and the other wanted to participant in another research study. At the end of June 2020, 20 participants had strictly observed the intervention protocol, completed the 1-year follow-up, and were enrolled in the PP analysis (11 in the RIS group and 9 in the control group). No participants needed the washout period. The participant flow diagram is shown in . And the relationship of AQI and asthma exacerbation events is shown in .
Figure 3. The relationship of AQI and asthma exacerbation events (AQI, air quality index. RIS group, rescue intervention strategy group. The blue line shows the value of daily AQI (daily AQI = (the sum of AQI of each participant on the day)/(the number of participants on the day)). The green line shows the daily number of asthma exacerbation events in the RIS group. The red line shows the daily number of asthma exacerbation events in the control group.).
There were no significant differences in baseline demographics and clinical characteristics between the two groups in the ITT analysis ( and ). Regarding primary outcomes, the frequency of asthma exacerbations per year was significantly lower in the RIS group than in the control group (RIS vs. control, 0.55 vs. 2.67; RR [95% CI], 0.21 [0.08–0.50]; p = 0.001). The frequencies of moderate and severe exacerbations were lower in the RIS group than in the control group (moderate, p = 0.010; severe, p = 0.019). Regarding secondary outcomes, the mean number of unplanned outpatient visits per person per year was lower in the RIS group than in the control group (RIS vs. control, 0.18 vs. 1.11; RR [95% CI], 0.16 [0.04–0.75]; p = 0.019). However, no difference was observed in the mean medical cost caused by exacerbations between the two groups. There were also no unplanned emergency visits, hospitalizations, or mortalities during the follow-up period in both groups. At the end of the follow-up period, the miniAQLQ score in the RIS group increased by 0.91 points compared with the baseline, while that in the control group increased by only 0.17 points (mean difference [95% CI], 0.67 [0.04–1.30]; p = 0.037). In addition, we calculated the doses and days of ICS or ICS/LABA use plus the original treatment per person per year, which included budesonide/formoterol plus the original treatment for intervention in the RIS group, and ICS or ICS/LABA plus the original treatment for relief therapy in both groups. Interestingly, only budesonide/formoterol (160 μg/4.5 μg/dose) was used as the ICS or ICS/LABA plus the original treatment for relief therapy during the follow-up period. There was no difference between the two groups with respect to the doses and days of budesonide/formoterol plus the original treatment per person per year. For other medications of exacerbations, no difference was observed between the two groups with respect to oral corticosteroid and leukotriene inhibitors. And no biologics have been used during the study. The details of outcomes are shown in .
Table 2. Baseline demographics and clinical characteristics of the participants in the ITT analysis.
Table 3. Primary and secondary outcomes in the ITT analysis.
The PP analysis revealed that there was no significant difference in baseline demographics and clinical characteristics between the two groups (). Because of the loss of participants who dropped out before the first follow-up visit and because we had no imputation plans for missing data, the results of the primary and secondary outcomes were the same as those of the ITT analysis.
In the SS analysis, only one participant in the RIS group reported an adverse event during the follow-up period. This patient complained of dizziness for a few days but quickly recovered after treatment. The adverse event did not result in a change in asthma treatment and intervention. The relationship between the adverse event and intervention was judged as “definitely unrelated.” No severe adverse events were reported, and no significant difference was observed in the rate of adverse events between the two groups (p = 1.000).
Discussion
We examined whether a rescue intervention strategy of budesonide/formoterol plus original treatments during periods of severe pollution would reduce the risk of asthma exacerbations caused by air pollution before patients experienced symptoms. The target population of this study were adult asthma patients in the non-exacerbation stage. A systematic review and meta-analysis of our previous research demonstrated that outdoor air pollution significantly increased the risk of asthma exacerbation in a single lag0 exposure pattern. Every increase of 100 units of the AQI increased the incidence of asthma exacerbations by 1.1–10.3% on the same day (Citation12). Several studies of animal models provide evidence that exposure to air pollutants modulates the airway epithelium and promotes the production of several cytokines within 24 h (Citation13–15). Budesonide/formoterol represents two classes of medications (budesonide–a synthetic corticosteroid has a wide range of inhibitory activities against multiple cell types and mediators involved in allergic and non-allergic-mediated inflammation; formoterol–a LABA with a rapid onset of action cause relaxation of bronchial smooth muscle and inhibition of the release of mediators of immediate hypersensitivity from cells) that have different effects on the clinical, physiological, and inflammatory indices of asthma (Citation16). Therefore, a rescue intervention strategy can stop the inflammation process on the day of severe air pollution onset, reducing asthma exacerbations and unplanned hospital visits.
Originally, we considered that the RIS group would require more doses of ICS because of the rescue intervention strategy, which could increase the risk of adverse events. To our surprise, no significant difference was observed in either the number of doses or days of ICS or ICS/LABA plus the original treatment per person per year between the two groups. The SS analysis also confirmed that there was no difference in the rates of adverse events. This suggests that the key to rescue intervention may be the timing of administering budesonide/formoterol during periods of severe air pollution. In the RIS group, the timing of administration was the day of severe pollution onset and before the appearance of asthma symptoms. In the control group, it was as needed for relief therapy. In these conditions, the patients in the control group may have had obvious symptoms for minutes or even hours, and damage to the airways caused by pollutants would have already occurred. The timing in the control group might have been too late to avoid the asthma becoming out of control, leading to unplanned hospital visits and administration of more ICS doses.
Our study found that the miniAQLQ scores at the end of follow-up period increased from the baseline scores and were much higher in the RIS group. This suggests that the asthma-related quality of life of participants improved in both groups, especially in the RIS group, at the end of the 1-year follow-up period. A possible explanation was that participants benefited from daily real-time AQI, warnings of severe air pollution, regular follow-ups for asthma, and opportune medical services when needed. The RIS group also had the added benefit of a decreased risk of asthma exacerbation owing to the rescue intervention strategy. The ACQ5 and ACT scores had a similar trend of improvement in both groups, especially in the RIS group; however, there was no statistically significant difference.
Only two participants (2/22, 9.1%) dropped out of the control group, and all participants of the RIS group strictly observed the intervention protocol and completed the follow-up. The rescue intervention strategy medication was consistent with the original treatment used by most participants, making the strategy easy to implement and could have played an important role in the compliance of participants in the RIS group.
This study had limitations. First, the study did not follow a double-blind design because participants in the control group were not administered a placebo. To decrease the potential bias, an independent group of researchers were responsible for the intervention and another group of researchers were responsible for randomization and grouping. The researchers responsible for providing medical services and using the asthma assessment scales at follow-up visits were blinded to the grouping results. Second, the study was not a multicenter study and was performed only in Beijing. Thus, selection bias could not be avoided. Third, the number of enrolled participants was less than expected, which could have affected the efficiency of the conclusions. Even so, we obtained positive results from the primary outcomes and some of the secondary outcomes. If the sample size was increased, other outcomes could have become positive. Fourth, because of the effect of coronavirus disease, most of the V4 and/or V5 follow-up visits were changed to online follow-up and the data related to physical examinations, lung function tests (bronchodilator reversibility tests), and ACQ7 and FeNO measurements were missed; thus, we could not analyze changes in these parameters from baseline.
Conclusions
A rescue intervention strategy may reduce the risk of air pollution-related asthma exacerbations before patients experience symptoms, thereby preventing airway damage. Our data also suggests an improvement of life quality score resulting from applying this novel strategy among asthma patients. In the future, larger studies are necessary and encouraged to provide multi-center and double-blind data to confirm the benefits.
Ethics approval and consent to participate
The first version of the study protocol was approved by the Peking University First Hospital Institutional Review Board (2018[268]) in December of 2018. Any protocol modifications were submitted for Institutional Review Board review and approval. Written informed consent was obtained from each participant.
Authors’ contributions
XY and JH are joint first authors. JH obtained the funding. GW and YH are joint corresponding authors. GW and JH conceived and designed the study. JH, XY, YH, and GW drafted the manuscript. JH, XY, CG, ZT, and YH were responsible for participant management and follow-up. JH, XY, CG, SZ, and XW were responsible for data management and performed the statistical analysis. ZY was responsible for administrative management. All authors have read and approved the final manuscript and are responsible for their contributions.
| Abbreviations | ||
| AQI | = | air quality index |
| ACT | = | Asthma Control Test |
| BMI | = | body mass index |
| CI | = | confidence interval |
| FeNO | = | exhaled nitric oxide |
| FEV1 | = | forced expiratory volume in one second |
| FVC | = | forced vital capacity |
| ICS | = | inhaled corticosteroids |
| ITT | = | intention-to-treat |
| LABA | = | long-acting β-agonists |
| miniAQLQ | = | Mini Asthma Quality of Life Questionnaire |
| ACQ | = | Numerical Control Questionnaire |
| PM | = | particulate matter |
| PP | = | per-protocol |
| Pre | = | prediastolic |
| Pred | = | predicted |
| Post | = | postdiastolic |
| RIS | = | Rescue Intervention Strategy |
| RR | = | risk rate |
| SS | = | safe set |
| SABA | = | short-acting β-agonists |
| SMART | = | single maintenance and relief therapy |
| SD | = | standard deviation |
Supplementary_Figure_1._The_flowchart_for_the_washout_period__SABA__short-acting__-agonists__LABA__long-acting__-agonists__ICS__inhaled_corticosteroids_..pdf
Download PDF (99.4 KB)Supplementary_table_3_The_other_baseline_demographic_and_clinical_characteristics_of_the_participants_in_the_PP_analysis.docx
Download MS Word (19.7 KB)Supplementary_table_2_Baseline_demographic_and_clinical_characteristics_of_the_participants_in_the_PP_analysis.docx
Download MS Word (23.7 KB)Supplementary_table_1_The_other_baseline_demographic_and_clinical_characteristics_of_the_participants_in_the_ITT_analysis.docx
Download MS Word (21.4 KB)Acknowledgements
The authors would like to acknowledge study participants.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Data availability
Data from the trial is available by request with privacy protection.
Additional information
Funding
References
- Guo Y, Li S, Tian Z, Pan X, Zhang J, Williams G. The burden of air pollution on years of life lost in Beijing, China, 2004-08: retrospective regression analysis of daily deaths. BMJ. 2013;347:f7139–f7139. doi:https://doi.org/10.1136/bmj.f7139.
- Huang K, Yang T, Xu J, Yang L, Zhao J, Zhang X, Bai C, Kang J, Ran P, Shen H, et al. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet. 2019;394(10196):407–418. doi:https://doi.org/10.1016/S0140-6736(19)31147-X.
- Martínez-Rivera C, Garcia-Olivé I, Stojanovic Z, Radua J, Ruiz Manzano J, Abad-Capa J. Association between air pollution and asthma exacerbations in Badalona, Barcelona (Spain), 2008-2016. Med Clin (Barc). 2019;152(9):333–338. doi:https://doi.org/10.1016/j.medcli.2018.06.027.
- Sobieraj DM, Weeda ER, Nguyen E, Coleman CI, White CM, Lazarus SC, Blake KV, Lang JE, Baker WL. Association of Inhaled corticosteroids and long-acting β-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma: A systematic review and meta-analysis. JAMA. 2018;319(14):1485–1496. doi:https://doi.org/10.1001/jama.2018.2769.
- Newton R, Giembycz MA. Understanding how long-acting β2 -adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids in asthma - an update. Br J Pharmacol. 2016;173(24):3405–3430. doi:https://doi.org/10.1111/bph.13628.
- Huang W, Wang G, Lu S-E, Kipen H, Wang Y, Hu M, Lin W, Rich D, Ohman-Strickland P, Diehl SR, et al. Inflammatory and oxidative stress responses of healthy young adults to changes in air quality during the Beijing Olympics. Am J Respir Crit Care Med. 2012;186(11):1150–1159. doi:https://doi.org/10.1164/rccm.201205-0850OC.
- Yang X, Huang J, Hu Y, Guo C, Wang X, Yang Z, Zhou T, Wang G. The rescue intervention strategy for asthma patients under severe air pollution: a protocol for a single-centre prospective randomized controlled trial. Trials. 2020;21(1):912. doi:https://doi.org/10.1186/s13063-020-04830-0.
- Qu Y, Zhang C, Gao W, et al. Validity of a Chinese version of the Mini Asthma Quality of Life Questionnaire (MiniAQLQ) and a comparison of completion by patients and relatives. J Asthma. 2017;12:1–7.
- Tian Y, Xiang X, Juan J, Sun K, Song J, Cao Y, Hu Y. Fine particulate air pollution and hospital visits for asthma in Beijing, China. Environ Pollut. 2017; 230:227–233. doi:https://doi.org/10.1016/j.envpol.2017.06.029.
- Weinmayr G, Romeo E, De Sario M, Weiland SK, Forastiere F. Short-term effects of PM 10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: a systematic review and meta-analysis. Environ Health Perspect. 2010;118(4):449–457. doi:https://doi.org/10.1289/ehp.0900844.
- Bureau BMEP. Beijing Environmental Statement. 2015. [accessed 2018 Feb 1]. http://hbdc.mep.gov.cn/hbdt/bjdt/201612/t20161219_371176.shtml.
- Huang J, Yang X, Fan F, Hu Y, Wang X, Zhu S, Ren G, Wang G. Outdoor air pollution and the risk of asthma exacerbation in single lag0 and lag1 exposure patterns a systematic review and meta-analysis. MedRxiv. 2021:21251113. doi:https://doi.org/10.1101/2021.02.04.21251113.
- Mathews JA, Krishnamoorthy N, Kasahara DI, Hutchinson J, Cho Y, Brand JD, Williams AS, Wurmbrand AP, Ribeiro L, Cuttitta F, et al. Augmented responses to ozone in obese mice require IL-17A and gastrin-releasing peptide. Am J Respir Cell Mol Biol. 2018;58(3):341–351. doi:https://doi.org/10.1165/rcmb.2017-0071OC.
- Willart MAM, Deswarte K, Pouliot P, Braun H, Beyaert R, Lambrecht BN, Hammad H. Interleukin-1α controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33. J Exp Med. 2012;209(8):1505–1517. doi:https://doi.org/10.1084/jem.20112691.
- Michaudel C, Maillet I, Fauconnier L, Quesniaux V, Chung KF, Wiegman C, Peter D, Ryffel B. Interleukin-1α mediates ozone-induced myeloid differentiation factor-88-dependent epithelial tissue injury and inflammation. Front Immunol. 2018;9:916.
- Symbicort – FDA prescribing information, side effects and uses. [accessed 2021 Feb 1]. https://www.drugs.com/pro/symbicort.html#s-34089-3.