Abstract
Objective
To assess feasibility of a novel video directly observed therapy (DOT)-based digital asthma program intended to support correct inhaled corticosteroid (ICS) use among children.
Methods
We conducted a 60-day pilot study among patients 2–18 years attending a primary care clinic with prescribed ICS and sub-optimally controlled asthma (recent hospitalization, ICS nonadherence, frequent rescue inhaler use, therapy escalation, or Asthma Control Test <20). Participants used a mobile application to receive reminders, submit videos of ICS doses (video DOT), and receive asynchronous feedback on adherence and inhaler technique. We assessed enrollment, engagement, program metrics, and user experience; adherence and inhaler errors were secondary outcomes.
Results
Of 26 eligible patients, 21 (81%) enrolled and submitted ≥1 video; median age was 11 years (8–15), 71% were male, 90% had Medicaid, and 62% experienced ≥1 exacerbation in the previous 6 months. Retention was 57% and 52% at week 5 and 8, respectively. Participants submitted 810 videos. Missed doses, inhaler errors (n = 247) and adherence issues (n = 107) prompted 543 communications; inadequate inspiration or holding breath were most common. Among 16 patients with engagement >7 days and >4 videos, median inhaler error rate (proportion of videos with ≥1 error) decreased from week 1 to week 2 (73% vs 8%, p ≤ 0.05) with median adherence >80%. Participants experienced the program as long, but easy to use; benefits included building routines, skill, and independence.
Conclusions
This pilot study suggests high program acceptability among our cohort. High engagement with improved inhaler technique over the first 14 days suggests shorter implementation.
Supplemental data for this article is available online at at
Introduction
Asthma is the most common pediatric chronic disease worldwide and currently affects an estimated 5.5 million children under age 18 years in the United States (Citation1). Daily controller therapy with inhaled corticosteroids (ICS) can reduce chronic airway inflammation, control asthma symptoms and prevent acute exacerbations (Citation2), yet prevalence of uncontrolled asthma remains above 50% in this age group (Citation1). Growing evidence implicates suboptimal adherence to controller inhalers, even when children have symptoms (Citation3–5), and improper inhaler technique among a majority of children, including lack of awareness of errors among children and their adult caregivers (Citation6–10). Although global and national asthma guidelines recommend regular assessment of adherence and inhaler technique plus optimization before any step up in therapy (Citation2,Citation11), traditional approaches are insufficient.
Patient-demonstrated inhaler technique and self-reported adherence or pharmacy refill data are feasible during time-constrained follow-up visits, but remain vulnerable to bias, fail to capture real-world behavior and are uncommonly documented in practice (Citation12,Citation13). Moreover, brief interventions do not translate to sustained behavior change. Inhaler errors commonly persist after in-person or video instruction (Citation14–16), and although various strategies appear to support inhaler adherence (e.g. education, reminders, feedback and incentives), no single strategy is effective across or within individuals over time (Citation3). Importantly, any improved adherence or technique fades over time without continuous or repeat intervention (Citation8,Citation17–21). Innovations in digital technology offer promising tools for objective, remote assessment and continuous intervention that could be used to support providers and patients.
A variety of mobile applications (app) have been developed to deliver interventions designed to support adolescents and children with asthma, including peer chat, daily symptom questionnaires, education, dose reminders, and allergen alerts (Citation22–27). In addition, video directly observed therapy (DOT) is particularly well suited for remote assessment of inhaler use. Originally developed to expand the scalability of in-person DOT, this approach requires patients to capture a video of each medication dose, enabling objective remote monitoring of dose-by-dose inhaler adherence and universal inhaler technique in a real-life setting. Mobile applications (app) supporting video DOT are recommended by the World Health Organization for monitoring patients taking anti-tuberculosis treatment (Citation28) and have been implemented among children receiving treatment for tuberculosis and sickle cell anemia, but have not been extensively used for inhaler use in adults or children (Citation29–31). As of this writing, two small studies report high feasibility and acceptability of using video DOT to monitor inhaler use in children (Citation31,Citation32). A comprehensive digital program combining video DOT with a multi-component intervention has not been evaluated.
We aimed to assess the feasibility of a novel 60-day digital pediatric asthma program comprising assessment of real-life ICS use via video DOT (adherence and technique) plus delivery of a multi-component intervention intended to support correct inhaler use. The program was delivered via a smartphone application; intervention components included reminders, feedback, on-demand nurse support, progress tracker and an incentive. We report enrollment, longitudinal engagement, program metrics and qualitative user experience among outpatients aged 2 to 18 years with asthma in Baltimore, MD.
Methods
Children aged 2 to 18 years with sub-optimally controlled asthma and prescribed ICS were recruited during outpatient appointments at Greenspring Pediatric Associates (GPA) at Sinai Hospital, an inner city residency continuity clinic in Baltimore, MD. Recruitment began in February 2020 and was prematurely terminated on March 13, 2020 due to a statewide COVID-19 lockdown. A GPA nurse identified all patients with an asthma follow-up appointment scheduled during the recruitment period (n = 67 patients). Pediatricians assessed patient eligibility during the visit and offered enrollment to those with indicators of: 1) Impairment (Asthma Control Test [ACT] score <20, reported rescue inhaler use >2 times per week, >1 rescue inhaler refill in the past 3 months or therapy escalation as per electronic medical record [EMR]); Risk (asthma-related hospitalization in the past 6 months as per EMR); or ICS nonadherence (patient report or missed ICS refill in the past 6 months as per EMR). Caregivers of eligible patients < 18 years provided written, informed consent prior to enrollment, and all patients aged ≥7 years and <18 years provided written assent. Participants were asked to maintain program engagement for 60 days. The study protocol was approved by the institutional review board at LifeBridge Health.
Digital asthma program
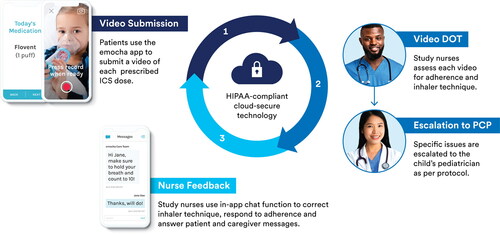
The program was delivered via the emocha mobile application (emocha Mobile Health Inc., Baltimore, MD). The emocha platform supports video capture of ICS doses and encrypted transmission to a secure server for remote viewing on a web portal (video DOT). The platform also supports delivery of the following intervention components: daily automatic and customizable dose reminders; two-way in-app chat function to facilitate nurse feedback as well as on-demand nurse support; and a progress tracker screen to visualize adherence over time (). Participants were asked to check in using the mobile app once or twice daily, depending on their prescribed regimen, and upload a video of each prescribed ICS dose for 60 consecutive days. emocha nurses reviewed uploaded videos using the emocha web portal and provided individualized feedback on inhaler technique and adherence via in-app chat and/or phone call. Participants could communicate with nurses through their video recordings or in-app chat. Nurses could escalate issues to a patient’s pediatrician according to protocol (). Participants were eligible to receive up to $60 in rewards – $1 per day of video submission.
Figure 1. Intervention schematic. Automated dose reminders prompt patients to check in to the mobile application and submit a video of each prescribed inhaled corticosteroid dose (dark blue). The emocha platform facilitates encrypted transmission of uploaded patient videos to a secure server for remote review by study nurses (video DOT). Nurses assess patient adherence and inhaler technique during video DOT (medium blue). Patients receive personalized feedback via the in-app chat function (light blue); nurses can also communicate with patients via phone call or escalate issues to the patient’s pediatrician, and patients can initiate chat communication with nurses for on-demand support.
Study procedures and data collection
Sociodemographic and clinical data were abstracted from the EMR. At enrollment, study staff downloaded the mobile app to each patient or caregiver smartphone and provided training on its use. All videos were timestamped when uploaded to the emocha server and reviewed by emocha nurses within 18 h. Nurses used an electronic form to record the following data: 1) dose-by-dose adherence (timestamp plus confirmed medication possession); 2) inhaler technique (“correct” or “incorrect”); 3) inhaler errors (forgets to shake inhaler before use; takes inadequate breath in; inadequate breath holding; no spacer used); 4) adherence issues – behavioral barriers (forgetfulness, lack of routine, disrupted routine, other), medication administration issues (takes more drug, takes less drug, takes at inappropriate time, other), social determinants of health (e.g. caregiver was unable to pick up child’s medication from the pharmacy), and patient-reported side effects; and 5) action taken (in-app chat, phone call, or escalation) with rationale. Program day one was defined by the first video timestamp or the first day coded as “missed dose,” whichever occurred first. Participants were dismissed if they did not upload any videos and did not respond to any program communications for 14 consecutive days; the final video timestamp was retrospectively considered the last day of program engagement. At the end of the program, patients and caregivers were contacted to participate in a semi-structured telephone interview intended to elicit user experience (Supplementary Table S2). The interviews were recorded; the interviewer field notes were used for thematic analysis.
Outcomes and definitions
Primary outcomes were feasibility and acceptability, as measured by enrollment, longitudinal program engagement (retention and adherence to video submission), program metrics, and user experience, including satisfaction (qualitative themes). Secondary outcomes were ICS adherence (proportion of video submissions to the number of prescribed doses – daily adherence could not exceed 100%) and inhaler error rate (proportion of video submissions with ≥1 error).
Statistical analysis
Baseline covariates and outcomes were summarized as frequency (percent) and median (interquartile range [IQR]) and examined by program week. Primary outcomes were assessed among participants with ≥1 video submitted. Interview data were coded, organized into sub-themes corresponding to feasibility, acceptability and satisfaction and integrated with quantitative findings. Secondary outcomes were assessed among participants with adequate program engagement, defined as participation >7 days and >4 videos submitted based on the data collected. These data were summarized and compared by program week using the Wilcoxon rank-sum test; p < 0.05 was considered significant. The relationship between program engagement and correct inhaler technique (proportion of video submissions with no errors) was explored using Spearman’s rank-order coefficient (rs). Strength of the correlation was interpreted as strong (0.70 ≤ rs ≤ 1.00), moderate (0.40 ≤ rs < 0.70) or weak (0.20 ≤ rs < 0.40). Data were analyzed using SPSS v 27 (IBM, Armonk, NY).
Results
Cohort characteristics
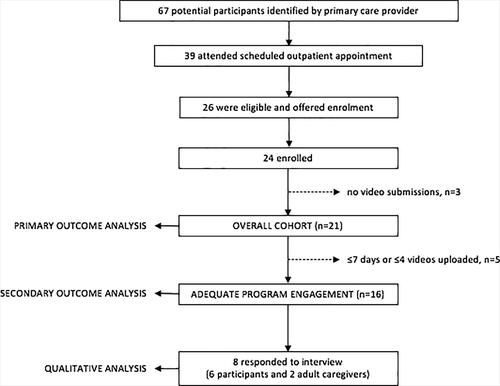
Of 26 patients meeting eligibility criteria, 24 (92%) enrolled. Of these, 21 submitted ≥1 video and were included in the primary outcome analysis, and 16 participated >7 days and submitted >4 videos and were included in the analysis of secondary outcomes. A total of eight participants (two patients and six adult caregivers) could be reached for the interview and contributed qualitative data ().
Figure 2. Study flow and analysis cohorts. Of 26 eligible patients, 24 enrolled. Of these, 21 submitted at least one video and comprised the overall cohort used for the feasibility analysis. Participants with adequate program engagement (participated >7 days and submitted >4 videos) were included in the analysis of preliminary program outcomes (inhaled corticosteroid adherence and inhaler error rate). A total of eight participants (2 patients and 6 adult caregivers) could be reached for the telephone interview at the end of the program and contributed qualitative data on feasibility, acceptability and user satisfaction.
Overall (n = 21), median age was 11 years (IQR 8–15). A majority were male (71%), had Medicaid insurance (90%) and enrolled during February 2020 (71%). A total of 13 (62%) children had at least one asthma exacerbation (composite of asthma-related emergency department visit or hospitalization or oral corticosteroid course) in the 6 months before enrollment. Among nine children with a baseline ACT available, the median score closest to enrollment was 18 (IQR 14–22). Analysis cohorts were generally similar among baseline characteristics (). All five children excluded from the secondary outcome analysis were male and enrolled in February with median program engagement of 7 days (IQR 7–9).
Table 1. Baseline characteristics among enrolled children with poorly controlled asthma by analysis cohort.
Primary outcomes
Quantitative
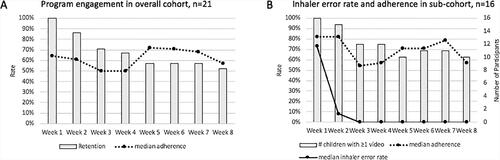
Initial program acceptability was high at 92%. Overall (n = 21), median program engagement was 53 days (IQR 14–59). Retention declined over the first 4 weeks to 57% (12 of 21) at week 5. After week 4, participants tended to remain engaged for the remainder of the program; retention was 52% (11 of 21) at week 8 (). Adherence to video submission also trended downward over the first 3 weeks, with median adherence decreasing from 64% to 50%. By comparison, the group that remained engaged after week 4 tended to maintain higher adherence, with median weekly adherence around 70% from week 5 to week 7 ().
Figure 3. Descriptive trends in (A) primary and (B) secondary outcomes by program week. Feasibility (A), as measured by program engagement, decreased over the first four weeks; 57% of enrolled children remained engaged at week 5, and median program adherence decreased from 64% to 50% over the first three weeks. Those that remained engaged past week 4 tended to complete the program and maintain higher program adherence. (B) During the first two weeks, over 90% of patients with adequate engagement submitted videos (grey bars), median inhaled corticosteroid adherence remained above 80% (dashed line) and inhaler technique improved with resolution of most errors (solid line).
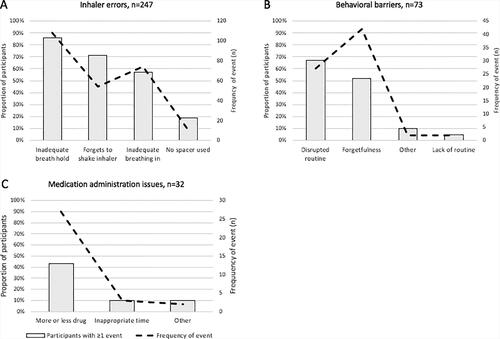
Nurses reviewed 810 videos, identified 247 inhaler errors and 107 adherence issues, and initiated 543 communications (in-app chat or phone call) in response to video DOT and missed doses; a minority of issues (n = 11) required escalation to healthcare providers. shows the frequency of individual inhaler errors (n = 247) and adherence issues (n = 107) and the proportion of participants experiencing at least one event. Inadequate breath holding (n = 108), forgetting to shake the inhaler before use (n = 54) and taking an inadequate breath in (n = 74) were most frequent, with 85% (18 of 21), 70% (15 of 21) and 57% (12 of 21) of participants demonstrating ≥1 event, respectively (). Behavioral barriers (n = 73) and medication administration issues (n = 32) were the most frequent adherence issues. Disrupted routine (n = 27) and forgetfulness (n = 42) were experienced by 67% (14 of 21) and 52% (11 of 21) of participants, respectively (). Notably, forgetfulness was by far the most frequent behavioral barrier, highlighting that remembering doses was a substantial challenge for at least some participants. Taking more or less drug than prescribed (n = 27) was the most common medication administration issue, demonstrated by 43% (10 of 21) of children (). Only two events related to social determinants of health were identified, and five participants reported ICS side effects during submitted videos (Supplementary Table S1).
Figure 4. Inhaler errors and adherence issues detected in the overall cohort (n = 21). Among 810 video submissions, nurses detected 247 inhaler errors and 107 adherence issues. (A) Errors in breath holding, forgetting to shake the inhaler before use and taking an adequate breath in were most common among children in our cohort. The majority of adherence issues were behavioral barriers (B), particularly disrupted routines and forgetfulness. The most common medication administration issue (C) was taking more or less drug than prescribed
Qualitative themes
Themes are presented in . The mobile app was considered easy to use, video DOT added little burden to the prescribed inhaler regimen, and children were generally eager participants. However, several respondents reported program fatigue over time, and for one adult caregiver, a busy work schedule limited video submissions. Technical issues also impacted some participants. Frustration mainly centered around sporadic delays in video upload. Over the course of the study, 11 technical issues were escalated to non-clinical study staff. Main program benefits included improved knowledge and skill concerning proper inhaler use, establishing daily routines, increased accountability for daily inhaler use, support for adult caregivers, and increased self-efficacy. Adult caregivers reported growing independence and confidence among patients both in remembering inhaler doses and using the inhaler correctly. Respondents felt that any age person with asthma could benefit from the program and were particularly satisfied with the amount of feedback, the detailed and personalized nature of the messages and the ability to reach out directly to a nurse outside of business hours and receive a “quick and individualized response.” Notably, all stated they would participate in the future. Suggestions for future modifications included gamification of the app, incorporating child-friendly features, offering a video call and video instruction option, and incorporating an icon badge to alert users of messages from the care team.
Table 2. Qualitative interview themes categorized according to study outcome (n = 8 respondents; two participants and six caregivers).a
Secondary outcomes
Among participants with adequate program engagement (participation >7 days and >4 videos, n = 16), median adherence was 68% (IQR 37–89), and median inhaler error rate was 18% (IQR 9–52). Weekly outcomes are shown in . Program engagement was highest during weeks 1 and 2; over 90% of participants submitted videos, and median adherence was above 80%. Inhaler technique also improved over this interval. On day one, 94% (15 of 16) of participants demonstrated at least one inhaler error. Most inhaler errors were resolved by week 3, and the median error rate remained at 0% from week 3 to week 8.
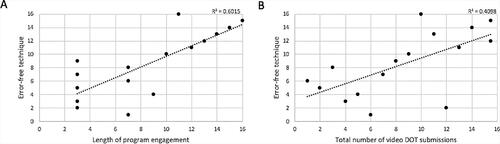
Overall, the median error rate decreased from 73% (IQR 18–85) at week 1 to 8% (IQR 0–50) at week 2 (Wilcoxon p < 0.05) (). Except for two participants (one made more inhaler errors over time and another remained error-free throughout the program), a majority (14 of 16, 88%) demonstrated improved inhaler technique by week 3 (). Three main patterns emerged: 1) Immediate improvement with most errors resolved (error rate <20%) after one week (6 of 16, 38%); 2) Gradual improvement with most errors resolved after two weeks (5 of 16, 31%); and 3) Consistent error rate (low <20%, n = 3 or high >80%, n = 1). Compared to the immediate improvement group, participants with gradual improvement tended to be male (100% vs 50%). Using Spearman’s rank-order correlation, there was a strong positive correlation between program engagement (total days) and correct technique (proportion of videos with error-free technique) (rs = 0.776, n = 16) and a moderate positive correlation between total video submissions and correct technique (rs = 0.640, n = 16) ().
Figure 5. Change in inhaler technique during the first three weeks in children with adequate engagement (n = 16). The box and whisker plot (A) summarizes inhaler errors by program week, illustrating significantly improved inhaler technique from week 1 to week 2 (median error rate 73% [IQR 18–85] vs 8% [IQR 0–50], p < 0.05). (B) Each colored line represents one participant. The majority of children (14 of 16, 88%) demonstrated improved inhaler technique over the first 3 weeks, as measured by proportion of videos with at least one error; one child made more errors over time, and one child maintained error-free technique throughout the program. Colors indicate three major patterns: orange indicates immediate resolution of most errors (error rate < 20%) within one week (6 of 16, 38%); blue indicates more gradual improvement with resolution of most errors by week 3 (5 of 16, 31%); and the green line indicates either consistently low (<20%) or high (>80%) error rate (4 of 16, 25%).
![Figure 5. Change in inhaler technique during the first three weeks in children with adequate engagement (n = 16). The box and whisker plot (A) summarizes inhaler errors by program week, illustrating significantly improved inhaler technique from week 1 to week 2 (median error rate 73% [IQR 18–85] vs 8% [IQR 0–50], p < 0.05). (B) Each colored line represents one participant. The majority of children (14 of 16, 88%) demonstrated improved inhaler technique over the first 3 weeks, as measured by proportion of videos with at least one error; one child made more errors over time, and one child maintained error-free technique throughout the program. Colors indicate three major patterns: orange indicates immediate resolution of most errors (error rate < 20%) within one week (6 of 16, 38%); blue indicates more gradual improvement with resolution of most errors by week 3 (5 of 16, 31%); and the green line indicates either consistently low (<20%) or high (>80%) error rate (4 of 16, 25%).](/cms/asset/3a63a11f-788c-40b3-88d5-9d840374e72d/ijas_a_1984525_f0005_c.jpg)
Figure 6. Relationship between program engagement and correct technique using Spearman’s rank order correlation (rs) in children with adequate engagement (n = 16). Correct technique was quantified as the proportion of video submissions with error-free technique. Scatter plots show (A) a strong positive correlation between length of program engagement and error-free technique (rs = 0.776) and (B) a moderate positive correlation between total number of video submissions and error-free technique (rs = 0.640).
Discussion
This pilot study of a 60-day digital video DOT-based asthma program found highest feasibility during the first 4 weeks and high overall acceptability and user satisfaction among a low socioeconomic status (SES) pediatric cohort with sub-optimally controlled asthma. Further, the analysis of preliminary program outcomes suggests good ICS adherence (>80%) and significantly improved inhaler technique during the first 14 days (mean difference 32%, p < 0.05) with a positive correlation between program engagement and error-free technique. Although three weeks may be required for maximal effect, shorter program implementation may offer highest feasibility while maintaining the potential to support correct inhaler use. Future studies are needed to determine a program length that optimizes desired outcomes with check-in fatigue and to formally evaluate program efficacy, including correlation with clinical outcomes.
Prior studies have reported high feasibility and acceptability of emocha (Citation33,Citation34) and other mobile video DOT platforms (Citation35,Citation36) as well as greater efficacy of video DOT compared to in-person DOT (Citation37). However, these studies have primarily focused on oral anti-tuberculosis treatment in adults. Our study is generally consistent with the few studies reporting high feasibility and acceptability of video DOT among children, including two studies that assessed asthma inhaler use (Citation29–32). To our knowledge, this is the first to assess a comprehensive pediatric asthma program combining video DOT-based monitoring of inhaler use with delivery of a multi-component intervention.
Although nearly 50% of children were able to complete the 60-day program, we observed a steady decline in program engagement during the first month. Moreover, inhaler errors were nearly completely resolved over the first three weeks. Another pediatric study also reported decreased feasibility of a mobile app-inhaler sensor intervention after the first month (Citation18), and a prior study of mobile DOT found that 90% of children achieved effective inhaler technique by week 4 (Citation30). Together, these findings suggest that a 60-day program may not be necessary to meaningfully resolve inhaler technique errors. To avoid deterioration of inhaler technique, a future implementation model could incorporate alternating phases, namely a shorter intensive phase (video DOT plus all intervention components) and an intervening maintenance phase with continuation of dose reminders via the mobile app. A larger, randomized study is needed to determine optimal program length, validate program efficacy and determine how long proper technique is sustained once achieved.
The emocha pediatric asthma program enabled nurses to identify specific inhaler errors and adherence barriers impacting effective asthma self-management. Consistent with a previous pilot of mobile DOT in children (Citation31), we found an initial high frequency of inhaler errors, particularly in the critical steps of breathing in the medication, holding breath and forgetting to shake the inhaler before use. However, a distinguishing feature of the emocha program is the ability to respond to inhaler errors, missed doses and adherence patterns with personalized, asynchronous feedback. This aspect enabled nurses to monitor and resolve issues, and the vast majority were handled remotely without involving the patient’s pediatrician. When escalations were necessary, they facilitated early linkage to care. In one case, visualization of inhaler technique and escalation to the patient’s provider resolved a unique face mask-spacer challenge that would have otherwise continued undetected.
The analysis of preliminary program outcomes suggests potential program efficacy. We found relatively high ICS adherence of 68%, which is consistent with prior studies reporting 73% adherence using video DOT among children (Citation31,Citation32). We also observed significantly improved inhaler technique and found a strong positive correlation between program engagement and mastery of inhaler technique. To date, heterogeneity has limited comparisons between studies and has impeded generating guidelines for best practices to improve asthma self-management among children (Citation16,Citation21,Citation38). In general, comprehensive multi-component interventions are recommended (Citation3) and have resulted in significant reductions in asthma-related emergency department visits, hospitalizations, and symptoms (Citation39). We were, however, unable to clearly interpret clinical outcomes due to the small sample size and confounding introduced by the statewide COVID-19 lockdown mandated in Maryland during the study in March 2020.
Overall, our findings support the feasibility and acceptability of the emocha asthma program in an urban pediatric primary care setting. There is also potential for future implementations in other settings, such as rural communities where access to an asthma specialist is limited. Other potential uses include program implementation within health plans or among case managers who could use identified adherence barriers to provide more patient-centered care. Importantly, children from low-income families are more likely to have asthma and poorly controlled asthma (Citation1) than higher SES counterparts. Although our low SES cohort was established in care with a prescribed ICS, the emocha asthma program could be implemented in school-based health centers (SBHC) intended to reach children without access to regular healthcare services. School asthma programs incorporating in-person DOT have achieved high inhaler adherence of 92% on school days (Citation40) as well as decreased oral corticosteroid courses and asthma-related hospitalizations (Citation41). The emocha program could potentially expand the scalability of in-person DOT in SBHCs while delivering other intervention components targeting asthma self-management.
Our study has several limitations. As our objective was to first assess program feasibility, the study design did not incorporate a randomized control group. In addition, our sample size was smaller than anticipated due to the COVID-19 lockdown. We were able to assess primary outcomes among 21 participants, but could only assess secondary outcomes among a smaller group (n = 16), as evaluation of adherence and inhaler technique over time depends on adequate video submission. Further, only eight participants could be reached for the interview, and the analysis relied on interviewer notes. Next, video submission was used as a proxy for ICS adherence, which may have underestimated adherence if patients used their inhaler without capturing a video. Nonetheless, we found at least 68% ICS adherence among our sub-cohort, which is consistent with previous video DOT studies (Citation31,Citation32) and well above national estimates of 50% adherence among children. Finally, the COVID-19 pandemic introduced a significant confounder, precluding reliable assessment of clinical outcomes and potentially influencing secondary outcomes. A recent multi-national study of childhood asthma during the first wave of the pandemic describes decreased healthcare utilization and asthma exacerbations as well as increased asthma control thought due to reduced exposure to asthma triggers and improved adherence (Citation42). We did observe longer engagement among children enrolled in March, perhaps due to being at home and being subjected to fewer life distractions (work, school, less complicated schedule), which could have resulted in better adherence than would have been observed in a “real-life” scenario. Despite these shortcomings, we were able to demonstrate program feasibility in our cohort along with improved inhaler technique and provide data to inform future implementations.
Conclusion
This pilot study suggests high feasibility and preliminary efficacy of a comprehensive, video DOT-based digital asthma program among children representing various stages of development from toddlers to adolescents. The program represents a promising, novel tool for objective assessment and optimization of inhaler adherence and technique, potentially filling an important void in asthma care, yet critical questions remain. A larger, randomized study would help refine a model of program implementation and allow for rigorous evaluation of program efficacy, including stratification by age group, as well as correlation with 12-month clinical outcomes. Future analyses should also include cost-effectiveness, health service utilization and examination of patterns of improvement with predictors.
Supplemental Material
Download MS Word (13.5 KB)Acknowledgements
The authors would like to acknowledge Margaret Broderick, LifeBridge Health-Greenspring Pediatric Associates, for her contributions regarding participant identification, subject recruitment, and coordination with the emocha team; the staff and providers at Greenspring Pediatric Associates; the LifeBridge Health Innovations Team; and the emocha team: Michelle Mendes, Christina Dahye Yoo, Maythana Paquete, Michael Lopez, Lauren Brown and Sebastian Seiguer.
Declaration of interest
LLY is an employee of emocha Mobile Health (emocha), a private company that licensed the video DOT technology. KM is a paid consultant to emocha. SDK and BW report no conflict of interest.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Centers for Disease Control. National Health Interview Survey. 2019. [accessed 2021 February 2]. www.cdc.gov/asthma/nhis/2019.htm.
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2020. [accessed 2021 February 2]. www.ginasthma.org.
- World Health Organization. Adherence to long-term therapies: evidence for action. WHO Geneva, 2003. [accessed 2021 February 2] www.who.int.
- Sullivan PW, Ghushchyan V, Navaratnam P, Friedman HS, Kavati A, Ortiz B, Lanier B. National prevalence of poor asthma control and associated outcomes among school-aged children in the United States. J Allergy Clin Immunol Pract. 2018;6(2):536–544. doi:10.1016/j.jaip.2017.06.039.
- Marckmann M, Hermansen MN, Hansen KS, Chawes BL. Assessment of adherence to asthma controllers in children and adolescents. Pediatr Allergy Immunol. 2020;31(8):930–937. doi:10.1111/pai.13312.
- Gillette C, Rockich-Winston N, Kuhn JA, Flesher S, Shepherd M. Inhaler technique in children with asthma: A systematic review. Acad Pediatr. 2016;16(7):605–615. doi:10.1016/j.acap.2016.04.006.
- Samady W, Rodriguez VA, Gupta R, Palac H, Karamanis M, Press VG. Critical errors in inhaler technique among children hospitalized with asthma. J Hosp Med. 2019;14(6):361–365. doi:10.12788/jhm.3195.
- Rodriguez-Garcia C, Barreiro E, Muñoz-Gall X, Bustamante-Madariaga V, de-Granda-Orive I, Gonzalez-Barcala FJ. Common errors in inhalation therapy: Impact and solutions. Clin Respir J. 2020;14(11):1001–1010. doi:10.1111/crj.13236.
- Litt HK, Press VG, Hull A, Siros M, Luna V, Volerman A. Association between inhaler technique and confidence among hospitalized children with asthma. Respir Med. 2020;174:106191. doi:10.1016/j.rmed.2020.106191.
- Almomani BA, Al-Qawasmeh BS, Al-Shatnawi SF, Awad S, Alzoubi SA. Predictors of proper inhaler technique and asthma control in pediatric patients with asthma. Pediatr Pulmonol. 2021;56(5):866–874. doi:10.1002/ppul.25263.
- National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda: US National Heart, Lung, and Blood Institute; 2007. [accessed 2021 February 2]. www.nhlbi.nih.gov.
- Anderson WC. Incorporating technology to advance asthma controller adherence. Curr Opin Allergy Clin Immunol. 2017;17(2):153–159. doi:10.1097/ACI.0000000000000343.
- Yawn BP, Rank MA, Cabana MD, Wollan PC, Juhn YJ. Adherence to asthma guidelines in children, tweens, and adults in Primary Care Settings: A Practice-Based Network Assessment. Mayo Clin Proc. 2016;91(4):411–421. doi:10.1016/j.mayocp.2016.01.010.
- Shaw N, Le Souëf P, Turkovic L, McCahon L, Kicic A, Sly PD, Devadason S, Schultz A. Pressurised metered dose inhaler-spacer technique in young children improves with video instruction. Eur J Pediatr. 2016;175(7):1007–1012. doi:10.1007/s00431-016-2738-2.
- Dhadge N, Shevade M, Kale N, Narke G, Pathak D, Barne M, Madas S, Salvi S. Monitoring of inhaler use at home with a smartphone video application in a pilot study. NPJ Prim Care Respir Med. 2020;30(1):46. doi:10.1038/s41533-020-00203-x.
- McCrossan P, Mallon O, Shields MD, O’Donoghue D. How we teach children with asthma to use their inhaler: a scoping review protocol. Syst Rev. 2020;9(1):178. doi:10.1186/s13643-020-01430-6.
- Brand PL. Key issues in inhalation therapy in children. Curr Med Res Opin. 2005;21 (suppl4):S27–S32. doi:10.1185/030079905X61767.
- Kenyon CC, Sundar KG, Gruschow SM, Quarshie WO, Feudtner C, Bryant-Stephens TC, Miller VA. Tailored medication adherence incentives for high-risk children with asthma: a pilot study. J Asthma. 2020;57(12):1372–1378. doi:10.1080/02770903.2019.1648503.
- Foster JM, Usherwood T, Smith L, Sawyer SM, Xuan W, Rand CS, Reddel HK. Inhaler reminders improve adherence with controller treatment in primary care patients with asthma. J Allergy Clin Immunol. 2014;134(6):1260–1268. doi:10.1016/j.jaci.2014.05.041.
- Chan AHY, Stewart AW, Harrison J, Black PN, Mitchell EA, Foster JM. Electronic adherence monitoring device performance and patient acceptability: a randomized control trial. Expert Rev Med Devices. 2017;14(5):401–411. doi:10.1080/17434440.2017.1322505.
- Normansell R, Kew KM, Stovold E. Interventions to improve adherence to inhaled steroids for asthma. Cochrane Database Syst Rev. 2017;4(4):CD012226. doi:10.1002/14651858.CD012226.pub2.
- Ferrante G, Licari A, Marseglia GL, La Grutta S. Digital health interventions in children with asthma. Clin Exp Allergy. 2021;51(2):212–220. doi:10.1111/cea.13793.
- Kagen S, Garland A. Asthma and allergy mobile apps in 2018. Curr Allergy Asthma Rep. 2019;19(1):6. doi:10.1007/s11882-019-0840-z.
- Teufel Ii RJ, Patel SK, Shuler AB, Andrews AL, Nichols M, Ebeling MD, Dawley E, Mueller M, Ruggiero KJ, Treiber FA. Smartphones for real-time assessment of adherence behavior and symptom exacerbation for high-risk youth with asthma: Pilot Study. JMIR Pediatr Parent. 2018;1(2):e8. doi:10.2196/pediatrics.9796.
- Kosse RC, Bouvy ML, Belitser SV, de Vries TW, van der Wal PS, Koster ES. Effective engagement of adolescent asthma patients with mobile health-supporting medication adherence. JMIR Mhealth Uhealth. 2019;7(3):e12411. doi:10.2196/12411.
- Cushing A, Manice MP, Ting A, Parides MK. Feasibility of a novel mHealth management system to capture and improve medication adherence among adolescents with asthma. Patient Prefer Adherence. 2016;10:2271–2275. doi:10.2147/PPA.S115713.
- Mayoral K, Garin O, Caballero-Rabasco MA, Praena-Crespo M, Bercedo A, Hernandez G, Castillo J, Lizano Barrantes C, Pardo Y, Ferrer M. Smartphone app for monitoring asthma in children and adolescents. Qual Life Res. 2021. doi:10.1007/s11136-020-02706-z.
- World Health Organization. Who guidelines for treatment of drug-susceptible tuberculosis and patient care (2017 update). Geneva: WHO; 2017. [accessed 2021 March 5]. www.who.int.
- Creary SE, Gladwin MT, Byrne M, Hildesheim M, Krishnamurti L. A pilot study of electronic directly observed therapy to improve hydroxyurea adherence in pediatric patients with sickle-cell disease. Pediatr Blood Cancer. 2014;61(6):1068–1073. doi:10.1002/pbc.24931.
- Hoffman JA, Cunningham JR, Suleh AJ, Sundsmo A, Dekker D, Vago F, Munly K, Igonya EK, Hunt-Glassman J. Mobile direct observation treatment for tuberculosis patients: a technical feasibility pilot using mobile phones in Nairobi, Kenya. Am J Prev Med. 2010;39(1):78–80. doi:10.1016/j.amepre.2010.02.018.
- Shields MD, ALQahtani F, Rivey MP, McElnay JC. Mobile direct observation of therapy (M-DOT) – a rapid systematic review and pilot study in children with asthma. PLoS One. 2018;13(2):e0190031. doi:10.1371/journal.pone.0190031.
- Makhecha S, Chan A, Pearce C, Jamalzadeh A, Fleming L. Novel electronic adherence monitoring devices in children with asthma: a mixed-methods study. BMJ Open Respir Res. 2020;7(1):e000589. doi:10.1136/bmjresp-2020-000589.
- Holzman SB, Zenilman A, Shah M. Advancing patient-centered care in tuberculosis management: a mixed-methods appraisal of video directly observed therapy. Open Forum Infect Dis. 2018;5(4):ofy046. doi:10.1093/ofid/ofy046.
- Holzman SB, Atre S, Sahasrabudhe T, Ambike S, Jagtap D, Sayyad Y, Kakrani AL, Gupta A, Mave V, Shah M. Use of smartphone-based video directly observed therapy (vDOT) in tuberculosis care: single-arm, prospective feasibility study. JMIR Form Res. 2019;3(3):e13411. doi:10.2196/13411.
- Garfein RS, Collins K, Muñoz F, Moser K, Cerecer-Callu P, Raab F, Rios P, Flick A, Zúñiga ML, Cuevas-Mota J, et al. Feasibility of tuberculosis treatment monitoring by video directly observed therapy: a binational pilot study. Int J Tuberc Lung Dis. 2015;19(9):1057–1064. doi:10.5588/ijtld.14.0923.
- Garfein RS, Liu L, Cuevas-Mota J, Collins K, Catanzaro DG, Muñoz F, Moser K, Chuck C, Higashi J, Bulterys MA, et al. Evaluation of recorded video-observed therapy for anti-tuberculosis treatment. Int J Tuberc Lung Dis. 2020;24(5):520–525. doi:10.5588/ijtld.19.0456.
- Story A, Aldridge RW, Smith CM, Garber E, Hall J, Ferenando G, Possas L, Hemming S, Wurie F, Luchenski S, et al. Smartphone-enabled video-observed versus directly observed treatment for tuberculosis: a multicentre, analyst-blinded, randomised, controlled superiority trial. Lancet. 2019;393(10177):1216–1224. doi:10.1016/S0140-6736(18)32993-3.
- Jeminiwa R, Hohmann L, Qian J, Garza K, Hansen R, Fox BI. Impact of eHealth on medication adherence among patients with asthma: a systematic review and meta-analysis. Respir Med. 2019;149:59–68. doi:10.1016/j.rmed.2019.02.011.
- Chan M, Gray M, Burns C, Owens L, Woolfenden S, Lingam R, Jaffe A, Homaira N. Community-based interventions for childhood asthma using comprehensive approaches: a systematic review and meta-analysis. Allergy Asthma Clin Immunol. 2021;17(1):19. doi:10.1186/s13223-021-00522-9.
- Holmes LC, Orom H, Lehman HK, Lampkin S, Halterman JS, Akiki V, Supernault-Sarker AA, Butler SB, Piechowski D, Sorrentino PM, et al. A pilot school-based health center intervention to improve asthma chronic care in high-poverty schools. J Asthma. 2021;6:1–18. doi:10.1080/02770903.2020.1864823.
- Pertzborn MC, Prabhakaran S, Hardy A, Baker D, Robinson MA, Hendeles L. Direct observed therapy of inhaled corticosteroids for asthma at school or daycare. Pediatr Allergy Immunol Pulmonol. 2018;31(4):226–229. doi:10.1089/ped.2018.0912.
- Papadopoulos NG, Mathioudakis AG, Custovic A, Deschildre A, Phipatanakul W, Wong G, Xepapadaki P, Abou-Taam R, Agache I, Castro-Rodriguez JA, et al. Childhood asthma outcomes during the COVID-19 pandemic: findings from the PeARL multi-national cohort. Allergy. 2021;76(6):1765–1775. doi:10.1111/all.14787.
