Abstract
Objective
Pregnant women with asthma have increased frequency of respiratory viral infections and exacerbations. Because of these risks, women with asthma may be subject to increased surveillance during pregnancy and may, therefore, be at increased risk of antibiotic receipt. The objective of this study was to assess the relationship between maternal asthma and outpatient prenatal antibiotic prescription fills to inform antibiotic stewardship.
Methods
We included women who delivered a singleton, term, non-low birthweight, and otherwise healthy infant enrolled in the Tennessee Medicaid Program. Maternal asthma and prenatal antibiotic fills were ascertained from healthcare encounters and outpatient pharmacy claims. We examined the association between maternal asthma and prenatal antibiotic fills using modified Poisson regression.
Results
Our study population included 168354 pregnant women, 4% of whom had asthma. Women with asthma had an increased risk of filling at least one prenatal antibiotic prescription (adjusted risk ratio [aRR] 1.27, 95% confidence interval [CI] 1.25-1.28) and had an increased number of fills during pregnancy (aRR 1.54, 95% CI 1.51-1.57) compared to women without asthma. Among those who filled at least one antibiotic prescription, women with asthma had earlier first prenatal antibiotic prescription fill and increased likelihood of filling at least one course of broad-spectrum antibiotics during pregnancy (versus narrow-spectrum).
Conclusions
Pregnant women with asthma had more outpatient antibiotic prescription fills than pregnant women without asthma. These findings highlight that pregnant women with asthma disproportionately fill more antibiotic prescriptions during pregnancy, providing data that may inform antibiotic stewardship.
Introduction
Antibiotics are the most commonly prescribed medication in the prenatal period, accounting for 80% of all prescriptions during pregnancy (Citation1,Citation2). Antibiotics are used to treat bacterial infections which can lead to adverse birth outcomes such as spontaneous abortion, prematurity, and low birth weight (Citation1). However, there is concern for overuse of antibiotics in obstetrics, particularly for the treatment of acute respiratory infections, as the focus has been on the benefits of antibiotics in the perinatal period as opposed to the risks (Citation3). Exposure to antibiotics can have major impacts on the health of both the mother and infant, including the development of multi-resistant bacteria and long-term effects on gut microbiota (Citation3).
Asthma affects 5-8% of pregnancies in the United States and is the most common respiratory disease complicating pregnancy (Citation4). Pregnant women with asthma have increased frequency and severity of respiratory viral infections compared to women without asthma (Citation4,Citation5). Asthma exacerbations are also of clinical concern during pregnancy, with up to 45% of women with asthma experiencing an exacerbation requiring medical attention (Citation6). Because of the known increased likelihood of asthma-related morbidity, pregnant women with asthma may be at increased risk of antibiotic receipt. The objective of this study was to assess the association between maternal asthma status and outpatient prenatal antibiotic prescription fills. This is a population that may disproportionately fill more antibiotic prescriptions during pregnancy on whom data on antibiotic utilization may improve how antibiotics are prescribed and used. This is critical for appropriate treatment, protecting pregnant women and their fetuses from harm and combating antibiotic resistance.
Materials and methods
Study population
Our study population included a subset of pregnant women in the Prevention of RSV: Impact on Morbidity and Asthma (PRIMA) cohort who delivered a singleton, term (gestational age ≥37 weeks), non-low birthweight (birthweight ≥2500 grams), and otherwise healthy (no chronic lung disease nor congenital heart disease defined by International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9] codes) infants from 1995-2007 and who were continuously enrolled in the Tennessee Medicaid Program (TennCare). This cohort has been described previously (Citation7). Data were obtained from linked TennCare administrative data files and Tennessee state vital records. Singleton birth, gestational age, and birthweight were ascertained from birth certificates. Continuous enrollment was defined as ≤45 days of non-enrollment during the period from 180 days prior to the last menstrual period (LMP) to the date of delivery. The study protocol was approved by the Vanderbilt University and Tennessee Department of Health Institutional Review Boards.
Maternal asthma ascertainment
Maternal asthma was ascertained from outpatient pharmacy claims and healthcare encounters using a previously validated algorithm (Citation8–14). Pregnant women with an ICD-9 diagnosis code of 493.xx (asthma) in any of the nine diagnostic fields for inpatient, other hospital care (23-h observation), emergency department visit, and/or two outpatient physician visit claims separated by at least 30 days were considered to have asthma. Pregnant women were also considered to have asthma if they had any of the following from 180 days prior to the LMP to the date of delivery: two prescription fills for any short-acting beta agonist, two prescription fills for montelukast in a 365-day period prior to Food and Drug Administration approval of montelukast for allergic rhinitis, or a single prescription fill for any other asthma medication (i.e. inhaled corticosteroid, long-acting beta agonist, combination corticosteroid/long-acting beta agonist, and leukotriene modifying agent).
Prenatal antibiotic prescription fills
Prenatal antibiotic prescription fills were ascertained from outpatient pharmacy claims. Antibiotic prescription fills, including number of fills and antibiotic spectrum, were assessed over the entire pregnancy and by trimester. As there is no standard classification of antibiotic spectrum, we classified an antibiotic fill as narrow- or broad-spectrum based on two recently published classification tables () (Citation13–16). Penicillins were classified as narrow-spectrum based on current literature (Citation14,Citation17,Citation18).
Table 1. Name and spectrum of prenatal antibiotic fills in a Tennessee Medicaid population identified through outpatient pharmacy claims.
Gestational age at prenatal antibiotic prescription fill was calculated using the date of the LMP for women in which this information was available on the birth certificate (84%). For women in which LMP was not available (16%), we calculated the LMP from the median gestational age in weeks for infants of the same race, birthweight, and birth year (Citation14,Citation19–21). When comparing pregnant women with and without asthma, we observed no difference in gestational age among women with and without LMP values. Pregnancy trimester was defined as LMP to 91 days (first trimester), 92-182 days (second trimester), and 183 days through delivery date (third trimester) (Citation14).
Covariates
Covariates were selected a priori based on clinical relevance or published evidence of their association with asthma or antibiotic use. Maternal age at delivery (years), parity (0, 1, ≥2), residence (urban, suburban, rural), maternal education level (<12 years, 12 years, >12 years), maternal race (White, Black, other), and maternal smoking during pregnancy (yes, no) were ascertained from birth certificates.
Statistical analysis
Analyses were carried out using all pregnant women or restricted to women with at least one prenatal antibiotic fill, when appropriate. The associations between maternal asthma status and prenatal antibiotic prescription fill (yes, no) and number of prenatal antibiotic prescription fills were examined among all pregnant women using modified Poisson regression with robust sandwich covariance estimator (Citation22). We used Kaplan-Meier curves to depict the relationship between maternal asthma and time to first prenatal antibiotic fill, defined by gestational age in days, among women with at least one prenatal antibiotic fill. We then used Cox proportional hazards regression to estimate the hazard ratio. We additionally assessed the association between maternal asthma status and broad-spectrum (versus narrow-spectrum) antibiotic prescription fills during pregnancy among women with at least one prenatal antibiotic fill using logistic regression. Analyses were performed using R software version 4.0.4 (R foundation for statistical computing, Vienna, Austria).
Results
Our study population included 168354 pregnant women, 4% of whom had asthma (). Pregnant women with asthma were more likely to be White, primiparous, reside in an urban area, have less than a high school degree, and deliver via cesarean section compared to pregnant women without asthma. Both groups were similar with regards to group B streptococcus positivity (10%). Among pregnant women with asthma, 38% filled at least one prescription of inhaled corticosteroids 180 days prior to the LMP to the date of delivery.
Table 2. Characteristics of pregnant women enrolled in Tennessee Medicaid, 1995–2007.
Overall, 62% of women filled at least one antibiotic prescription during pregnancy (). Among women with at least one prenatal antibiotic fill, the median number of antibiotic fills during pregnancy was 2 (interquartile range [IQR] 1-2), and the median gestational age at first fill was 91 days (IQR 40-163 days). Antibiotic fills were evenly spaced throughout pregnancy, with 33% of fills occurring in the first and second trimester and 34% of fills occurring in the third trimester. Most antibiotic prescription fills were narrow-spectrum (77%).
Table 3. Characteristics of prenatal antibiotic use among women with at least one fill during pregnancy.
Among the entire study population, the proportion of women with at least one prenatal antibiotic prescription fill increased from 1995 to 2007 (60% to 63%), with a dip from 2003 to 2004 (65% to 54%) (). The proportion of pregnant women with at least one antibiotic fill was consistently higher for women with asthma compared to women without asthma. Overall, the proportion of women with asthma who had at least one prenatal antibiotic fill decreased over the study period (82% to 75%), while the proportion of women without asthma who had at least one prenatal antibiotic fill increased (59% to 63%).
Figure 1. Proportion of pregnant women in the Tennessee Medicaid (TennCare) population with at least one outpatient antibiotic fill from 1995 to 2007.
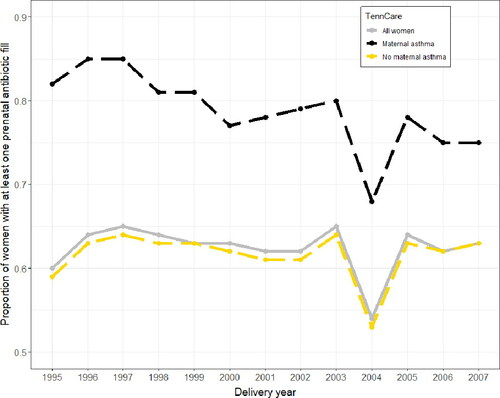
A larger proportion of women with asthma filled at least one antibiotic prescription during pregnancy than women without asthma (79% vs. 62%). Women with asthma had an increased risk of filling at least one antibiotic prescription during pregnancy compared to women without asthma (adjusted risk ratio [aRR] 1.27, 95% confidence interval [CI] 1.25–1.28) (), as well as an increased number of antibiotic fills during pregnancy (aRR 1.54, 95% CI 1.51–1.57). Women with asthma had a median of 2 (IQR 1-3) antibiotic fills during pregnancy, while women without asthma had a median of 1 (IQR 0-2) (). Among women with at least one prenatal antibiotic fill (n = 105139), women with asthma also had a significantly earlier first prenatal antibiotic prescription fill than women without asthma (median 68 days gestation [95% CI 65-70 days] vs. median 92 days gestation [95% CI 91-93 days]; adjusted hazard ratio [aHR] 1.26 [95% CI 1.23-1.29]) (). Additionally, among women with at least one prenatal antibiotic fill, women with asthma were more likely to fill at least one course of broad-spectrum antibiotics during pregnancy (versus narrow-spectrum) than women without asthma (aOR 1.52, 95% CI 1.44-1.61).
Figure 2. Number of prenatal outpatient antibiotic fills among pregnant women with and without asthma.
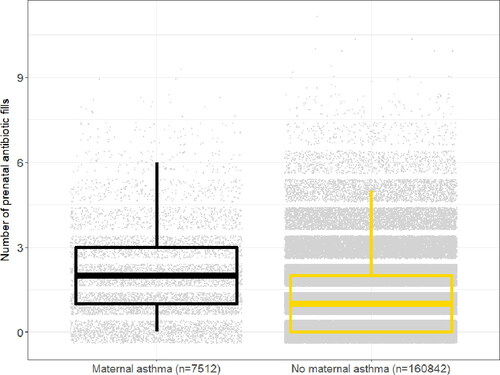
Figure 3. Cumulative incidence of first prenatal outpatient antibiotic fill among pregnant women with and without asthma who had at least one prenatal antibiotic fill.
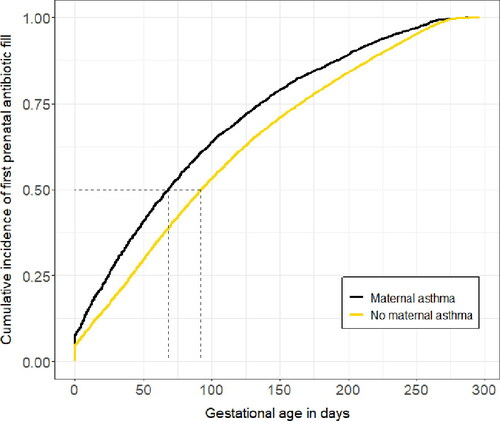
Table 4. Associations between maternal asthma and prenatal antibiotic prescription fills among women enrolled in the Tennessee Medicaid Program, 1995–2007.
Discussion
In this large, population-based cohort study, we observed an increased risk of outpatient antibiotic prescription fills during pregnancy among women with asthma compared to women without asthma. Maternal asthma was associated with an increased number of antibiotic fills during pregnancy, earlier first prenatal antibiotic fill, and increased likelihood of filling at least one course of broad-spectrum antibiotics during pregnancy (versus narrow-spectrum). These findings highlight that pregnant women with asthma disproportionately fill more antibiotic prescriptions during pregnancy, providing data that may inform antibiotic stewardship.
Few studies have assessed groups of pregnant women who may be at increased risk for antibiotic use. Several studies have found an increased risk of antibiotic prescriptions among women who smoke during pregnancy (Citation5,Citation23,Citation24). We similarly showed that a larger proportion of pregnant women with asthma smoked during pregnancy than women without asthma, and that these women had more outpatient antibiotic prescription fills. Namazy et al. showed that pregnant women with a diagnosis of asthma, rhinitis, or chronic sinusitis prior to pregnancy had a 2-fold increased risk of antibiotic administration for an upper respiratory tract infection during pregnancy (Citation5). We have also previously shown that maternal asthma was more common among children with prenatal antibiotic exposure than children without prenatal antibiotic exposure (Citation14). However, other studies have found no significant influence of asthma status on prenatal antibiotic use (Citation23). Pregnant women with asthma have greater frequency and severity of respiratory viral infections than pregnant women without asthma (Citation4,Citation5). Pregnant women with asthma also experience higher rates of exacerbations that non-pregnant women with asthma (Citation4). It is known that antibiotics may be inappropriately used to treat respiratory viral infections and asthma exacerbations (Citation25), which could potentially contribute to the association observed in the present study. Our comprehensive assessment of the relationship between maternal asthma and antibiotic prescription fills during pregnancy may inform antibiotic stewardship among those with disproportionately high use.
Concerns about antibiotic resistant bacterial strains and potential risk for birth defects have led to increased scrutiny of antibiotic use during pregnancy, particularly for broad-spectrum antibiotics (Citation26–28). While we observed a decrease in antibiotic prescription fills among pregnant women with asthma over the study period, the proportion of pregnant women with antibiotic fills was consistently higher for women with asthma compared to women without asthma. Overall prevalence rates of antibiotic fills during pregnancy in this Medicaid population were higher than previous studies conducted in the United States (Citation29,Citation30), which may reflect clinical practices in a population vulnerable to increased antibiotic prescribing and who are located in a region with known high antibiotic utilization (Citation31,Citation32). We observed a dip in the proportion of pregnant women with antibiotic fills from 2003 to 2004, which is consistent with previously reported antibiotic prescribing patterns in the United States (Citation33).
Antibiotic exposure during pregnancy may contribute to adverse long-term effects on child health. Both maternal asthma and prenatal antibiotic use have been associated with the development of childhood asthma (Citation14,Citation34). One of the strongest predictors of viral-induced lower respiratory tract infections in infancy and childhood onset asthma is a maternal history of asthma (Citation34). Further, we have previously shown that prenatal antibiotic exposure is associated with development of childhood asthma, and maternal asthma may act as an effect modifier of this association (Citation14). Although whether a causal association exists between maternal antibiotic use and childhood asthma development is unknown, maternal antibiotics are a well-established risk factor with a dose-dependent relationship with childhood asthma (Citation14,Citation35). Because antibiotics are known to be inappropriately used to treat respiratory viral infections and asthma exacerbations and antibiotics may play a role the development of childhood asthma, pregnant women with asthma represent a target group for antibiotic stewardship.
Our study is strengthened by the use of a large patient population with linked vital records and administrative data files. This included a comprehensive outpatient pharmacy claims database, which allowed us to effectively capture prenatal antibiotic prescription fills, and use of a previously validated algorithm to define maternal asthma (Citation8–14).
While our study had many strengths, there are several important limitations. We were unable to determine the indication for antibiotic prescription fills. Thus, it is unknown whether antibiotic fills were related to inappropriate treatment of asthma exacerbations or respiratory viral infections as hypothesized, or whether increased risk of bacterial infection among pregnant women with asthma contributed to the relationship between maternal asthma and prenatal antibiotic fills. Future assessment to determine whether antibiotic use among pregnant women with asthma is a result of higher bacterial infection risk or if antibiotics are being used to inappropriately treat asthma exacerbations or viral respiratory infections will better inform clinical decision making. We used antibiotic prescription fills as a surrogate measure of antibiotic exposure. However, antibiotic fill does not necessarily equate with antibiotic administration. In addition, our study population included primarily low-income pregnant women, and our results may not be generalizable to the larger US population. Lastly, as antibiotic use has changed over time, the association between maternal asthma status and antibiotic use should be assessed in more contemporary cohorts.
Conclusions
Pregnant women with asthma had more outpatient antibiotic prescription fills than pregnant women without asthma, with an increased number of antibiotic fills, earlier first prenatal antibiotic fill, and increased likelihood of filling at least one course of broad-spectrum antibiotics during pregnancy (versus narrow-spectrum). Pregnant women with asthma represent an ideal target population for antibiotic stewardship, including critical assessment of clinical decision making regarding antibiotic use to prevent overutilization and potential adverse maternal-child health outcomes.
Acknowledgements
The authors are indebted to the Division of TennCare in the Tennessee Department of Finance and Administration for providing the data.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Bookstaver PB, Bland CM, Griffin B, Stover KR, Eiland LS, McLaughlin M. A review of antibiotic use in pregnancy. Pharmacotherapy. 2015;35(11):1052–1062. doi:10.1002/phar.1649.
- Palmsten K, Hernández-Díaz S, Chambers CD, Mogun H, Lai S, Gilmer TP, Huybrechts KF. The most commonly dispensed prescription medications among pregnant women enrolled in the U.S. Medicaid program. Obstet Gynecol. 2015;126(3):465–473. doi:10.1097/AOG.0000000000000982.
- Martinez de Tejada B. Antibiotic use and misuse during pregnancy and delivery: benefits and risks. Int J Environ Res Public Health. 2014;11(8):7993–8009. doi:10.3390/ijerph110807993.
- Bonham CA, Patterson KC, Strek ME. Asthma outcomes and management during pregnancy. Chest. 2018;153(2):515–527. doi:10.1016/j.chest.2017.08.029.
- Namazy JA, Schatz M, Yang SJ, Chen W. Antibiotics for respiratory infections during pregnancy: prevalence and risk factors. J Allergy Clin Immunol Pract. 2016;4(6):1256–1257.e2. doi:10.1016/j.jaip.2016.06.015.
- Murphy VE. Managing asthma in pregnancy. Breathe. 2015;11(4):258–267. doi:10.1183/20734735.007915.
- Escobar GJ, Gebretsadik T, Carroll K, Li SX, Walsh EM, Wu P, Mitchel E, Sloan C, Hartert T. Adherence to immunoprophylaxis regimens for respiratory syncytial virus infection in insured and medicaid populations. J Pediatr Infect Dis Soc. 2013;2(3):205–214. doi:10.1093/jpids/pit007.
- Wu P, Dupont WD, Griffin MR, Carroll KN, Mitchel EF, Gebretsadik T, Hartert TV. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med. 2008;178(11):1123–1129. doi:10.1164/rccm.200804-579OC.
- Wakefield DB, Cloutier MM. Modifications to HEDIS and CSTE algorithms improve case recognition of pediatric asthma. Pediatr Pulmonol. 2006;41(10):962–971. doi:10.1002/ppul.20476.
- Hartert TV, Togias A, Mellen BG, Mitchel EF, Snowden MS, Griffin MR. Underutilization of controller and rescue medications among older adults with asthma requiring hospital care. J Am Geriatr Soc. 2000;48(6):651–657. doi:10.1111/j.1532-5415.2000.tb04723.x.
- Hartert TV, Speroff T, Togias A, Mitchel EF, Snowden MS, Dittus RS, Griffin MR. Risk factors for recurrent asthma hospital visits and death among a population of indigent older adults with asthma. Ann Allergy Asthma Immunol. 2002;89(5):467–473. doi:10.1016/S1081-1206(10)62083-2.
- Talbot TR, Hartert TV, Mitchel E, Halasa NB, Arbogast PG, Poehling KA, Schaffner W, Craig AS, Griffin MR. Asthma as a risk factor for invasive pneumococcal disease. N Engl J Med. 2005;352(20):2082–2090. doi:10.1056/NEJMoa044113.
- Donovan BM, Abreo A, Ding T, Gebretsadik T, Turi KN, Yu C, Ding J, Dupont WD, Stone CA, Hartert TV, et al. Dose, timing, and type of infant antibiotic use and the risk of childhood asthma. Clin Infect Dis. 2020;70(8):1658–1665. doi:10.1093/cid/ciz448.
- Turi KN, Gebretsadik T, Ding T, Abreo A, Stone C, Hartert TV, Wu P. Dose, timing, and spectrum of prenatal antibiotic exposure and risk of childhood asthma. Clin Infect Dis. 2021;72(3):455–462. doi:10.1093/cid/ciaa085.
- Sarpong EM, Miller GE. Narrow- and broad-spectrum antibiotic use among U.S. children. Health Serv Res. 2015;50(3):830–846. doi:10.1111/1475-6773.12260.
- King LM, Bartoces M, Fleming-Dutra KE, Roberts RM, Hicks LA. Changes in US outpatient antibiotic prescriptions from 2011–2016. Clin Infect Dis. 2020;70(3):370–377.
- Gerber JS, Hersh AL, Kronman MP, Newland JG, Ross RK, Metjian TA. Development and application of an antibiotic spectrum index for benchmarking antibiotic selection patterns across hospitals. Infect Control Hosp Epidemiol. 2017;38(8):993–997. doi:10.1017/ice.2017.94.
- Gerber JS, Ross RK, Bryan M, Localio AR, Szymczak JE, Wasserman R, Barkman D, Odeniyi F, Conaboy K, Bell L, et al. Association of broad- vs narrow-spectrum antibiotics with treatment failure, adverse events, and quality of life in children with acute respiratory tract infections. JAMA. 2017;318(23):2325–2336. doi:10.1001/jama.2017.18715.
- Hayes RM, Wu P, Shelton RC, Cooper WO, Dupont WD, Mitchel E, Hartert TV. Maternal antidepressant use and adverse outcomes: a cohort study of 228,876 pregnancies. Am J Obstet Gynecol. 2012;207(1):49.e1-9–49.e9.
- Carroll KN, Griffin MR, Gebretsadik T, Shintani A, Mitchel E, Hartert TV. Racial differences in asthma morbidity during pregnancy. Obstet Gynecol. 2005;106(1):66–72. doi:10.1097/01.AOG.0000164471.87157.4c.
- Cooper WO, Hernandez-Diaz S, Arbogast PG, Dudley JA, Dyer S, Gideon PS, Hall K, Ray WA. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med. 2006;354(23):2443–2451. doi:10.1056/NEJMoa055202.
- Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi:10.1093/aje/kwh090.
- Stokholm J, Schjørring S, Pedersen L, Bischoff AL, Følsgaard N, Carson CG, Chawes BLK, Bønnelykke K, Mølgaard A, Krogfelt KA, et al. Prevalence and predictors of antibiotic administration during pregnancy and birth. PLOS One. 2013;8(12):e82932. doi:10.1371/journal.pone.0082932.
- Miller JE, Wu C, Pedersen LH, de Klerk N, Olsen J, Burgner DP. Maternal antibiotic exposure during pregnancy and hospitalization with infection in offspring: a population-based cohort study. Int J Epidemiol. 2018;47(2):561–571. doi:10.1093/ije/dyx272.
- Fleming-Dutra KE, Hersh AL, Shapiro DJ, Bartoces M, Enns EA, File TM, Finkelstein JA, Gerber JS, Hyun DY, Linder JA, Jr, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA. 2016;315(17):1864–1873. doi:10.1001/jama.2016.4151.
- Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin No. 199: Use of prophylactic antibiotics in labor and delivery. Obstet Gynecol. 2018;132(3):e103–e119.
- Ailes EC, Summers AD, Tran EL, Gilboa SM, Arnold KE, Meaney-Delman D, Reefhuis J. Antibiotics dispensed to privately insured pregnant women with urinary tract infections - United States, 2014. MMWR Morb Mortal Wkly Rep. 2018;67(1):18–22. doi:10.15585/mmwr.mm6701a4.
- Norwitz ER, Greenberg JA. Antibiotics in pregnancy: are they safe? Rev Obstet Gynecol. 2009;2(3):135–136.
- Heerman WJ, Daley MF, Boone-Heinonen J, Rifas-Shiman SL, Bailey LC, Forrest CB, Young JG, Gillman MW, Horgan CE, Janicke DM, et al. Maternal antibiotic use during pregnancy and childhood obesity at age 5 years. Int J Obes (Lond). 2019;43(6):1202–1209. doi:10.1038/s41366-018-0316-6.
- Poulsen MN, Pollak J, Bailey-Davis L, Hirsch AG, Glass TA, Schwartz BS. Associations of prenatal and childhood antibiotic use with child body mass index at age 3 years. Obesity (Silver Spring). 2017;25(2):438–444. doi:10.1002/oby.21719.
- Hicks LA, Bartoces MG, Roberts RM, Suda KJ, Hunkler RJ, Taylor TH, Jr, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60(9):1308–1316.
- Fischer MA, Mahesri M, Lii J, Linder JA. Non-infection-related and non-visit-based antibiotic prescribing is common among medicaid patients. Health Aff (Millwood)). 2020;39(2):280–288. doi:10.1377/hlthaff.2019.00545.
- Lee GC, Reveles KR, Attridge RT, Lawson KA, Mansi IA, Lewis JS, Frei CR. Outpatient antibiotic prescribing in the United States: 2000 to 2010. BMC Med. 2014;12(1):96. doi:10.1186/1741-7015-12-96.
- Lim RH, Kobzik L, Dahl M. Risk for asthma in offspring of asthmatic mothers versus fathers: a meta-analysis. PLoS One. 2010;5(4):e10134. doi:10.1371/journal.pone.0010134.
- Loewen K, Monchka B, Mahmud SM, T Jong G, Azad MB. Prenatal antibiotic exposure and childhood asthma: a population-based study. Eur Respir J. 2018;52(1):1702070. doi:10.1183/13993003.02070-2017.
