Abstract
Background
Asthma is a common, chronic inflammatory airway disorder, with up to 1,177,000 people receiving asthma treatment in Japan. Dupilumab is a first-in-class, monoclonal antibody for the treatment of atopic diseases, including persistent asthma. The objective of this study was to assess the cost-effectiveness of dupilumab, compared with other biologics, as add-on treatment to background therapy in patients aged ≥12 years with uncontrolled, persistent asthma in Japan.
Methods
A life-time Markov cohort model was used to conduct cost-effectiveness analysis from the Japanese healthcare payer perspective with an annual discount rate of 2%. Dupilumab was compared with benralizumab and mepolizumab, and against omalizumab (as a hypothetical scenario). Inputs were informed by dupilumab clinical trials (VENTURE [NCT02528214] and QUEST [NCT02414854] trials), the literature, official Japanese sources and expert opinions.
Results
The base case results suggest that treatment with dupilumab leads to fewer severe exacerbations and increased life-years (LYs) and quality-adjusted LYs (QALYs) than benralizumab and mepolizumab. At a willingness-to-pay (WTP) threshold of ¥5,000,000 per QALY gained, dupilumab was the dominant strategy (lower cost, increased QALYs) versus benralizumab, and cost-effective versus mepolizumab with an incremental cost-effectiveness ratio (ICER) of ¥1,010,921 (US$9,190, US$1 = ¥110). Versus omalizumab, dupilumab was not cost-effective (ICER of ¥10,802,368 [US$98,203]).
Conclusions
In Japan, dupilumab, as an add-on to background therapy, is economically dominant compared with benralizumab, and cost-effective versus mepolizumab.
Introduction
Asthma is a common, chronic disorder of the airways, characterized by recurring symptoms such as shortness of breath, wheezing and coughing, and inflammation of the airways (Citation1). In Japan, the prevalence of asthma is estimated to be 8.1% (Citation2) with up to 1,177,000 people receiving asthma treatment, as based on 2014 estimates made by The Japanese Ministry of Health, Labor, and Welfare (MHLW) (Citation3).
Dupilumab is a first-in-class, monoclonal antibody indicated for the treatment of atopic diseases, including persistent asthma. Based on the mechanism of action, the indication of dupilumab covers a broader population of type 2 asthma patients compared with other biologics (i.e. in terms of blood eosinophilic count and patients with an allergic disease) (Citation4,Citation5). Dupilumab was licensed in Japan in April 2018 for treatment of patients with atopic dermatitis who were uncontrolled with existing treatment. In March 2019, dupilumab was then granted an indication extension for uncontrolled asthma in adults and adolescents (i.e. ≥12 years of age) corresponding to step 3 and 4 treatment of the Japanese guidelines (Citation2). Other novel therapeutic strategies with biologics like omalizumab and mepolizumab for severe asthma, also have been approved in Japan (Citation6).
Given the increasing healthcare costs in Japan attributed to the expenditure on new technologies, including biologics, and the rapidly aging population, evidence from cost-effectiveness analyses (CEA) are needed to inform healthcare decision-making. Such evidence is important, not only for government/payer policy-making, but also for physicians and patients in Japan. Therefore, the objective of this study was to assess the cost-effectiveness of dupilumab compared with other biologics (benralizumab, mepolizumab and omalizumab) in patients with uncontrolled persistent asthma in Japan. In this analysis, all the biologics were considered to be add-on treatment to the background (i.e. existing) therapy, including inhaled corticosteroid (ICS) combined with another controller medication, but not other biologics, for asthma treatment (Citation2). While CEA of biologics in treating severe asthma has been investigated in other countries (Citation7–9) or from different perspectives (Citation10), this is the first such study for dupilumab from the Japanese public payer perspective.
Methods
Model overview
A Markov cohort approach was adopted for the model for consistency with the previous economic evaluations (Citation11–15). This model could reflect both, the chronic day-to-day asthma symptoms that patients with uncontrolled persistent asthma experience which influences their health-related quality of life (HRQoL), as well as the risks these patients may experience during intermittent asthma exacerbations that can vary in severity and in some instances, lead to death.
A public healthcare payer perspective and 2% annual discount rate for both cost and effectiveness (0%–4% in the sensitivity analysis) were used, as recommended by the Japanese guideline for cost-effectiveness evaluation (Citation16). A lifetime model time horizon applied. The model was programmed in Microsoft Excel® using macros and the Visual Basic for Applications platform integrated to Excel®.
Intervention and comparators
Dupilumab (300 mg, q2w, first dose 600 mg) as an add-on to the background therapy was the reference intervention, using only the comparable data of the comparators for each respective evaluation. The comparators included a selection of other biologics, including benralizumab (based on the ZONDA trial) (Citation17); mepolizumab (based on the SIRIUS trial) (Citation18) and omalizumab (based on the INNOVATE trial) (Citation19), each added to a background therapy. Therefore, for dupilumab, only the benralizumab-like, mepolizumab-like, and omalizumab-like population data were applied, respectively.
Patient population
The model considered a population of patients aged ≥12 years with uncontrolled asthma despite treatment with a medium-to-high dose of ICS and a second controller medication (including those on systemic corticosteroid treatment). The dupilumab trial had the broadest eligibility criteria, covering the phenotypes observed across most of the comparator trials. To make a fair indirect comparison in similar patients, data for a dupilumab subgroup were generated by considering the available data from the VENTURE/QUEST trial that best matched the patient phenotypes of the approved labels for the comparator biologics creating mepolizumab-, benralizumab- and omalizumab-like subgroups. In each subgroup, a pairwise Bucher ITC was performed since only one study was available for each pair of treatment comparisons (i.e. each comparator biologic versus placebo). These were identified based on the inclusion criteria of other monoclonal antibodies and the conditions used in respective trials, similar to the approach taken for the indirect treatment comparison (ITC) of dupilumab (Citation20). The baseline characteristics are shown in .
Table 1. Model cohort characteristics at model start.†
Benralizumab like population in the VENTURE [NCT02528214] trial (Citation21) (according to the population in the ZONDA [NCT02075255] trial) (Citation17): age ≥18 years; high ICS, eosinophil count ≥150 cells/µL, number of severe exacerbations in the previous 12 months ≥1; with the population 100% on a maintenance oral corticosteroid (mOCS);
Mepolizumab like population in the VENTURE trial (Citation21) (according to the population in the SIRIUS [NCT01691508] trial) (Citation18): age ≥12 years; high ICS, eosinophil count ≥150 cells/µL; with the population 100% on a mOCS;
Omalizumab like population in the QUEST [NCT02414854] trial (Citation22) (according to the population in the INNOVATE [NCT00046748] trial) (Citation19): age ≥12 years; high ICS, eosinophil count ≥150 cells/µL, total IgE ≥30 IU/mL, allergic phenotype; without the assumption of the population 100% on mOCS because we do not have mOCS-tapering clinical trial using omalizumab such as the VENTURE, ZONDA or SIRIUS trials (Citation17,Citation18,Citation21).
Having the eosinophil count of ≥150 cells/µL as an inclusion criterion ensured the exclusion of patients with low eosinophil count from the analyses. This may be meaningful as mean eosinophil count varied greatly for some populations, namely the mepolizumab-like population. Furthermore, while there is evidence on fractional exhaled nitric oxide (FeNO) as a predictor of efficacy of dupilumab but not in other biologics, adjustments were not made since there were no inclusion criteria on FeNO, nor any FeNO values reported in the trials. Unlike the benralizumab- and mepolizumab-like populations, the omalizumab-like population is analyzed for patients not on mOCS as data on the corticosteroid tapering study of omalizumab in the clinical trial is not available. Therefore, omalizumab-like population is a hypothetical scenario.
Model structure
The model structure is shown in . The model for treatment-related health states considered background therapy alone and with add-on treatment as separate health states to reflect the influence of discontinuation of an add-on treatment. Patients starting in the “add-on treatment + background therapy” arm are separated into responders and non-responders after an initial response assessment. Responders remain on add-on treatment (plus background therapy) after the assessment, and non-responders are assumed to move to the “background therapy alone” health state after the assessment.
Figure 1. Model structure.
Color coding: Black: Absorbing health state; Dark gray: Treatment-related health state; White: Sub-states within treatment-related health states.
*Add-on treatment may be any one of the following monoclonal antibodies: dupilumab, mepolizumab, benralizumab.
†Moderate exacerbations are defined based on the loss of asthma control (LOAC) events (excluding severe exacerbation events) as collected in the QUEST trial. As such, at least one of the following criteria must be satisfied to count as a moderate exacerbation: ≥6 additional reliever puffs of salbutamol/albuterol or levosalbutamol/levalbuterol in a 24-hour period (compared with the baseline) on two consecutive days; ≥20% decrease in pre-bronchodilator forced expiratory volume in one second (FEV1) compared with the baseline; Increase in inhaled corticosteroid (ICS) dose ≥4 times than the dose at Visit 2; A decrease in AM or PM peak flow of 30% or more on two consecutive days of treatment, based on the defined stability limit. The treatment period stability limit is defined as the respective mean AM or PM peak expiratory flow (PEF) obtained over the last seven days prior to randomization (Day 1). ΔSevere exacerbations are defined based on the severe exacerbation events as collected in the dupilumab trials. As such, at least one of the following criteria must be satisfied to count as a severe exacerbation: Use of systemic corticosteroids for ≥3 days; Hospitalization or emergency room visit because of asthma, requiring systemic corticosteroids.
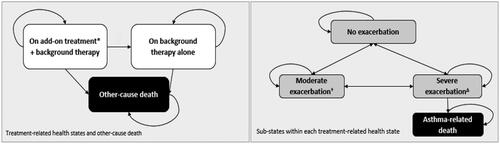
Beyond the initial response assessment (or if excluded this would apply from the model start), a proportion of patients may also discontinue add-on treatment and move to the “background therapy alone” health state (long-term discontinuation). Patients starting in the “background therapy alone” arm are assumed to remain on background therapy alone until death or the end of the time horizon, whichever occurs first.
Transitions from the “On add-on treatment + background therapy” to the “On background therapy only” health state were modeled as a function of (Citation1) response and (Citation2) long-term continuation rules and other reasons for discontinuation. In the aforementioned treatment-related health states, the other-caused death was considered due to the possibility of a non-asthma death.
The following definitions of response were used as follows:
For patients not on mOCS:
At least a 50% reduction in the severe exacerbation rate during the treatment period (∼12 months) as compared to the severe exacerbation rate in the preceding year;
For patients on mOCS:
At least a 50% reduction in the baseline mOCS dose at 12 weeks, or
At least a 50% reduction in the baseline mOCS dose at 24 weeks or at least a 50% reduction in the severe exacerbation rate during the treatment period as compared to the severe exacerbation rate in the preceding year. This definition of response was used as the base case for patients on mOCS.
Non-responders were assumed to discontinue the add-on treatment and transition to the “On background therapy only” arm. After discontinuation, patients could not revert to receiving the add-on treatment and no residual treatment effect was assumed.
Within each of these two treatment-related health states, disease-related sub-states were considered. Five health states defined by the occurrence of exacerbations and the level of asthma control were included in the model: “Controlled asthma,” “Uncontrolled asthma,” “Moderate exacerbation,” “Severe exacerbation” and “Asthma-related death” (. All patients entered the model in the “Uncontrolled asthma” sub-state which was consistent with the inclusion criteria in the VENTURE and QUEST trials (Citation21,Citation22). Patients were assumed to be receiving background therapy plus initiating one of the add-on treatments at the model start.
Patients were able to progress between states according to transition probabilities depending on the treatment modeled at four-week cycles. When accumulating costs and utilities, a half-cycle correction was applied to correct for discrete time.
Model input parameters
Inputs were derived from dupilumab clinical trials (Citation21,Citation22), the literature, official Japanese sources including the package inserts by the Pharmaceutical and Medical Devices Agency (PMDA), Drug master list, National Health Insurance Fee List (NHI-Fee List), and life tables by the MHLW. Opinions from Japanese clinical experts also informed some of the inputs. Japanese data were used whenever possible. If not available, global data were used with validation by Japanese experts.
Clinical inputs
Global data from the VENTURE and QUEST trials (Citation21,Citation22) were mainly used as no evident heterogeneity was observed between Japanese and non-Japanese patient populations. Transition probabilities used in the base case were considered based on post-hoc analyses of the VENTURE and QUEST trials (Citation21,Citation22). Estimation of transition probabilities involved counting the number of patients in each health state every four weeks, along with the frequency of transitions to other health states from that health state. Separate transition probabilities were derived for the period before and beyond Week 12 for dupilumab (Supplementary Table 1). Transition probabilities for responders were derived based on the period beyond Week 12. Considering responders for the VENTURE trial (Citation21), transition probabilities to exacerbation states were based on the entire treatment period (i.e. 0–24 weeks) while transitions between controlled states were based on observations between 12–24 weeks. For transition probabilities beyond the trials, an increased risk of severe exacerbations was assumed because the inclusion criteria and potential improvement in monitoring in clinical trials may have resulted in a lower severe exacerbation rate. Severe exacerbation rates were adjusted by a factor of 1.35 (SE: 0.237), based on the ratio of the severe exacerbation rate observed among mepolizumab studies (Citation23).
The proportion of patients achieving a response was obtained from post-hoc analyses of the VENTURE and QUEST trials (Citation21,Citation22) for patients on mOCS (Supplementary Table 2).
Table 2. Overview of the main cost inputs.
Changes in the mOCS use were considered because a certain proportion of patients on mOCS at the beginning of the model may completely discontinue mOCS. Patients who do not discontinue mOCS completely may experience a reduction in their daily mOCS dose to a dose of below 5 mg. For the model, changes in mOCS use at weeks 12 and 24, compared with the baseline, were analyzed from the post-hoc analyses of the VENTURE trial (Citation21).
Treatment persistence was also considered in the model. The annual rates of discontinuation were based on data from the VENTURE trial (Citation21). These rates were applied to add-on treatment responders after the initial response assessment. The treatment setting for exacerbations was determined based on severity. Moderate exacerbations do not require emergency department (ED) visits nor hospitalizations. As in the trial, exacerbations leading to an ED visit or hospitalization or with ≥3 days of corticosteroid use were classified as severe. The proportion of patients moving to the “Severe exacerbation” state in a given cycle was further distributed into “Severe exacerbation—Office visit,” “Severe exacerbation—ED visit” and “Severe exacerbation—Hospitalization” categories, using the respective proportions of patients being treated in each type of setting. These proportions were assumed not to vary by treatment, due to a limited number of events observed to inform otherwise (Supplementary Table 3).
Table 3. Base case results for the benralizumab like population.
Clinical efficacy of treatments, other than dupilumab, were incorporated in the model via use of relative rates versus dupilumab for all patients and for responders. Thus, the relative rates of each biologic versus dupilumab for all patients (regardless of the response status) receiving the add-on biologic were obtained via means of ITC (data on file), whereas those for responders were obtained from reimbursement submissions. The ITC results for relative rates between dupilumab and other biologics are not presented here as they have not been published. Dupilumab trials had the broadest eligibility criteria, covering the phenotypes observed across most of the comparator trials. Thus, a series of Bucher pairwise ITCs were performed, whereby subgroup dupilumab data were generated by considering the available data that best matched the patient phenotypes of the approved US/global labels for the comparator biologics where available, for relevant clinical efficacy endpoints, to reduce the observed heterogeneity (Citation20).
Safety and mortality
The model only considered adverse events (AE) related to the use of mOCS from the study of Bloechliger et al., as long-term corticosteroid use was associated with significant AEs and costs in severe asthma (Citation24). Monoclonal antibody-related AEs were excluded due to their rare occurrence and small differences across trial arms. All-cause and asthma-related mortality of the general population were based on the life tables of the MHLW (Citation25,Citation26). The fatality rate associated with severe exacerbations was based on a benralizumab report submitted to The National Institute for Health and Care Excellence (Citation27). Annual mortality rates for other-cause deaths were calculated by excluding asthma-related deaths from the general population mortality data to avoid the double-counting of asthma-related mortality. In the scenario, other-cause mortality was adjusted based on lung function using hazard ratios of other-cause mortality by percent predicted forced expiratory volume in 1 s (ppFEV1) level.
Resource use and costs
For the economic evaluation, costs for the following healthcare resources were considered: drug acquisition; drug administration (including outpatient visits); routine visits and disease management; exacerbations; and mOCS- related AEs.
Drug prices were obtained from the Drug master list by MHLW (2018) (Citation27). Drug strengths and doses for background therapy and add-on treatments were obtained from the package inserts of biologics by the PMDA and Japanese guidelines (Citation2,Citation28). Drug acquisition costs for background therapy were estimated by the proportions of patients using controller medications from the VENTURE and QUEST trials (Citation21,Citation22).
No drug administration cost was assumed for background therapies, as these are either inhaled or taken orally. Patients were administered monoclonal antibodies during office visits and in hospital outpatient settings, both of which required the same unit cost per drug administration. Unit costs on the administration of monoclonal antibodies, routine visits, disease management, and exacerbation were based on medical fee points [converted to monetary value by multiplying ¥10] from the NHI-Fee List (2018) (Citation29). Considering the actual practice in Japan, outpatient visit, blood collection, oxygen administration, hepatic function check, corticosteroid medication, short-acting inhaled beta-agonists, emergency treatment, and hospitalization were included in the unit costs of exacerbation, and mOCS-related AE costs were obtained from the literature. The main cost inputs are summarized in .
Utilities
Utility values associated with controlled and uncontrolled health states were informed by post-hoc analyses of the VENTURE and QUEST trials (Citation21,Citation22) data based on the EQ-5D-5L UK-weighted utility index derived by the Dolan and van Hout et al. cross-walk algorithm (30,31). Disutilities (i.e. utility decrements) including duration, associated with exacerbations were also informed by the QUEST trial (Citation22). Disutilities associated with mOCS-related events were identified by the EQ-5D-3L utility catalogue from Sullivan’s studies and verified by experts (Citation32,Citation33). Utility values are presented in Supplementary Table 4. While other factors such as frequency of injection may impact the HRQoL of patients, these factors were not considered in the model.
Table 4. Base case results for the mepolizumab like population.
Base case analyses
The base case analyses were performed on the dupilumab group compared with other biologics such as benralizumab, mepolizumab and omalizumab. The outcomes included a number of exacerbations, number of deaths (number of exacerbation-related deaths and number of non-asthma deaths), life-years (LYs), quality-adjusted life-years (QALYs), total costs, and incremental cost-effectiveness ratio (ICER) per QALY gained.
Sensitivity analyses
Sensitivity analyses evaluated the impact of model assumptions on the results and the uncertainty surrounding model inputs. A series of one-way deterministic sensitivity analyses, illustrated with a Tornado diagram, were run for the base case to determine the significant drivers of cost-effectiveness and assess the robustness of the base case. All parameters subject to parameter uncertainty (transition probabilities, settings of exacerbation treatment, probabilities of asthma-related death, changes in mOCS, utility values for each health state, costs related to disease management, exacerbations, monitoring and AEs) were included.
A probabilistic sensitivity analysis, illustrated with cost-effectiveness acceptability curve, was performed using a second-order Monte Carlo simulation with 1,000 iterations. All parameters subject to parameter uncertainty were included. The Dirichlet distribution was used for transition probabilities and proportions of severe exacerbations treated in each setting. The Gamma distribution was used for resource use, costs, discontinuation rates, AE incidences and the duration of time for which to apply utility decrements. The Beta distribution was used for utilities and proportion estimates, such as proportion of responders. The Log-normal distribution was used for relative effects. For baseline characteristics, normal distribution was assumed.
Scenario analysis
Several scenarios for each subgroup were considered to examine the influence of model structural uncertainties including:
Severe exacerbations after a trial period: increased risk based on severe exacerbations prior to enrollment;
Alternative model structure: four health states defined by combining the “Controlled asthma” and “Uncontrolled asthma” states into one “No exacerbation” state;
Other cause mortality: considering the influence of lung function, which was categorized into three levels: ppFEV1 ≥ 80% (reference category), ppFEV1 between 50% and 79%, and ppFEV1 < 50%;
Distribution of exacerbation treatment: clinical trial data of the equivalent population;
Alternative utility measure, i.e. Asthma Quality of Life utility index 5 dimensions (AQL-5D).
Results
Base case
Dupilumab versus benralizumab ()
The numbers of severe exacerbations per patient over a lifetime were 43.55 for dupilumab with background therapy and 44.83 for benralizumab with background therapy. Compared with benralizumab, dupilumab was associated with 1.27 fewer severe exacerbations per patient over a lifetime. Dupilumab was also associated with 0.13 more LYs and 0.17 more QALYs per patient compared with benralizumab and decrease of ¥2 316 832 total costs per patient. As a result, dupilumab was a dominant strategy compared with benralizumab as the add-on treatment over a lifetime horizon.
Dupilumab versus mepolizumab ()
The number of severe exacerbations per patient over a lifetime were 34.42 for dupilumab with background therapy and 40.57 for mepolizumab with background therapy. Compared with mepolizumab, dupilumab was associated with 6.15 fewer severe exacerbations per patient over a lifetime, and thus, associated with a 0.59 increase in LY and 0.61 increase in QALYs per patient compared with mepolizumab. With an increase of ¥619 179 per patient in the total cost, dupilumab was, thus, found to be cost-effective versus mepolizumab as add-on treatment based on an ICER of ¥1 010 921 (US$9,190) per patient, which was lower than the Japan-specific willingness-to-pay (WTP) threshold of ¥5 000 000 per QALY gained in Japan (Citation34).
Dupilumab versus omalizumab (Supplementary Table 5)
The numbers of severe exacerbations per patient over a lifetime were 65.59 dupilumab and 70.54 for and omalizumab. Compared with omalizumab, dupilumab was associated with 4.95 fewer severe exacerbations per patient over a lifetime. Dupilumab was associated with increased 0.49 and increased QALYs by 0.44 per patient compared with omalizumab, but the total cost of omalizumab was much lower than that of dupilumab (¥12 177 512 versus ¥16 892 170). Based on the ICER of ¥10 802 368 (US$98 203) and a WTP threshold of ¥5 000 000 per QALY gained in Japan, dupilumab was not cost-effective compared with omalizumab.
Deterministic sensitivity analyses
Dupilumab versus benralizumab
Incremental costs for dupilumab versus benralizumab varied between ¥−19 417 976 and ¥13 805 386 (Supplementary Figure 1). Incremental costs were most influenced by the discontinuation rates associated with benralizumab and dupilumab, and the cost for a vial of benralizumab.
Dupilumab versus mepolizumab
Incremental costs for dupilumab versus mepolizumab varied between ¥−15 084 938 and ¥17 284 994 (Supplementary Figure 2). Incremental costs were most influenced by the discontinuation rates associated with mepolizumab and dupilumab, and the cost for a vial of mepolizumab.
Dupilumab versus omalizumab
The key drivers on the incremental costs for dupilumab versus omalizumab were the cost for vial of dupilumab, and the discontinuation rates associated with dupilumab and omalizumab.
Probabilistic sensitivity analyses
Dupilumab versus benralizumab
For dupilumab versus benralizumab, there was a 68.9% probability for cost-effectiveness based on a WTP threshold of ¥5 000 000 per QALY (Supplementary Figure 3). The probabilistic mean incremental costs and QALYs for dupilumab versus benralizumab were ¥−2 987 881 (95% CI: ¥−24 202 756–¥18 515 998) and 0.219 (95% CI: −1.552 to 2.049), respectively (Supplementary Table 6).
Dupilumab versus mepolizumab
For dupilumab versus mepolizumab, there was a 60.4% of cost-effectiveness (Supplementary Figure 4). The probabilistic mean incremental costs and QALYs for dupilumab versus mepolizumab were ¥675 177 (95% CI: ¥−20 199 142–¥20 813 905) and 0.650 (95% CI: −0.669–2.312), respectively (Supplementary Table 6).
Dupilumab versus omalizumab
For dupilumab versus omalizumab, there was a probability of 7.0% to be cost-effective. The probabilistic mean values of incremental costs and QALY for dupilumab versus omalizumab were ¥4 645 732 (95% CI: ¥2 073 691–¥7 450 942) and 0.371 (95% CI: 0.026–1.529), respectively (Supplementary Table 6).
Scenario analysis
Dupilumab versus benralizumab
Across all scenarios, dupilumab versus benralizumab was consistently associated with decreased costs, increased QALYs and fewer severe exacerbations, and therefore, consistently economically dominant (Supplementary Table 7).
Dupilumab versus mepolizumab
Across all scenarios, dupilumab versus mepolizumab was associated with increased costs and QALYs, and fewer severe exacerbations. The ICER for dupilumab was always lower than the WTP threshold of ¥5 000 000 per QALY gained (Supplementary Table 8).
Dupilumab versus omalizumab
Across all scenarios, dupilumab versus omalizumab was associated with increased QALYs and fewer severe exacerbations, but with a higher cost. A change of distribution of exacerbation treatment decreased the ICER by 13.9%, to ¥9 296 403. A change of the model structure increased the ICER by 47.0%, to ¥15 879 702 (Supplementary Table 9).
Discussion
This CEA suggested that treatment with dupilumab in addition to background therapy is associated with fewer severe exacerbations, and increased LYs and QALYs compared with other biologics, respectively added to background therapy. Treatment with dupilumab resulted in cost savings versus benralizumab; as a result, dupilumab was the dominant strategy. Compared with mepolizumab (¥175 684 per cycle), dupilumab had a slightly lower cost per cycle (¥163 280) but a higher cost for the initial cycle (¥244 920). In our model, dupilumab was found to have a higher total drug acquisition cost (¥31 842 405), especially a higher add-on treatment costs (¥28 069 875 versus ¥27 355 262) as a response rate was higher for dupilumab than for mepolizumab (90.1% versus 83%) which lead to a longer mean time on-treatment for dupilumab (13.1 years) compared with mepolizumab (11.9 years). While treatment with dupilumab resulted in higher costs versus mepolizumab, dupilumab was cost-effective versus mepolizumab at the Japanese WTP threshold of ¥5 000 000 per QALY gained. However, in the hypothetical analyses in comparison with omalizumab, due to relatively small differences in outcomes and higher costs, dupilumab resulted in an ICER higher than the ¥5 000 000 threshold.
Between 2000 and 2018, 20 CEAs on asthma biologics were published (19 studies on omalizumab, 1 study on mepolizumab) (Citation35). Most of these studies included usual care or standard therapy as a comparator, and only a few compared the cost-effectiveness between biologics. This analysis is the first CEA for dupilumab in comparison with other biologics as an add-on to background therapy.
The use of inputs adapted to the Japanese setting, whenever feasible, catered the model to the Japanese population and should be considered a strength of the model. The latest unit cost data available at the time of analyses (2018) were used. As drug costs of dupilumab decreased from 2018 to 2020, the use of 2018 costs can be considered to have led to conservative results. While global data were used when domestic data were unavailable (e.g. clinical inputs and utility data), they were verified by the Japanese experts, a standard approach taken by previous CEA studies (Citation36–38). The use of domestic data whenever available allowed customization of the analyses to the Japanese healthcare setting enabling the model to provide results that are specific to Japan. The efficacy estimates were based on data from clinical trials, not observational studies or real-world data, which could limit the generalizability to real-world practice. A further study is needed with the use of real-world evidence.
Furthermore, given the limitations associated with data to inform relative efficacy for other biologics (i.e. alternative definitions of response, missing data), ITC between dupilumab and other monoclonal antibodies were conducted in the model utilizing numerous assumptions. The use of the pairwise Bucher ITC approach helped reduce, albeit not completely, the issue of heterogeneity across trial populations as the dupilumab trials contained a broader patient population. Uncertainty in terms of the proportion of responders and incomparable relative effects may have increased due to underlying assumption and the use of naïve comparison between treatments for responders. Nevertheless, this uncertainty seemed to have minimal impact on model results for all populations based on deterministic sensitivity analyses. In Japan, patients usually start a biologic therapy in addition to mOCS first. However, unlike the benralizumab- and mepolizumab-like populations, the omalizumab-like population was analyzed for patients not on mOCS because data on the corticosteroid tapering study of omalizumab in the clinical trial was not available. Lastly, a comparison of the results of omalizumab-like population with other biologics should be avoided as it is a hypothetical scenario.
In this CEA of dupilumab versus other biologics at the Japan-specific WTP of ¥5,000 000 per QALY gained, the base case analyses indicated that dupilumab was cost-effective compared with benralizumab and mepolizumab when considering a lifetime horizon of analysis. Further studies using more up-to-date drug and resource cost data and with considerations for other factors such as treatment persistence and comorbidities may be beneficial in further understanding the cost-effectiveness of dupilumab in Japan.
Authors’ contributions
YTa and MM had planned this study. YTo, HM, MM, YTa, MU, FJ, and IA have contributed to the conduct of the study, data collection, interpretation of the study results, and development of the manuscript. All authors gave approval on the submission of the manuscript.
| Abbreviations | ||
| AE: | = | adverse events |
| AQL-5D: | = | Asthma Quality of Life utility index-5 dimensions |
| CEA: | = | cost-effectiveness analysis |
| CEAC: | = | cost-effectiveness acceptability curve |
| CI: | = | confidence interval |
| ED: | = | emergency department |
| FeNO: | = | Fractional exhaled nitric oxide |
| HRQoL: | = | health-related quality of life |
| ICER: | = | incremental cost-effectiveness ratio |
| ICS: | = | inhaled corticosteroid |
| ITC: | = | indirect treatment comparison |
| LABA: | = | long-acting beta-agonists |
| LAMA: | = | long-acting muscarinic antagonists |
| LOAC: | = | loss of asthma control |
| LTRA: | = | leukotriene receptor antagonists |
| LY: | = | life-year |
| MHLW: | = | Ministry of Health, Labor, and Welfare |
| NHI-Fee List: | = | National Health Insurance Fee List |
| mOCS: | = | maintenance oral corticosteroid |
| OCS: | = | oral corticosteroid |
| ppFEV1: | = | percent predicted forced expiratory volume in 1 second |
| PMDA: | = | Pharmaceutical and Medical Devices Agency |
| QALY: | = | quality-adjusted life-year |
| QoL: | = | quality of life |
| SABA: | = | short-acting inhaled beta-agonists |
| SE: | = | standard error |
| TP: | = | transition probability |
| WHO: | = | World Health Organization |
| WTP: | = | willingness-to-pay |
Suppl_Tables1-9_and_Suppl_Figures1-4__v.Final_20210609_STC.docx
Download MS Word (839 KB)Acknowledgements
Anna Tytula and Yitong Wang of Creativ-Ceutical provided technical support.
Declaration of interest
YTo has received honoraria from AstraZeneca K.K., Kyorin Pharmaceutical Co., Ltd., and Sanofi K.K. HM has received honoraria from AstraZeneca K.K., Kyorin Pharmaceutical Co., Ltd., GlaxoSmithKline K.K., Novartis Pharma K.K. and Sanofi K.K and research funding from Novartis Pharma K.K. YTo, HM and IA had received consultancy fees from Sanofi K.K. for this study. IA is a chairperson of the society for pharmacoeconomics and YTa is a councilor of ISPOR-Japan. MM, YTa and FJ are Sanofi employees and may hold shares and/or stock options in the company. All authors respect the Ethical Guidelines for Medical and Health Research Involving Human Subjects.
Data availability statement
All data supporting the findings of this study are available within the article and its supplementary materials.
Additional information
Funding
References
- Expert Panel Report 3 (EPR-3): Guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–S138. doi:10.1016/j.jaci.2007.09.043.[published Online First: 2007/12/06]
- Ichinose M, Sugiura H, Nagase H, Yamaguchi M, Inoue H, Sagara H, Tamaoki J, Tohda Y, Munakata M, Yamauchi K, et al. Japanese guidelines for adult asthma 2017. Allergol Int. 2017;66(2):163–189. doi:10.1016/j.alit.2016.12.005.
- Sato K, Ohno T, Ishii T, Ito C, Kaise T. The prevalence, characteristics, and patient burden of severe asthma determined by using a Japan health care claims database. Clin Ther. 2019;41(11):2239–2251. doi:10.1016/j.clinthera.2019.08.015.
- Sanofi/Regeneron Global Brand Team. Asthma Brand Plan 2018. 2017.
- Vatrella A, Fabozzi I, Calabrese C, Maselli R, Pelaia G. Dupilumab: A novel treatment for asthma. J Asthma Allergy. 2014;7:123–130. doi:10.2147/jaa.s52387.
- Nagase H. Severe asthma in Japan. Allergol Int. 2019;68(2):167–171. doi:10.1016/j.alit.2019.02.004.
- Wu AC, Fuhlbrigge AL, Robayo MA, Shaker M. Cost-effectiveness of biologics for allergic diseases. J Allergy Clin Immunol Pract. 2021;9(3):1107–1117. doi:10.1016/j.jaip.2020.10.009.[published Online First: 2020/10/19]
- Anderson WC, III, Szefler SJ. Cost-effectiveness and comparative effectiveness of biologic therapy for asthma: To biologic or not to biologic? Ann Allergy Asthma Immunol. 2019;122(4):367–372. doi:10.1016/j.anai.2019.01.018. [published Online First: 2019/02/01]
- Agache I, Song Y, Rocha C, Beltran J, Posso M, Steiner C, Alonso-Coello P, Akdis C, Akdis M, Canonica GW, et al. Efficacy and safety of treatment with dupilumab for severe asthma: A systematic review of the EAACI guidelines-Recommendations on the use of biologicals in severe asthma. Allergy. 2020;75(5):1058–1068. doi:10.1111/all.14268. [published Online First: 2020/03/11]
- Morishima T, Ikai H, Imanaka Y. Cost-effectiveness analysis of omalizumab for the treatment of severe asthma in Japan and the value of responder prediction methods based on a multinational trial. Value Health Reg Issues. 2013;2(1):29–36. doi:10.1016/j.vhri.2013.01.007. [published Online First: 2013/05/01]
- National Institute for Health and Care Excellence (NICE). Reslizumab for treating eosinophilic asthma inadequately controlled on inhaled corticosteroids [ID872]. London, UK: National Institute for Health and Care Excellence; 2016 Nov [accessed 2019 Oct 10]. https://www.nice.org.uk/guidance/ta479/documents/committee-papers-4.
- Gerzeli S, Rognoni C, Quaglini S, Cavallo MC, Cremonesi G, Papi A. Cost-effectiveness and cost-utility of beclomethasone/formoterol versus fluticasone propionate/salmeterol in patients with moderate to severe asthma. Clin Drug Investig. 2012;32(4):253–265. doi:10.2165/11598940-000000000-00000.
- Norman G, Faria R, Paton F, Llewellyn A, Fox D, Palmer S, Clifton I, Paton J, Woolacott N, McKenna C. Omalizumab for the treatment of severe persistent allergic asthma: A systematic review and economic evaluation. Health Technology Assessment (Winchester, England). 2013;17(52):1–342. doi:10.3310/hta17520.
- National Institute for Health and Care Excellence (NICE). Mepolizumab for treating severe eosinophilic asthma [ID798]. London, UK: National Institute for Health and Care Excellence; 2017 Jan [accessed 2019 October 10]. https://www.nice.org.uk/guidance/ta431.
- Rodriguez-Martinez CE, Sossa-Briceno MP, Castro-Rodriguez JA. Cost-utility analysis of the inhaled steroids available in a developing country for the management of pediatric patients with persistent asthma. J Asthma. 2013;50(4):410–418. [published Online First: 2013/01/30] doi:10.3109/02770903.2013.767909.
- Shiroiwa T, Fukuda T, Ikeda S, Takura T, Moriwaki K. Development of an official guideline for the economic evaluation of drugs/medical devices in Japan. Value Health. 2017;20(3):372–378. [published Online First: 2017/03/16] doi:10.1016/j.jval.2016.08.726.
- Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, Barker P, Sproule S, Ponnarambil S, Goldman M, et al. Oral glucocorticoid–sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376(25):2448–2458. doi:10.1056/NEJMoa1703501.
- Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, Ortega HG, Pavord ID. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–1197. doi:10.1056/NEJMoa1403291.
- Humbert M, Beasley R, Ayres J, Slavin R, Hébert J, Bousquet J, Beeh K-M, Ramos S, Canonica GW, Hedgecock S, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309–316. doi:10.1111/j.1398-9995.2004.00772.x. [published Online First: 2005/02/01]
- Bateman ED, Khan AH, Xu Y, Guyot P, Chao J, Kamat S, Rowe P, Burnett H, Msihid J, Weinreich D, et al. Pairwise indirect treatment comparison of dupilumab versus other biologics in patients with uncontrolled persistent asthma. Respir Med. 2020:105991. doi:10.1016/j.rmed.2020.105991.
- Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, Zhu H, Hamilton JD, Swanson BN, Khan A, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378(26):2475–2485. doi:10.1056/NEJMoa1804093.
- Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, Busse WW, Ford L, Sher L, FitzGerald JM, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–2496. doi:10.1056/NEJMoa1804092.
- National Institute for Health and Care Excellence (NICE). Mepolizumab for treating severe refractory eosinophilic asthma in adults [ID798]. Company response to the second ACD. London, UK: National Institute for Health and Care Excellence; 2016 Aug [accessed 2019 Oct 10]. https://www.nice.org.uk/guidance/ta431/documents/committee-papers.
- Bloechliger M, Reinau D, Spoendlin J, Chang S-C, Kuhlbusch K, Heaney LG, Jick SS, Meier CR. Adverse events profile of oral corticosteroids among asthma patients in the UK: Cohort study with a nested case–control analysis. Respir Res. 2018;19(1):75. [published Online First: 2018/04/28]. doi:10.1186/s12931-018-0742-y.
- Ministry of Health, Labor and Welfare. The 22nd life tables. Tokyo, Japan: Ministry of Health, Labor and Welfare [accessed 2019 Oct 10]. https://www.mhlw.go.jp/english/database/db-hw/lifetb22nd/index.html.
- Zafari Z, Sadatsafavi M, Mark FitzGerald J for the Canadian Respiratory Research Network. Cost-effectiveness of tiotropium versus omalizumab for uncontrolled allergic asthma in US. Cost Eff Res Alloc. 2018;16(1):3. [published Online First: 2018/02/10] doi:10.1186/s12962-018-0089-8.
- National Institute for Health and Care Excellence (NICE). Benralizumab for treating severe eosinophilic asthma [TA565]. London, UK: National Institute for Health and Care Excellence; 2019 Sep [accessed 2019 Oct 10]. https://www.nice.org.uk/guidance/ta565.
- Ministry of Health, Labor and Welfare. Drug Master Search. Various information on medical fees. Tokyo, Japan: Ministry of Health, Labor and Welfare [accessed 2018 Apr]. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000078916.html.
- Pharmaceuticals and Medical Devices Agency. Search of package inserts. Tokyo, Japan: Pharmaceuticals and Medical Devices Agency [accessed 2019 Oct 10]. http://www.info.pmda.go.jp/psearch/html/menu_tenpu_base.html.
- Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35(11):1095–1108. doi:10.1097/00005650-199711000-00002. [published Online First: 1997/11/21]
- van Hout B, Janssen MF, Feng Y-S, Kohlmann T, Busschbach J, Golicki D, Lloyd A, Scalone L, Kind P, Pickard AS, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15(5):708–715. [published Online First: 2012/08/08] doi:10.1016/j.jval.2012.02.008.
- Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making. 2011;31(6):800–804. [published Online First: 2011/03/23] doi:10.1177/0272989X11401031.
- Sullivan PW, Ghushchyan VH, Globe G, Sucher B. Health-related quality of life associated with systemic corticosteroids. Qual Life Res. 2017;26(4):1037–1058. [published Online First: 2016/10/21] doi:10.1007/s11136-016-1435-y.
- Hasegawa M, Komoto S, Shiroiwa T, Fukuda T. Formal implementation of cost-effectiveness evaluations in Japan: A unique health technology assessment system. Value Health. 2020;23(1):43–51. doi:10.1016/j.jval.2019.10.005.
- McQueen RB, Sheehan DN, Whittington MD, van Boven JFM, Campbell JD. Cost-effectiveness of biological asthma treatments: A systematic review and recommendations for future economic evaluations. Pharmacoeconomics. 2018;36(8):957–971. doi:10.1007/s40273-018-0658-x.
- Hernandez L, Kuwabara H, Shah A, Yamabe K, Burnett H, Fahrbach K, Koufopoulou M, Iwakiri R. Cost-effectiveness analysis of vedolizumab compared with other biologics in anti-TNF-naïve patients with moderate-to-severe ulcerative colitis in Japan. Pharmacoeconomics. 2020;38(1):69–84. doi:10.1007/s40273-019-00841-1. [published Online First: 2019/09/26]
- Kobayashi M, Kudo M, Izumi N, Kaneko S, Azuma M, Copher R, Meier G, Pan J, Ishii M, Ikeda S, et al. Cost-effectiveness analysis of lenvatinib treatment for patients with unresectable hepatocellular carcinoma (uHCC) compared with sorafenib in Japan. J Gastroenterol. 2019;54(6):558–570. doi:10.1007/s00535-019-01554-0. [published Online First: 2019/02/23]
- Sakamaki H, Nakao K, Matsumoto T, Inoue S. Cost-effectiveness analysis of percutaneous mitral valve repair with the MitraClip delivery system for patients with mitral regurgitation in Japan. J Med Econ. 2019;22(12):1312–1320. doi:10.1080/13696998.2019.1668132.
