Abstract
Objective: To synthesize evidence regarding the relationship between outdoor air pollution and risk of asthma exacerbations in single lag0 and lag1 exposure patterns.
Methods: We performed a systematic literature search using PubMed, Embase, Cochrane Library, Web of Science, ClinicalTrials, China National Knowledge Internet, Chinese BioMedical, and Wanfang databases. Articles published until August 1, 2020 and the reference lists of the relevant articles were reviewed. Two authors independently evaluated the eligible articles and performed structured extraction of the relevant information. Pooled relative risks (RRs) and 95% confidence intervals (CIs) of lag0 and lag1 exposure patterns were estimated using random-effect models.
Results: Eighty-four studies met the eligibility criteria and provided sufficient information for meta-analysis. Outdoor air pollutants were associated with increased risk of asthma exacerbations in both single lag0 and lag1 exposure patterns [lag0: RR (95% CI) (pollutants), 1.057(1.011, 1.103) (air quality index, AQI), 1.007 (1.005, 1.010) (particulate matter of diameter ≤ 2.5 μm, PM2.5), 1.009 (1.005, 1.012) (particulate matter of diameter, PM10), 1.010 (1.006, 1.014) (NO2), 1.030 (1.011, 1.048) (CO), 1.005 (1.002, 1.009) (O3); lag1:1.064(1.022, 1.106) (AQI), 1.005 (1.002, 1.008) (PM2.5), 1.007 (1.004, 1.011) (PM10), 1.008 (1.004, 1.012) (NO2), 1.025 (1.007, 1.042) (CO), 1.010 (1.006, 1.013) (O3)], except SO2 [lag0: RR (95% CI), 1.004 (1.000, 1.007); lag1: RR (95% CI), 1.003 (0.999, 1.006)]. Subgroup analyses revealed stronger effects in children and asthma exacerbations associated with other events (including symptoms, lung function changes, and medication use).
Conclusion: Outdoor air pollution increases the asthma exacerbation risk in single lag0 and lag1 exposure patterns.
Trial registration: PROSPERO, CRD42020204097. https://www.crd.york.ac.uk/.
Supplemental data for this article is available online at https://doi.org/10.1080/02770903.2021.2008429 .
Introduction
Asthma is a common chronic airway inflammatory disease that affects > 300 million people worldwide (Citation1). The global prevalence of clinical/treated asthma in adults is 4.5%; moreover, it varies among 70 countries by as much as 21-fold (Citation2). Epidemiologic studies have revealed an increasing prevalence of asthma; in addition, asthma contributes to functional loss, increased healthcare costs, and severe medical complications (Citation3). Exacerbation of asthma accelerates disease progression and increases the incidence of hospitalization and death (Citation4).
Outdoor air pollution has received increasing attention owing to its serious consequences. Air pollution can cause critical public health problems. A retrospective study of 80 515 deaths in Beijing during 2004–2008 found that a reduction in life expectancy was associated with increased air pollution (Citation5). Exposure to air pollution also increases the risk of asthma exacerbations (Citation6–8). The air pollution components are complex, including particulate matter of diameters ≤ 2.5 μm (PM2.5) and ≤ 10 μm (PM10), sulfur dioxide (SO2), carbon monoxide (CO), nitrogen dioxide (NO2), and ozone (O3), among others. Martinez-Rivera et al. (Citation6) reported a positive association of the levels of NO2, but not of SO2, and CO, with the number of accident and emergency room (ER) visits and hospitalizations for asthma exacerbations. However, another study reported a positive association of SO2 levels, but not of NO2 levels, with pediatric asthma exacerbations (Citation7). Additionally, Ostro et al. (Citation8) reported that new coughing episodes were associated with exposure to PM10, PM2.5, and NO2, but not O3. Studies on different pollutants have reported inconsistent findings.
A meta-analysis confirmed the association between the aforementioned six air pollutants and increased risk of ER visits and hospitalizations for asthma (Citation9). Nonetheless, there are numerous pollutant types to be monitored, and each pollutant has different effects on asthma exacerbation. Therefore, there is a need for a comprehensive pollution index that can represent the various pollutant effects and facilitate estimation of the air pollution level by the general public. The air quality index (AQI) is a useful comprehensive index that was adopted by the US Environmental Pollution Administration for daily air quality reporting to the general public in 1999 (Citation10). The AQI includes sub-indices for PM2.5, PM10, SO2, NO2, CO, and O3 (Citation11). Pan et al. (Citation12) reported an association between AQI and increased risk of hospitalization for childhood asthma. In contrast, Letz et al. (Citation13) reported no significant correlation between AQI and ER visits for asthma in the basic military trainee population. There have been inconsistent findings regarding AQI and the risk of asthma exacerbation. In addition, multiple lag estimates, including single and cumulative lags, were selected for the overall analysis of the aforementioned meta-analysis. Lag exposure sensitivity analyses have employed different single-lag patterns for different pollutants (Citation9). However, there is a need to understand if pollutants contribute to asthma exacerbation on the same day or have lag effects. Furthermore, other asthma exacerbation outcomes (e.g. symptoms) should be considered.
This systematic review and meta-analysis of time-series and case-crossover studies aimed to summarize existing evidence regarding the relationships of various pollutants (AQI, PM2.5, PM10, SO2, NO2, CO, and O3) with the risk of asthma exacerbation (exacerbation-associated outpatient visits; ER visits; hospitalizations; deaths, and other events, including symptoms, lung function changes, and medication use as needed) in single lag0 and lag1 exposure patterns.
Methods
We followed the standardized Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (Citation14). There is no review protocol for this study.
Eligibility criteria and search strategy
The inclusion criteria were as follows: 1) a time-series or case-crossover study with original data; 2) asthma exacerbations associated with outpatient visits, ER visits, hospitalizations, deaths, or other events (symptoms, lung function changes, and medication use as needed) as outcomes; 3) inclusion of a study population of children, adults, or both; 4) use of AQI, PM2.5, PM10, SO2, NO2, CO, or O3 as measures of outdoor air pollution; and 5) report of the relationship between outdoor air pollution and asthma exacerbations.
The exclusion criteria were as follows: 1) no single-pollutant model; 2) no data regarding single lag0 or lag1 exposure patterns; and 3) data that could not be recalculated into study-specific relative risks (RRs) and 95% confidence intervals (CIs) to a 100-unit increase in AQI, a 1 mg/m3 increase in CO, and a 10 μg/m3 increase in the other pollutants (PM2.5, PM10, SO2, NO2, and O3) by assuming a linear relationship of all pollutants.
We conducted a systematic literature search of the PubMed, Embase, Cochrane Library, Web of Science, ClinicalTrials, China National Knowledge Internet, Chinese BioMedical, and Wanfang databases until August 1, 2020 (no start date specified and no language limitation). We used a combination of keywords associated with the exposure types (e.g. “AQI”) and asthma exacerbation outcomes (e.g. “symptom increase”)
We excluded irrelevant articles by screening their title. Articles meeting the inclusion criteria were included in abstract reading. Moreover, we included the references of articles that met the inclusion criteria. Articles that did not meet the exclusion criteria were included in this systematic review and meta-analysis through full-text reading.
Quality assessment and data extraction
Quality assessment was conducted based on Mustafic’s study with a maximum score of 5 (Citation15). One point was assigned if asthma exacerbations were coded by the International Classification of Diseases, American Thoracic Society, National Asthma Education or Prevention Program, or International Classification of Primary Care 2. One point was assigned if pollutant measurements were performed daily with < 25% of missing data. One point was assigned if long-term trends, seasonality, and temperature were all adjusted. One point was assigned if the humidity or the day of the week was adjusted. One point was assigned if influenza epidemics or holidays were adjusted.
Data extraction was performed using a standardized form, including the main characteristics (author, publication year, location, subgroup, study population, sample size, period, lag pattern, and quality score), outcome measures (asthma exacerbations associated with outpatient visits; ER visits; hospitalizations; deaths; and other events, including symptoms, lung function changes, and medication use as needed), exposure measures (AQI, PM2.5, PM10, SO2, NO2, CO, and O3), and study-specific RRs (95%CIs) of the single lag0 or lag1 exposure patterns. Eligible studies were examined and their relevant characteristics were independently recorded by two authors (XW and JH) in this standardized form. Any disagreements were resolved by consensus after discussion with an additional author (YH). When the same population was used in several publications, we included only the largest and most complete study.
Statistical analysis and data synthesis
Standardized effect estimates are expressed as RRs and 95% CIs. The study-specific RRs were derived from single-pollutant models reporting RRs or percentage changes. We recalculated the study-specific RRs to a 100-unit increase in AQI, a 1 mg/m3 increase in CO, and a 10 μg/m3 increase in the other pollutants (PM2.5, PM10, SO2, NO2, and O3), by assuming a linear relationship of all pollutants. We calculated the summary RRs of the single lag0 and lag1 exposure patterns. Heterogeneity was evaluated using Q and I2-statistics through the random-effect model. An I2 statistic > 50%, 25%-50%, and < 25% indicate high, moderate, and low heterogeneity, respectively (Citation16). Publication bias was assessed using Begg’s and Egger’s tests (Citation17,Citation18). To explore the heterogeneity in our pooled analysis, we applied sensitivity analyses based on studies with a quality score of 4–5. Various outcome analyses were conducted to combine the effects of evaluating differences in outpatient visits, ER visits, hospitalizations, deaths, and other events. Moreover, age-based subgroup analyses were conducted to evaluate the differences between children (0–14 years) and adults (> 14 years). Statistical analyses were conducted using Stata/MP 14.0 (Stata, College Station, TX, USA). All tests were two-sided, and statistical significance was defined as P < 0.05.
Results
Study selection and eligible study characteristics
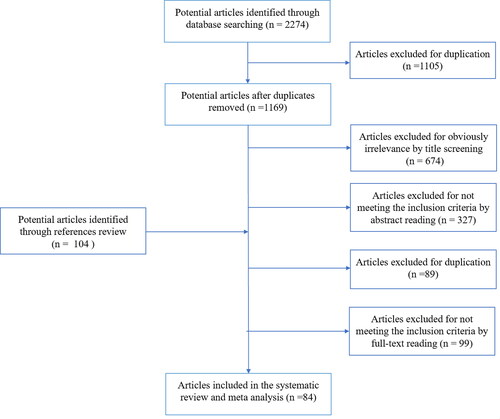
We initially identified 1169 articles. After reading the title, abstract, and full text, we included 84 articles. presents the approach to study selection.
Supplementary Table S2 presents the quality scores and main characteristics of the eligible studies (Citation12,Citation19–101). The outcomes were asthma exacerbations associated with outpatient visits (9 studies), ER visits (44 studies), hospitalizations (29 studies), deaths (2 studies), and other events (7 studies). Additionally, one study did not specify separate data regarding ER visits and hospitalizations (Citation96); consequently, it was classified as ER visits or hospitalization outcomes. Regarding the single lag exposure pattern, 68 and 63 studies reported on lag0 and lag1 exposure patterns, respectively. Regarding the age subgroups, 34 and 21 studies investigated children and adults, respectively.
Overall and quality sensitivity analyses
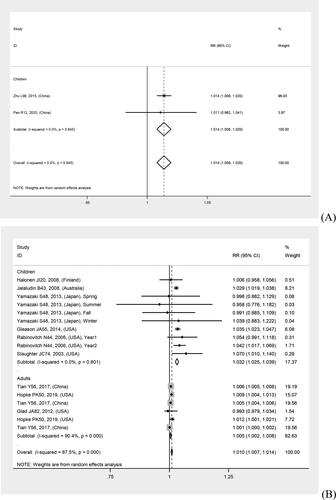
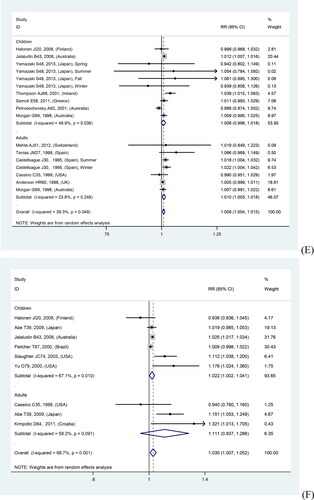
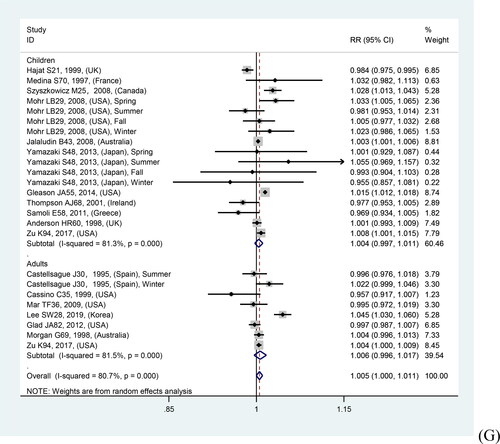
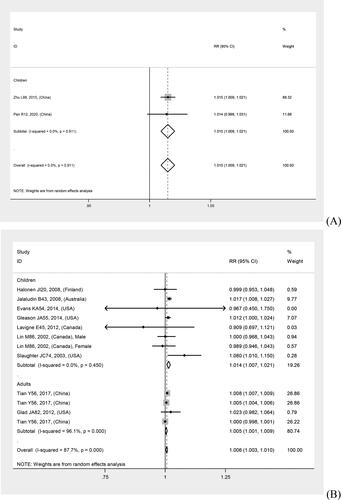
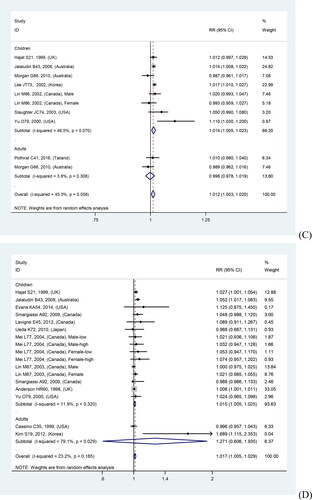
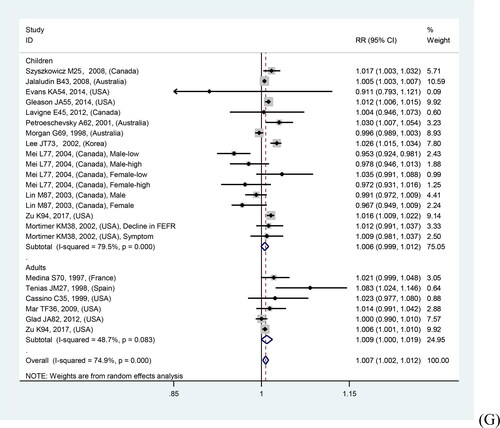
In overall analyses, air pollutants were associated with increased risks of asthma exacerbations in both of the single lag0 and lag1 exposure patterns [lag0: RR (95% CI) (pollutants), 1.057 (1.011, 1.103) (AQI), 1.007 (1.005, 1.010) (PM2.5), 1.009 (1.005, 1.012) (PM10), 1.010 (1.006, 1.014) (NO2), 1.030 (1.011, 1.048) (CO), 1.005 (1.002, 1.009) (O3); lag1: RR (95% CI) (pollutants), 1.064 (1.022, 1.106) (AQI), 1.005 (1.002, 1.008) (PM2.5), 1.007 (1.004, 1.011) (PM10), 1.008 (1.004, 1.012) (NO2), 1.025 (1.007, 1.042) (CO), 1.010 (1.006, 1.013) (O3)], except for SO2 [lag0: RR (95% CI), 1.004 (1.000, 1.007); lag1: RR (95% CI), 1.003 (0.999, 1.006)] (). The study-specific RRs presented high heterogeneity for all pollutants except for CO in the single lag1 exposure pattern. shows the p values of the Begg’s and Egger’s tests. There was no publication bias for AQI, PM2.5, NO2, and CO in the lag0 pattern, as well as AQI, PM2.5, SO2, and NO2 in the lag1 pattern. shows the forest plot for the association between air pollutants and asthma exacerbations, while shows the Begg’s funnel plot.
Figure 2. Forest plot for relationships between air pollutants ((A) AQI, (B) PM2.5, (C) PM10, (D) SO2, (E) NO2, (F) CO, (G) O3) and asthma exacerbations with lag0 exposure in age subgroup analyses.
Table 1. Relationships between air pollutants and asthma exacerbations in overall and quality sensitivity analyses.
Moreover, quality sensitivity analyses revealed a significant positive association of PM2.5, PM10, NO2, CO, and O3 with risk of asthma exacerbation in both single lag0 and lag1 exposure patterns (). The study-specific RRs showed high heterogeneity for all pollutants, except for CO. Publication bias was detected using Begg’s test for O3 in the lag0 pattern and Egger’s test for PM10 and O3 in the lag1 pattern (). shows the forest plot for relationships between air pollutants and asthma exacerbations, while Supplementary Figure S4 shows the Begg’s funnel plot.
Figure 3. Forest plot for relationships between air pollutants ((A) AQI, (B) PM2.5, (C) PM10, (D) SO2, (E) NO2, (F) CO, (G) O3) and asthma exacerbations with lag1 exposure in age subgroup analyses.
Various outcome and age subgroup analyses
Various outcome analyses revealed more pronounced relationships with other event outcomes [lag0: RR (95% CI) (pollutants), 1.201 (1.155, 1.247) (AQI), 1.047 (1.024, 1.069) (PM2.5), 1.119 (1.018, 1.220) (PM10), 1.033 (1.000, 1.066) (NO2), 1.124(1.051, 1.197) (CO); lag1: RR (95% CI) (pollutants), 1.204 (1.158, 1.251) (AQI), 1.080 (1.010, 1.150) (PM2.5), 1.122 (1.015, 1.230) (PM10), 1.046 (1.013, 1.079) (NO2), 1.155 (1.037, 1.274) (CO)], except for SO2 and O3. Supplementary Table S3 presents details regarding the relationship between air pollutants and asthma exacerbations in various outcome analyses. shows the forest plot.
Age subgroup analyses revealed stronger relations in children than in adults, except for NO2 in the lag1 pattern and O3 in both patterns. There was no tendency toward a stronger relation for AQI, given that the AQI adult subgroup lacked eligible studies. Supplementary Table S3 presents the details regarding the relationship between air pollutants and asthma exacerbations in various outcomes and age subgroup analyses. and show the forest plots.
Discussion
Summary of evidence
Our study provides novel evidence that air pollution exposure increases the risk of asthma exacerbation; specifically, AQI, PM2.5, PM10, NO2, CO, and O3 contribute to asthma exacerbations with single lag0 and lag1 exposure patterns. This suggests that these pollutants cause asthma exacerbation on the day of air pollution onset. Furthermore, AQI may be a good indicator of asthma exacerbation risk during air pollution. Mechanisms underlying the association between air pollution and asthma exacerbations include stimulation of airway epithelium and inflammatory cells, oxidative stress responses, respiratory cells, respiratory reflex responses, and epigenetic modifications (Citation102). Exposure to PM modulates the airway epithelium and promotes the production of several cytokines, including IL-1, IL-6, IL-8, IL-25, IL-33, TNF-α, and GM-CSF (Citation103). In a mouse model, there was an ozone-induced increase in bronchoalveolar lavage (BAL) levels of IL-23, which is an important cytokine for IL-17A + cell recruitment and activation, 24 h after ozone exposure (2 ppm for 3 h) (Citation104). Willart et al. (Citation105) reported an increase in lung IL-1α levels in naïve mice at 2 and 24 h after exposure. Another study reported an increase in IL-1α levels within the first 24 h after ozone exposure (1 ppm for 1 h) (Citation106). One study assessed sensitized mice before (0 h) and 1, 6, 12, 24, and 72 h after exposure and reported that the 12- and 24-h groups had the highest cytokine levels and cell counts in BAL fluid (Citation107). Diesel exhaust particles were found to induce an increase in IL-17A levels in primary bronchial epithelial cells of patients with asthma at 2 h post-exposure (Citation108). In addition, Zhang et al. (Citation109) reported that diesel exhaust particles induced increased TET1 expression in human bronchial epithelial cells at 1 h post-exposure. Therefore, if air pollution exposure cannot be avoided, patients with asthma should employ adequate strategies (including asthma medications) to reduce damage on the same day.
In lag0 and lag1 exposure patterns, we found that the RRs of air pollution and asthma exacerbations associated with other events (including symptoms, lung function changes, and medication use as needed) were much higher than those of outpatient visits, ER visits, hospitalizations, and deaths. There have been no studies on the relationship between outdoor air pollution and other events, as well as outpatient visits, ER visits, hospitalizations, or deaths within the same population. However, a prospective case-control study on children reported an association of AQI, PM2.5, NO2, and O3 with increased expression of multiple inflammatory airway epithelial responses, as well as a decline in FEV1%predicted in the lead up to clinical asthma exacerbations on the exacerbation-onset day without a viral trigger (Citation110). Pan et al. (Citation12) reported a positive correlation between AQI and childhood asthma hospitalizations that appeared and peaked on lag3 and lag9 days, respectively. Additionally, there were positive correlations between asthma deaths and PM2.5 on lag3 day (Citation32) or SO2 and NO2 on lag2 day (Citation79). This could be attributed to changes in symptoms and lung function, which are the initial manifestations of airway inflammation caused by air pollution. Subsequently, patients use relief medications for these changes, and if they do not improve, they seek medical help and are recorded as outpatient visits, ER visits, or hospitalizations. Therefore, when patients with asthma present with symptoms or lung function changes during air pollution, they should receive active treatment to block asthma exacerbation progression.
It has been confirmed that children with asthma are more susceptible to outdoor air pollution. This could be attributed to immature lung growth and host defense capacity in children (Citation9). Since children spend more time outdoors than adults, they inhale more air pollutants per pound of body weight (Citation111). Moreover, children may have low symptom tolerance, and parents may be more active in taking their children to hospitals.
Validity of results
This study has several strengths, including the identification of potential articles based on a systematic literature search of six databases (no start date specified and no language limitation) and inclusion of secondary references. Furthermore, two authors examined the eligible studies based on the inclusion and exclusion criteria, followed by independent data extraction using a priori set method in the standardized form. Finally, disagreements were discussed with a third author.
Limitations
This study has several limitations. First, there are few available studies on AQI, other events, and death analyses. Therefore, findings regarding the AQI, other events, and death analyses should be cautiously interpreted. Second, there was a high degree of heterogeneity in most of the analyses. This may be associated with varying study outcomes and design qualities. Nonetheless, we reduced the degree of heterogeneity in the quality sensitivity and various outcome analyses.
Conclusions
This systematic review and meta-analysis provides new evidence indicating that air pollution exposure increases the risk of asthma exacerbation. Specifically, AQI, PM2.5, PM10, NO2, CO, and O3 contribute to asthma exacerbations with single lag0 and lag1 exposure patterns. If air pollution exposure cannot be avoided, patients with asthma should employ adequate strategies (including asthma medications) to reduce same-day damage.
Authors’ contributions
JH and XY are joint first authors. JH obtained funding. JH, YH, and GW conceived and designed the study. JH and XY performed a systematic literature search under the supervision of GR and YH. JH, YH, and XW reviewed the articles and performed data extraction. JH and FF analyzed the data under the supervision of SZ, YH, and GW. JH, YH, XY, and FF wrote the manuscript. All authors read and approved the final manuscript and were responsible for their contributions.
The authors thank Dr. Pengkang He from Peking University First Hospital for his help.
| Abbreviations | ||
| AQI | = | air quality index |
| BAL | = | bronchoalveolar lavage |
| CIs | = | confidence intervals |
| CO | = | carbon monoxide |
| ER | = | emergency room |
| NO2 | = | nitrogen dioxide |
| O3 | = | ozone |
| PM2.5 | = | particulate matter diameter ≤ 2.5 μm |
| PM10 | = | particulate matter diameter ≤ 10 μm |
| RRs | = | relative risks |
| SO2 | = | sulfur dioxide |
Figure_S4._Begg_s_funnel_plot_for_relationships_between_air_pollutants_and_asthma_exacerbations_in_quality_sensitivity_analyses.pdf
Download PDF (78.5 KB)Figure_S3._Forest_plot_for_relationships_between_air_pollutants_and_asthma_exacerbations_in_quality_sensitivity_analyses.pdf
Download PDF (87.4 KB)Figure_S2._Begg_s_funnel_plot_for_relationships_between_air_pollutants_and_asthma_exacerbations_in_overall_analyses.pdf
Download PDF (89.7 KB)Figure_S1._Forest_plot_for_relationships_between_air_pollutants_and_asthma_exacerbations_in_overall_and_various_outcomes_analyses.pdf
Download PDF (144.8 KB)Table_S3._Relationships_between_air_pollutants_and_asthma_exacerbations_in__various_outcomes_and_age_subgroup_analyses.pdf
Download PDF (139.4 KB)Table_S2.__The_quality_scores_and_the_main_characteristics_of_the_eligible_studies.xlsx
Download MS Excel (24.4 KB)Table_S1._Search_strategies.pdf
Download PDF (256.4 KB)Data availability
Data are available on reasonable request (contact Yan Hu, [email protected]).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Global Initiative for Asthma. From the Global Strategy for Asthma Management and Prevention (GINA) 2020. update [OL] [accessed May 2020]. http://ginasthma.org.
- To T, Stanojevic S, Moores G, Gershon AS, Bateman ED, Cruz AA, Boulet L-P. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health. 2012;12(2012):204. doi:10.1186/1471-2458-12-204.
- Ledford R. Adult asthma. Allergy and Asthma. 2019:289–304. doi:10.1007/978-3-030-05147-1_13.
- Rennard SI, Farmer SG. Exacerbations and progression of disease in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1(2):88–92. doi:10.1513/pats.2306026.
- Guo Y, Li S, Tian Z, Pan X, Zhang J, Williams G. The burden of air pollution on years of life lost in Beijing, China, 2004-08: retrospective regression analysis of daily deaths. BMJ. 2013;347(7):f7139. doi:10.1136/bmj.f7139.
- Martínez-Rivera C, Garcia-Olivé I, Stojanovic Z, Radua J, Ruiz Manzano J, Abad-Capa J. Association between air pollution and asthma exacerbations in Badalona, Barcelona (Spain), 2008-2016. Med Clin (Barc)). 2019;152(9):333–338. doi:10.1016/j.medcli.2018.06.027.
- Chew FT, Goh DY, Ooi BC, Saharom R, Hui JK, Lee BW. Association of ambient air-pollution levels with acute asthma exacerbation among children in Singapore. Allergy. 1999;54(4):320–329. doi:10.1034/j.1398-9995.1999.00012.x.
- Ostro B, Lipsett M, Mann J, et al. Air pollution and exacerbation of asthma in African-American communities. Epidemiology. 2001;12(2):200–208.
- Zheng X-y, Ding H, Jiang L-n, Chen S-w, Zheng J-p, Qiu M, Zhou Y-x, Chen Q, Guan W-j. Association between air pollutants and asthma emergency room visits and hospital admissions in time series studies: a systematic review and meta-analysis. PLoS One. 2015;10(9):e0138146. doi:10.1371/journal.pone.0138146.
- USEPA. Getting started: emission inventory methods for PM 2.5. Emission inventory improvement program. 1999; Volume IX, Chapter 1:1–72. https://www.epa.gov/sites/default/files/2015-08/documents/ix01.pdf.
- Ministry of Environmental Protection of the People’s Republic of China. Technical Regulation on Ambient Air Quality Index (on Trial): HJ 633-2012. Beijing, China: China Environmental Science Press; 2012.
- Pan R, Wang X, Yi W, Wei Q, Gao J, Xu Z, Duan J, He Y, Tang C, Liu X, et al. Interactions between climate factors and air quality index for improved childhood asthma self-management. Sci Total Environ. 2020;723(Suppl. 2):137804. doi:10.1016/j.scitotenv.2020.137804.
- Letz AG, Quinn JM. Relationship of basic military trainee emergency department visits for asthma and San Antonio air quality. Allergy Asthma Proc. 2005;26(6):463–467.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097.
- Mustafic H, Jabre P, Caussin C, Murad MH, Escolano S, Tafflet M, Périer M-C, Marijon E, Vernerey D, Empana J-P, et al. Main air pollutants and myocardial infarction: a systematic review and meta-analysis. JAMA. 2012;307(7):713–721. doi:10.1001/jama.2012.126.
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi:10.1136/bmj.327.7414.557.
- Begg CB, Mazumdar M. Operating characteristics of a RANK correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. doi:10.2307/2533446.
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi:10.1136/bmj.315.7109.629.
- Kim S, Kim Y, Lee M-R, Kim J, Jung A, Park JS, Jang A-S, Park S-W, Uh S-T, Choi JS, et al. Winter season temperature drops and sulfur dioxide levels affect on exacerbation of refractory asthma in South Korea: a time-trend controlled case-crossover study using soonchunhyang asthma cohort data. J Asthma. 2012;49(7):679–687. doi:10.3109/02770903.2012.702839.
- Halonen JI, Lanki T, Yli-Tuomi T, Kulmala M, Tiittanen P, Pekkanen J. Urban air pollution and asthma and COPD hospital emergency room visits. Thorax. 2008;63(7):635–641. doi:10.1136/thx.2007.091371.
- Hajat S, Haines A, Goubet SA, Atkinson RW, Anderson HR. Association of air pollution with daily GP consultations for asthma and other lower respiratory conditions in London. Thorax. 1999;54(7):597–605. doi:10.1136/thx.54.7.597.
- Galán I, Tobías A, Banegas JR, Aránguez E. Short-term effects of air pollution on daily asthma emergency room admissions. Eur Respir J. 2003;22(5):802–808. doi:10.1183/09031936.03.00013003.
- Stieb DM, Szyszkowicz M, Rowe BH, Leech JA. Air pollution and emergency department visits for cardiac and respiratory conditions: a multi-city time-series analysis. Environ Health. 2009;8(1):25–38. doi:10.1186/1476-069X-8-25.
- Wilson AM, Wake CP, Kelly T, Salloway JC. Air pollution, weather, and respiratory emergency room visits in two northern New England cities: an ecological time-series study. Environ Res. 2005;97(3):312–321. doi:10.1016/j.envres.2004.07.010.
- Szyszkowicz M. Ambient air pollution and daily emergency department visits for asthma in Edmonton, Canada. Int J Occup Med Environ Health. 2008;21(1):25–30.
- Norris G, YoungPong SN, Koenig JQ, Larson TV, Sheppard L, Stout JW. An association between fine particles and asthma emergency department visits for children in Seattle. Environ Health Perspec. 1999;107(6):489–493. doi:10.1289/ehp.99107489.
- Tenias JM, Ballester F, Rivera ML. Association between hospital emergency visits for asthma and air pollution in Valencia, Spain. Occup Environ Med. 1998;55(8):541–547. doi:10.1136/oem.55.8.541.
- Lee SW, Yon DK, James CC, Lee S, Koh HY, Sheen YH, Oh J-W, Han MY, Sugihara G. Short-term effects of multiple outdoor environmental factors on risk of asthma exacerbations: age-stratified time-series analysis. J Allergy Clin Immunol. 2019;144(6):1542–1550. doi:10.1016/j.jaci.2019.08.037.
- Mohr LB, Luo S, Mathias E, Tobing R, Homan S, Sterling D. Influence of season and temperature on the relationship of elemental carbon air pollution to pediatric asthma emergency room visits. J Asthma. 2008;45(10):936–943. doi:10.1080/02770900802404082.
- Castellsague J, Sunyer J, Sáez M, Antó JM. Short-term association between air pollution and emergency room visits for asthma in Barcelona. Thorax. 1995;50(10):1051–1056. doi:10.1136/thx.50.10.1051.
- Jaffe DH, Singer ME, Rimm AA. Air pollution and emergency department visits for asthma among Ohio Medicaid recipients, 1991–1996. Environ Res. 2003;91(1):21–28. doi:10.1016/S0013-9351(02)00004-X.
- Liu Y, Pan J, Zhang H, Shi C, Li G, Peng Z, Ma J, Zhou Y, Zhang L. Short-term exposure to ambient air pollution and asthma mortality. Am J Respir Crit Care Med. 2019;200(1):24–32. doi:10.1164/rccm.201810-1823OC.
- Cirera L, García-Marcos L, Giménez J, Moreno-Grau S, Tobías A, Pérez-Fernández V, Elvira-Rendeles B, Guillén JJ, Navarro C. Daily effects of air pollutants and pollen types on asthma and COPD hospital emergency visits in the industrial and Mediterranean Spanish city of Cartagena. Allergol Immunopathol (Madr)). 2012;40(4):231–237. doi:10.1016/j.aller.2011.05.012.
- Babin S, Burkom H, Holtry R, Tabernero N, Davies-Cole J, Stokes L, Dehaan K, Lee D. Medicaid patient asthma-related acute care visits and their associations with ozone and particulates in Washington, DC, from 1994–2005. Int J Environ Health Res. 2008;18(3):209–221. doi:10.1080/09603120701694091.
- CASSINO CARA, Ito K, Bader IRA, Ciotoli C, Thurston G, Reibman JOAN. Cigarette smoking and ozone-associated emergency department use for asthma by adults in New York City. Am J Respir Crit Care Med. 1999;159(6):1773–1779. doi:10.1164/ajrccm.159.6.9809042.
- Mar TF, Koenig JQ. Relationship between visits to emergency departments for asthma and ozone exposure in greater Seattle, Washington. Ann Allergy Asthma Immunol. 2009;103(6):474–479. doi:10.1016/S1081-1206(10)60263-3.
- Cadelis G, Tourres R, Molinie J. Short-term effects of the particulate pollutants contained in Saharan dust on the visits of children to the emergency department due to asthmatic conditions in Guadeloupe (French Archipelago of the Caribbean). PLoS One. 2014;9(3):e91136. doi:10.1371/journal.pone.0091136.
- Mortimer KM, Neas LM, Dockery DW, Redline S, Tager IB. The effect of air pollution on inner-city children with asthma. Eur Respir J. 2002;19(4):699–705. doi:10.1183/09031936.02.00247102.
- Abe T, Tokuda Y, Ohde S, Ishimatsu S, Nakamura T, Birrer RB. The relationship of short-term air pollution and weather to ED visits for asthma in Japan. Am J Emerg Med. 2009;27(2):153–159. doi:10.1016/j.ajem.2008.01.013.
- Chimonas MAR, Gessner BD. Airborne particulate matter from primarily geologic, non-industrial sources at levels below National Ambient Air Quality Standards is associated with outpatient visits for asthma and quick-relief medication prescriptions among children less than 20 years old enrolled in Medicaid in Anchorage, Alaska. Environ Res. 2007;103(3):397–404. doi:10.1016/j.envres.2006.08.013.
- Pothirat C, Tosukhowong A, Chaiwong W, et al. Effects of seasonal smog on asthma and COPD exacerbations requiring emergency visits in Chiang Mai, Thailand. Asian Pac J Allergy Immunol. 2016;34(4):284–289.
- Paulu C, Smith AE. Tracking associations between ambient ozone and asthma-related emergency department visits using case-crossover analysis. J Public Health Manag Pract. 2008;14(6):581–591. doi:10.1097/01.PHH.0000338371.53242.0e.
- Jalaludin B, Khalaj B, Sheppeard V, Morgan G. Air pollution and ED visits for asthma in Australian children: a case-crossover analysis. Int Arch Occup Environ Health. 2008;81(8):967–974. doi:10.1007/s00420-007-0290-0.
- Rabinovitch N, Strand M, Gelfand EW. Particulate levels are associated with early asthma worsening in children with persistent disease. Am J Respir Crit Care Med. 2006;173(10):1098–1105. doi:10.1164/rccm.200509-1393OC.
- Lavigne E, Villeneuve PJ, Cakmak S. Air pollution and emergency department visits for asthma in Windsor, Canada. Can J Public Health. 2012;103(1):4–8. doi:10.1007/BF03404060.
- Tolbert PE, Mulholland JA, Macintosh DL, Xu F, Daniels D, Devine OJ, Carlin BP, Klein M, Butler AJ, Nordenberg DF, et al. Air quality and pediatric emergency room visits for asthma in Atlanta, Georgia, USA. Am J Epidemiol. 2000;151(8):798–810. doi:10.1093/oxfordjournals.aje.a010280.
- Boutin-Forzano S, Adel N, Gratecos L, Jullian H, Garnier JM, Ramadour M, Lanteaume A, Hamon M, Lafay V, Charpin D, et al. Visits to the emergency room for asthma attacks and short-term variations in air pollution a case-crossover study. Respiration. 2004;71(2):134–137. doi:10.1159/000076673.
- Yamazaki S, Shima M, Yoda Y, Oka K, Kurosaka F, Shimizu S, Takahashi H, Nakatani Y, Nishikawa J, Fujiwara K, et al. Association of ambient air pollution and meteorological factors with primary care visits at night due to asthma attack. Environ Health Prev Med. 2013;18(5):401–406. doi:10.1007/s12199-013-0339-5.
- Santus P, Russo A, Madonini E, Allegra L, Blasi F, Centanni S, Miadonna A, Schiraldi G, Amaducci S. How air pollution influences clinical management of respiratory diseases. A case-crossover study in Milan. Respir Res. 2012;13(1):95. doi:10.1186/1465-9921-13-95.
- Hopke PK, Croft D, Zhang W, Lin S, Masiol M, Squizzato S, Thurston SW, van Wijngaarden E, Utell MJ, Rich DQ, et al. Changes in the acute response of respiratory diseases to PM2.5 in New York State from 2005 to 2016. Sci Total Environ. 2019;677(10):328–339. doi:10.1016/j.scitotenv.2019.04.357.
- Mehta AJ, Schindler C, Perez L, et al. Acute respiratory health effects of urban air pollutants in adults with different patterns of underlying respiratory disease. Swiss Med Wkly. 2012;142(142):w13681. doi:10.4414/smw.2012.13681.
- Laurent O, Pedrono G, Segala C, Filleul L, Havard S, Deguen S, Schillinger C, Rivière E, Bard D. Air pollution, asthma attacks, and socioeconomic deprivation: a small-area case-crossover study. Am J Epidemiol. 2008;168(1):58–65. doi:10.1093/aje/kwn087.
- Pereira G, Cook A, Vos AJBM, Holman CDJ. A case-crossover analysis of traffic-related air pollution and emergency department presentations for asthma in Perth, Western Australia. Med J Aust. 2010;193(9):511–514. doi:10.5694/j.1326-5377.2010.tb04034.x.
- Evans KA, Halterman JS, Hopke PK, Fagnano M, Rich DQ. Increased ultrafine particles and carbon monoxide concentrations are associated with asthma exacerbation among urban children. Environ Res. 2014;129(2):11–19. doi:10.1016/j.envres.2013.12.001.
- Gleason JA, Bielory L, Fagliano JA. Associations between ozone, PM2.5, and four pollen types on emergency department pediatric asthma events during the warm season in New Jersey: a case-crossover study. Environ Res. 2014;132(7):421–429. doi:10.1016/j.envres.2014.03.035.
- Tian Y, Xiang X, Juan J, Sun K, Song J, Cao Y, Hu Y. Fine particulate air pollution and hospital visits for asthma in Beijing, China. Environ Pollut. 2017;230(11):227–233. doi:10.1016/j.envpol.2017.06.029.
- Raun LH, Ensor KB, Persse D. Using community level strategies to reduce asthma attacks triggered by outdoor air pollution: a case crossover analysis. Environ Health. 2014;13(7):58. doi:10.1186/1476-069X-13-58.
- Samoli E, Nastos PT, Paliatsos AG, Katsouyanni K, Priftis KN. Acute effects of air pollution on pediatric asthma exacerbation: evidence of association and effect modification. Environ Res. 2011;111(3):418–424. doi:10.1016/j.envres.2011.01.014.
- Fusco D, Forastiere F, Michelozzi P, Spadea T, Ostro B, Arcà M, Perucci CA. Air pollution and hospital admissions for respiratory conditions in Rome, Italy. Eur Respir J. 2001;17(6):1143–1150. doi:10.1183/09031936.01.00005501.
- Anderson HR, de Leon AP, Bland JM, Bower JS, Emberlin J, Strachan DP. Air pollution, pollens, and daily admissions for asthma in London 1987–92. Thorax. 1998;53(10):842–848. doi:10.1136/thx.53.10.842.
- Lee SL, Wong WHS, Lau YL. Association between air pollution and asthma admission among children in Hong Kong. Clin Exp Allergy. 2006;36(9):1138–1146. doi:10.1111/j.1365-2222.2006.02555.x.
- Petroeschevsky A, Simpson RW, Thalib L, Rutherford S. Associations between outdoor air pollution and hospital admissions in Brisbane, Australia. Arch Environ Health. 2001;56(1):37–52. doi:10.1080/00039890109604053.
- Ko FWS, Tam W, Wong TW, Lai CKW, Wong GWK, Leung T-F, Ng SSS, Hui DSC. Effects of air pollution on asthma hospitalization rates in different age groups in Hong Kong. Clin Exp Allergy. 2007;37(9):1312–1319. doi:10.1111/j.1365-2222.2007.02791.x.
- Krmpotic D, Luzar-Stiffler V, Rakusic N, Stipic Markovic A, Hrga I, Pavlovic M. Effects of traffic air pollution and hornbeam pollen on adult asthma hospitalizations in Zagreb. Int Arch Allergy Immunol. 2011;156(1):62–68. doi:10.1159/000322177.
- Romero-Placeres M, Más-Bermejo P, Lacasaña-Navarro M, Téllez Rojo-Solís MM, Aguilar-Valdés J, Romieu I. Air pollution, bronchial asthma, and acute respiratory infections in minors, Habana City. Salud Publ Mex. 2004;46(3):222–233. doi:10.1590/s0036-36342004000300012.
- Morgan G, Sheppeard V, Khalaj B, Ayyar A, Lincoln D, Jalaludin B, Beard J, Corbett S, Lumley T. Effects of bushfire smoke on daily mortality and hospital admissions in Sydney, Australia. Epidemiology. 2010;21(1):47–55. doi:10.1097/EDE.0b013e3181c15d5a.
- Fletcher T, Gouveia N. Respiratory diseases in children and outdoor air pollution in San Paulo, Brazil a time series analysis. Occup Environ Med. 2000;57(7):477–483. doi:10.1136/oem.57.7.477.
- Thompson AJ, Shields MD, Patterson CC. Acute asthma exacerbations and air pollutants in children living in Belfast, Northern Ireland. Arch Environ Health. 2001;56(3):234–241. doi:10.1080/00039890109604447.
- Morgan G, Corbett S, Wlodarczyk J. Air pollution and hospital admissions in Sydney, Australia, 1990 to 1994. Am J Public Health. 1998;88(12):1761–1766. doi:10.2105/ajph.88.12.1761.
- Medina S, Le Tertre A, Quénel P, Le Moullec Y, Lameloise P, Guzzo JC, Festy B, Ferry R, Dab W. Air pollution and doctors’ house calls: results from the ERPURS system for monitoring the effects of air pollution on public health in Greater Paris, France, 1991-1995. Evaluation des Risques de la Pollution Urbaine pour la Santé. Environ Res. 1997;75(1):73–84. doi:10.1006/enrs.1997.3773.
- Son J-Y, Lee J-T, Park YH, Bell ML. Short-term effects of air pollution on hospital admissions in Korea. Epidemiology. 2013;24(4):545–554. doi:10.1097/EDE.0b013e3182953244.
- Ueda K, Nitta H, Odajima H. The effects of weather, air pollutants, and Asian dust on hospitalization for asthma in Fukuoka. Environ Health Prev Med. 2010;15(6):350–357. doi:10.1007/s12199-010-0150-5.
- Lee J-T, Kim H, Song H, Hong Y-C, Cho Y-S, Shin S-Y, Hyun Y-J, Kim Y-S. Air pollution and asthma among children in Seoul, Korea. Epidemiology. 2002;13(4):481–484.
- Slaughter JC, Lumley T, Sheppard L, Koenig JQ, Shapiro GG. Effects of ambient air pollution on symptom severity and medication use in children with asthma. Ann Allergy Asthma Immunol. 2003;91(4):346–353. doi:10.1016/S1081-1206(10)61681-X.
- Ye F, Piver WT, Ando M, Portier CJ. Effects of temperature and air pollutants on cardiovascular and respiratory diseases for males and females older than 65 years of age in Tokyo, July and August 1980-1995. Environ Health Perspect. 2001;109(4):355–359. doi:10.1289/ehp.01109355.
- Castner J, Guo L, Yin Y. Ambient air pollution and emergency department visits for asthma in Erie County, New York 2007-2012. Int Arch Occup Environ Health. 2018;91(2):205–214. doi:10.1007/s00420-017-1270-7.
- Lin M, Chen Y, Villeneuve PJ, Burnett RT, Lemyre L, Hertzman C, McGrail KM, Krewski D. Gaseous air pollutants and asthma hospitalization of children with low household income in Vancouver, British Columbia, Canada. Am J Epidemiol. 2004;159(3):294–303.
- Schouten JP, Vonk JM, Graaf AD. Short term effects of air pollution on emergency hospital admissions for respiratory disease: results of the APHEA project in two major cities in The Netherlands, 1977-89. J Epidemiol Community Health. 1996;50(Suppl 1):S22–S29. doi:10.1136/jech.50.Suppl_1.s22.
- Yu O, Sheppard L, Lumley T, Koenig JQ, Shapiro GG. Effects of ambient air pollution on symptoms of asthma in seattle-area children enrolled in the CAMP study. Environ Health Perspect. 2000;108(12):1209–1214. doi:10.1289/ehp.001081209.
- Amancio CT, Nascimento LFC. Asthma and air pollutants: a time series study. Rev Assoc Med Bras. 2012;58(3):302–307.
- Lu P, Zhang Y, Lin J, Xia G, Zhang W, Knibbs LD, Morgan GG, Jalaludin B, Marks G, Abramson M, et al. Multi-city study on air pollution and hospital outpatient visits for asthma in China. Environ Pollut. 2020;257(2):113638. doi:10.1016/j.envpol.2019.113638.
- Glad JA, Brink LL, Talbott EO, Lee PC, Xu X, Saul M, Rager J. The relationship of ambient ozone and PM2.5 levels and asthma emergency department visits: possible influence of gender and ethnicity. Arch Environ Occup Health. 2012;67(2):103–108. doi:10.1080/19338244.2011.598888.
- Hwang S-L, Lin Y-C, Guo S-E, Chi M-C, Chou C-T, Lin C-M. Emergency room visits for respiratory diseases associated with ambient fine particulate matter in Taiwan in 2012: a population-based study. Atmos Pollut Res. 2017;8(3):465–473. doi:10.1016/j.apr.2016.11.008.
- Schwartz J, Slater D, Larson TV, Pierson WE, Koenig JQ. Particulate air pollution and hospital emergency room visits for asthma in Seattle. Am Rev Respir Dis. 1993;147(4):826–831. doi:10.1164/ajrccm/147.4.826.
- Zuo B, Liu C, Chen R, Kan H, Sun J, Zhao J, Wang C, Sun Q, Bai H. Associations between short-term exposure to fine particulate matter and acute exacerbation of asthma in Yancheng, China. Chemosphere. 2019;237(12):124497. doi:10.1016/j.chemosphere.2019.124497.
- Lin M, Chen Y, Burnett RT, Villeneuve PJ, Krewski D. The influence of ambient coarse particulate matter on asthma hospitalization in children: case-crossover and time-series analyses. Environ Health Perspect. 2002;110(6):575–581. doi:10.1289/ehp.02110575.
- Lin M, Chen Y, Burnett RT, Villeneuve PJ, Krewski D. Effect of short-term exposure to gaseous pollution on asthma hospitalisation in children: a bi-directional case-crossover analysis. J Epidemiol Community Health. 2003;57(1):50–55. doi:10.1136/jech.57.1.50.
- Byers N, Ritchey M, Vaidyanathan A, et al. Short-term effects of ambient air pollutants on asthma-related emergency department visits in Indianapolis, Indiana, 2007-2011. J Asthma. 2016;53(3):245–252. doi:10.3109/02770903.2015.1091006.
- Chi R, Li H, Wang Q, Zhai Q, Wang D, Wu M, Liu Q, Wu S, Ma Q, Deng F, et al. Association of emergency room visits for respiratory diseases with sources of ambient PM2.5. J Environ Sci (China). 2019;86(12):154–163. doi:10.1016/j.jes.2019.05.015.
- Cai J, Zhao A, Zhao J, Chen R, Wang W, Ha S, Xu X, Kan H. Acute effects of air pollution on asthma hospitalization in Shanghai, China. Environ Pollut. 2014;191(8):139–144. doi:10.1016/j.envpol.2014.04.028.
- Slaughter JC, Kim E, Sheppard L, Sullivan JH, Larson TV, Claiborn C. Association between particulate matter and emergency room visits, hospital admissions and mortality in Spokane, Washington. J Expo Anal Environ Epidemiol. 2005;15(2):153–159. doi:10.1038/sj.jea.7500382.
- Smargiassi A, Kosatsky T, Hicks J, Plante C, Armstrong B, Villeneuve PJ, Goudreau S. Risk of asthmatic episodes in children exposed to sulfur dioxide stack emissions from a refinery point source in Montreal, Canada. Environ Health Perspect. 2009;117(4):653–659. doi:10.1289/ehp.0800010.
- Zhang Y, Xiang Q, Yu C, Yang Z. Asthma mortality is triggered by short-term exposures to ambient air pollutants: evidence from a Chinese urban population. Atmos Environ. 2020;223(2):117271. doi:10.1016/j.atmosenv.2020.117271.
- Zu K, Liu X, Shi L, Tao G, Loftus CT, Lange S, Goodman JE. Concentration-response of short-term ozone exposure and hospital admissions for asthma in Texas. Environ Int. 2017;104(7):139–145. doi:10.1016/j.envint.2017.04.006.
- Sunyer J, Atkinson R, Ballester F, Le Tertre A, Ayres JG, Forastiere F, Forsberg B, Vonk JM, Bisanti L, Anderson RH, et al. Respiratory effects of sulphur dioxide: a hierarchical multicity analysis in the APHEA 2 study. Occup Environ Med. 2003;60(8):e2. doi:10.1136/oem.60.8.e2.
- James KA, Strand M, Hamer MK, Cicutto L. Health services utilization in asthma exacerbations and PM10 levels in rural Colorado. Ann Am Thorac Soc. 2018;15(8):947–954. doi:10.1513/AnnalsATS.201804-273OC.
- Kim H, Kim H, Park Y-H, Lee J-T. Assessment of temporal variation for the risk of particulate matters on asthma hospitalization. Environ Res. 2017;156(7):542–550. doi:10.1016/j.envres.2017.04.012.
- Zhu L. Time series analysis on association between air pollution and outpatient visits for respiratory diseases of pediatrics in a hospital in Hefei. Doctoral Dissertation Database of Anhui Medical University. 2015. http://cdmd.cnki.com.cn/Article/CDMD-10366-1016139520.htm.
- Su JG, Barrett MA, Henderson K, Humblet O, Smith T, Sublett JW, Nesbitt L, Hogg C, Van Sickle D, Sublett JL, et al. Feasibility of deploying inhaler sensors to identify the impacts of environmental triggers and built environment factors on asthma short-acting bronchodilator use. Environ Health Perspect. 2017;125(2):254–261. doi:10.1289/EHP266.
- Romieu I, Meneses F, Sienra-Monge JJ, Huerta J, Ruiz Velasco S, White MC, Etzel RA, Hernandez-Avila M. Effects of urban air pollutants on emergency visits for childhood asthma in Mexico City. Am J Epidemiol. 1995;141(6):546–553. doi:10.1093/oxfordjournals.aje.a117470.
- Zhang H, Liu S, Chen Z, Zu B, Zhao Y. Effects of variations in meteorological factors on daily hospital visits for asthma: a time-series study. Environ Res. 2020;182(3):109115. doi:10.1016/j.envres.2020.109115.
- Bontinck A, Maes T, Joos G. Asthma and air pollution: recent insights in pathogenesis and clinical implications. Curr Opin Pulm Med. 2020;26(1):10–19. doi:10.1097/MCP.0000000000000644.
- De Grove KC, Provoost S, Brusselle GG, Joos GF, Maes T. Insights in particulate matter-induced allergic airway inflammation: focus on the epithelium. Clin Exp Allergy. 2018;48(7):773–786. doi:10.1111/cea.13178.
- Mathews JA, Krishnamoorthy N, Kasahara DI, Hutchinson J, Cho Y, Brand JD, Williams AS, Wurmbrand AP, Ribeiro L, Cuttitta F, et al. Augmented responses to ozone in obese mice require IL-17A and gastrin-releasing peptide. Am J Respir Cell Mol Biol. 2018;58(3):341–351. doi:10.1165/rcmb.2017-0071OC.
- Willart MAM, Deswarte K, Pouliot P, Braun H, Beyaert R, Lambrecht BN, Hammad H. Interleukin-1α controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33. J Exp Med. 2012;209(8):1505–1517. doi:10.1084/jem.20112691.
- Michaudel C, Maillet I, Fauconnier L, Quesniaux V, Chung KF, Wiegman C, Peter D, Ryffel B. Interleukin-1α mediates ozone-induced myeloid differentiation factor-88-dependent epithelial tissue injury and inflammation. Front Immunol. 2018;9(5):916.
- Haczku A, Cao Y, Vass G, Kierstein S, Nath P, Atochina-Vasserman EN, Scanlon ST, Li L, Griswold DE, Chung KF, et al. IL-4 and IL-13 form a negative feedback circuit with surfactant protein-D in the allergic airway response. J Immunol. 2006;176(6):3557–3565. doi:10.4049/jimmunol.176.6.3557.
- Weng C-M, Lee M-J, He J-R, Chao M-W, Wang C-H, Kuo H-P. Diesel exhaust particles up-regulate interleukin-17A expression via ROS/NF-κB in airway epithelium. Biochem Pharmacol. 2018;151(5):1–8. doi:10.1016/j.bcp.2018.02.028.
- Zhang X, Chen X, Weirauch MT, Zhang X, Burleson JD, Brandt EB, Ji H. Diesel exhaust and house dust mite allergen lead to common changes in the airway methylome and hydroxymethylome. Environ Epigenet. 2018;4(3):dvy020–15. doi:10.1093/eep/dvy020.
- Jackson DJ, Gill MA, Liu AH, Gruchalla RS, O’Connor GT, Pongracic JA, Kercsmar CM, Khurana Hershey GK, Zoratti EM, Teach S, et al. Air pollution levels drive inflammatory epithelial responses in the pathogenesis of non-viral asthma exacerbations in urban children. Am J Respir Crit Care Med. 2020;201(1):A1036–A1036. doi:10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A1036.
- Hashemi A, Shirkani A, Hashemi M, et al. Role of air pollution on pathogenesis of asthma and allergic diseases. J Air Pollut Health. 2017;2(4):205–210.