Abstract
Objective
The efficacy and safety of mepolizumab in patients with severe eosinophilic asthma in randomized controlled trials is well established. Following approval of mepolizumab as add-on therapy for severe eosinophilic asthma in multiple regions worldwide, it is now important to determine its impact in real-world settings in which patients are not subject to stringent eligibility criteria. This systematic literature review assessed published evidence of clinical outcomes, safety, and healthcare resource use among patients with severe asthma receiving mepolizumab in real-world settings.
Data sources
Searches were conducted in Embase, MEDLINE, and MEDLINE In-Process via Ovid.
Study selections
Eligible studies were observational, and enrolled ≥10 patients with asthma who received mepolizumab 100 mg subcutaneously. Data extracted included annualized exacerbation rate, mean daily oral corticosteroid (OCS) dose, proportion of patients using OCS, several measures of lung function, patient-reported asthma control and health-related quality of life (HRQoL), safety, and economic burden.
Results
Twenty-three articles (22 unique studies; 2,040 patients with severe asthma on mepolizumab) were identified. Mepolizumab use was associated with a reduction in annualized exacerbation rates (requiring OCS) of 54–97% (p < 0.05 in all studies), reduced mean/median daily OCS doses, and OCS discontinuation during follow-up (27–84% of patients). Improvements in lung function, asthma control, and HRQoL were also observed. The most commonly reported adverse events included headache and arthralgia; discontinuation of mepolizumab due to adverse events occurred in 0–10.6% of patients.
Conclusion
Findings show that patients with severe asthma consistently demonstrate clinically relevant benefits with mepolizumab treatment in a real-world setting.
Supplemental data for this article is available online at at www.tandfonline.com/ijas .
Introduction
Asthma is a heterogeneous respiratory condition affecting more than 339 million people globally (Citation1). Patients with severe asthma typically have poorly controlled disease and experience frequent asthma exacerbations, impaired lung function and reduced health-related quality of life (HRQoL), despite standard of care therapy, which consists of medium to high doses of inhaled corticosteroids (ICS) plus one or more additional controller medications (such as a long-acting beta agonist [LABA]) (Citation2–4). Patients whose symptoms persist despite optimization (i.e. good adherence and correct inhaler technique) of medium to high dose ICS/LABA treatment should be considered for add-on therapy such as biologics that target the inflammatory signaling pathways seen in asthma (Citation4).
A subset of patients with severe asthma have severe eosinophilic asthma, characterized by elevated levels of eosinophils in the blood and/or airways (Citation3). Mepolizumab is a humanized monoclonal antibody that binds to and neutralizes interleukin (IL)-5, thus inhibiting IL-5 signaling and blocking eosinophil proliferation, activation, and survival (Citation5,Citation6). It is approved as an add-on therapy for patients with severe eosinophilic asthma and eosinophilic granulomatosis with polyangiitis in multiple regions worldwide, and for patients with hypereosinophilic syndrome in the US (Citation7–9). Randomized controlled trials (RCTs) (Citation10–13) and real-world studies (Citation14–16) have demonstrated that compared with placebo, mepolizumab reduces exacerbations and oral corticosteroid (OCS) dependence, in addition to improving lung function, asthma control and HRQoL in patients with severe eosinophilic asthma. As real-world use of mepolizumab increases, the population of patients with access to mepolizumab is more diverse than those included in the mepolizumab RCTs, which have strict eligibility criteria that often lead to the exclusion of patients with respiratory comorbidities and other characteristics, such as a recent smoking history and lack of adherence to controller medications, frequently found in the real-world severe asthma population (Citation11,Citation13,Citation17–19). It is therefore of clinical interest to fully understand the extent to which the clinical benefits associated with mepolizumab are observed in routine clinical practice.
The aim of this systematic literature review (SLR) was to assess the published evidence relating to a broad range of clinical outcomes, safety, and healthcare resource utilization (HCRU) among patients with severe asthma receiving add-on therapy with mepolizumab in a real-world setting.
Methods
This SLR was conducted and reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Citation20). All SLR methodology followed the established best methods used in systematic review research (Citation21,Citation22).
Search strategy
The SLR was designed to identify studies reporting data on the effectiveness and safety of mepolizumab in patients with asthma in a real-world setting. Systematic literature searches were conducted in Embase, MEDLINE and MEDLINE In-Process via Ovid, using a combination of free-text terms and medical subject headings for asthma combined with terms for mepolizumab (). Searches were limited to articles published in the English language and publications reporting the results of clinical trials were excluded. A hand search of conference proceedings for several key 2018, 2019, and 2020 meetings (as available) was also conducted. This included the following congresses: American Academy of Allergy, Asthma, and Immunology (2018, 2019); American College of Allergy, Asthma, and Immunology (2018, 2019); American College of Chest Physicians (2018, 2019); American Thoracic Society (2018, 2019, 2020); British Thoracic Society (2018, 2019); European Academy of Allergy and Clinical Immunology (2018, 2019); European Respiratory Society (2018, 2019); International Society for Pharmacoeconomics and Outcomes Research (2018, 2019).
Table 1. Patient baseline characteristics.
Study selection and data extraction
The eligibility criteria for abstracts and full-text articles were based on the Population, Intervention, Comparison, Outcomes, and Study (PICOS) design framework, and are described in full in . Briefly, eligible publications were published prior to 10 July, 2020 (with no lower limit applied to the publication date) and reported data from observational studies (prospective and retrospective cohort studies, registry and claims database analyses, cross-sectional studies) that enrolled ≥10 patients ≥6 years of age with asthma who had been treated with the approved dose of mepolizumab (100 mg, administered subcutaneously). Eligible publications also had to include data on at least one outcome of interest, including clinical outcomes (such as exacerbation frequency or lung function), disease control or HRQoL outcomes, or economic outcomes (). The data could be from the overall study population, or for subgroups of patients stratified by baseline blood eosinophil count. Other subgroups (such as patients deemed to be treatment responders/ non-responders) were not included, owing to inconsistent definitions of treatment response across studies.
Table 2. Exacerbation data.
Any duplicate publications identified across databases were removed from the initial search results and all remaining unique publications were screened using Distiller Systematic Review software. Titles/abstracts and then full-text publications were screened for inclusion by two independent investigators and any discrepancies between investigators were resolved by a third investigator. Once all abstracts and full-text articles meeting the eligibility criteria were identified, data from these publications were extracted by a single investigator into a data extraction template. The data were then independently validated by a second, senior investigator.
Data synthesis
The results of the SLR were summarized qualitatively and no statistical analyses were planned. With regards to safety data, adverse events (AEs) that occurred during the follow-up period of the included studies were captured by reported event type. The results reported in this publication focus on the available evidence from full-text, peer-reviewed journal articles, with the findings of gray literature (including letters to the editor and congress abstracts) summarized only briefly. For all key outcomes of interest, results have been summarized for prospective and retrospective studies, separately.
Ethics
Only publicly available summary-level data were included in this SLR; ethical approval was therefore not required.
Results
Characteristics of the included studies
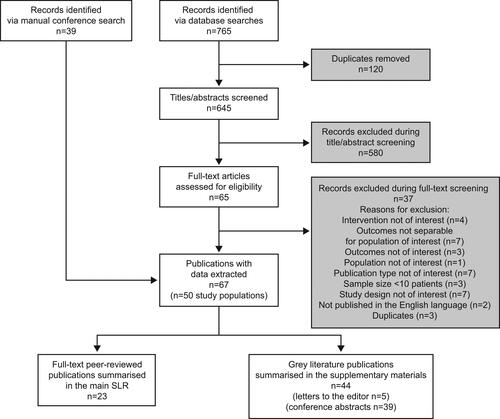
The electronic database searches identified 645 unique titles and abstracts; 580 of these were excluded during eligibility screening. The remaining 65 abstracts were retrieved for full-text review, and following screening, 67 publications reporting data in 50 unique study populations met the criteria for inclusion. Among these, 23 full-text peer-reviewed articles reported data from 22 unique study populations and 44 gray literature publications reported data from 28 unique study populations ().
The 22 unique study populations included in the full-text publications (Citation14–16,Citation23–41) included a total of 2,040 patients and were conducted in Italy (n = 7) (Citation28–31,Citation33,Citation37,Citation39), Australia (n = 3) (Citation23–25), United Kingdom (n = 2) (Citation34,Citation35), United States (n = 1) (Citation14), France (n = 1) (Citation40), Netherlands (n = 1) (Citation16), Spain (n = 1) (Citation32), Germany (n = 1) (Citation27), Belgium (n = 1) (Citation26), Portugal (n = 1) (Citation38), Japan (n = 1) (Citation36), Turkey (n = 1) (Citation41) and Israel (n = 1) (Citation15). Most (n = 12) studies were conducted at a single centre (Citation15,Citation16,Citation23,Citation25–28,Citation33–36,Citation41), while five enrolled patients across multiple centers (Citation29,Citation32,Citation37,Citation38,Citation40) and five analyzed data from database/registry sources (Citation14,Citation24,Citation30,Citation31,Citation39). Full study details are described in . Details of the gray literature publications identified by this SLR are described in Supplementary Table 4.
Table 3. OCS data.
Findings of studies reported in full-text peer-reviewed publications
Patient characteristics
Patient baseline characteristics from the 22 studies reported in full-text publications are shown in . The mean/median age of recruited patients ranged from 49 (Citation14) to 67 (Citation31) years across the studies and the proportion of female patients ranged from 35% (Citation33) to 90% (Citation38). Eighteen studies reported information on maintenance oral corticosteroid (mOCS) use at baseline, with between 22.2% (Citation33) and 100% (Citation28,Citation34,Citation41) of patients using mOCS; three studies enrolled only patients with OCS-dependent asthma (Citation28,Citation34,Citation41). Fourteen studies reported smoking status, with the proportion of patients who never smoked ranging from 42% (Citation33) to 88% (Citation41) and the proportion of current smokers ranging from 0% (Citation23) to 26% (Citation37). Eight studies reported mean baseline blood eosinophil counts, ranging from 647 (Citation28) to 1,228 cells/µL (Citation36); two studies reported median (interquartile range) baseline blood eosinophil counts of 630 (400–900) (Citation15) and 590 (400–830) (Citation24) cells/µL (Supplementary Table 5). Among the two studies enrolling only patients with OCS-dependent asthma and with blood eosinophil count data available, mean baseline blood eosinophil counts were 580 (Citation41) and 647 (Citation28) cells/µL. Across the few studies that reported asthma-related comorbidities, 22% (Citation14) to 100% (Citation41) of patients had nasal polyps (study reporting the upper range recruited only patients with comorbid nasal polyps), 18% (Citation40) to 100% (Citation41) of patients had allergic rhinitis/chronic rhinosinusitis, and 8% (Citation16) to 58% (Citation26) had gastroesophageal reflux disease.
Exacerbations
A total of 16 studies (three prospective and 13 retrospective) reported annualized exacerbation rates. Although the definitions of an exacerbation varied across the studies, common factors across the definitions used included worsening asthma symptoms requiring emergency department (ED) or hospital visits, treatment with systemic corticosteroids, and/or increases in mOCS doses. Three studies (Citation28,Citation32,Citation34) did not provide a definition for exacerbations. The exacerbation definition(s) adopted by each study as well as changes in exacerbation rate with mepolizumab are described in .
Three prospective studies, all with 12 months of follow-up data, reported statistically significant (p < 0.001) reductions in OCS-requiring exacerbations with mepolizumab. These studies reported reductions from baseline to follow-up of 66% (4.16 vs 1.41 events/year) (Citation24), 68% (2.80 vs 0.90 events/year) (Citation25) and 73% (3.14 vs 0.85 events/year) (Citation15), with one study also reporting a 54% reduction in the annualized rate of exacerbations requiring hospitalization (0.57 vs 0.26 events/year at baseline vs follow-up; p < 0.001) (Citation24) (). The findings of the prospective studies were supported by data from the 13 retrospective studies, which included between 6 and 24 months of follow-up data (). Six of the retrospective studies reported the rate of exacerbations requiring at least treatment with OCS (Citation14,Citation16,Citation35,Citation39–41), showing reductions of 54% (4.04 vs 1.86 events/year at baseline vs follow-up; p < 0.001) (Citation35) to 97% (2.10 vs 0.07 events/year at baseline vs follow-up; p = 0.012) (Citation41) (). Three retrospective studies also reported reductions in the rate of exacerbations requiring hospitalization of 55% (0.77 vs 0.35 events/year at baseline vs follow-up; p = 0.001) (Citation16), 73% (0.11 vs 0.03 events/year at baseline vs follow-up; p = 0.004) (Citation14), and 83% (0.6 vs 0.1 events/year at baseline vs follow-up; p values not reported) (Citation40) events/year (). Two of the retrospective studies presented results in subgroups according to baseline blood eosinophil count (Citation29,Citation40). In both studies, exacerbation rates were reduced with mepolizumab across all blood eosinophil count groups; of note, the majority of patients from both studies (80% and 93%) were receiving mOCS at baseline.
Figure 2. Summary of study outcomes.
Darker spheres indicate prospective studies and paler spheres indicate retrospective studies; sphere size is proportional to study patient population size; for studies reporting statistical analyses, statistical significance information was extracted and has been reported in and (exacerbation and OCS data), and supplementary Tables 5–7 (FEV1, blood eosinophil count, ACQ and ACT data).
*Exacerbations defined as worsening of asthma that required at least treatment with OCS; †Farah (2019), Harvey (2020) and Langton (2020) reported ACQ-5 score, d’Ancona (2020), Kavanagh (2020) and van Toor (2020) reported ACQ-6 score, Schleich (2020) did not disclose the version of the ACQ used.
ACT, asthma control test; ACQ-5, asthma control questionnaire – 5 item; ACQ-6, asthma control questionnaire – 6 item; FEV1, forced expiratory volume in 1 second; mOCS, maintenance oral corticosteroids.
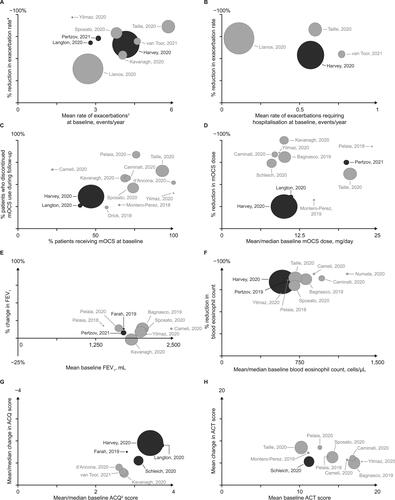
The proportion of patients with exacerbations (any definition) before and after mepolizumab initiation was reported by one prospective (Citation15) and three retrospective (Citation14,Citation30,Citation35) studies (). The prospective study reported an 85% relative risk reduction from baseline to follow-up in the proportion of patients with ≥1 exacerbation (85% in the 12 months preceding vs 13% in the 12 months following mepolizumab initiation). The three retrospective studies all reported reductions from baseline to follow-up in the proportion of patients with ≥1 exacerbation, ranging from 24% (Citation14) to 85% (Citation15); one of these also showed a 65% relative risk reduction in the proportion of patients with an exacerbation requiring hospitalization (Citation14).
Reduction in maintenance OCS
Eighteen studies enrolled patients who were receiving mOCS at baseline, and 16 (three prospective (Citation15,Citation24,Citation25) and 13 retrospective (Citation14,Citation16,Citation27–30,Citation32,Citation33,Citation35,Citation37,Citation39–41)) of these reported either the percentage of patients who remained on mOCS following mepolizumab initiation or the proportion of patients who discontinued mOCS use during the follow-up period ().
In the three prospective studies, 40% (Citation25) to 53% (Citation15) of patients were receiving mOCS at baseline; after 6 months to 1 year of follow-up, the two studies with data available showed that 36% (Citation24) and 26% (Citation25) of the patients who were using mOCS at baseline had discontinued mOCS (; ). Among the retrospective studies, 22% (Citation33) to 100% (Citation41) of patients were receiving mOCS at baseline. Among those studies with follow-up data available at 6 months (n = 4) (Citation28,Citation30,Citation33,Citation41), 12 months (n = 5) (Citation29,Citation32,Citation34,Citation35,Citation37), and 24 months (n = 1) (Citation40), 40% (Citation41) to 79% (Citation28) of the patients receiving mOCS at baseline had discontinued mOCS after 6 months of mepolizumab treatment, 27% (Citation32) to 84% (Citation37) had discontinued mOCS after 12 months, and 65% (Citation40) had discontinued mOCS after 24 months (; ). There was no clear association between the duration of follow-up and the degree of reduction in mOCS use.
Three prospective (Citation15,Citation24,Citation25) and eight retrospective (Citation26,Citation28–30,Citation32,Citation35,Citation40,Citation41) studies reported mOCS doses at baseline and at follow-up, among patients who were using mOCS at baseline. In the prospective studies, reductions from baseline in mOCS dose ranged from 2.5 mg/day (p = 0.001) (Citation24) to 15 mg/day (p values not recorded) (Citation15) during follow-up, translating to a 25% (Citation24) to 75% (Citation15) reduction from baseline in mOCS dose with mepolizumab (; ). In the retrospective studies, reductions from baseline in mOCS dose ranged from 4.8 mg/day (p values not recorded) (Citation32) to 22 mg/day (p < 0.0001) (Citation28) during follow-up, translating to a 32% (Citation32) to 100% (Citation35) reduction from baseline mOCS dose with mepolizumab (; ).
Lung function
Of nine studies (two prospective (Citation15,Citation23) and seven retrospective (Citation28,Citation29,Citation33,Citation35,Citation37,Citation39,Citation41)) reporting changes in FEV1 from baseline to follow-up, both prospective studies and the majority (n = 6 (Citation28,Citation29,Citation33,Citation37,Citation39,Citation41)) of the retrospective studies reported improvements in forced expiratory volume in 1 s (FEV1) with mepolizumab (; Supplementary Table 6). Mean changes in FEV1 ranged from +0.15 (Citation15) to +0.27 L (Citation23) in the prospective studies and −0.03 (Citation35) to +0.40 L (Citation33) in the retrospective studies. Results were statistically significant (p < 0.05) in all but two (Citation35,Citation41) of the studies in which statistical analyses were reported.
Of the 10 studies (two prospective (Citation24,Citation25) and eight retrospective (Citation16,Citation30,Citation33–36,Citation39,Citation41)) with available data, both prospective studies and six retrospective studies (Citation16,Citation30,Citation33,Citation36,Citation39,Citation41) reported improvements in % predicted FEV1 with mepolizumab (Supplementary Table 5). The remaining two retrospective studies reported reductions in % predicted FEV1 of 1.5% (p = 0.438) (Citation35) and 2.3% (p values not reported) (Citation34). Improvements from baseline ranged from 6.1% (Citation24) to 6.3% (Citation25) in the prospective studies and 3.2% (Citation36) to 8.1% (Citation30) in the retrospective studies; statistical significance (p < 0.05) was shown in the one prospective study and two of the five retrospective studies that showed improvements and reported statistical analyses (Citation16,Citation25,Citation34,Citation35,Citation39).
Three studies (one prospective (Citation23) and two retrospective (Citation29,Citation33)) evaluated mean changes in forced vital capacity (FVC); an improvement from baseline to follow-up in FVC was observed with mepolizumab in all three studies (mean change from baseline in the prospective study: +0.15 L [no p values reported]; mean change from baseline in the retrospective studies: +0.16 L [p < 0.0001] and +0.03 L [p = 0.7857]; Supplementary Table 5). All five studies with FEV1/FVC data (one prospective (Citation23) and four retrospective (Citation28,Citation29,Citation33,Citation36)) reported improvements following 6 or 12 months of mepolizumab treatment, with mean FEV1/FVC ratios of 61% (mean change of 6%) in the prospective study and 66–72% (Citation28,Citation33) (mean change of 2.1–4.8% (Citation28,Citation36)) in the retrospective studies (Supplementary Table 5).
Blood eosinophil counts
Ten studies (two prospective (Citation15,Citation24) and eight retrospective (Citation28–30,Citation33,Citation36,Citation39–41)) reported the impact of mepolizumab on blood eosinophil counts. All studies reported lower mean blood eosinophil counts following mepolizumab treatment, with post-treatment mean (SD) absolute blood eosinophil counts ranging from 75–177 cells/µL. Percentage reductions from baseline to follow-up in blood eosinophil counts ranged from 83% (Citation24) to 84% (Citation15) in the prospective studies and 69% (Citation41) to 92% (Citation36) in the retrospective studies (; Supplementary Table 6). Reductions were statistically significant in both prospective studies and in all six of the retrospective studies that reported statistical analyses (Citation28,Citation29,Citation33,Citation36,Citation39,Citation41).
Asthma control and HRQoL
All 16 studies reporting Asthma Control Questionnaire (ACQ) or Asthma Control Test (ACT) scores (four prospective (Citation23–26) and 12 retrospective (Citation26,Citation28–30,Citation32–35,Citation37,Citation39–41)) showed improvements with mepolizumab (; Supplementary Table 7). Among the included studies, mean baseline ACQ and ACT scores ranged from 2.6 (Citation16) to 3.7 (Citation25) and 10.2 (Citation40) to 18.0 (Citation41) points, respectively. Mean changes in ACQ score from baseline to follow-up ranged from −1.1 (Citation26) to −1.9 (Citation24) points among the prospective studies (n = 4) and −0.5 (Citation35) to −0.8 (Citation16) points among the retrospective studies (n = 3) with ACQ data available. These differences were all equal to or greater than the established minimal clinically important difference (MCID) of 0.5 points (Citation42). Changes were statistically significant (p < 0.001) in all five studies that reported statistical analyses (Citation16,Citation24–26,Citation35). With regards to ACT score, mean changes from baseline to follow-up were 5.31 points (p < 0.0001) (Citation26) in one prospective study and 5.00 (Citation29) to 8.53 (Citation37) points in nine retrospective studies with data available. These differences all exceeded the established MCID of 3 points (Citation43). Changes were statistically significant (p ≤ 0.006) in the eight retrospective studies that reported statistical analyses (Citation28–30,Citation33,Citation37,Citation39,Citation41).
Three studies (two prospective (Citation24,Citation26) and one retrospective (Citation35)) showed that mepolizumab had a statistically significant (p < 0.001) beneficial impact on Asthma Quality of Life Questionnaire (AQLQ) or mini-AQLQ score. Mean baseline scores ranged from 3.48 (Citation26) to 4.05 (Citation35) points; the prospective studies reported AQLQ improvements of 1.5 (Citation24) and 1.24 (Citation26) points, and the retrospective study reported a 0.86-point improvement (Citation35) in mini-AQLQ score (Supplementary Table 7). One retrospective study (Citation33) also reported Sino-nasal Outcome Test-22 scores among 26 patients with severe eosinophilic asthma, and showed a statistically significant improvement of 16.9 points (from a mean baseline score of 40.5 points) following mepolizumab treatment, almost double the MCID of 8.9 points (Citation44) (Supplementary Table 7).
Safety and treatment discontinuation
Across 10 studies with available data (three prospective (Citation24–26) and seven retrospective (Citation16,Citation27,Citation33,Citation35,Citation37,Citation40,Citation41)), the overall rate of mepolizumab discontinuation ranged from 7% (6 months of follow-up) (Citation26) to 26% (12 months of follow-up) (Citation25) in the prospective studies and from 0% (7–15 months of follow-up) (Citation27) to 33% (24 months of follow-up) (Citation40) in the retrospective studies (Supplementary Table 8). Treatment discontinuation due to AEs was uncommon, occurring in 2% (Citation24) to 11% (Citation25) of patients in the prospective studies (12 months of follow-up) and 0% (Citation27,Citation37) to 6% (Citation41) of patients in the retrospective studies (6–24 months of follow-up). Fifteen studies (three prospective (Citation24–26) and 12 retrospective (Citation16,Citation27–33,Citation37,Citation38,Citation40,Citation41)) reported the proportions of patients with AEs whilst receiving mepolizumab treatment. Reported AEs in order of frequency included headache (0–40%), arthralgia (0–35%), fatigue (0–30%), dizziness (0–26%), injection-site reactions (0–7%), urticaria or hypersensitivity reactions (0–5%), and cardiac events (0–1%) (Supplementary Table 9).
Economic outcomes: HCRU and cost
Two retrospective studies presented data on HCRU before and after mepolizumab treatment. Both of these studies, one from Italy (Citation29) and one from the US (Citation14), reported a statistically significant decrease in the mean number of hospitalizations in the 12 months following versus the 12 months preceding mepolizumab initiation. The US study also reported a significant reduction from baseline to follow-up in mean exacerbation-related costs per patient ($5,178 vs $2,383; p < 0.001) and a numerical reduction in the mean number of OCS pharmacy claims during the follow-up period (3.9 claims) compared with the baseline period (5.5 claims) (Citation14).
Findings from the gray literature
Results reported in the gray literature generally supported those of the full-text peer-reviewed publications. Where data were available, overall improvements in exacerbation rates, mOCS use, lung function, blood eosinophil count, and asthma control were observed. Rates of mepolizumab discontinuation reported in the gray literature were also similar to those reported in the full-text peer-reviewed publications.
Discussion
Across the current respiratory treatment landscape, there is an important need for real-world studies with high external validity to support the efficacy results of RCTs (Citation45). This SLR aimed to provide a broad picture of the full range of clinical impacts seen with mepolizumab in a real-world setting, in order to determine whether the clinical benefits shown in RCTs of mepolizumab in severe eosinophilic asthma, namely reduced exacerbation rates, reduced OCS use, improved asthma control, and improved HRQoL (Citation10–13), are also seen in more diverse, real-world populations. Our findings indicate that patients with severe asthma receiving mepolizumab treatment in real-world clinical practice experience important health benefits, including reductions in exacerbations, OCS use and blood eosinophil counts, plus improvements in lung function, asthma control and HRQoL.
The observational real-world studies identified by the SLR included a heterogeneous patient population, with a broader spectrum of disease severity, comorbidities and concomitant medications compared with those typically enrolled in RCTs. Compared with patients enrolled in previous mepolizumab RCTs (Citation10–13,Citation46,Citation47), the patients included in real-world studies were generally older, with more severe asthma and a larger number of comorbid conditions. Overall, 40–93% of patients included in this SLR were using mOCS prior to mepolizumab initiation, compared with 23–33% in the RCTs of mepolizumab in patients with severe asthma (where mOCS use was not a requirement for patient eligibility) (Citation11–13). Although definitions varied, the annualized rate of exacerbations requiring at least OCS treatment was similar in the real-world literature prior to mepolizumab initiation compared with previous RCTs (2.1–5.8 events/year in this SLR vs 2.7–3.8 events/year in RCTs). Only a few of the studies included in this SLR reported on asthma-specific comorbidities; among these, the proportion of patients with comorbidities was high, and generally exceeded the proportion reported in a recent open-label extension study of mepolizumab (22% to 100% in this SLR vs 7% in RCT with nasal polyps; 49% to 100% in this SLR vs 12% in RCT with chronic rhinosinusitis) (Citation47). This is expected, since interventional studies in patients with severe asthma typically exclude those with comorbidities associated with respiratory symptoms, such as chronic obstructive pulmonary disease. The studies identified by this SLR included a wide selection of geographic locations, with publications from the United States, Japan, Australia, and multiple European countries. Although no studies from China (where mepolizumab is not licensed) or South America were identified as meeting the eligibility criteria, the geographical spread of the available data appears to be sufficiently representative of real-world use of mepolizumab.
Despite the range of differences between the real-world populations included in this SLR and the populations included in RCTs of mepolizumab in patients with severe asthma, the clinical benefits shown in real-world settings were consistent with those shown previously in RCTs. These included a significant reduction in asthma exacerbations, which is a primary goal of asthma treatment, and indeed, reductions in exacerbation rates were among the most important efficacy results of the landmark mepolizumab RCTs. The SLR also demonstrated mepolizumab treatment to have a significant OCS-sparing effect in clinical practice, with 24–84% of patients able to discontinue OCS use completely with mepolizumab. Reducing OCS dependence is another key goal of asthma treatment, owing to multiple negative side effects associated with long-term OCS use (Citation48). The real-world data reported here build on the results of a formal OCS-sparing trial, which reported 23% of patients receiving mepolizumab to have reduced their mOCS use by 90–100% by study end (Citation10). It should be noted that the ability to reduce OCS was not at the expense of loss of disease control; moreover, irrespective of their improvements in disease control with mepolizumab, some patients may not have been able to discontinue mOCS entirely due to adrenal insufficiency (Citation49). Finally, the published real-world evidence also indicated that geometric mean absolute blood eosinophil counts were reduced to levels within the identified normal range following mepolizumab initiation (Citation50). Despite variations in the assessment tools used, improvements in asthma control and HRQoL were also observed with mepolizumab therapy. It is important to consider that since mepolizumab specifically targets IL-5, patients who had any respiratory comorbidities that were not associated with eosinophilia may have experienced smaller improvements in their asthma control than those without (Citation24). Together the data from the large number of studies included in the SLR demonstrate that the broad range of clinical benefits shown with mepolizumab in patients with severe asthma during RCTs was mirrored in the real-world population of patients with asthma receiving mepolizumab.
In the SLR studies with available safety data, headache was the most commonly reported AE, followed by injection site reactions and arthralgia. These AEs were reported in the mepolizumab clinical trials, where headache was often also the most commonly reported AE (Citation11–13,Citation46,Citation47). Mepolizumab discontinuation rates due to AEs were typically low in the studies identified by the SLR, and broadly similar to those reported in RCTs (Citation10,Citation11,Citation47,Citation51).
With regards to the impact of mepolizumab on economic burden, only one study reporting exacerbation-related costs and two studies reporting exacerbation-related HCRU were identified by the SLR. The findings of these studies associated mepolizumab treatment with substantial reductions in exacerbation-related costs for patients with severe uncontrolled asthma, compared with baseline values. In addition, hospitalizations due to asthma exacerbations were significantly decreased following mepolizumab treatment.
There are several limitations that must be considered when interpreting the findings of this SLR. Firstly, differences in study design may limit comparison of the data from real-world studies with those from RCTs. For example, restrictions in OCS tapering protocols may have prevented patients from substantially tapering or discontinuing OCS in the RCT setting; conversely, RCTs with rapid reduction algorithms may have reduced OCS use more quickly than in real-world clinical practice. Second, the absence of a control group in the included studies makes them prone to bias; however, the general consistency of the extracted data does not suggest that the results were confounded. Moreover, results of the single-centre clinical studies included in this SLR (n = 12) are likely to be less generalizable to real-world clinical practice than those of the multi-centre registry/national database studies (n = 5) (Citation52). Third, SLRs are by nature limited to published data (and in this case those published in the English language) only. In addition, the risk of bias within individual studies was not assessed. Finally, we observed considerable heterogeneity in study design, patient cohort, sample size and outcome definitions across the included studies. In particular, the small sample size of some of the studies included in this SLR may have resulted in a lack of power to identify statistical differences in specific outcomes. Furthermore, the definitions of exacerbation varied across the studies, as did the patient reported outcomes instruments used to assess HRQoL. Owing to this between-study heterogeneity, the SLR did not pool results to perform meta-analyses. Nonetheless, the data identified by this SLR consistently demonstrated clinical benefits in patients with severe asthma receiving mepolizumab treatment. Notably, since the search was conducted in July 2020, several additional real-world studies of mepolizumab have been published, all of which report clinical benefits with mepolizumab (Citation53–55) and therefore support the findings of this SLR.
Conclusions
Despite substantial heterogeneity observed in the available literature, mepolizumab was consistently associated with clinically relevant benefits in eligible patients with severe asthma receiving treatment in real-world clinical practice. It is therefore reasonable to conclude that the benefits of mepolizumab demonstrated in previous RCTs extend to patients with heterogeneous demographics and characteristics, treated in a real-world setting.
Author contributions
SY, PHH, ALM, MK, SGS and RAC all contributed to the conception or design of this study and were involved in the acquisition of data. All authors were involved in the data analysis and interpretation, contributed to the development of the manuscript, and approved the final version for submission. All authors agree to be accountable for all aspects of the work.
Disclosure of interest
EI reports consultancy fees from AB Science, Allergy and Asthma Network, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Equillium, Genentech, GSK, Merck, NHLBI, Novartis, Pneuma Respiratory, PPS Health, Regeneron, Sanofi Genzyme, Sienna Biopharmaceuticals and Teva; receipt of study drug/equipment from Boehringer Ingelheim, Circassia, Genentech, GSK and Teva; clinical research grants from AstraZeneca, Avillion, Circassia, Gossamer Bio, NIH, Novartis and PCORI; advisory board participation for Novartis; royalties from UpToDate and stock options from Vorso; he was a co-ordinating committee member for the National Asthma Education Prevention Program. GWC reports research grants and fees from A.Menarini, Alk-Abello’, Allergy Therapeutics, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, Genentech, Guidotti-Malesci, GSK, Hal Allergy, Mylan, Merck, Merck Sharp & Dome, Mundipharma, Novartis, Regeneron, Roche, Sanofi-Aventis, Sanofi-Genzyme, Stallergenes-Greer, UCB Pharma, Uriach Pharma, Valeas and Vibor-Pharma. GB has received honoraria for lectures from AstraZeneca, Boehringer-Ingelheim, Chiesi, GSK, Novartis, Teva and Sanofi; he is a member of advisory boards for Amgen, AstraZeneca, Boehringer-Ingelheim, Chiesi, GSK, Novartis, Sanofi/Regeneron and Teva. SY, PHH, SGS and RAC are employees of GSK and hold stocks/shares. ALM is an employee of Evidera and MK is a former employee of Evidera and a current employee of Xcenda UK Ltd; as Evidera employees, they did not receive any direct payments or honoraria for their services.
213095_ms_SUPPLEMENTARY_Updated_1.docx
Download MS Word (71.8 KB)Acknowledgements
This study was funded by GlaxoSmithKline (GSK; ID: 213095). Analyses were performed by Evidera, funded by GSK; employees did not receive funding for manuscript development. Editorial support (in the form of writing assistance, including development of the initial draft from the author discussions, assembling tables and figures, collating authors comments, grammatical editing and referencing) was provided by Bianca Paris, PhD, at Fishawack Indicia Ltd, UK, part of Fishawack Health, and was funded by GSK.
Data availability statement
Data recorded in the data extraction template for this systematic literature review are available upon reasonable request from GlaxoSmithKline
Additional information
Funding
References
- Vos T, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, Abdulkader RS, Abdulle AM, Abebo TA, Abera SF, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. 2017; 390(10100):1211–1259. doi:10.1016/S0140-6736(17)32154-2.
- Wenzel S. Severe asthma: from characteristics to phenotypes to endotypes. Clin Exp Allergy. 2012; 42(5):650–658. doi:10.1111/j.1365-2222.2011.03929.x.
- Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014; 43(2):343–373. doi:10.1183/09031936.00202013.
- Global Initiative for Asthma. Global Strategy for Asthma management and prevention. 2021. Available from: https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf. Accessed Oct 2021
- Menzella F, Lusuardi M, Galeone C, Taddei S, Zucchi L. Profile of anti-IL-5 mAb mepolizumab in the treatment of severe refractory asthma and hypereosinophilic diseases. J Asthma Allergy. 2015; 8:105–114. doi:10.2147/JAA.S40244.
- Busse WW. Biological treatments for severe asthma: a major advance in asthma care. Allergol Int. 2019; 68(2):158–166. doi:10.1016/j.alit.2019.01.004.
- European Medicines Agency. Nucala summary of product characteristics. 2021. Available from: https://www.ema.europa.eu/en/documents/product-information/nucala-epar-product-information_en.pdf. Accessed Jan 2021
- Food and Drug Administration. Mepolizumab (NUCALA) prescribing information. 2020. Available from: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Nucala/pdf/NUCALA-PI-PIL.PDF. Accessed Jan 2021
- GlaxoSmithKline. Nucala: basic product information. 2020. Available from: https://gskpro.com/ja-jp/products-info/nucala/index/. Accessed Jan 2021
- Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, Ortega HG, Pavord ID, SIRIUS Investigators Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–1197. doi:10.1056/NEJMoa1403291.
- Chupp GL, Bradford ES, Albers FC, Bratton DJ, Wang-Jairaj J, Nelsen LM, Trevor JL, Magnan A, ten Brinke A. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017; 5(5):390–400. doi:10.1016/S2213-2600(17)30125-X.
- Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, Humbert M, Katz LE, Keene ON, Yancey SW, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014; 371(13):1198–1207. doi:10.1056/NEJMoa1403290.
- Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, Ortega H, Chanez P. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012; 380(9842):651–659. doi:10.1016/S0140-6736(12)60988-X.
- Llanos J-P, Ortega H, Bogart M, Packnett ER, Manjelievskaia J, Bell CF, Hahn B. Real-World Effectiveness of Mepolizumab in Patients with Severe Asthma: An Examination of Exacerbations and Costs. JAA. 2020;Volume 13:77–87. doi:10.2147/JAA.S236609.
- Pertzov B, Unterman A, Shtraichman O, Shitenberg D, Rosengarten D, Kramer MR. Efficacy and safety of mepolizumab in a real-world cohort of patients with severe eosinophilic asthma. J Asthma. 2021; 58(1):79–84. doi:10.1080/02770903.2019.1658208.
- van Toor JJ, van der Mark SC, Kappen JH, In ‘t Veen JCCM, Braunstahl GJ. Mepolizumab add-on therapy in a real world cohort of patients with severe eosinophilic asthma: response rate, effectiveness, and safety. J Asthma. 2021; 58(5):651–658. doi:10.1080/02770903.2020.1723623.
- Boulet LP. Influence of comorbid conditions on asthma. Eur Respir J. 2009; 33(4):897–906. doi:10.1183/09031936.00121308.
- Battaglia S, Basile M, Spatafora M, Scichilone N. Are asthmatics enrolled in randomized trials representative of real-life outpatients? Respiration. 2015; 89(5):383–389. doi:10.1159/000375314.
- Chatkin JM, Dullius CR. The management of asthmatic smokers. Asthma Res Pract. 2016; 2(1):10–18. doi:10.1186/s40733-016-0025-7.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009; 62(10):e1–e34. doi:10.1016/j.jclinepi.2009.06.006.
- Cook DJ, Mulrow CD, Haynes RB. Systematic reviews: synthesis of best evidence for clinical decisions. Ann Intern Med. 1997; 126(5):376–380. doi:10.7326/0003-4819-126-5-199703010-00006.
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions. Sheffield, UK: John Wiley & Sons, 2019.
- Farah CS, Badal T, Reed N, Rogers PG, King GG, Thamrin C, Peters MJ, Seccombe LM. Mepolizumab improves small airway function in severe eosinophilic asthma. Respir Med. 2019; 148:49–53. doi:10.1016/j.rmed.2019.01.016.
- Harvey ES, Langton D, Katelaris C, Stevens S, Farah CS, Gillman A, Harrington J, Hew M, Kritikos V, Radhakrishna N, et al. Mepolizumab effectiveness and identification of super-responders in severe asthma. Eur Respir J. 2020; 55(5):1902420. doi:10.1183/13993003.02420-2019.
- Langton D, Sha J, Guo S, Sharp J, Banks C, Wang W, Plummer V, Thien F. Bronchial thermoplasty versus mepolizumab: Comparison of outcomes in a severe asthma clinic. Respirology. 2020; 25(12):1243–1249. doi:10.1111/resp.13830.
- Schleich F, Graff S, Nekoee H, Moermans C, Henket M, Sanchez C, Paulus V, Guissard F, Donneau A‐F, Louis R, et al. Real-word experience with mepolizumab: Does it deliver what it has promised? Clin Exp Allergy. 2020; 50(6):687–695. doi:10.1111/cea.13601.
- Drick N, Seeliger B, Welte T, Fuge J, Suhling H. Anti-IL-5 therapy in patients with severe eosinophilic asthma - clinical efficacy and possible criteria for treatment response. BMC Pulm Med. 2018; 18(1):119. doi:10.1186/s12890-018-0689-2.
- Pelaia C, Busceti MT, Solinas S, Terracciano R, Pelaia G. Real-life evaluation of the clinical, functional, and hematological effects of mepolizumab in patients with severe eosinophilic asthma: Results of a single-centre observational study. Pulm Pharmacol Ther. 2018; 53:1–5. doi:10.1016/j.pupt.2018.09.006.
- Bagnasco D, Caminati M, Menzella F, Milanese M, Rolla G, Lombardi C, Bucca C, Heffler E, Paoletti G, Testino E, et al. One year of mepolizumab. Efficacy and safety in real-life in Italy. Pulm Pharmacol Ther. 2019; 58:101836. doi:10.1016/j.pupt.2019.101836.
- Caminati M, Cegolon L, Vianello A, Chieco Bianchi F, Festi G, Marchi MR, Micheletto C, Mazza F, Tognella S, Senna G, et al. Mepolizumab for severe eosinophilic asthma: a real-world snapshot on clinical markers and timing of response. Expert Rev Respir Med. 2019; 13(12):1205–1212. doi:10.1080/17476348.2019.1676734.
- Lombardi C, Bagnasco D, Caruso C, D’Amato M, Menzella F, Milanese M, Senna G, Canonica GW, Passalacqua G. Analysis of the drop-out rate in patients receiving mepolizumab for severe asthma in real life. Pulm Pharmacol Ther. 2019; 54:87–89. doi:10.1016/j.pupt.2018.12.003.
- Montero-Perez O, Contreras-Rey MB, Sanchez-Gomez E. Effectiveness and safety of mepolizumab in severe refractory eosinophilic asthma: results in clinical practice. Drugs Context. 2019; 8:212584. doi:10.7573/dic.212584.
- Cameli P, Bergantini L, d’Alessandro M, Perruzza M, Cekorja B, Perillo F, Massa E, Ruzza A, Fossi A, Beltrami V, et al. A Comprehensive Evaluation of Mepolizumab Effectiveness in a Real-Life Setting. Int Arch Allergy Immunol. 2020; 181(8):606–612. doi:10.1159/000507996.
- d’Ancona G, Kavanagh J, Roxas C, Green L, Fernandes M, Thomson L, Dhariwal J, Nanzer AM, Jackson DJ, Kent BD, et al. Adherence to corticosteroids and clinical outcomes in mepolizumab therapy for severe asthma. Eur Respir J. 2020; 55(5):1902259. doi:10.1183/13993003.02259-2019.
- Kavanagh JE, d’Ancona G, Elstad M, Green L, Fernandes M, Thomson L, Roxas C, Dhariwal J, Nanzer AM, Kent BD, et al. Real-World Effectiveness and the Characteristics of a "Super-Responder" to Mepolizumab in Severe Eosinophilic Asthma. Chest. 2020; 158(2):491–500. doi:10.1016/j.chest.2020.03.042.
- Numata T, Miyagawa H, Kawamoto H, Yoshida M, Utsumi H, Hashimoto M, Minagawa S, Hara H, Araya J, Kuwano K, et al. Predictors of the enhanced response to mepolizumab treatment for severe eosinophilic asthma: A retrospective, long-term study. Cogent Medicine. 2020; 7(1):1776468. doi:10.1080/2331205X.2020.1776468.
- Pelaia C, Crimi C, Pelaia G, Nolasco S, Campisi R, Heffler E, Valenti G, Crimi N. Real-life evaluation of mepolizumab efficacy in patients with severe eosinophilic asthma, according to atopic trait and allergic phenotype. Clin Exp Allergy. 2020; 50(7):780–788. doi:10.1111/cea.13613.
- Sousa J, Taborda-Barata L, Monteiro C. Biological therapy-associated adverse reactions in asthma: analysis of reporting to the Portuguese pharmacovigilance system. Expert Opin Drug Saf. 2020; 19(1):99–106. doi:10.1080/14740338.2020.1686481.
- Sposato B, Camiciottoli G, Bacci E, Scalese M, Carpagnano GE, Pelaia C, Santus P, Maniscalco M, Masieri S, Corsico A, et al. Mepolizumab effectiveness on small airway obstruction, corticosteroid sparing and maintenance therapy step-down in real life. Pulm Pharmacol Ther. 2020; 61:101899. doi:10.1016/j.pupt.2020.101899.
- Taillé C, Chanez P, Devouassoux G, Didier A, Pison C, Garcia G, Charriot J, Bouée S, Gruber A, Pribil C, et al. Mepolizumab in a population with severe eosinophilic asthma and corticosteroid dependence: results from a French early access programme. Eur Respir J. 2020; 55(6):1902345. doi:10.1183/13993003.02345-2019.
- Yilmaz İ, Türk M, Bahçecioğlu S, Tutar N, Gülmez İ. Efficacy of mepolizumab treatment in oral corticosteroid-dependent severe eosinophilic asthma patients with chronic rhinosinusitis with nasal polyps: single center, real life study. Turk J Med Sci. 2020; 44(2):433–441. doi:10.3906/sag-1912-62.
- Juniper EF, Guyatt GH, Willan A, et al. Determining a minimal important change in a disease-specific Quality of Life Questionnaire. J Clin Epidemiol. 1994; 47(1):81–87. doi:10.1016/0895-4356(94)90036-1.
- Schatz M, Kosinski M, Yarlas AS, Hanlon J, Watson ME, Jhingran P. The minimally important difference of the Asthma Control Test. J Allergy Clin Immunol. 2009; 124(4):719–723. e711. doi:10.1016/j.jaci.2009.06.053.
- Chowdhury NI, Mace JC, Bodner TE, Alt JA, Deconde AS, Levy JM, Smith TL. Investigating the minimal clinically important difference for SNOT-22 symptom domains in surgically managed chronic rhinosinusitis. Int Forum Allergy Rhinol. 2017; 7(12):1149–1155. doi:10.1002/alr.22028.
- Roche N, Anzueto A, Bosnic Anticevich S, Kaplan A, Miravitlles M, Ryan D, Soriano JB, Usmani O, Papadopoulos NG, Canonica GW, et al. The importance of real-life research in respiratory medicine: manifesto of the Respiratory Effectiveness Group: Endorsed by the International Primary Care Respiratory Group and the World Allergy Organization. Eur Respir J. 2019; 54(3):1901511. doi:10.1183/13993003.01511-2019.
- Lugogo N, Domingo C, Chanez P, Leigh R, Gilson MJ, Price RG, Yancey SW, Ortega HG. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Ther. 2016; 38(9):2058–2070.e2051. doi:10.1016/j.clinthera.2016.07.010.
- Khatri S, Moore W, Gibson PG, Leigh R, Bourdin A, Maspero J, Barros M, Buhl R, Howarth P, Albers FC, et al. Assessment of the long-term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma. J Allergy Clin Immunol. 2019; 143(5):1742–1751. e1747. doi:10.1016/j.jaci.2018.09.033.
- Suehs CM, Menzies-Gow A, Price D, Bleecker ER, Canonica GW, Gurnell M, Bourdin A. Expert Consensus on the Tapering of Oral Corticosteroids for the Treatment of Asthma: A Delphi Study. Am J Respir Crit Care Med. 2021;203(7):871–881. doi:10.1164/rccm.202007-2721OC.
- Nicolaides NC, Pavlaki AN, Alexandra M, Chrousos GP. Glucocorticoid therapy and adrenal suppression. In Endotext. South Dartmouth (MA): MDText.com, Inc.; 2018.
- Hartl S, Breyer M-K, Burghuber OC, Ofenheimer A, Schrott A, Urban MH, Agusti A, Studnicka M, Wouters EFM, Breyer-Kohansal R, et al. Blood eosinophil count in the general population: typical values and potential confounders. Eur Respir J. 2020; 55(5):1901874. doi:10.1183/13993003.01874-2019.
- Ortega H, Chupp G, Bardin P, Bourdin A, Garcia G, Hartley B, Yancey S, Humbert M. The role of mepolizumab in atopic and nonatopic severe asthma with persistent eosinophilia. Eur Respir J. 2014; 44(1):239–241. doi:10.1183/09031936.00220413.
- Lacombe D, O’Morain C, Casadei B, Hill K, Mateus E, Lories R, Brusselle G. Moving forward from drug-centred to patient-centred research: a white paper initiated by EORTC and developed together with the BioMed Alliance members. Eur Respir J. 2019; 53(2):1801870. doi:10.1183/13993003.01870-2018.
- Crimi C, Campisi R, Nolasco S, Cacopardo G, Intravaia R, Porto M, Impellizzeri P, Pelaia C, Crimi N. Mepolizumab effectiveness in patients with severe eosinophilic asthma and co-presence of bronchiectasis: A real-world retrospective pilot study. Respir Med. 2021; 185:106491. doi:10.1016/j.rmed.2021.106491.
- Kayser MZ, Drick N, Milger K, Fuge J, Kneidinger N, Korn S, Buhl R, Behr J, Welte T, Suhling H, et al. Real-World Multicenter Experience with Mepolizumab and Benralizumab in the Treatment of Uncontrolled Severe Eosinophilic Asthma Over 12 Months. J Asthma Allergy. 2021; 14:863–871. doi:10.2147/JAA.S319572.
- Li H, Zhang Q, Wang J, Gao S, Li C, Wang J, Zhang S, Lin J. Real-world Effectiveness of Mepolizumab in Severe Eosinophilic Asthma: A Systematic Review and Meta-analysis. Clin Ther. 2021; 43(6):e192–e208. doi:10.1016/j.clinthera.2021.03.023.