Abstract
Objectives
Mepolizumab treatment provides clinical benefits for patients with severe eosinophilic asthma in randomized controlled trials. However, real-world data for patients in Finland are lacking.
Methods
This retrospective, non-interventional, chart review study included patients with severe eosinophilic asthma ≥18 years of age initiating mepolizumab between January 1, 2016 and January 31, 2019 at three investigational sites in Finland. Patient characteristics during the 12 months prior to mepolizumab initiation (baseline) were recorded and primary and secondary endpoints included changes from baseline in disease outcomes during follow-up (up to 24 months following mepolizumab initiation). Exploratory endpoints included association between patient characteristics and exacerbation frequency/annual cumulative oral corticosteroid (OCS) dose.
Results
Overall, 51 patients were included (mean 17.8 months follow-up). At baseline, patients had a mean (standard deviation) blood eosinophil count of 550 (410) cells/µL; impaired lung function and health-related quality of life; poor symptom control; frequent exacerbations (2.78/year); and 90% were using OCS (mean: 9.80 mg/day). At the last follow-up visit, reductions from baseline in blood eosinophil count (84%) and fractional exhaled nitric oxide (26%) were observed, as were improvements in Asthma Quality of Life Questionnaire score (36%) and Asthma Control Test score (34%). Reductions in the mean number of annual exacerbations (82%) and mean daily OCS dose (39%) were also seen; reductions were observed even after adjustment for several patient baseline characteristics.
Conclusions
Results are consistent with previous randomized clinical trials, indicating that Finnish patients experience clinically relevant improvements when treated with mepolizumab in real-world clinical practice.
Introduction
Patients with severe eosinophilic asthma, characterized by elevated eosinophil counts in the blood and airways (Citation1), typically have poorly controlled disease and often have associated comorbidities such as chronic rhinosinusitis with nasal polyps (Citation2–4). Consequently, these patients experience recurrent exacerbations and lung function decline, and have a reduced health-related quality of life (HRQoL), despite using one or more maintenance medications (Citation1,Citation3,Citation5–7). Standard of care therapies for severe asthma include high-dose inhaled corticosteroids (ICS) with long-acting bronchodilators and other add-on therapies (Citation3). Short courses of oral corticosteroids (OCS) and/or maintenance OCS may also be used in patients with frequent exacerbations and poor asthma control (Citation3). However, chronic use of corticosteroids, particularly OCS, is associated with a variety of side effects limiting their therapeutic usefulness (Citation8,Citation9). Moreover, despite these treatments, approximately 30% of patients are estimated to have uncontrolled asthma (Citation10,Citation11). The development of targeted biological anti-interleukin (IL)-5 therapies has started to change the landscape of severe asthma treatment and these therapies are now available and routinely recommended for patients with severe eosinophilic asthma (Citation3).
The humanized monoclonal antibody mepolizumab binds to and inactivates IL-5, which is essential for the proliferation, activation and survival of eosinophils (Citation12,Citation13). Mepolizumab is approved for the treatment of severe eosinophilic asthma and eosinophilic granulomatosis with polyangiitis in multiple regions worldwide and for hypereosinophilic syndrome in the US (Citation14–16). Randomized controlled trials (RCTs) in patients with severe eosinophilic asthma have demonstrated that mepolizumab treatment reduces blood and sputum eosinophil counts, exacerbation rates, asthma symptoms and OCS dependence, and improves lung function and HRQoL compared with placebo, with a similar safety profile (Citation1,Citation17–19). Moreover, mepolizumab treatment benefits have been demonstrated to be durable over 3 years of treatment, with a favorable long-term safety profile shown over this period compared with placebo (Citation20–22).
Although RCTs can evaluate the efficacy and safety of a therapy with high internal validity, data from these trials do not necessarily reflect real-world patient populations (Citation23–25). Patients receiving new therapies in clinical practice can have more severe disease than those included in RCTs; they may also have comorbidities that exclude them from RCT eligibility, which could affect treatment options and outcomes (Citation26,Citation27). Therefore, it is important to validate and complement the results of RCTs with real-world data, which can provide valuable information for clinical decision making (Citation23). Although real-world data on mepolizumab are available from several countries (Citation28–34), data are currently limited in Finland (Citation35), where approximately 6% of patients with adult-onset asthma fulfill the European Respiratory Society/American Thoracic Society criteria (Citation1) for severe uncontrolled asthma (Citation11).
The objective of this study was to determine treatment outcomes following mepolizumab therapy in real-world clinical practice in Finland, to determine if results are consistent with those reported in clinical trials.
Methods
Study design and patients
This was a multicenter, retrospective, non-interventional, chart review study (GSK study HO-19–20034) of real-world data from patients initiating mepolizumab. The study utilized electronic health records from three investigational sites in Finland (University Hospitals of Turku, Tampere and Helsinki). Mepolizumab initiation was defined as the date of first mepolizumab injection. The baseline period was defined as the 12 months preceding mepolizumab initiation; the follow-up period was defined as the period from mepolizumab initiation until the last visit during mepolizumab therapy (up to 24 months following mepolizumab initiation) or until mepolizumab discontinuation, whichever came first. Included data were from patients ≥18 years of age with a diagnosis of asthma (International Classification of Diseases-10th edition [ICD-10] code J45.xx) who initiated mepolizumab treatment between January 1, 2016 and January 31, 2019 and received at least one dose. At least 6 months of mepolizumab use was required for the specific analysis. Data from patients enrolled in a clinical trial were excluded. Data were collected from medical records using pre-established standardized case report forms, completed by healthcare professionals in participating clinics or members of the research group.
This study was conducted in accordance with the Declaration of Helsinki, Good Pharmacoepidemiology Practices, the Data Protection Directive, and all additional local requirements. Each participating University Hospital granted permission to use medical chart data. Ethics committee review was not required, in accordance with the Finnish Medicines Agency regulations for retrospective non-interventional registry studies.
Endpoints and assessments
The demographics and clinical characteristics of patients recorded during the baseline period included age, sex, body mass index (BMI), comorbidities, smoking status, prior biologic use, blood eosinophil count, fractional exhaled nitric oxide (FeNO), forced expiratory volume in 1 second (FEV1), Asthma Quality of Life Questionnaire (AQLQ) score, Asthma Control Test (ACT) score, exacerbation history and controller medication use (OCS, ICS, long-acting β2-agonists [LABAs]; long-acting muscarinic receptor antagonists [LAMAs]; leukotriene receptor antagonists). The duration of mepolizumab treatment and treatment persistence (persistent treatment was defined as a <4-month gap between mepolizumab administrations) during the follow-up period were also assessed.
Primary endpoints included changes from baseline in blood eosinophil count, FeNO, FEV1 z-score, AQLQ score and ACT score during the follow-up period. The AQLQ is a tool used to measure asthma-related quality of life, with scores ranging from 1 to 7 and lower scores indicating poorer quality of life; the minimal clinically important difference (MCID) is 0.5 points between repeated measurements (Citation36). For ACT, scores ≥20 indicate well-controlled asthma and the MCID is 3 points (Citation37).
In patients who received mepolizumab for ≥6 months, the following secondary endpoints were assessed during baseline versus follow-up: change in exacerbation frequency, mean daily prednisone-equivalent OCS dose, annual cumulative OCS dose, the proportion of patients who were OCS-dependent (defined as ≥5 mg/day prednisone-equivalent for ≥6 out of 12 months in a year, or ≥900 mg/year), mean daily fluticasone propionate-equivalent ICS dose and annual cumulative fluticasone propionate-equivalent ICS dose. Asthma exacerbations were defined as events requiring an emergency department (ED) visit, inpatient admission, or sick leave with a primary diagnosis of asthma (ICD-10: J45.xx, J46.xx), influenza (J09.xx), pneumonia (J09.xx) or acute bronchitis (J20.xx), or a ≥3-day burst of OCS or an increase in current maintenance OCS dose.
Exploratory endpoints included the association between patient characteristics and exacerbation frequency and annual cumulative OCS dose in patients who received mepolizumab for ≥6 months. In addition, controller medication use during the follow-up period, reasons for mepolizumab discontinuation, and subsequent treatment received following mepolizumab discontinuation were examined.
Sample size and statistical analysis
The sampled population was estimated to include approximately half of all mepolizumab users in Finland. Endpoints were analyzed using paired t-tests unless otherwise specified. The association between patient characteristics and exacerbations, and annual cumulative OCS dose was performed using a Poisson regression model with covariates of gender (male vs female), BMI (kg/m2, ≥30 vs <30), age at diagnosis (years, >40 vs ≤40), rhinosinusitis and/or nasal polyps (yes vs no), smoking status (yes vs no) and time period (after mepolizumab initiation vs prior). Treatment persistence was calculated using the Kaplan–Meier method. Statistical analyses were performed using R version 3.4.0.
Results
Patient characteristics at baseline
Data from 51 patients were included. The mean (standard deviation [SD]) age at first mepolizumab injection was 54.8 (12.24) years, 59% of patients were female and 45% had a BMI ≥30 (). The most common comorbidities at baseline were rhinosinusitis (73%), nasal polyps (49%), hypertension (37%) and sleep apnea (22%). Most (63%) patients had never smoked, with 4% (n = 2) being current smokers.
Table 1. Patient demographics and clinical characteristics.
As expected, baseline blood eosinophil counts and FeNO levels were high (mean [SD] values: 550 [410] cells/µL and 58.49 [35.06] parts per billion [ppb], respectively; and )); lung function was impaired (mean [SD] values: FEV1 z-score -2.60 [1.31]; )), and HRQoL and asthma control were poor (mean [SD] values: AQLQ score 3.85 [1.00] and ACT score 14.2 [5.45], respectively; and )). Patients experienced a mean (range) of 2.78 (0–10) exacerbations during the 12-month baseline period, with 78% of patients having at least one exacerbation (). All patients were using ICS to control their asthma, and the majority were also using OCS (90%) and LABAs (90%) ().
Figure 1. (A) Blood eosinophil counts, (B) FeNO, (C) FEV1, (D) AQLQ score, and (E) ACT score during the baseline and follow-up periods.
All patients with available data were included; all follow-up values represent the last recorded value during the follow-up period. Error bars represent SD. Z-score represents the SDs of the measured FEV1 value from the reference value. p-values were calculated using paired t-tests, including only patients with values available at both baseline and follow-up (n = 47 for blood eosinophil counts; n = 28 for FeNO; n = 46 for FEV1; n = 7 for AQLQ score; n = 40 for ACT score).
ACT, asthma control test; AQLQ, asthma quality of life questionnaire; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 second; OCS, oral corticosteroids; SD, standard deviation.
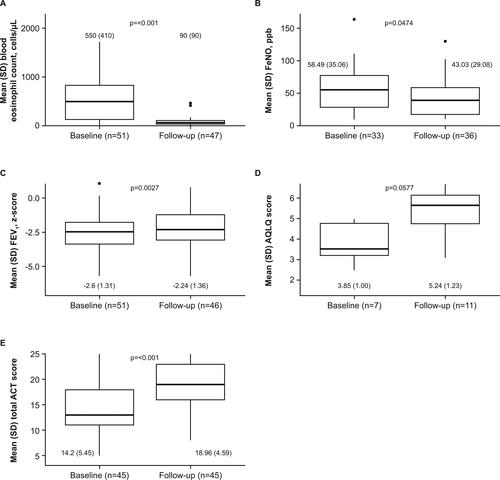
Figure 2. (A) Exacerbation frequency and (B) cumulative OCS dose during the study follow-up versus baseline periods, adjusting for baseline patient characteristics.
These analyses included only patients with ≥6 months follow-up data. Arrows denote whether patients reported more/fewer exacerbations and higher/lower annual cumulative OCS doses during the follow-up period compared with the baseline period, when adjusting for each of the baseline patient characteristics listed.
BMI, body mass index; CI, confidence interval; IRR, incidence rate ratio; NP, nasal polyps; OCS, oral corticosteroids.
Table 2. Asthma exacerbations and OCS/ICS dose during the baseline and follow-up periods.
Table 3. Maintenance medication use during the baseline and follow-up periods.
Patient outcomes at follow-up
Patients were followed for a mean of 17.8 months (range: 3.0–24.0) after mepolizumab initiation. At 6 months (range: ≥4–<9 months) and 12 months (≥9–<15 months) of follow-up, data were available for 49 (96%) and 38 (86%) patients, respectively.
From baseline to the last follow-up visit, patients demonstrated reductions in mean (SD) blood eosinophil counts of 84% (550 [410] to 90 [90] cells/µL; p < 0.001) and reductions in mean (SD) FeNO of 26% (58.49 [35.06] to 43.03 [29.08] ppb; p = 0.047; and )). A small (14%) improvement in mean (SD) FEV1 z-score was also observed (−2.60 [1.31] to −2.24 [1.36] units; p = 0.003; ). Patients also demonstrated improvements in HRQoL and asthma control with mepolizumab; mean (SD) AQLQ scores increased by 36% (3.85 [1.00] vs 5.24 [1.23] units; p = 0.06; ). The MCID of 0.5 was reached by 57% (4/7) patients, though only seven patients had AQLQ scores available at baseline and during the follow-up period. Mean (SD) ACT scores increased by 34% (14.20 [5.45] vs 18.96 [4.59] units; p < 0.001; ) from baseline compared with the last follow-up visit. At baseline and during follow-up, 22% (10/45) and 64% (29/45) of patients had ACT scores ≥19, respectively. The MCID of 3 points was reached by 70% (28/40) of those who had ACT scores available at baseline and during the follow-up period.
Patients who received ≥6 months of mepolizumab treatment demonstrated significant reductions in exacerbations and maintenance medication use (). The mean number of exacerbations per patient-year decreased by 82%, from 2.78/year during the baseline period to 0.51/year during the first year of follow-up (p < 0.001; ). In addition, the proportion of patients with ≥1 exacerbation was reduced from 78% to 37% during the baseline versus the follow-up period (p < 0.001; ). The proportion of patients using OCS decreased from 90% at baseline to 82% and 67% at 12 and 24 months of mepolizumab treatment, respectively (). Although the proportion of OCS-dependent patients only decreased from 88% to 76% between baseline and the first year of follow-up, there was a 39% reduction in both the mean (SD) daily OCS dose (9.80 [5.66] mg/day vs 5.97 [4.49] mg/day; p < 0.001; ) and the mean (SD) annual cumulative OCS dose (3579.5 [2066.1] mg vs 2179.2 [1638.4] mg; p < 0.001). Half of the patients reduced their annual cumulative OCS dose by at least 1300 mg (). During the baseline and follow-up periods mean (SD) daily ICS fluticasone propionate-equivalent doses were 0.90 (0.29) mg/day and 0.85 (0.34) mg/day, respectively (); annual cumulative doses were 329.1 (107.4) mg and 310.2 (125.6) mg, respectively. A majority of the patients (>50%) did not reduce their annual cumulative ICS dose, and 25% of patients reduced it by ≥55 mg.
Association between patient characteristics and exacerbation frequency and OCS dose
The frequency of exacerbations was lower during mepolizumab treatment compared with the baseline period (incidence rate ratio [IRR]: 0.19; 97.5% confidence interval [CI]: 0.12, 0.29), as was the annual cumulative OCS dose (IRR [97.5% CI]: 0.62 [0.61, 0.62]; and ). Higher BMI was associated with more frequent on-treatment exacerbations, while being a current or former smoker was associated with fewer exacerbations (). Male sex, higher BMI, comorbid rhinosinusitis and/or nasal polyps, and being a current or former smoker were associated with higher on-treatment annual cumulative OCS dose ().
Treatment discontinuation
Overall, mepolizumab was well tolerated; a total of nine (18%) patients ended follow-up due to treatment discontinuation, of whom three (6%) had a suspected adverse event, two (4%) did not respond sufficiently to treatment, and four (8%) discontinued due to other reasons. Of the patients who discontinued mepolizumab, one started replacement therapy with reslizumab.
Discussion
Data from RCTs have shown clinically relevant treatment benefits with mepolizumab in patients with severe eosinophilic asthma (Citation17–19,Citation38); real-world studies are needed to confirm that these findings are transferrable to the broader patient population, outside of a clinical trial setting (Citation23). The objective of this study was to determine treatment outcomes following mepolizumab therapy in real-world clinical practice in Finland, and to look at consistency of the data with those reported in clinical trials to date.
The patient population included in this Finnish study displayed a broader range of demographic and clinical characteristics than those included in the mepolizumab RCTs, highlighting the unmet need for treatments to reduce asthma burden in a real-world setting. For example, a total of 25 patients included in this study would have been excluded from the Phase III MENSA RCT of mepolizumab in severe eosinophilic asthma (Citation18), on the basis of their baseline comorbidities. At baseline, the majority of patients had severe uncontrolled disease, as evidenced by three-quarters of patients experiencing at least one asthma exacerbation per year despite 90% of patients using OCS. Patients typically had multiple comorbidities, two of the most common being rhinosinusitis and nasal polyps. This is consistent with previous studies that have demonstrated an association between severe eosinophilic asthma and chronic rhinosinusitis with nasal polyps (CRSwNP) (Citation2,Citation39), where patients frequently demonstrate evidence of eosinophilic inflammation (Citation4,Citation40). Indeed, therapies targeting the cytokines that drive eosinophilic inflammation have been approved or are being investigated for use in patients with several different eosinophilic diseases. For example, among the eosinophil-targeted biologics currently approved for the treatment of severe asthma or severe eosinophilic asthma (Citation14,Citation15,Citation41–44), omalizumab (anti-immunoglobulin E) and dupilumab (anti-IL-4/IL-13) have also been approved for the treatment of CRSwNP (Citation41,Citation42). Benralizumab (anti-IL-5 alpha receptor) and mepolizumab are both currently under investigation for use in patients with CRSwNP (Citation45,Citation46), eosinophilic chronic obstructive pulmonary disease (Citation47,Citation48) and eosinophilic esophagitis (Citation49,Citation50). In addition, mepolizumab is being explored in patients with eosinophilic pneumonia (Citation51) and eosinophilic otitis media (Citation52). Almost half of the patients in this study were obese. Additionally, as is characteristic of severe eosinophilic asthma with a history of frequent exacerbations, patients included in this study had impaired lung function and HRQoL at baseline (Citation6,Citation7). In contrast with mepolizumab RCTs, the current study did not exclude patients based on criteria such as the presence of bronchiectasis and patients were included regardless of smoking history (Citation1,Citation17–19).
In the real-world setting, Finnish patients with severe eosinophilic asthma experienced reductions in blood eosinophil counts and improvements in several clinically important outcomes following mepolizumab treatment, consistent with the demonstrated clinical benefit of mepolizumab in previous RCTs and open-label studies (Citation1,Citation17–22). Patients receiving mepolizumab for ≥6 months demonstrated an 82% reduction in annual exacerbation rate compared with the year prior to treatment initiation. This was coupled with an approximate halving in the proportion of patients with ≥1 annual exacerbation and an approximately 40% decrease in daily OCS use, with similar reductions in the annual cumulative OCS dose and proportion of patients who were OCS dependent. This is consistent with the results of the SIRIUS RCT, which demonstrated an OCS-sparing effect of mepolizumab in patients with at least a 6-month history of systemic corticosteroid therapy at a dose of ≥5 mg/day (Citation1). Results are also in accordance with current treatment goals for asthma management, which include improving symptom control, reducing the risk of future exacerbations and minimizing OCS use (due to the side effects associated with chronic use) (Citation3,Citation8,Citation9). Similarly, blood eosinophil counts were reduced from baseline by 84% at the last follow-up visit and mepolizumab treatment was associated with improvements in HRQoL and asthma control. Although not statistically significant, small improvements in lung function were also seen.
In this study, obesity was associated with higher numbers of on-treatment exacerbations, in line with previously reported data showing that obesity in patients with asthma is associated with poor asthma control, more frequent exacerbations and higher OCS use (Citation39,Citation53). In addition, lower BMI is a predictor of patients more likely to respond to mepolizumab treatment (Citation30). Being a smoker was associated with fewer exacerbations than not smoking in the current study; however, it should be noted that patients who were smokers had higher total OCS use than non-smokers, which may account for this difference, or indeed this finding may be a result of the small sample size in this analysis. Likely owing to the small sample size and the low number of exacerbations during the follow-up period, no significant associations between other baseline patient characteristics and on-treatment exacerbations were observed. Importantly, significant reductions in exacerbations and cumulative OCS use were demonstrated in the overall study population during the follow-up versus baseline period, despite the presence of a broad number of factors that may influence disease course. Overall, these efficacy results suggest that mepolizumab is likely to provide clinical benefit to Finnish patients with severe eosinophilic asthma and a broader range of demographic and clinical characteristics than those included in RCTs.
Our results are consistent with other real-world studies, which have included patients with a variety of clinical characteristics indicative of similar or more severe disease compared with the current study (Citation28–33). For example, a real-world study conducted by Taillé et al. included patients with ACT symptom scores indicative of more severe disease than in the current study (10 vs 14) as well as higher blood eosinophil counts (721 vs 550 cells/µL) and a history of more exacerbations (5.8 vs 2.8/year) (Citation33). By contrast, Kavanagh et al. included patients with a mean blood eosinophil count of 200 cells/µL and a baseline exacerbation rate of 4.0/year (Citation30). Despite between-study differences such as these, other real-world studies have demonstrated blood eosinophil count reductions from baseline of 50–90% (vs an 84% reduction in the current study), annual exacerbation rate reductions of 54–87% (vs an 82% reduction in the current study) and daily OCS dose reductions of 32–100% (vs a 39% reduction in the current study) (Citation28–33). Improvements in blood eosinophil count, exacerbation rate and OCS dose were also generally accompanied by improvements in lung function, HRQoL and symptom scores (Citation28,Citation30–33), though it should be noted that one study did not find any improvement in FEV1, likely due to this endpoint being assessed in only nine out of the 25 patients enrolled in the study (Citation31). In addition, the results of a retrospective database study suggest that these treatment benefits may translate into reduced asthma-related costs, showing a 54% decrease in asthma exacerbation-related costs with a 38% decrease in annual exacerbation rate (Citation54).
With regards to mepolizumab treatment persistence, 82% of patients remained on treatment during the study period; this compares favorably with the 69% and 87% of patients with treatment persistence in real-world studies conducted in France (Citation33) and Spain (Citation31), in addition to the 81% reported in a real-world global study (Citation29,Citation31).
The current study has several limitations, which should be considered when interpreting results. Firstly, data were collected from patient medical records, which meant that the availability of some data including AQLQ scores was limited. However, this limitation also meant that all data were manually collected and recorded as written in patient charts, which may be more reliable than the automated collection utilized by many other real-world studies. Secondly, the frequency of comorbidities was based on diagnosis codes recorded in patient files; although respiratory comorbidities related to asthma (e.g. rhinosinusitis and sleep apnea) are typically coded for all patients, codes may be missing for other comorbidities such as gastroesophageal reflux disease in a small number of cases, leading to an underestimation of their prevalence. Thirdly, the total number of patients included in this study was relatively small, limiting the ability to make statistical comparisons between groups for some of the analyses performed. Moreover, the study was initiated at a time when few patients had been treated with mepolizumab for more than 12 months, further limiting the size of the sample with long-term data available. Finally, data up to 24 months were not available for all patients due to some of the included patients having initiated mepolizumab treatment close to the study end date. Nevertheless, our findings support those of previous RCTs and real-world studies of mepolizumab in patients with severe eosinophilic asthma.
Conclusions
Despite the availability of advanced inhaled combination asthma therapies, there remains a subpopulation of patients with severe asthma with poor asthma control, impaired lung function and frequent exacerbations, who need either recurrent courses of (or maintenance) OCS treatment. The results of this real-world study are consistent with previous clinical trials, showing that mepolizumab treatment is associated with improvements in several clinically important outcomes including asthma symptom score, exacerbation frequency and OCS dose in a cohort of Finnish patients with severe eosinophilic asthma. Overall, these data support the efficacy and safety data from RCTs, which have been consistently demonstrated across several real-world studies in different geographic locations.
Authors contributions
VK, PK, II-M and MK were involved in conception and design, acquisition of data, and data analysis and interpretation. JI-H, LV, TY-O and JM were involved in conception and design, and data analysis and interpretation. AV was involved in the acquisition of data, and data analysis and interpretation. All authors provided final approval of the version submitted for publication and agreed to be accountable for the accuracy and integrity of the work.
Disclosures
VK and MK have no conflicts to declare; PK declares consultancy and lecturing fees for GSK, Novartis and Sanofi; JI-H is an employee of GSK and owns GSK stocks/shares; LV is a former employee of GSK; II-M is a former employee of MedEngine Oy; JM is an employee of MedEngine Oy; TY-O is the owner of MedEngine Oy; AV declares consultancy for GSK and Novartis, lecturing fees from ALK, AstraZeneca, Boehringer-Ingelheim, Chiesi, GSK and Novartis and meeting participation fees from Boehringer-Ingelheim and Sanofi.
Acknowledgments
Analyses were performed by MedEngine Oy, funded by GSK; employees of MedEngine Oy did not receive payment for manuscript development. Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing and referencing) was provided by Bianca Paris PhD, of Fishawack Indicia Ltd, part of Fishawack Health, and was funded by GSK.
Data availability statement
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies which evaluate medicines, upon approval of proposals submitted to www.clinicalstudydatarequest.com. To access data for other types of GSK sponsored research, for study documents without patient-level data and for clinical studies not listed, please submit an enquiry via the website. The sharing of a de-identified dataset of this study is restricted by Finnish law (Data Protection Act [1050/2018]).
Additional information
Funding
References
- Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi:10.1183/09031936.00202013.
- Chan R, RuiWen Kuo C, Lipworth B. Pragmatic clinical perspective on biologics for severe refractory type 2 asthma. J Allergy Clin Immunol Pract. 2020;8(10):3363–3370. doi:10.1016/j.jaip.2020.06.048.
- Global Initiative for Asthma. Global strategy for asthma management and prevention 2020. Available from: https://ginasthma.org/wp-content/uploads/2020/04/GINA-2020-full-report_-final-_wms.pdf [last accessed Jan 2021].
- Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, Toppila-Salmi S, Bernal-Sprekelsen M, Mullol J, Alobid I, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(Suppl S29):1–464. doi:10.4193/Rhin20.600.
- Wenzel S. Severe asthma: from characteristics to phenotypes to endotypes. Clin Exp Allergy. 2012;42(5):650–658. doi:10.1111/j.1365-2222.2011.03929.x.
- Ortega H, Yancey SW, Keene ON, Gunsoy NB, Albers FC, Howarth PH. Asthma exacerbations associated with lung function decline in patients with severe eosinophilic asthma. J Allergy Clin Immunol Pract. 2018;6(3):980–986. e981. doi:10.1016/j.jaip.2017.12.019.
- Luskin AT, Chipps BE, Rasouliyan L, Miller DP, Haselkorn T, Dorenbaum A. Impact of asthma exacerbations and asthma triggers on asthma-related quality of life in patients with severe or difficult-to-treat asthma. J Allergy Clin Immunol Pract. 2014;2(5):544–552. doi:10.1016/j.jaip.2014.02.011.
- Volmer T, Effenberger T, Trautner C, Buhl R. Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: a focused review of the impact data in the literature. Eur Respir J. 2018;52(4):1800703. doi:10.1183/13993003.00703-2018.
- Dahl R. Systemic side effects of inhaled corticosteroids in patients with asthma. Respir Med. 2006;100(8):1307–1317. doi:10.1016/j.rmed.2005.11.020.
- Tomisa G, Horváth A, Szalai Z, Müller V, Tamási L. Prevalence and impact of risk factors for poor asthma outcomes in a large, specialist-managed patient cohort: a real-life study. J Asthma Allergy. 2019;12:297–307. doi:10.2147/jaa.s211246.
- Ilmarinen P, Tuomisto LE, Niemelä O, Kankaanranta H. Prevalence of patients eligible for anti-IL-5 Treatment in a cohort of adult-onset asthma. J Allergy Clin Immunol Pract. 2019;7(1):165–174. e164. doi:10.1016/j.jaip.2018.05.032.
- Menzella F, Lusuardi M, Galeone C, Taddei S, Zucchi L. Profile of anti-IL-5 mAb mepolizumab in the treatment of severe refractory asthma and hypereosinophilic diseases. J Asthma Allergy. 2015;8:105–114. doi:10.2147/JAA.S40244.
- Busse WW. Biological treatments for severe asthma: a major advance in asthma care. Allergol Int. 2019;68(2):158–166. doi:10.1016/j.alit.2019.01.004.
- GlaxoSmithKline. Mepolizumab prescribing information (US). 2020. Available from: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Nucala/pdf/NUCALA-PI-PIL.PDF [last accessed Jan 2021].
- European Medicines Agency. 2021. Nucala EU prescribing information. Available from: https://www.ema.europa.eu/en/documents/product-information/nucala-epar-product-information_en.pdf [last accessed Jan 2021].
- GlaxoSmithKline. Nucala: basic product information (Japan) 2020. Available from: https://gskpro.com/ja-jp/products-info/nucala/index/ [last accessed Jan 2021].
- Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, Ortega H, Chanez P. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. doi:10.1016/S0140-6736(12)60988-X.
- Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, Humbert M, Katz LE, Keene ON, Yancey SW, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207. doi:10.1056/NEJMoa1403290.
- Chupp GL, Bradford ES, Albers FC, Bratton DJ, Wang-Jairaj J, Nelsen LM, Trevor JL, Magnan A, ten Brinke A. Ten Brinke A. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5(5):390–400. doi:10.1016/S2213-2600(17)30125-X.
- Lugogo N, Domingo C, Chanez P, Leigh R, Gilson MJ, Price RG, Yancey SW, Ortega HG. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Ther. 2016;38(9):2058–2070. e2051. doi:10.1016/j.clinthera.2016.07.010.
- Khatri S, Moore W, Gibson PG, Leigh R, Bourdin A, Maspero J, Barros M, Buhl R, Howarth P, Albers FC, et al. Assessment of the long-term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma. J Allergy Clin Immunol. 2019;143(5):1742–1751. doi:10.1016/j.jaci.2018.09.033.
- Khurana S, Brusselle GG, Bel EH, FitzGerald JM, Masoli M, Korn S, Kato M, Albers FC, Bradford ES, Gilson MJ, et al. Long-term safety and clinical benefit of mepolizumab in patients with the most severe eosinophilic asthma: the COSMEX study. Clin Ther. 2019;41(10):2041–2056. doi:10.1016/j.clinthera.2019.07.007.
- Roche N, Anzueto A, Bosnic Anticevich S, Kaplan A, Miravitlles M, Ryan D, Soriano JB, Usmani O, Papadopoulos NG, Canonica GW. The importance of real-life research in respiratory medicine: manifesto of the Respiratory Effectiveness Group: endorsed by the international primary care respiratory group and the world allergy organization. Eur Respir J. 2019;54(3):1901511. doi:10.1183/13993003.01511-2019.
- Pahus L, Alagha K, Sofalvi T, Vachier I, Bourdin A, Molinari N, Chanez P, COBRA Consortium. External validity of randomized controlled trials in severe asthma. Am J Respir Crit Care Med. 2015;192(2):259–261. doi:10.1164/rccm.201502-0391LE.
- Brown T, Jones T, Gove K, Barber C, Elliott S, Chauhan A, Howarth P. Wessex Severe Asthma Cohort team, Members of the Wessex Severe Asthma Cohort team. Randomised controlled trials in severe asthma: selection by phenotype or stereotype. Eur Respir J. 2018;52(6):1801444. doi:10.1183/13993003.01444-2018.
- Battaglia S, Basile M, Spatafora M, Scichilone N. Are asthmatics enrolled in randomized trials representative of real-life outpatients? Respiration. 2015;89(5):383–389. doi:10.1159/000375314.
- Kennedy-Martin T, Curtis S, Faries D, Robinson S, Johnston J. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials. 2015;16:495. doi:10.1186/s13063-015-1023-4.
- Bagnasco D, Caminati M, Menzella F, Milanese M, Rolla G, Lombardi C, Bucca C, Heffler E, Paoletti G, Testino E, et al. One year of mepolizumab. Efficacy and safety in real-life in Italy. Pulm Pharmacol Ther. 2019;58:101836. doi:10.1016/j.pupt.2019.101836.
- Harrison T, Canonica GW, Chupp G, Lee J, Schleich F, Welte T, Valero A, Gemzoe K, Maxwell A, Joksaite S, et al. Real-world mepolizumab in the prospective severe asthma REALITI-A study: initial analysis. Eur Respir J. 2020;56(4):2000151. doi:10.1183/13993003.00151-2020.
- Kavanagh JE, d’Ancona G, Elstad M, Green L, Fernandes M, Thomson L, Roxas C, Dhariwal J, Nanzer AM, Kent BD, et al. Real-world effectiveness and the characteristics of a “super-responder” to mepolizumab in severe eosinophilic asthma. Chest. 2020;158(2):491–500. doi:10.1016/j.chest.2020.03.042.
- Montero-Pérez O, Contreras-Rey MB, Sánchez-Gómez E. Effectiveness and safety of mepolizumab in severe refractory eosinophilic asthma: results in clinical practice. Drugs Context. 2019;8:212584 doi:10.7573/dic.212584.
- Pertzov B, Unterman A, Shtraichman O, Shitenberg D, Rosengarten D, Kramer MR. Efficacy and safety of mepolizumab in a real-world cohort of patients with severe eosinophilic asthma. J Asthma. 2021;58(1):79–84. doi:10.1080/02770903.2019.1658208.
- Taillé C, Chanez P, Devouassoux G, Didier A, Pison C, Garcia G, Charriot J, Bouée S, Gruber A, Pribil C, et al. Mepolizumab in a population with severe eosinophilic asthma and corticosteroid dependence: results from a French early access programme. Eur Respir J. 2020;55(6):1902345. doi:10.1183/13993003.02345-2019.
- Strauss RA, Jawhari N. Mepolizumab in the treatment of severe eosinophilic asthma: results from a physician in the field. Ann Allergy Asthma Immunol. 2018;121(1):121–123. doi:10.1016/j.anai.2018.04.016.
- Kotisalmi E, Hakulinen A, Mäkelä M, Toppila-Salmi S, Kauppi P. A comparison of biologicals in the treatment of adults with severe asthma – real-life experiences. Asthma Res Pract. 2020;6(2). doi:10.1186/s40733-020-00055-9.
- Juniper EF, Guyatt GH, Willan A, Griffith L. Determining a minimal important change in a disease-specific quality of life questionnaire. J Clin Epidemiol. 1994;47(1):81–87. doi:10.1016/0895-4356(94)90036-1.
- Schatz M, Kosinski M, Yarlas AS, Hanlon J, Watson ME, Jhingran P. The minimally important difference of the Asthma Control Test. J Allergy Clin Immunol. 2009;124(4):719–723. doi:10.1016/j.jaci.2009.06.053.
- Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, Ortega HG, Pavord ID, SIRIUS Investigators. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–1197. doi:10.1056/NEJMoa1403291.
- Tay TR, Hew M. Comorbid “treatable traits” in difficult asthma: current evidence and clinical evaluation. Allergy. 2018;73(7):1369–1382. doi:10.1111/all.13370.
- Enache I, Ioniță E, Mitroi M, Anghelina F, MogoantĂ C, Ciolofan S, CĂpitĂnescu A, Stepan A, Simionescu C. Histopathological features of chronic rhinosinusitis with nasal allergic polyps. Curr Health Sci J. 2020;46(1):66–71. doi:10.12865/chsj.46.01.09.
- Regeneron. Dupilumab prescribing information (US) 2021. Available from: https://www.regeneron.com/sites/default/files/Dupixent_FPI.pdf. [last accessed Jan 2021].
- Genentech. Omalizumab prescribing information (US) 2019. Available from: https://www.gene.com/download/pdf/xolair_prescribing.pdf. [last accessed Jan 2021].
- AstraZeneca. Benralizumab prescribing information (US) 2021. Available from: https://www.azpicentral.com/fasenra/fasenra.pdf#page=1. [last accessed Jan 2021].
- Teva Pharmaceuticals. Reslizumab prescribing information (US) 2019. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/0761033s010lbl.pdf. [last accessed Jan 2021].
- Tversky J, Lane AP, Azar A. Benralizumab effect on severe chronic rhinosinusitis with nasal polyps (CRSwNP): a randomized double–blind placebo–controlled trial. Clin Exp Allergy. 2021;51(6):836–844. doi:10.1111/cea.13852.
- Han JK, Bachert C, Fokkens W, Desrosiers M, Wagenmann M, Lee SE, Smith SG, Martin N, Mayer B, Yancey SW. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021.
- Pavord ID, Chanez P, Criner GJ, Kerstjens HAM, Korn S, Lugogo N, Martinot J-B, Sagara H, Albers FC, Bradford ES, et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med. 2017;377(17):1613–1629. doi:10.1056/NEJMoa1708208.
- Criner GJ, Celli BR, Singh D, Agusti A, Papi A, Jison M, Makulova N, Shih VH, Brooks L, Barker P, et al. Predicting response to benralizumab in chronic obstructive pulmonary disease: analyses of GALATHEA and TERRANOVA studies. Lancet Respir Med. 2020;8(2):158–170. doi:10.1016/S2213-2600(19)30338-8.
- Straumann A, Conus S, Grzonka P, Kita H, Kephart G, Bussmann C, Beglinger C, Smith DA, Patel J, Byrne M, et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut. 2010;59(1):21–30. doi:10.1136/gut.2009.178558.
- U.S. National Library of Medicine. A study of benralizumab in patients with eosinophilic esophagitis (MESSINA) 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT04543409. [last accessed Apr 2021].
- Brenard E, Pilette C, Dahlqvist C, Colinet B, Schleich F, Roufosse F, Froidure A. Real-life study of mepolizumab in idiopathic chronic eosinophilic pneumonia. Lung. 2020;198(2):355–360. doi:10.1007/s00408-020-00336-3.
- Iino Y, Takahashi E, Ida S, Kikuchi S. Clinical efficacy of anti-IL-5 monoclonal antibody mepolizumab in the treatment of eosinophilic otitis media. Auris Nasus Larynx. 2019;46(2):196–203. doi:10.1016/j.anl.2018.07.011.
- Juel CT-B, Ali Z, Nilas L, Ulrik CS. Asthma and obesity: does weight loss improve asthma control? a systematic review. J Asthma Allergy. 2012;5:21–26. doi:10.2147/jaa.s32232.
- Llanos JP, Ortega H, Bogart M, Packnett ER, Manjelievskaia J, Bell CF, Hahn B. Real-world effectiveness of mepolizumab in patients with severe asthma: an examination of exacerbations and costs. JAA. 2020;13:77–87. doi:10.2147/JAA.S236609.
