Abstract
Objective: Large international comparisons describing the clinical characteristics of patients with COVID-19 are limited. The aim of the study was to perform a large-scale descriptive characterization of COVID-19 patients with asthma.
Methods: We included nine databases contributing data from January to June 2020 from the US, South Korea (KR), Spain, UK and the Netherlands. We defined two cohorts of COVID-19 patients (‘diagnosed’ and ‘hospitalized’) based on COVID-19 disease codes. We followed patients from COVID-19 index date to 30 days or death. We performed descriptive analysis and reported the frequency of characteristics and outcomes in people with asthma defined by codes and prescriptions.
Results: The diagnosed and hospitalized cohorts contained 666,933 and 159,552 COVID-19 patients respectively. Exacerbation in people with asthma was recorded in 1.6–8.6% of patients at presentation. Asthma prevalence ranged from 6.2% (95% CI 5.7–6.8) to 18.5% (95% CI 18.2–18.8) in the diagnosed cohort and 5.2% (95% CI 4.0–6.8) to 20.5% (95% CI 18.6–22.6) in the hospitalized cohort. Asthma patients with COVID-19 had high prevalence of comorbidity including hypertension, heart disease, diabetes and obesity. Mortality ranged from 2.1% (95% CI 1.8–2.4) to 16.9% (95% CI 13.8–20.5) and similar or lower compared to COVID-19 patients without asthma. Acute respiratory distress syndrome occurred in 15–30% of hospitalized COVID-19 asthma patients.
Conclusion: The prevalence of asthma among COVID-19 patients varies internationally. Asthma patients with COVID-19 have high comorbidity. The prevalence of asthma exacerbation at presentation was low. Whilst mortality was similar among COVID-19 patients with and without asthma, this could be confounded by differences in clinical characteristics. Further research could help identify high-risk asthma patients.
KEY MESSAGES
Asthma prevalence in COVID-19 patients varied internationally (5.2–20.5%).The prevalence of asthma exacerbation at presentation with COVID-19 in diagnosed and hospitalized patients was low.Comorbidities were common in COVID-19 patients with asthma.
Supplemental data for this article is available online at https://doi.org/10.1080/02770903.2021.2025392 .
Keywords:
Background
Coronavirus disease 2019 (COVID-19) causes severe lung injury and pneumonia as well as other complications including acute kidney injury, cardiovascular complications and death. In people with asthma, viral respiratory tract infections exacerbate the underlying disease and are associated with higher rates of morbidity and mortality (Citation1–3). COVID-19 has been compared to seasonal influenza in terms of symptoms and complications, resulting in asthmatic individuals being classed as high risk. National policy measures consequently stipulated the need for asthmatic patients to shield or shelter during the COVID-19 pandemic (Citation4).
Although COVID-19 causes greater morbidity and mortality than influenza among the general population, further information is required describing the characteristics of people with asthma and COVID-19 and their outcomes (Citation5,Citation6). It has been speculated that reduced susceptibility to COVID-19 may exist as a result of type 2 immunity linked to atopy and reduced expression of the angiotensin converting enzyme-2 receptor (ACE2) (Citation7,Citation8). In this regard, ACE2 is required by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to enter human cells. Concerns have also been raised over the safety of inhaled corticosteroids (Citation9).
Patients with severe COVID-19 have comorbidities including cardiovascular disease, diabetes and obesity (Citation10,Citation11). Some studies have found that asthma is a common comorbidity among severe and fatal COVID-19 cases (Citation12,Citation13). However, studies examining the association between asthma and risk of COVID-19 related death have been inconsistent (Citation11,Citation13–15). Existing studies are typically limited to smaller hospitalized COVID-19 populations, with uncertain generalizability across data sources and/or countries. Further detailed information on comorbidities, medication use and outcomes in people with asthma with milder and more severe COVID-19 would therefore be of value. The aim of this study was to describe the demographics, comorbidities, and outcomes of patients with COVID-19 and co-morbid asthma.
Methods
Study design
The Characterizing Health Associated Risks, and Your Baseline Disease In SARS-COV-2 (CHARYBDIS) study is a multinational cohort study using retrospective electronic health records and claims data on COVID-19 patients from the United States (US), Europe, and Asia. All data were standardized to the Observational Medical Outcomes Partnership (OMOP) Common Data Model (CDM) (Citation16,Citation17). This allowed contributing centers to execute analytical code in a distributed/federated fashion without sharing patient-level data. The Charybdis protocol and source code can be found at (https://github.com/ohdsi-studies/Covid19CharacterizationCharybdis) (Citation18).
Data sources
Of the sixteen available databases on 4th August 2020, nine were included that had a minimum pre-specified sample size of 140 COVID-19 patients with asthma. This was considered necessary to estimate the prevalence of a previous condition or 30-day risk of an outcome affecting 10% of the study population. presents the database selection process for this study. Data from the US included: the Columbia University Irving Medical Center data warehouse (CUIMC), Stanford Medicine Research Data Repository (STARR-OMOP) (Citation19), Health Verity, IQVIA Open Claims, and the United States Department of Veterans Affairs (VA-OMOP). Data from South Korea included the Health Insurance Review and Assessment Service (HIRA) database. Data from Europe included the Spanish Information System for Research in Primary Care (SIDIAP) database (Citation20); the Integrated Primary Care Information (IPCI) database from the Netherlands (Citation21), and the UK Clinical Practice Research Datalink (CPRD) (Citation22). Further information about databases considered for inclusion is contained in Appendix 1.
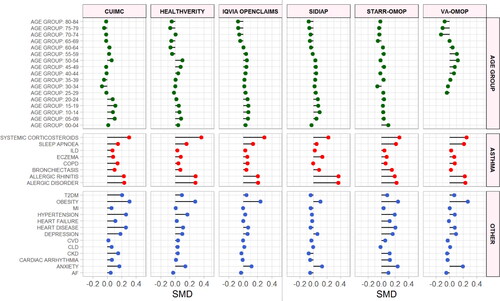
Figure 1. Standardized mean differences of characteristics between diagnosed COVID-19 patients with and without asthma. SMD < 0 = prevalence greater in COVID-19 patients without asthma. SMD > 0 = prevalence greater in COVID-19 patients with asthma. SMD greater or less than 0.1 suggests significant imbalance between groups. Only databases providing sufficient comparative information included. Asthma = Conditions related to asthma. SMD = Standardized mean difference. ILD = Interstitial lung disease. COPD = Chronic obstructive pulmonary disease. CVD = Cerebrovascular disease. CLD = Chronic liver disease. CKD = Chronic kidney disease. AF = Atrial fibrillation. T2DM = Type 2 diabetes mellitus. Asthma = Asthma related comorbidities. Other = Non-asthma related comorbidities. Top row = Name of the database.
Study participants and follow-up
COVID-19 cohorts: Two non-mutually exclusive cohorts were defined. Patients “diagnosed” with COVID-19 were defined by patients having a clinical diagnosis and/or positive test for SARS-CoV-2 from outpatient and inpatient records. In the diagnosed cohort, index date was the earliest date of COVID-19 diagnosis or a first positive test. Patients “hospitalized” with COVID-19 were defined by a hospitalization episode with a clinical diagnosis or positive SARS-CoV-2 test within 21 days prior to admission and up to the end of hospitalization. This time window was chosen to include patients with a diagnosis prior to hospitalization and to allow for delays in recording of test results. In the hospitalized cohort, index date was the day of hospitalization. All patients were required to have at least a year of observation time prior to index date. Patients were followed from the index date to the earliest of death, end of the study (30 days after index date) or end of data capture.
Asthma definition: Asthma was defined as either: a) an occurrence of an asthma diagnosis code along with prescription or administration of asthma medications using any time before the COVID-19 index date or b) by at least two prescriptions or administration of asthma medications at least six months apart within the year prior to index date in patients younger than 55 years old (Appendix 3). We excluded patients with a diagnosis of chronic obstructive pulmonary disease (COPD) or prescription of a long-acting muscarinic antagonist (LAMA) prior to the asthma definition. Patients with a COPD diagnosis occurring after asthma diagnosis were included to measure COPD comorbidity. Codes used to define these cohorts are described in Appendix 2.
Baseline characteristics
Conditions and procedures were identified within day −365 to −1 prior to the index date using Systematized Nomenclature of Medicine (SNOMED CT) codes with all descendent codes included. We report pre-specified demographics and conditions related to asthma and COVID-19. Other conditions analyzed as part of the larger CHARYBDIS project are reported in the accompanying website (https://data.ohdsi.org/Covid19CharacterizationCharybdis/). Asthma exacerbation was defined by an asthma exacerbation code at index date.
The following medication use (recent use) was identified within day −30 to −1 prior to index date to characterize how patients were managed prior to the COVID-19 index date: systemic corticosteroids, inhaled corticosteroids (ICS), short-acting beta2-agonists (SABA), long-acting beta2-agonists (LABA), leukotriene receptor antagonists (LTRA), methylxanthines, decongestants and other nasal preparations for topical use (comprising preparations for local treatment in case of nasal congestion (e.g. sympathomimetics) or for prophylaxis and treatment of allergic rhinitis (e.g. corticosteroids, cromoglicate preparations)), antibiotics (beta-lactam penicillins, macrolides, fluoroquinolones), acetaminophen, NSAIDs and opioids. Medication use was calculated using drug eras that began on the start date of the first drug exposure medication record and ended on the observed end date of the last concatenated medication record. A grace period of 30 days between medication records allowed for sequential medication records to be considered as continuous drug exposure.
Outcomes
We report the following outcomes within 30 days after hospitalization: death, use of intensive services (identified by a recorded mechanical ventilation and/or a tracheostomy and/or extracorporeal membrane oxygenation procedure), acute respiratory distress syndrome (ARDS), acute renal failure syndrome (ARFS), cardiac arrhythmia, heart failure, myocardial infarction, sepsis, bleeding, venous thromboembolism (VTE), pulmonary embolism (PE) and stroke (ischaemic and haemorrhagic). Other conditions analyzed as part of the larger CHARYBDIS project are reported in the accompanying website.
Analysis
A common analytical code for Charybdis was developed for the Observational Health Data Science and Informatics (OHDSI) Methods library and was run locally in each database. Only aggregate results from each database were then shared for characteristics and cohort that it captured. We report the number and proportion by socio-demographics, comorbidities, and commonly used medications in each population with 95% confidence intervals (CI) calculated using the Wilson score method. Crude comparisons in the characteristics of study cohorts were undertaken by calculating standardized mean differences (SMD). SMD are a commonly used statistic to examine the distribution of covariate balance between groups and are not influenced by differences in sample size. A SMD threshold of 0.1 or greater indicates potential imbalance in the prevalence of a binary covariate between groups (Citation23). In the main text we report the median prevalence and range across all databases per cohort, with detailed individual database results presented in the online supplementary. We used R version 3.6.0 for data visualization. All the data partners obtained Institutional Review Board (IRB) approval or equivalent governance approval to conduct this study.
Results
Prevalence of asthma and asthma exacerbation at presentation
The study included 666 933 patients diagnosed with COVID-19 and 159 552 patients hospitalized with COVID-19. The prevalence of asthma ranged from 6.2% (95% CI 5.7–6.8) to 18.5% (95% CI 18.2–18.8) among diagnosed COVID-19 patients and 5.2% (95% CI 4.0–6.8) to 20.5% (95% CI 18.6–22.6) among hospitalized COVID-19 patients (). The prevalence of asthma exacerbation ranged from 1.6% (95% CI 1.2–2.1) to 5.3% (95% CI 4.2–6.6) among asthma patients diagnosed with COVID-19 and 0.8% (95% CI 0.5–1.4) to 8.6% (95% CI 6.1–12.0) among hospitalized COVID-19 patients with asthma (Supplementary Table S1).
Table 1. Prevalence of asthma among the COVID-19 patients in the diagnosed and hospitalized cohorts by country and database.
Demographics
Children aged 0 to 14 years old accounted for less than 5% of COVID-19 patients with asthma. Asthma patients diagnosed and hospitalized with COVID-19 were more commonly female (range 60.8–69.2%, respectively), apart from VA-OMOP ().
Figure 2. Standardized mean differences of characteristics between hospitalized COVID-19 patients with and without asthma. SMD < 0 = prevalence greater in patients without asthma. SMD > 0 = prevalence greater in patients with asthma. SMD greater or less than 0.1 suggests significant imbalance between groups. Only databases providing sufficient comparative information included. Asthma = Conditions related to asthma. SMD = Standardized mean difference. ILD = Interstitial lung disease. COPD = Chronic obstructive pulmonary disease. CVD = Cerebrovascular disease. CLD = Chronic liver disease. CKD = Chronic kidney disease. AF = Atrial fibrillation. T2DM = Type 2 diabetes mellitus. Asthma = asthma related comorbidities. Other = Non-asthma related comorbidities. Top row = Name of the database.
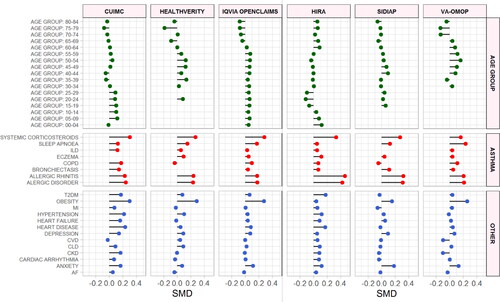
Table 2. Comorbidities across databases in diagnosed and hospitalized COVID-19 patients with and without asthma.
Table 3. Treatments across databases in patients with asthma 30 days before COVID-19 diagnosis or hospitalization.
Table 4. Outcomes across databases in diagnosed and hospitalized COVID-19 patients with and without asthma.
Prevalence of comorbidities
The prevalence of comorbidities is shown in
Compared to patients in the diagnosed cohort, hospitalized asthma patients with COVID-19 had a greater prevalence of hypertension (median 46.9% vs 76%, mSMD 0.28), heart disease (median 38.1% vs 64.9%, mSMD 0.21), obesity (median 48.1% vs 55.9%, mSMD 0.14), T2DM (median 20.6% vs 497%, mSMD 0.25), chronic kidney disease (CKD) (median 16% vs 25.5%, mSMD 0.24) and comorbid COPD (median 5.9% vs 14%, mSMD 0.12) (Supplementary Figure S2, Supplementary Table S9).
Medication use
The median systemic corticosteroid use was 14.6% (range 6.1–17.5%) 30 days prior to COVID-19 diagnosis, and 17.9% (range 9.2–19.9%) 30 days prior to hospitalization (
Mortality
The median 30-day mortality among asthma patients diagnosed with COVID-19 was 6.1% (range 2.1–9.6%) and 6.3% (range 4.5–12.5%) in those without asthma (
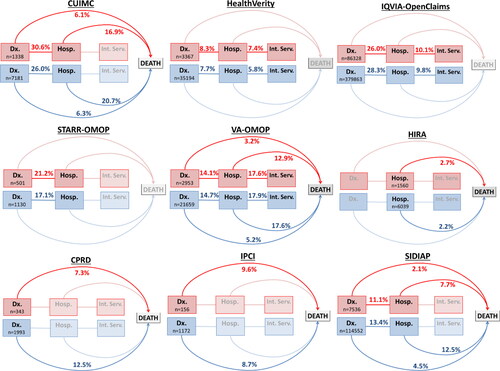
Figure 3. Hospitalization, intensive service use and death among COVID-19 patients with asthma (red) compared to those without asthma (blue) by database. Faded lines and boxes = No data available. Hospitalization = On or within 30 days of COVID-19 diagnosis. Dx = Diagnosis. Hosp = Hospitalization. Int. Serv = Intensive services. Top row = Name of the database.
Other outcomes
The medina prevalence of intensive services use in asthma patients hospitalized COVID-19 was similar to those without asthma (10.1% (range 2.7–17.6) vs 9.8% (range 1.4–17.9) (
Discussion
In this international descriptive cohort study, the prevalence of asthma among patients diagnosed and hospitalized with COVID-19 patients was similar. Asthma prevalence was lowest in Spain and highest in US COVID-19 patients, which may be related to differences in shielding recommendations between countries during wave one of the pandemic. COVID-19 patients with asthma were mainly adults, with hospitalized patients being expectedly older. Asthma exacerbation at presentation with COVID-19 diagnosis or hospitalization was relatively low among asthma patients and varied by type of data source and cohort, generally being lower among diagnosed patients and primary care databases. This may be associated with differences in the severity of symptoms at presentation, increased likelihood of detecting asymptomatic SARS-CoV-2 infection as a result of testing due to the nature of overlapping symptoms or differences in the coding of asthma exacerbations.
In contrast, the prevalence of comorbidity in COVID-19 patients with asthma was generally high, particularly for hypertension, heart disease, T2DM and obesity, and was modestly greater than in COVID-19 patients without asthma. COVID-19 patients with asthma had a greater prevalence of allergic disorder and allergic rhinitis compared to those without asthma, but prevalence of these conditions was similar among hospitalized asthma patients with COVID-19 and those in the diagnosed cohort. Between one third to a half of asthma patients with COVID-19 had been prescribed systemic corticosteroids in the year prior to diagnosis. In both diagnosed and hospitalized asthma patients with COVID-19, use of nasal preparations, which included nasal corticosteroid and decongestants, was highly prevalent. Mortality in COVID-19 patients with asthma ranged from 2.1 to 16.9% depending upon the cohort and was similar or lower compared to those without asthma despite asthma patients appearing to have moderately greater prevalence of comorbidity. These may be related to differences in age as asthma patients were more commonly under 60 years of age compared to patients without asthma. In this regard, comorbidity and age are risk factors for poor COVID-19 outcomes.
Early studies from China reported that <1% of COVID-19 patients had asthma but studies from the US have since reported a prevalence of between 7.4 and 17%, which are in line with our findings (Citation24–28). This difference in prevalence could be due to China having a lower prevalence of asthma, were previous estimates from epidemiological studies have ranged from 12 to 58%, and/or lower testing and diagnosis of obstructive airways disease (Citation28–30).
Hospitalized COVID-19 patients with asthma in our cohort consisted of very few children. This suggests that COVID-19 may have a milder course in children with asthma compared to adults with asthma, similar to that seen in children and adults without asthma elsewhere (Citation31). There was some evidence of a higher proportion of children with asthma may have been hospitalized with COVID-19 compared to children without asthma but differences were modest. A greater proportion of hospitalized asthma patients with COVID-19 were aged between 40 and 70 years old compared to COVID-19 patients without asthma. Whilst these differences could partly be related to difficulties in detecting older patients with asthma within routine health databases, this may also suggest that having asthma led to hospitalization with COVID-19 at a modestly younger age. This could be due to asthma patients having more severe COVID-19 illness or having a lower threshold for referral due to be being considered high risk, particularly during wave one of the pandemic.
Comorbidity is common in patients with asthma with 62.6% of asthma patients reported to have ≥1 comorbidity compared to 46.2% in the general population (Citation32–35). Comorbidities such as cardiovascular disease and diabetes may complicate asthma management, with men and obese patients being at greater risk of COVID-19 mortality (Citation11,Citation36). Recognized risk factors such as obesity was highly prevalent in our population, although COVID-19 patients with asthma were more commonly female. There have been inconsistent reports as to whether COVID-19 patients with asthma are at higher risk of death (Citation11,Citation13–15). Among a large UK general population, patients with asthma had an 11–25% increased risk of COVID-19 death depending on whether patients had recently been prescribed oral corticosteroids (Citation11). However, among 1,298 hospitalized patients in New York aged ≤65 years with severe COVID-19, a diagnosis of asthma was not associated with worse outcomes (Citation13).
Respiratory viruses are a known trigger for asthma exacerbations; however, the risk of asthma exacerbations with COVID-19 is uncertain. We observed a low prevalence of asthma exacerbation at presentation with COVID-19, even if prior corticosteroid use is taken into account. Interestingly, among 768 hospitalized COVID-19 patients with asthma from France, none of them presented an asthma exacerbation (Citation14). Also, in a case series involving 106 patients hospitalized with severe COVID-19, asthma appeared not to be a risk factor for severe SARS-CoV-2 pneumonia and did not induce severe asthma exacerbation (Citation15). Furthermore, among 304 hospitalized COVID-19 patients in the Chinese Nanfang Hospital COVID-19 Research Database that contributed to Charybdis but was ineligible for our study, no patients had a reported history of asthma. This suggests that the mechanism of morbidity and mortality associated with COVID-19 may not be strongly related to worsening airway hyperresponsiveness.
It has been hypothesized that patients with type 2 immunity linked to atopy may have reduced susceptibility to COVID-19 as a result of reduced ACE2 expression (Citation7,Citation8). We observed only modest differences in the prevalence of allergic disorder and allergic rhinitis between hospitalized and diagnosed COVID-19 patients that could be explained by age, whilst the prevalence of eczema and urticaria within our cohorts was low limiting comparisons. The exception was among hospitalized COVID-19 patients in South Korea where the recorded prevalence of these conditions among COVID-19 patients was high. Whilst our data do not exclude the possibility of a protective mechanism associated with type 2 immunity, they do not provide strong evidence supporting this.
This study has several limitations. Firstly, the study utilizes routine electronic health data that is dependent upon the extent and quality of recording that could vary between the databases. Some COVID-19 symptoms may overlap with those of asthma and we cannot exclude the potential for detection bias. People with asthma may therefore have presented more frequently with symptoms and be tested for SARS-CoV-2, which may influence the prevalence of asthma particularly when policy measures consider people with asthma as high risk. This may be one reason for the difference in prevalence between databases and countries where testing approaches differed, particularly during wave one. Differences may also be related to differences in shielding policies during wave one of the pandemic and/or recording within routine databases. Data were unavailable to characterize blood test results and some comorbidities were only identified in the year prior to diagnosis that may cause under-ascertainment in absolute prevalence. Furthermore, we were restricted to crude comparisons and further causal inference modeling studies would be required to adjust for important potential confounders such as age. Different data sources ranged from primary care health records to health insurance claims data that could result in heterogeneity. However, the vast majority of observations were informative and consistent across the databases. A major strength of this study is its size and international comparability, reporting standardized data comparisons on COVID-19 patients with asthma from the US, South Korea and several European countries.
The prevalence of asthma among patients with COVID-19 is variable, which may have related to differences in policy intervention at the beginning of the pandemic. People with asthma and comorbidity are similarly at high risk of COVID-19 hospitalization and should therefore be prioritized for policy interventions including vaccination programmes, particularly as asthma patients are at higher risk of developing comorbidities compared to the general population. Further research is required to identify higher risk asthma patients with COVID-19.
Ethical approval
All the data partners received Institutional Review Board (IRB) approval or exemption. STARR-OMOP had approval from IRB Panel #8 (RB-53248) registered to Leland Stanford Junior University under the Stanford Human Research Protection Program (HRPP). The use of VA data was reviewed by the Department of Veterans Affairs Central Institutional Review Board (IRB), was determined to meet the criteria for exemption under Exemption Category 4(3), and approved for Waiver of HIPAA Authorization. The research was approved by the Columbia University Institutional Review Board as an OHDSI network study. The use of SIDIAP was approved by the Clinical Research Ethics Committee of the IDIAPJGol (project code: 20/070-PCV). The use of CPRD was approved by the Independent Scientific Advisory Committee (ISAC) (protocol number 20_059RA2). IQVIA OpenClaims is ADE-identified commercially available data product that could be purchased and licensed by any researcher. As these data are deemed commercial assets, there is no Institutional Review Board applicable to the usage and dissemination of these result sets or required registration of the protocol with additional ethics oversight. Compliance with Data Use Agreement terms, which stipulate how these data can be used and for what purpose, is sufficient for the licensing commercial entities. Further inquiry related to the governance oversight of these assets can be made to IQVIA (iqvia.com). At no point in the course of this study were the authors of this study exposed to identified patient-level data.
Competing interest statement
Transparency declaration
Lead authors affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Data sharing statement
Analyses were performed locally in compliance with all applicable data privacy laws. Although the underlying data is not readily available to be shared, authors contributing to this paper have direct access to the data sources used in this study. All results (e.g. aggregate statistics, not presented at a patient-level with redactions for minimum cell count) are available for public inquiry. These results are inclusive of site-identifiers by contributing data sources to enable interrogation of each contributing site. All analytic code and result sets are made available at: https://github.com/ohdsi-studies/Covid19CharacterizationCharybdis
Supplemental Material
Download MS Word (572.3 KB)2_Asthma_Manuscript_Tables_Supplement-JoA-CLEAN.docx
Download MS Word (130.8 KB)4_Asthma_Appendix_JoA.docx
Download MS Word (65.4 KB)2_Asthma_Manuscript_Tables_Supplement-JoA-Marked.docx
Download MS Word (139.4 KB)Funding
This study was supported by Wellcome Trust (Grant 214588/Z/18/Z), Bill & Melinda Gates Foundation (Investment ID INV-016201 and INV-019257), Innovative Medicines Initiative 2 Joint Undertaking (No 806968).
References
- Sears MR. Epidemiology of asthma exacerbations. J Allergy Clin Immunol. 2008;122(4):662–668. doi:10.1016/j.jaci.2008.08.003.
- Wark PA, Tooze M, Powell H, Parsons K. Viral and bacterial infection in acute asthma and chronic obstructive pulmonary disease increases the risk of readmission. Respirology. 2013;18(6):996–1002. doi:10.1111/resp.12099.
- Ko FW-S, Chan PK-S, Chan RWY, Chan K-P, Ip A, Kwok A, Ngai JC-L, Ng S-S, On CT, Hui DS-C, et al. Molecular detection of respiratory pathogens and typing of human rhinovirus of adults hospitalized for exacerbation of asthma and chronic obstructive pulmonary disease. Respir Res. 2019;20(1):210. doi:10.1186/s12931-019-1181-0.
- British Thoracic Society. BTS guidance for health care professionals in relation to shielding. 2020. https://www.brit-thoracic.org.uk/about-us/covid-19-identifying-patients-for-shielding/ Accessed 22/09/20.
- Trinh P, Jung TH, Keene D, Demmer RT, Perzanowski M, Lovasi G. Temporal and spatial associations between influenza and asthma hospitalisations in New York City from 2002 to 2012: a longitudinal ecological study. BMJ Open. 2018;8(9):e020362. doi:10.1136/bmjopen-2017-020362.
- Puig-Barberà J, Natividad-Sancho A, Trushakova S, Sominina A, Pisareva M, Ciblak MA, Badur S, Yu H, Cowling BJ, El Guerche-Séblain C, Global Influenza Hospital Surveillance Study Group (GIHSN), et al. Epidemiology of hospital admissions with Influenza during the 2013/2014 Northern Hemisphere Influenza Season: results from the Global Influenza Hospital Surveillance Network. PLoS One. 2016;11(5):e0154970 doi:10.1371/journal.pone.0154970
- Wang H, Song J, Yao Y, Deng Y‐K, Wang Z‐C, Liao B, Ma J, He C, Pan L, Liu Y, et al. Angiotensin-converting enzyme II expression and its implication in the association between COVID-19 and allergic rhinitis [published online ahead of print, 2020 Aug 27]. Allergy. 2021;76(3):906–910. doi:10.1111/all.14569.
- Shi W, Gao Z, Ding Y, Zhu T, Zhang W, Xu Y. Clinical characteristics of COVID-19 patients combined with allergy. Allergy. 2020;75(9):2405–2408. doi:10.1111/all.14434
- Halpin DMG, Singh D, Hadfield RM. Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective. Eur Respir J. 2020;55(5):2001009 (Published 2020 May 7. doi:10.1183/13993003.01009-2020.
- Burn E, You SC, Sena AG, Kostka K, Abedtash H, Abrahão MTF, Alberga A, Alghoul H, Alser O, Alshammari TM, et al. Deep phenotyping of 34,128 adult patients hospitalised with COVID-19 in an international network study. Nat Commun. 2020;11(1):5009. doi:10.1038/s41467-020-18849-z.
- Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi:10.1038/s41586-020-2521-4
- Chhiba KD, Patel GB, Vu THT, Chen MM, Guo A, Kudlaty E, Mai Q, Yeh C, Muhammad LN, Harris KE, et al. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146(2):307–314.e4. doi:10.1016/j.jaci.2020.06.010
- Lovinsky-Desir S, Deshpande DR, De A, et al. Asthma among hospitalized patients with COVID-19 and related outcomes [published online ahead of print, 2020 Aug 6]. J Allergy Clin Immunol. 2020;S0091–6749(20):31100–31103. doi:10.1016/j.jaci.2020.07.026.
- Beurnier A, Jutant E-M, Jevnikar M, Boucly A, Pichon J, Preda M, Frank M, Laurent J, Richard C, Monnet X, et al. Characteristics and outcomes of asthmatic patients with COVID-19 pneumonia who require hospitalisation [published online ahead of print, 2020 Jul 30]. Eur Respir J. 2020;56(5):2001875. doi:10.1183/13993003.01875-2020.
- Grandbastien M, Piotin A, Godet J, Abessolo-Amougou I, Ederlé C, Enache I, Fraisse P, Tu Hoang TC, Kassegne L, Labani A, et al. SARS-CoV-2 pneumonia in hospitalized asthmatic patients did not induce severe exacerbation. J Allergy Clin Immunol Pract. 2020;8(8):2600–2607. doi:10.1016/j.jaip.2020.06.032.
- Voss EA, Makadia R, Matcho A, Ma Q, Knoll C, Schuemie M, DeFalco FJ, Londhe A, Zhu V, Ryan PB, et al. Feasibility and utility of applications of the common data model to multiple, disparate observational health databases. J Am Med Inform Assoc. 2015;22(3):553–564. doi:10.1093/jamia/ocu023.
- Hripcsak G, Duke JD, Shah NH, Reich CG, Huser V, Schuemie MJ, Suchard MA, Park RW, Wong IC, Rijnbeek PR, et al. Observational health data sciences and informatics (OHDSI): opportunities for observational researchers. Stud Health Technol Inform. 2015;216:574–578.
- Sena A, Kostka K, Schuemie M, Posada JD. ohdsi-studies/Covid19CharacterizationCharybdis: Charybdis v1.1.1-Publication Package (Version v1.1.1). Zenodo. 2020. doi:10.5281/zenodo.4033034.
- Datta S, Posada J, Olson G, et al. A new paradigm for accelerating clinical data science at Stanford Medicine. Published Online March 17. http://arxiv.org/abs/2003.10534 (Accessed Aug 20, 2020).
- Del Mar García-Gil M, Hermosilla E, Prieto-Alhambra D, et al. Construction and validation of a scoring system for the selection of high-quality data in a Spanish population primary care database (SIDIAP). Inform Prim Care. 2012;19:135–145.
- Engelkes M, de Ridder MA, Svensson E, Berencsi K, Prieto-Alhambra D, Lapi F, Giaquinto C, Picelli G, Boudiaf N, Albers FC, et al. Multinational cohort study of mortality in patients with asthma and severe asthma. Respir Med. 2020;Apr–May165:105919. doi:10.1016/j.rmed.2020.105919.
- Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, Smeeth L. Data resource profile: clinical practice research datalink (CPRD). Int J Epidemiol. 2015;44(3):827–836. doi:10.1093/ije/dyv098.
- Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(6):1228–1234. doi:10.1080/03610910902859574.
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi:10.1016/S0140-6736(20)30211-7.
- Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. doi:10.1016/j.jaci.2020.04.006.
- Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, Greninger AL, Pipavath S, Wurfel MM, Evans L, et al. Covid-19 in critically ill patients in the Seattle region – case series. N Engl J Med. 2020;382(21):2012–2022. doi:10.1056/NEJMoa2004500.
- Garg S. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 states, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020:69.
- Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA. 2020;323(21):2195–2198. doi:10.1001/jama.2020.7202.
- Brusselle GG, Ko FW. Prevalence and burden of asthma in China: time to act. Lancet. 2019;394(10196):364–366. doi:10.1016/S0140-6736(19)31349-2.
- The Global Asthma Report. 2018. http://www.globalasthmareport.org/burden/burden.php. Accessed 30/09/2020.
- Centres for Disease Control and Prevention (CDC). COVID-19 hospitalization and death by age. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html. Accessed 16 Sep 2020.
- Weatherburn CJ, Guthrie B, Mercer SW, Morales DR. Comorbidities in adults with asthma: population-based cross-sectional analysis of 1.4 million adults in Scotland. Clin Exp Allergy. 2017;Oct47(10):1246–1252. doi:10.1111/cea.12971.
- Morales DR, Lipworth BJ, Donnan PT, Jackson C, Guthrie B. Respiratory effect of beta-blockers in people with asthma and cardiovascular disease: population-based nested case control study. BMC Med. 2017;Jan 15(1):18. doi:10.1186/s12916-017-0781-0.
- Morales DR, Dreischulte T, Lipworth BJ, Donnan PT, Jackson C, Guthrie B. Respiratory effect of beta-blocker eye drops in asthma: population-based study and meta-analysis of clinical trials. Br J Clin Pharmacol. 2016;Sep82(3):814–822. doi:10.1111/bcp.13006.
- Morales DR, Lipworth BJ, Guthrie B, Jackson C, Donnan PT, Santiago VH. Safety risks for patients with aspirin-exacerbated respiratory disease after acute exposure to selective nonsteroidal anti-inflammatory drugs and COX-2 inhibitors: meta-analysis of controlled clinical trials. J Allergy Clin Immunol. 2014;Jul134(1):40–45. doi:10.1016/j.jaci.2013.10.057.
- Recalde M, Roel E, Pistillo A, et al. Characteristics and outcomes of 627 044 COVID-19 patients with and without obesity in the United States, Spain, and the United Kingdom. Int J Obes. 2021;45(11):2347–2357. doi:10.1101/2020.09.02.2018517.
