Abstract
Objective
In order to understand the role of regular controller inhaled corticosteroids (ICS) versus as-needed ICS-formoterol in managing mild asthma, we performed a modified Delphi procedure.
Methods
Opinions from 16 respiratory experts to three surveys and during a virtual scientific workshop helped to develop final consensus statements (pre-defined as 70% agreement).
Results
Thirteen participants completed all rounds (response rate 81%). At the end of the procedure, there was final consensus on: regular daily ICS being the recommended treatment approach in mild persistent asthma, with better symptom control and robust long-term clinical data compared with as-needed ICS-formoterol (85%); to avoid noncompliance, frequently seen in mild asthma patients, regular ICS dosing should be accompanied by ongoing education on treatment adherence (100%); treatment aims should be targeting asthma control (92%) and reduction of exacerbation risk (85%). No consensus was reached on whether GINA or national guidelines most influence prescribing decisions.
Conclusions
It is important to encourage patients to be adherent and to target both asthma control and exacerbation risk reduction. There is robust clinical evidence to support proactive regular dosing with ICS controller therapy plus as-needed short-acting beta-agonists for the management of patients with mild asthma.
Abbreviations.
Supplemental data for this article is available online at https://doi.org/10.1080/02770903.2022.2034850 .
Introduction
Asthma is a heterogenous, chronic, respiratory disease characterized by airway inflammation, a history of respiratory symptoms and variable airway obstruction (Citation1). Although the majority of patients with asthma (50–75%) are reported to have mild asthma (Citation2,Citation3), there have been multiple definitions of mild asthma in the past based either on symptoms, airflow limitation and activity limitations or more recently on the intensity of treatment required to control symptoms and prevent future risks (Citation4,Citation5). The definition of mild asthma is still debated and in need of further clarification (Citation1,Citation6). It is well recognized that airway inflammation is present in mild asthma and patients with mild asthma often have suboptimal asthma control, putting them at risk of disease progression, severe exacerbations and an impaired quality of life (Citation2–5,Citation7–9). A recent large US database study reported that 19.8% of patients with mild asthma (defined as Global Initiative for Asthma (GINA) treatment step 2) had uncontrolled asthma (Citation10).
Inhaled corticosteroids (ICS) are the cornerstone of treatment across all severities of asthma (Citation1,Citation11,Citation12), and early intervention with regular ICS treatment shows benefits in term of achieving asthma control, and in reducing the risk of asthma-related exacerbations, hospitalizations and death (Citation1,Citation13,Citation14). However, adherence with asthma medications in the community is poor (Citation1), and patients with mild asthma are particularly at risk of poor adherence (Citation15). Another challenge in treating patients with mild asthma is that patients frequently do not report their symptoms (Citation16), or have poor perception of their airways obstruction, which may result in under treatment with appropriate ICS or ICS/long-acting beta-agonist regular controller medication and an increased risk of exacerbation (Citation17).
The GINA report recommends two treatment tracks for mild asthma, low dose as-needed ICS-formoterol (preferred option) or regular low dose ICS plus as-needed short-acting beta-agonist (SABA) (Citation1). The 1-year, randomized, double-blind, double-dummy, studies in adults evaluating the as-needed ICS-formoterol treatment strategy demonstrated that treatment with as-needed ICS-formoterol resulted in similar annual severe exacerbation rates but worse asthma symptom control (between treatment difference in mean change from baseline: Asthma Control Questionnaire-5 (ACQ-5): 0.11–0.15) and lower change from baseline in pre-bronchodilator forced expiratory volume in 1 s (FEV1) (between treatment difference: 33–54 ml) compared with regular maintenance daily ICS and as-needed SABA (Citation18–20). Subsequently, in two 1-year, randomized, open label, controlled trials, one showed better symptom control and one showed similar symptom control with regular maintenance treatment compared with as-needed ICS/formoterol, whilst both showed less severe exacerbations in the ICS-formoterol group as compared to daily ICS and as-needed SABA (Citation20–22). However, around the world, there is not full agreement among several recommendations for the management of mild asthma with some recommending regular daily low dose ICS and as-needed SABA (Citation11,Citation23), and others recommending as-needed ICS-formoterol as an alternative to regular ICS (Citation24–26).
In view of these recommendations, we undertook a modified Delphi procedure, incorporating three rounds of surveys and a scientific workshop, with the aims of understanding asthma specialists’ goals in managing and treating mild asthma, focusing on the role of regular controller ICS in managing mild asthma as well as in the use of GINA and other national guidelines.
Methods
Study design
This study used a modified Delphi technique to develop consensus-based statements on factors determining the management of patients with mild asthma. The Delphi procedure is a facilitated group technique that uses structured questionnaires, usually delivered via mail or email, to reach a consensus on a specific topic among a panel of selected experts (Citation27–29). The technique uses anonymity, controlled feedback and group responses to reach consensus. In this study, in addition to the structured, anonymous, Delphi questionnaires, an on-line, virtual scientific workshop was conducted which involved open discussion between the participants and hence we employed a ‘modified’ Delphi method.
The Delphi survey was run by an independent specialist market research team at Ashfield MedComms, part of Ashfield Health (Macclesfield, United Kingdom). Following a literature search, the research team prepared the survey questionnaire, hosted the online surveys using Decipher software, and analyzed data generated in each survey. The research was designed in compliance with UK General Data Protection Regulation (GDPR) and with the British Healthcare Business Intelligence Association’s (BHBIA) Legal & Ethical Guidelines, along with the European Pharmaceutical Market Research Association’s (EphMRA). No formal ethical approval was required as this non-interventional physician survey fell outside the remit of the Research Government Framework. All participants provided their informed consent for each survey and any information provided was kept confidential and anonymous.
Participants
Twenty respiratory experts were invited to participate in all rounds of the process. Respiratory physicians with expertise and experience in managing patients with asthma were invited to participate, representing 10 countries (Argentina, Indonesia, Japan, Mexico, Malaysia, Philippines, Spain, Thailand, Turkey, and Vietnam). Participants were selected due to their knowledge of asthma and having an interest in improving patient outcomes, for having experience as an investigator in asthma studies or being a member of international and/or national guideline committees, and, in some cases, due to their own country having national asthma guidelines in place.
The industry sponsors organized and facilitated the scientific workshop but did not complete any of the Delphi surveys.
Stages of the Delphi procedure
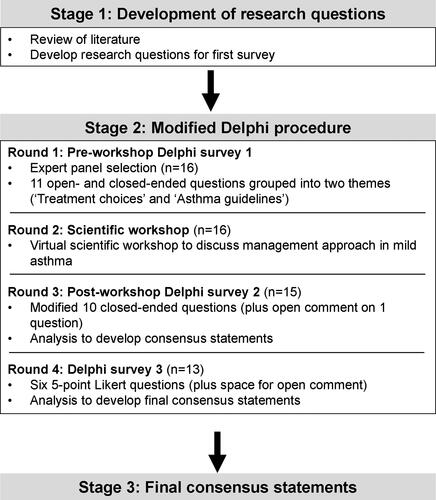
The Delphi procedure comprised three stages: (1) development of research questions; (2) Delphi surveys and scientific workshop; and (3) development of final post-survey consensus statements ().
Survey questionnaire development
A review of the literature on management of mild asthma in adults, including treatment and management guidelines was used to develop the first survey questionnaire. Subsequent questionnaires were developed based on participant group responses.
Delphi surveys and scientific workshop
Participants were emailed the first Delphi survey which was completed prior to the scientific workshop. The survey consisted of 11 questions (7 close-ended questions and 4 open-ended questions). The objectives of this survey were to: (a) understand prescribers’ preferences for the treatment and management of patients with mild asthma; (b) explore the advantages and disadvantages of prescribing daily ICS treatment vs. as-needed ICS-formoterol treatment to patients with mild asthma; and (c) to understand how guidelines and other factors impact the prescribing choices of healthcare professionals.
Participants in the round 1 Delphi survey were invited to participate in an on-line, virtual scientific workshop to discuss the scientific and clinical data for regular ICS dosing versus as-needed ICS/formoterol as well as GINA and national guidelines with respect to management of mild asthma.
Following the scientific workshop, participants were emailed the second Delphi survey which consisted of 10 close-ended questions with space for open comment following 1 question. The objective of the post-workshop survey was to assess level of consensus on key topics following survey 1 results and workshop discussions, specifically, (a) the clinical evidence on the different treatment regimens for controlling mild asthma and differences in outcomes; (b) the extent to which treatment adherence and patient perception of symptoms impact treatment choices and asthma control; and (c) to understand views on using GINA treatment recommendations or local country guidelines for the holistic management of patients with mild asthma
In the last step of the Delphi procedure, participants were emailed final follow-up questions consisting of six 5-point Likert-type questions (Strongly disagree, somewhat disagree, neither agree nor disagree, somewhat agree, and strongly agree). Space for open comment followed each question. The objectives were to obtain a consensus amongst the participants on their understanding and beliefs on, (a) the reasons for recommending regular daily dosing for patients with mild asthma and (b) targeting asthma control or severe exacerbations reduction primarily for asthma management
Data analysis
All data were analyzed descriptively. The results of the first survey and the scientific workshop were subjected to a content analysis to identify and classify common elements in the overall opinion of the panel, and for development of survey 2. A similar approach was used to develop survey 3, the final set of questions. Full feedback on the results of each survey was given to the panelists for consideration and discussion before the next survey was sent out. The phrasing of questions and the rating of responses using a Likert scale were decided by market research experts in line with best practice and other Delphi surveys (Citation27,Citation28,Citation30). For data quality checks, all responses were checked by a data analyst at Ashfield Health to ensure consistency and quality of response.
For the results of surveys 2 and 3, a consensus was pre-defined as achieving 70% agreement. This proportion has been considered appropriate in previous studies that used the Delphi procedure (Citation31–33).
Results
Participants
Twenty respiratory experts were invited to participate and 16 accepted, completed the first Delphi survey and attended the scientific workshop (response rate of 80%) (). Of the 16 who accepted the initial invitation, 15 completed the post-workshop Delphi survey 2 (response rate 94%) and 13 completed Delphi survey 3 (response rate 81%). The participant characteristics are summarized in . Among participants, there was an equal distribution of males and females, their mean age was 56.8 years and they resided across 10 countries. All participants had medical degrees and had been working in the respiratory field for ≥ 15 years.
Table 1. Participants’ current and preferred treatments for patients with mild asthma (Delphi survey 1).
Delphi survey 1 results
Participants defined mild asthma in a range of different ways, most frequently referring to the GINA definition and based on infrequent symptoms, no impact on daily life, and exacerbations easily controlled by Step 1 or 2 treatment. Participants indicated that 45% of patients with mild asthma currently receive daily ICS (alone or plus another controller) plus as-needed SABA and 25% receive as-needed ICS-formoterol (). Participants’ preferred treatment regimen in mild asthma, was indicated as daily ICS (alone or plus another controller) plus as-needed SABA for 44% and as-needed ICS-formoterol for 44% ().
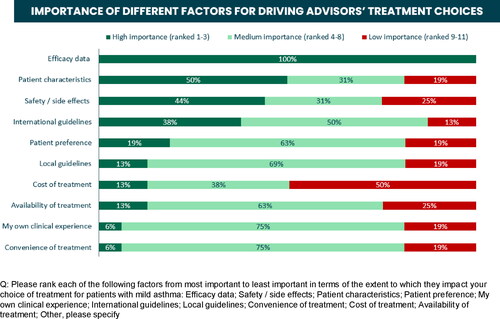
The most important factors driving treatment choices for mild asthma were efficacy (). When considering the advantages and disadvantages of daily ICS versus as-needed ICS-formoterol, the main advantage of daily ICS was efficacy (69% of participants) and the main disadvantage was adherence (56%); whereas the main advantages of as-needed ICS-formoterol were reported as efficacy (31%), convenience (25%) and adherence (25%), and the main disadvantages were cost (44%) and, also, level of efficacy (31%). When asked if there are situations/particular patients for whom as needed ICS-formoterol would be recommended over daily treatment, 56% agreed, while 44% voted against.
Eighty-eight percent of participants strongly or somewhat agreed that there are clear guidelines on the recommended approach to achieving good asthma control in their own country. GINA was most commonly cited as the global document used to guide prescribing decisions for asthma management and most participants also listed their own local/national guidelines as influencing their prescribing decisions.
Scientific workshop summary
The main findings of the scientific workshop are summarized in with examples of individual feedback presented in . There was general agreement amongst the panel that regular maintenance dosing with ICS is the best asthma management strategy in mild asthma and that the key studies evaluating as-needed ICS-formoterol showed that regular ICS was superior or equivalent to as-needed ICS-formoterol for asthma control parameters, but not superior for reducing the risk of severe exacerbations. Adherence with regular ICS dosing was raised as an issue and it was agreed by most that it is yet unknown whether a variable dosing regimen would ensure real life adherence in the community.
Table 2. Summary of scientific workshop discussions.
Most participants agreed that GINA is a good document about asthma, based on the assessment of published literature, although it does not use GRADE methodology (Citation1), and would benefit from being more balanced in this respect. It was also acknowledged that GINA is an outlier in terms of recommending as-needed ICS-formoterol as the preferred treatment option in mild asthma and national treatment guidelines have taken a consensus- and evidence-based approach, with some not recommending as-needed ICS-formoterol at all for mild asthma (Citation11,Citation12,Citation23), some recommending as-needed ICS-formoterol as an alternative to regular ICS in mild persistent asthma (Citation24–26), and some recommending either regular ICS plus as-needed SABA or as-needed ICS-formoterol as a treatment option for mild asthma (Citation34).
Delphi survey 2 results
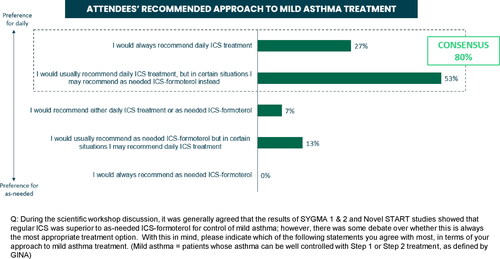
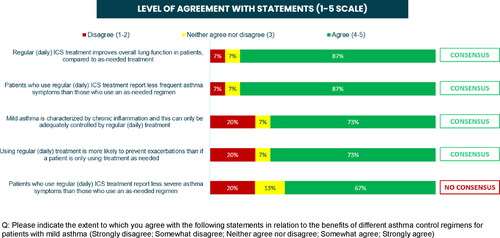
Following the scientific workshop and completion of the second survey, a number of consensus statements were reached. There was a consensus among participants (80% agreed somewhat or strongly) that they would usually or always recommend daily ICS for patients with mild asthma (). In addition, 87% agreed that daily ICS improves lung function and reduces symptom frequency, 73% agreed that the chronic inflammation in mild asthma can only be controlled by regular daily ICS, and 73% agreed that regular ICS is more likely to prevent exacerbations compared with as-needed treatment (). Eighty seven percent of physicians reported that the proportion of patients with mild asthma who should be prescribed as-needed ICS-formoterol rather than daily ICS ranges between 0% and 40% (), with the main reason given as ‘lack of adherence to daily ICS’.
No consensus was reached on which ICS treatment regimen is believed to be most successful for controlling mild asthma (60% favored regular ICS over as-needed ICS-formoterol and 33% regarded both options equally effective), or on the statement, ‘as-needed treatment is unlikely to be successful as it is dependent on patients’ own accurate perception of symptoms’ (53% agreed).
Physicians somewhat or strongly agreed that low adherence is seen across all treatment regimens in asthma management (87%), that patients are less likely to be adherent to treatment if they are feeling well (100%) or to a treatment they need to use every day versus a treatment used less frequently (73%), and that there is wide variation in patients’ abilities to accurately assess/perceive their own symptoms (73%).
A consensus was reached on the following statements about GINA: GINA should be treated as an opinion statement rather than a guideline (87%), GINA is a useful document for physicians to refer to for the management of patients with asthma (80%) and there is a need for GINA to include more appropriate clinical evidence in its strategy (73%).
Delphi survey 3 results
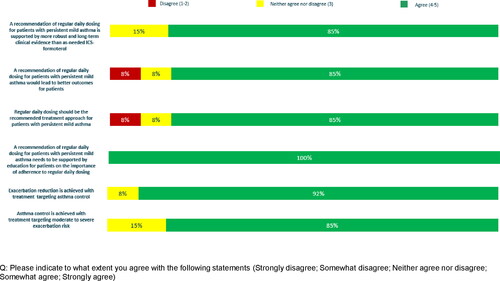
A consensus was reached on all six of the final Likert-style questions with physicians agreeing that regular daily dosing with ICS should be the recommended treatment approach in mild persistent asthma, supported by better outcomes and robust long-term clinical data compared with as-needed ICS-formoterol (85%); that a regular daily dosing treatment regimen should be accompanied by ongoing education in the importance of adherence to treatment (100%); that exacerbation reduction is achieved with treatment targeting asthma control (92%) and that asthma control is achieved with treatment targeting moderate to severe exacerbation risk (85%) ().
Discussion
The aim of this modified Delphi procedure was to develop consensus-based statements on factors determining the management of patients with mild asthma. After the four-round process, a consensus was reached that, in terms of long-term clinical data and clinical outcomes, regular maintenance dosing with ICS should be the preferred treatment option in mild persistent asthma to achieve symptom control and reduce exacerbations, supported by patient education to encourage treatment adherence. It was also agreed that effective treatment results from targeting both asthma exacerbation reduction and asthma control. There was agreement at the scientific workshop that GINA would benefit from being more methodological in its evaluation of the evidence to reach a consensus on recommendations. Although traditionally GINA recommendations about asthma have been widely accepted, the last proposal regarding step 1 and 2 have not been incorporated by many medicine agencies such as the European Medicines Agency. No consensus was reached on whether GINA or country-specific guidelines are more rigorous or objective, or which have the most influence in driving prescribing habits.
Airway inflammation and airway remodeling are present even in mild asthma (Citation2,Citation35,Citation36), and treatment with ICS leads to decreases in airway inflammation and the airway wall structural changes observed in mild asthma, supporting a treatment strategy for regular daily ICS (Citation35,Citation37). Support for regular treatment also comes from long-term clinical studies that show the benefit of early intervention with ICS on asthma symptoms, in reducing the risk of exacerbations, and on functional changes associated with asthma, (lung function and airway responsiveness) (Citation13,Citation14,Citation38,Citation39). Inflammation and bronchial responsiveness can persist even in the absence of asthma symptoms (Citation40,Citation41), both of which are related to lung function decline (Citation42,Citation43). Jayaram et al. showed in patients with very mild or mild asthma that a clinical control strategy to guide ICS maintenance treatment (based on symptoms and spirometry) was similar to a strategy guided by inflammation (sputum cell counts) in reducing the number of exacerbations (Citation44). A risk of symptom-driven, as-needed therapy is that inflammation and remodeling may persist in periods when symptoms are not apparent. The long-term impact of this strategy on airway inflammation, hyper-responsiveness, remodeling and asthma mortality compared with regular ICS usage is as yet unknown, and further research would be warranted (Citation45). Regular daily ICS therapy with as-needed SABA resulted in better asthma symptom control and similar risk of severe exacerbations compared with as-needed ICS-formoterol, in two double-blind, randomized, controlled trials (Citation18,Citation19). In an open-label, randomized, controlled trial, regular, daily ICS therapy with as-needed SABA was associated with better suppression of inflammation (fractional exhaled nitric oxide (FeNO), similar symptom control and lung function, but less reduction in the moderate/severe exacerbation rate, compared with as-needed ICS-formoterol (Citation22). In another study comparing regular maintenance dosing with budesonide with intermittent, symptom-guided short courses of corticosteroids (inhaled budesonide or oral prednisolone), Boushey et al. reported similar rates of exacerbations for the two groups but significantly greater improvements in markers of inflammation (bronchial reactivity, the percentage of eosinophils in sputum, and FeNO) following regular daily maintenance treatment (Citation46).
The uncertainty surrounding the long-term impacts of as-needed ICS-formoterol treatment in mild asthma is reflected in the differing treatment recommendations by national guidelines compared with GINA (Citation1,Citation11,Citation12,Citation23–26,Citation34,Citation47), some of which do not recommend or mention as-needed ICS-formoterol at all for the treatment of mild asthma. This indicates a need for clarity in defining mild asthma and in determining the most appropriate treatment for these patients, and may require further development of national guidelines and adaptations to local healthcare systems. The collection of real-life data in different countries will contribute to these discussions. It seems clear that achieving asthma symptom control is not the key objective of as-needed ICS-formoterol therapy, it being more focused on the reduction of severe exacerbations, and there appears to be some acceptance that this treatment strategy would be expected to result in worse symptom control versus regular daily maintenance treatment (Citation21,Citation22). Individual patient characteristics, risk factors, comorbidities and pheno- and endotypes are important factors when considering the effects of a particular treatment on a patient’s symptoms and exacerbation risk (Citation1), as well as practical issues such as inhaler technique, adherence and affordability (Citation1,Citation48). Recently, the benefits of type 2 inflammation assessment (FeNO and blood eosinophils) in addition to symptoms and spirometry were reported as useful to determine the effective use of ICS in asthma (Citation49), but these tools are not routinely available in general practice. Certainly, a blanket strategy to treat all mild asthma patients with as-needed ICS-formoterol is not supported by the current available evidence (Citation20).
One of the main problems in maintaining a treatment regimen with regular ICS is that treatment adherence is poor, with reported adherence rates with controller therapies ranging between 22% and 78% (Citation15,Citation50–52), and having mild asthma is particularly associated with poor adherence (Citation15), putting patients at risk of poorer outcomes, including death due to underuse of ICS (Citation1,Citation4,Citation9). The anti-inflammatory protective effect of ICS treatment builds up over time; this benefit has been shown when ICS are taken regularly and consistently over the long-term (Citation53). Because of the central role ICS play in achieving effective asthma control, addressing poor adherence continues to be of paramount importance. This involves an understanding of a patient’s beliefs and perceptions about their asthma and the reasons for their poor adherence – which could be related to technical, behavioral or educational reasons – so that adherence interventions can be implemented and tailored to individual patients (Citation1,Citation15,Citation51,Citation54). Moreover, in some situations primary care physicians are still reluctant to prescribe ICS for prolonged periods, making ‘continuing medical education’ (CME) an important tool as well. For patients with adherence issues, regular asthma reviews of inhaler technique and adherence (Citation2), and using a personalized action plan (agreed between the patient and healthcare professional) can empower patients to make short-term changes to their medication in response to changes in their symptoms and when to seek professional help (Citation55). Real life adherence to an as-needed dosing strategy in the community is not yet known. A major concern is that as-needed treatment relies on patients perceiving their symptoms and patients who are under-perceivers may delay starting appropriate treatment, increasing the risk of an exacerbation (Citation17). Interestingly, in a one-year open-label study in a real-world setting, regular ICS therapy resulted in better symptom control measured by ACQ-5 and a numerically lower annualized exacerbation rate, despite a low adherence of 56% in this group, compared with as-needed ICS-formoterol (annualized exacerbation rates of 0.175 versus 0.195, respectively) (Citation21).
The Delphi procedure has been well-described as a useful technique for obtaining a group consensus on a specific topic, particularly in circumstances where there is contradictory or insufficient information (Citation27–29). The method is based on the assumption that the opinions of a group are more valid than that of an individual and the use of an independent facilitator and anonymous controlled feedback ensures a robustness in the process. Previous studies in asthma and COPD suggest that a panel with at least 12 members is sufficient to reach a meaningful consensus (Citation56–58). The Delphi procedure used in the current study has some strengths and limitations. A strength of this study is that response rates were relatively high among the selected panel with 81% of participants completing all four rounds, indicating a good level of interest and engagement in the process. Another strength of this study is that physicians were invited to participate from a broad range of countries and backgrounds (public and private health care), representing different viewpoints. Industry sponsors organized and facilitated the scientific workshop but did contribute to the consensus statements derived from the Delphi surveys. A limitation of this study is that only respiratory specialists were included and their views do not reflect physicians in primary care where most patients with mild asthma are treated. Another limitation is that there is no evidence of reliability with the Delphi methods i.e. that other expert panels would reach the same conclusions (Citation29), and so these results should be interpreted accordingly. Whilst all participants were experts in asthma, their views were not homogenous, suggesting the panel represented a range of views.
Conclusions
The objective of this study was to understand the role of proactive regular controller ICS in the management of mild asthma. The participants in this Delphi study reached the following consensus recommendations: They agreed that regular maintenance dosing with ICS should be the preferred initial treatment option in mild persistent asthma, supported by patient education to encourage treatment adherence. It was also agreed that effective treatment should target both asthma severe exacerbation reduction and asthma control. As no consensus was reached on whether GINA or country-specific guidelines are more rigorous or objective, or which have the most influence in driving prescribing habits, this highlights a need for the development of national guidelines in each country or region which consider local healthcare systems, with a specific focus on mild asthma.
Author contributions
Conceived and designed the study: NB, EP, BA, VE, GL. Statistical analyses: BA, VE, supported by Ashfield MedComms, Ashfield Health. All authors contributed to the interpretation of results, drafting, reviewing, and critically revising the paper, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
| ACQ-5 | = | Asthma Control Questionnaire-5 |
| BHBIA | = | British Healthcare Business Intelligence Association |
| CME | = | continuing medical education |
| EphMRA | = | European Pharmaceutical Market Research Association |
| FeNO | = | fractional exhaled nitric oxide |
| GDPR | = | General Data Protection Regulation |
| GINA | = | Global Initiative for Asthma |
| ICS | = | inhaled corticosteroid |
| SABA | = | short-acting beta-agonist |
R1_Delphi_workshop_Manuscript_Supplementary_materials_J_Asthma_clean.docx
Download MS Word (103.7 KB)R1_Delphi_workshop_Manuscript_Supplementary_materials_J_Asthma_tracked_submitted.docx
Download MS Word (105.4 KB)Acknowledgements
The authors would like to thank all the scientific workshop participants for their helpful contributions to the discussions. This study (GSK study 217879) was funded by GlaxoSmithKline (GSK). Editorial support was provided by Kate Hollingworth of Continuous Improvement Ltd and was funded by GSK.
Declaration of interest
CD has received personal grants from Novartis, Sanofi, GSK, TEVA, MSD, Esteve, AstraZeneca, Chiesi, Menarini, Ferrer, Stallergenes, ALK-Abelló, Allergy therapeutics, Hall Allergy, Inmunotek and Roxall; a grant from GSK paid to the Parc Tauli Foundation; consulting fees from Novartis, Sanofi, GSK, TEVA, MSD, AstraZeneca, Menarini, Pfizer, Ferrer, Stallergenes and ALK-Abelló; payment for expert testimony and/or honoraria for presentations, speakers bureaus, manuscript writing or educational events from Novartis, Sanofi, GSK, MSD, Esteve, TEVA, Almiral, AstraZeneca, Chiesi, Menarini, Pfizer, Ferrer, ALK-Abelló; and support for travel/attending meetings from Novartis, Sanofi, GSK, Chiesi, MSD. BG’s institution has received honoraria for presentations, speakers’ bureaus, manuscript writing or educational events from Deva, Sandoz, Abdi Ibrahim, AstraZeneca, Chiesi; and has received support for travel/attending meetings from GSK, Astra, Chiesi. GVV has received honoraria for presentations, speakers’ bureaus, manuscript writing or educational events from GSK, Boehringer Ingelheim, Novartis, AstraZeneca, MSD, Pfizer, Sanofi, Chiesi, Bayer. DLL’s institution has received grants from GSK, Novartis, Sanofi, AstraZeneca, Circassia; has received speaker’s fees and/or fees for participation in AdBoards from AstraZeneca, GSK Mexico, Novartis, Sanofi, Chiesi, Carnot, DBV Technologies; and support for travel/attending meetings from Sanofi, AstraZeneca, Novartis. GG, HN, OP and HS have no competing interests. The following are GSK employees and hold GSK shares: NB (part-time), EP, EI (part-time), BA, VE, GL.
Data sharing statement
Information on GlaxoSmithKline R&D’s data sharing commitments and requesting access can be found at: https://www.clinicalstudydatarequest.com.
References
- Global Initiative for Asthma. Global strategy for asthma management and prevention. Global Initiative for Asthma (GINA); 2021. https://ginasthma.org/gina-reports/ [last accessed 7 September 2021].
- Dusser D, Montani D, Chanez P, de Blic J, Delacourt C, Deschildre A, Devillier P, Didier A, Leroyer C, Marguet C, et al. Mild asthma: an expert review on epidemiology, clinical characteristics and treatment recommendations. Allergy. 2007;62(6):591–604. doi:10.1111/j.1398-9995.2007.01394.x.
- Rabe KF, Adachi M, Lai CKW, Soriano JB, Vermeire PA, Weiss KB, Weiss ST. Worldwide severity and control of asthma in children and adults: the global asthma insights and reality surveys. J Allergy Clin Immunol. 2004;114(1):40–47.
- Mulgirigama A, Barnes N, Fletcher M, Pedersen S, Pizzichini E, Tsiligianni I. A review of the burden and management of mild asthma in adults - implications for clinical practice. Respir Med. 2019;152:97–104. doi:10.1016/j.rmed.2019.04.024.
- FitzGerald JM, Barnes PJ, Chipps BE, Jenkins CR, O’Byrne PM, Pavord ID, Reddel HK. The burden of exacerbations in mild asthma: a systematic review. ERJ Open Res. 2020;6(3):00359-2019. doi:10.1183/23120541.00359-2019.
- Krishnan JA, Cloutier MM, Schatz M. National Asthma Education and Prevention Program 2020 guideline update: where do we go from here? Am J Respir Crit Care Med. 2021;203(2):164–167.
- Ding B, Small M. Disease burden of mild asthma: findings from a cross-sectional real-world survey. Adv Ther. 2017;34(5):1109–1127.
- Suruki RY, Daugherty JB, Boudiaf N, Albers FC. The frequency of asthma exacerbations and healthcare utilization in patients with asthma from the UK and USA. BMC Pulm Med. 2017;17(1):74.
- Royal College of Physicians. Why asthma still kills: the National Review of Asthma Deaths (NRAD) Confidential Enquiry report; 2014. http://www.rcplondon.ac.uk/sites/default/files/why-asthma-still-kills-full-report.pdf [last accessed 7 September 2021].
- Busse WW, Fang J, Marvel J, Tian H, Altman P, Cao H. Uncontrolled asthma across GINA treatment steps 2 - 5 in a large US patient cohort. J Asthma. 2021:1–13. doi:10.1080/02770903.2021.1897834.
- The British Thoracic Society. BTS/SIGN British guideline on the management of asthma; 2019. https://www.brit-thoracic.org.uk/standards-of-care/guidelines/btssign-british-guideline-on-the-management-of-asthma/ [last accessed 7 September 2021].
- Cloutier MM, Baptist AP, Blake KV, Brooks EG, Bryant-Stephens T, DiMango E, Dixon AE, Elward KS, Hartert T, Krishnan JA, et al. 2020 focused updates to the asthma management guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol. 2020;146(6):1217–1270. doi:10.1016/j.jaci.2020.10.003.
- Busse WW, Pedersen S, Pauwels RA, Tan WC, Chen YZ, Lamm CJ, O’Byrne PM, START Investigators Group. The inhaled steroid treatment as regular therapy in early asthma (START) study 5-year follow-up: effectiveness of early intervention with budesonide in mild persistent asthma. J Allergy Clin Immunol. 2008;121(5):1167–1174. doi:10.1016/j.jaci.2008.02.029.
- O’Byrne PM, Pedersen S, Lamm CJ, Tan WC, Busse WW. START Investigators Group. Severe exacerbations and decline in lung function in asthma. Am J Respir Crit Care Med. 2009;179(1):19–24. doi:10.1164/rccm.200807-1126OC.
- Bårnes CB, Ulrik CS. Asthma and adherence to inhaled corticosteroids: current status and future perspectives. Respir Care. 2015;60(3):455–468. doi:10.4187/respcare.03200.
- Bellamy D, Harris T. Poor perceptions and expectations of asthma control: results of the International Control of Asthma Symptoms (ICAS) survey of patients and general practitioners. Prim Care Respir J. 2005;14(5):252–258.
- Barnes PJ, Szefler SJ, Reddel HK, Chipps BE. Symptoms and perception of airway obstruction in asthmatic patients: clinical implications for use of reliever medications. J Allergy Clin Immunol. 2019;144(5):1180–1186. doi:10.1016/j.jaci.2019.06.040.
- O’Byrne PM, FitzGerald JM, Bateman ED, Barnes PJ, Zhong N, Keen C, Jorup C, Lamarca R, Ivanov S, Reddel HK. Inhaled combined budesonide-formoterol as needed in mild asthma. N Engl J Med. 2018;378(20):1865–1876.
- Bateman ED, Reddel HK, O’Byrne PM, Barnes PJ, Zhong N, Keen C, Jorup C, Lamarca R, Siwek-Posluszna A, FitzGerald JM. As-needed budesonide-formoterol versus maintenance budesonide in mild asthma. N Engl J Med. 2018;378(20):1877–1887. doi:10.1056/NEJMoa1715275.
- Domingo C, Rello J, Sogo A. As-needed ICS-LABA in mild asthma: what does the evidence say? Drugs. 2019;79(16):1729–1737. doi:10.1007/s40265-019-01202-0.
- Beasley R, Holliday M, Reddel HK, Braithwaite I, Ebmeier S, Hancox RJ, Harrison T, Houghton C, Oldfield K, Papi A, et al. Controlled trial of budesonide-formoterol as needed for mild asthma. N Engl J Med. 2019;380(21):2020–2030. doi:10.1056/NEJMoa1901963.
- Hardy J, Baggott C, Fingleton J, Reddel HK, Hancox RJ, Harwood M, Corin A, Sparks J, Hall D, Sabbagh D, et al. Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (PRACTICAL): a 52-week, open-label, multicentre, superiority, randomised controlled trial. Lancet. 2019;394(10202):919–928. doi:10.1016/S0140-6736(19)31948-8.
- Nakamura Y, Tamaoki J, Nagase H, Yamaguchi M, Horiguchi T, Hozawa S, Ichinose M, Iwanaga T, Kondo R, Nagata M, et al. Japanese guidelines for adult asthma 2020. Allergol Int. 2020;69(4):519–548.
- Spanish guide for asthma management (GEMA) 5.0; 2020. https://www.gemasma.com/ [last accessed 7 September 2021].
- Yang CL, Hicks EA, Mitchell P, Licskai C, Dell SD, Rowe BH, Fitzgerald M, Leigh R, Watson W, Boulet LP. 2021 Canadian Thoracic Society guideline – a focused update on the management of very mild and mild asthma. Can J Respir Crit Care Sleep Med. 2021;5(4):205–245. doi:10.1080/24745332.2021.1877043.
- Kawamatawong T, Sangasapaviriya A, Saiphoklang N, Oer-Areemitr N, Sriprasart T, Kamalaporn H, Amnuaypattanapon K, Rerkpattanapipat T, Chirakalwasan N, Kulpraneet M, et al. Guidelines for the management of asthma in adults: evidence and recommendations. Asian Pac J Allergy Immunol. 2021. doi:10.12932/AP-210421-1118.
- Boulkedid R, Abdoul H, Loustau M, Sibony O, Alberti C. Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLoS One. 2011;6(6):e20476. doi:10.1371/journal.pone.0020476.
- Thompson M. Considering the implication of variations within Delphi research. Fam Pract. 2009;26(5):420–424. doi:10.1093/fampra/cmp051.
- Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32(4):1008–1015.
- Beiderbeck D, Frevel N, von der Gracht HA, Schmidt SL, Schweitzer VM. Preparing, conducting, and analyzing Delphi surveys: cross-disciplinary practices, new directions, and advancements. MethodsX. 2021;8:101401. doi:10.1016/j.mex.2021.101401.
- Slade SC, Dionne CE, Underwood M, Buchbinder R. Standardised method for reporting exercise programmes: protocol for a modified Delphi study. BMJ Open. 2014;4(12):e006682. doi:10.1136/bmjopen-2014-006682.
- Diamond IR, Grant RC, Feldman BM, Pencharz PB, Ling SC, Moore AM, Wales PW. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014;67(4):401–409. doi:10.1016/j.jclinepi.2013.12.002.
- Vogel C, Zwolinsky S, Griffiths C, Hobbs M, Henderson E, Wilkins E. A Delphi study to build consensus on the definition and use of big data in obesity research. Int J Obes (Lond). 2019;43(12):2573–2586. doi:10.1038/s41366-018-0313-9.
- Larenas-Linneman D, Salas-Hernández J, Del Río-Navarro BE, Luna-Pech JA, Navarrete-Rodríguez EM, Gochicoa L, Cano-Salas MC, García-Ramírez UN, López-Estrada C, Ortega-Martell JA, et al. Manejo Integral del Asma. Lineamientos para México (Mexico Asthma Guidelines). Rev Alerg Mex. 2021;68(Suppl 1):s1–s122.
- Shiba K, Kasahara K, Nakajima H, Adachi M. Structural changes of the airway wall impair respiratory function, even in mild asthma. Chest. 2002;122(5):1622–1626. doi:10.1378/chest.122.5.1622.
- Chetta A, Foresi A, Del Donno M, Bertorelli G, Pesci A, Olivieri D. Airways remodeling is a distinctive feature of asthma and is related to severity of disease. Chest. 1997;111(4):852–857. doi:10.1378/chest.111.4.852.
- Sandström T. Effects of pharmacological and non-pharmacological interventions. Clin Respir J. 2010;4 (Suppl 1):41–48. doi:10.1111/j.1752-699X.2010.00196.x.
- The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343:1054–1063.
- Haahtela T, Järvinen M, Kava T, Kiviranta K, Koskinen S, Lehtonen K, Nikander K, Persson T, Selroos O, Sovijärvi A. Effects of reducing or discontinuing inhaled budesonide in patients with mild asthma. N Engl J Med. 1994;331(11):700–705. doi:10.1056/NEJM199409153311103.
- Boulet LP. Asymptomatic airway hyperresponsiveness: a curiosity or an opportunity to prevent asthma? Am J Respir Crit Care Med. 2003;167(3):371–378. doi:10.1164/rccm.200111-084PP.
- Woolcock AJ. What are the important questions in the treatment of asthma? Clin Exp Allergy Rev. 2001;1(2):62–64. doi:10.1046/j.1472-9725.2001.00007.x.
- Bergeron C, Tulic MK, Hamid Q. Airway remodelling in asthma: from benchside to clinical practice. Can Respir J. 2010;17(4):e85–e93. doi:10.1155/2010/318029.
- Harmsen L, Ulrik CS, Porsbjerg C, Thomsen SF, Holst C, Backer V. Airway hyperresponsiveness and development of lung function in adolescence and adulthood. Respir Med. 2014;108(5):752–757.
- Jayaram L, Pizzichini MM, Cook RJ, Boulet LP, Lemière C, Pizzichini E, Cartier A, Hussack P, Goldsmith CH, Laviolette M, et al. Determining asthma treatment by monitoring sputum cell counts: effect on exacerbations. Eur Respir J. 2006;27(3):483–494. doi:10.1183/09031936.06.00137704.
- Muneswarao J, Hassali MA, Ibrahim B, Saini B, Ali Hyder Ali I, Verma AK. It is time to change the way we manage mild asthma: an update in GINA 2019. Respir Res. 2019;20(1):183.
- Boushey HA, Sorkness CA, King TS, Sullivan SD, Fahy JV, Lazarus SC, Chinchilli VM, Craig TJ, Dimango EA, Deykin A, et al. National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Daily versus as-needed corticosteroids for mild persistent asthma. N Engl J Med. 2005;352(15):1519–1528. doi:10.1056/NEJMoa042552.
- Turkish Thoracic Society and Turkish Society of Allergy and Clinical Immunology Guideline on asthma diagnosis and management, 2020 update. Çelik GE Ed., Buluş Tasarım Matbaacılık. Ankara-Turkey. SBN: 978-605-74980-0-7; 2020. https://www.toraks.org.tr/site/community/library/ZNCCbNLFjQgfmgnI [last accessed 24 August 2021].
- Aggarwal B, Shantakumar S, Hinds D, Mulgirigama A. Asia-Pacific Survey of Physicians on Asthma and Allergic Rhinitis (ASPAIR): physician beliefs and practices about diagnosis, assessment, and treatment of coexistent disease. J Asthma Allergy. 2018;11:293–307. doi:10.2147/JAA.S180657.
- Shaw DE, Heaney LG, Thomas M, Beasley R, Gibson PG, Pavord ID. Balancing the needs of the many and the few: where next for adult asthma guidelines? Lancet Respir Med. 2021;9(7):786–794.
- Cerveri I, Locatelli F, Zoia MC, Corsico A, Accordini S, de Marco R. International variations in asthma treatment compliance: the results of the European Community Respiratory Health Survey (ECRHS). Eur Respir J. 1999;14(2):288–294. doi:10.1034/j.1399-3003.1999.14b09.x.
- Eakin MN, Rand CS. Improving patient adherence with asthma self-management practices: what works? Ann Allergy Asthma Immunol. 2012;109(2):90–92. doi:10.1016/j.anai.2012.06.009.
- Mäkelä MJ, Backer V, Hedegaard M, Larsson K. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med. 2013;107(10):1481–1490. doi:10.1016/j.rmed.2013.04.005.
- Ward C, Pais M, Bish R, Reid D, Feltis B, Johns D, Walters EH. Airway inflammation, basement membrane thickening and bronchial hyperresponsiveness in asthma. Thorax. 2002;57(4):309–316. doi:10.1136/thorax.57.4.309.
- Gibbons DC, Aggarwal B, Fairburn-Beech J, Hinds D, Fletcher M, Bosnic-Anticevich S, Price D. Treatment patterns among non-active users of maintenance asthma medication in the United Kingdom: a retrospective cohort study in the Clinical Practice Research Datalink. J Asthma. 2021;58(6):793–804. doi:10.1080/02770903.2020.1728767.
- Dhruve H. Management of asthma: adherence, inhaler technique and self-management. Pract Nursing. 2018;29(10):465–468. doi:10.12968/pnur.2018.29.10.465.
- Bousquet J, Winchester C, Papi A, Virchow JC, Haughney J, Costa D, Usmani O, Bjermer L, Price D, Global Allergy and Asthma European Network (GA²LEN). Inhaled corticosteroid/long-acting β2-agonist combination therapy for asthma: attitudes of specialists in Europe. Int Arch Allergy Immunol. 2012;157(3):303–310. doi:10.1159/000329519.
- Harding G, Leidy NK, Meddis D, Kleinman L, Wagner S, O’Brien CD. Interpreting clinical trial results of patient-perceived onset of effect in asthma: methods and results of a Delphi panel. Curr Med Res Opin. 2009;25(6):1563–1571. doi:10.1185/03007990902914403.
- Sheikh A, Major P, Holgate ST. Developing consensus on national respiratory research priorities: key findings from the UK Respiratory Research Collaborative’s e-Delphi exercise. Respir Med. 2008;102(8):1089–1092. doi:10.1016/j.rmed.2008.03.006.