Abstract
Introduction
Over the last decades, dietary habits in developing countries have been characterized by low intake of fruits and vegetables and high consumption of sweetened drinks. Most of the evidence linking carbohydrate intake and asthma comes from children over 6 years of age. The aim of this study was to examine the association between macronutrient intake and the severity of asthma exacerbations in children aged 2–6 years.
Methods
We performed a cross-sectional study that included all children aged 2–6 years hospitalized due to an asthma exacerbation. Dietary data were collected using a food frequency questionnaire (FFQ) validated in the Colombian population. The GINA classification of acute asthma was used to define the severity. To identify factors independently associated with asthma exacerbation severity, we fit the data to ordinal logistic regression.
Results
During the study period, 228 cases of patients with asthma exacerbation were included. Asthma severity was dose-dependently associated with protein and carbohydrate-rich intake. The variables included in the multivariable analysis included reactive C protein (OR 1.05, CI 95% (1.03–1.07)), smoking at home (OR 3.92 (1.82–8.44)), atopic dermatitis (OR 3.82 (1.59–9.21)), and protein and carbohydrate-rich food intake (OR 0.11 (0.03–0.33)) and (OR 2.42 (1.09–5.80)), respectively.
Conclusion
High carbohydrate-rich food intake is associated with the severity of asthma exacerbation adjusted by other known risk factors such as atopy, smoking, and reactive C protein. This evidence should motivate the development of public health policies to control the consumption of sugar-rich products in children under 6 years.
Keywords:
Introduction
Asthma is the most frequent chronic disease in children, and its prevalence is increasing in low- and middle-income countries (Citation1). Changes in environmental factors and dietary patterns can explain this trend over the past few decades (Citation2). The consumption of a healthy diet of fruits, vegetables, fish, and olive oil can moderate airway inflammation and protect against asthmatic symptoms in children (Citation3,Citation4). Obesity is associated with an increased risk of asthma and more frequent and severe exacerbations, reduced response to several asthma medications, and decreased quality of life (Citation3,Citation4).
Over the last few decades, dietary habits in developing countries have been characterized by low intake of fruits and vegetables and high consumption of sugary drinks (Citation5). In children older than 6 years, the frequent consumption of vegetables and grains has been shown to be associated with a lower risk of asthma, these findings explained by dietary effects on interleukin-17F-dependent pathways (Citation6). A healthy diet, with frequent consumption of vegetables and grains and low consumption of dairy products and sweets, has been shown to be associated with higher lung function (as measured by FEV1 and FVC) (Citation7). Current wheeze in children between 6 and 7 years has been shown to be associated with a high-carbohydrate diet (a selection of foods in which more than 65% of the caloric composition comes from carbohydrates) (OR: 1.76, 95% CI: 1.32–2.35) (Citation8). In children under 2 years of age, severe bronchiolitis has been shown to be dose-dependently associated with ingestion four or more times per week of bread, pastries, sugar-sweetened beverages, sweetened infusions, pasta, rice, and potatoes during pregnancy (OR 3.29 95% CI, 1.15–9.44] (Citation9). A systematic review of observational studies in children older than 6 years reported that the consumption of a fast-food diet high in fats and carbohydrates also increased the risk of recurrent wheezing (OR: 1.21; 95% CI: 1.16–1.27) (Citation10). In conclusion, there is evidence of the negative role of high carbohydrate intake on the severity of asthma in children older than 6 years and even in wheezers less than 2 years of age. However, the population between 2 and 6 years of age constitutes a diagnostic challenge, since not all patients who wheeze are as associated with a specific respiratory virus, as are those under 1 year of age, nor do they necessarily become true asthmatics in the future. It is possible that this association between macronutrients and asthma is diluted because of this etiological heterogeneity. The relevance of clarifying the role of diet in asthma is of great importance, since it is potentially a modifiable risk factor. The aim of this study was to examine the association between macronutrient intake and the severity of asthma exacerbations in children aged 2–6 years.
Methods
Subjects
We performed a cross-sectional study that included all children aged 2–6 years hospitalized due to an asthma exacerbation in a tertiary center in Rionegro, Colombia between January 2019 and December, 2019. Rionegro is Colombian municipality of the department of Antioquia, at 2200 meters above sea level located in the subregion of Eastern Antioquia. According to the figures presented by the National Administrative Department of Statistics (DANE) in the 2015 census, the municipality of Rionegro had a total population of 101,046 inhabitants, the sixth largest populated area in Antioquia., with two tertiary referral hospitals (Citation11). The ethnicity of the population is almost entirely of Mestizo and White persons (98.9%) with 1.1% Afro Colombian. 48.6% of the population are men and 51.4% women. Public services in Rionegro are of a relatively high standard with 98.7% of houses supplied with electricity, 95.6% with a water supply and 87.1% (Citation11).
The inclusion criteria were defined as children aged 2–6 years who were admitted to the emergency department and diagnosed with an asthma exacerbation. Patients without respiratory symptoms or with positive bacterial cultures on admission, or a confirmed whooping cough (culture or PCR) were excluded. The study protocol was approved by the Institutional Review Board of the University of Antioquia (No 18/2015). Informed consent was obtained from the parents. The privacy and the confidentiality of the information were guaranteed.
Study procedures and outcome definition
We collected the following variables: age, sex, weight, height, signs, and symptoms on admission related to bronchiolitis (including fever, chest auscultation, oxygen saturation, etc.), complete vaccination record, current exposure (maternal or paternal) to cigarette smoking, history of prematurity and bronchopulmonary dysplasia confirmed by a neonatologist upon discharge from the neonatal intensive care unit, comorbidities (congenital heart disease, neurological disease), diagnostic tools such as chest X-rays, complete blood count, bacterial cultures, etc. In our hospitals, bronchodilators and systemic steroids are used at the discretion of attending physicians, in accordance with national clinical bronchiolitis and asthma guidelines (Citation12). Dietary data were collected for all children, using a food frequency questionnaire (FFQ) in Spanish, validated in children aged between 3 and 6 years (Citation13). This tool is a quantitative FFQ, the Spanish version can be accessed directly at the publication (Citation13). This questionnaire was completed by the parent or caretaker living with the patient under the supervision of a trained member of the research team. The foods included in the tool reflect those that are traditionally consumed in urban and rural areas in Colombia. This questionnaire version requests that respondents estimate their daily, weekly, monthly, and rarely/never consumption frequency of individual foods (e.g. bread). The participants were categorized into quartiles based on their reported intake frequency, with the lowest quartile serving as a reference group for each of the food categories. Given the 90-day period of reference and consequent concerns about recall of portion size (Citation14), our study did not collect information about daily portion sizes. Vitamin D levels were assessed by measuring serum 25-hydroxycholecalciferol (25-OH vitamin D). Serum 25-OH vitamin D was measured using a Liaison® 25-OH Vitamin D Total assay kit with the Liaison® rapid automated assay system (DiaSorin, Stillwater, MN, USA). 25-OH vitamin D levels were categorized as deficient (<20 ng/ml), insufficient (20–29 ng/ml), or sufficient (>29 ng/ml) (Citation15). To determine the frequency of malnutrition or obesity, the z-score was calculated and then the nutritional classification was determined according to WHO reference standards for BMI/age, using the WHO ANTHRO software for children under 5 years of age. Asthma exacerbation was defined as a visit to the ED/urgent care or hospitalization for asthma, requiring treatment with systemic corticosteroids (oral, intramuscular, or intravenous) for at least 3 days of hospitalization. The GINA classification of acute asthma in children 5 years and younger was used to define the severity levels of the asthma (Citation12).
Statistical analysis
Clinical and sociodemographic variables information was compared between groups (asthma exacerbation: mild, moderate, or severe) with Student’s t-tests or Kruskal-Wallis tests for continuous variables and the Chi-squared test or Fisher’s exact test for categorical variables. To identify factors independently associated with asthma exacerbation severity, we fit the data to ordinal logistic regression. Initially, the regression model only included variables associated with asthma exacerbation severity, with values of p < 0.2 or that changed the effect estimate by over 10% after their inclusion. Ordinal logistic regression was performed using a p values of 0.05 as the limit value for the model entry. To test the goodness of fit of the model, we calculated the ordinal version of the Hosmer-Lemeshow test, the Pulkstenis-Robinson chi-squared and deviance tests, and the Lipsitz likelihood-ratio test with a single “ologitgof” command. The regression results are reported as odds ratios and their respective 95% confidence intervals (CI). All statistical tests were two-tailed, and the significance level used was p < 0.05. The data were analyzed with the Statistical Package Stata 17.0 (Stata Corporation, College Station, TX, USA).
Results
Study population
During the study period, 228 cases of patients with asthma exacerbation were included. The mean age of patients was 3.8 years (SD 0.42), with 51% female, 98% were whites and mestizos. The median of hospitalization stay was 5.40 days. Twenty-four patients (10.5%) were admitted to PICU, 7 of them needed mechanical ventilation, and no deaths were observed. The mean of previous hospitalization during the preceding year for asthma exacerbation was 1.5 (SD 1.04). Seventy-nine percent of the patients reported having used some inhaled corticosteroids as a controller (93% beclometasone and 7% fluticasone), and 93% were classified as inadequate due to use as needed or sub-therapeutic dose. Eleven percent of the patients had symptoms or a previous history of gastroesophageal reflux disease, and eight patients reported some familiar contact with tuberculosis, all with a negative tuberculin and bacteriological diagnosis. None reported parents or siblings with cystic fibrosis, and eight patients were in a study for some primary immunodeficiency, but none with a confirmed diagnosis. Twenty-eight percent of the patients had atopic dermatitis, and 77% allergic rhinitis. Forty-four patients (22%) had their exacerbation classified as mild, 45% moderate, and 35% severe, .
Table 1. Sociodemographic and clinical characteristics of the study population.
Multivariable analysis
There were statistical differences between asthma exacerbation severity levels in the proportion of patients with premature birth, atopic dermatitis, smoking at home, previous hospitalization for asthma, complete vaccination for age, school attendance, protein- and carbohydrate-rich intake, and reactive C protein. Asthma severity was dose-dependently associated with protein- and carbohydrate-rich intake, and . As was expected, the proportion of pneumonia associated with exacerbation and median hospitalization stay was higher in patients with severe and moderate exacerbation. After control for age, weight, and underlying chronic illness, the variables included in the multivariable analysis were reactive C protein (OR 1.05, CI 95% (1.03–1.07)), smoking at home (OR 3.92, CI 95% (1.82–8.44)), atopic dermatitis (OR 3.82, CI 95% (1.59–9.21)), and protein- and carbohydrate-rich food intake (OR 0.11, CI 95% (0.03–0.33)) and (OR 2.42, CI 95% (1.09–5.80)), respectively, .
Figure 1. Carbohydrate intake and asthma severity. Carbohydrate-rich food groups: bread, pastries, sugar-sweetened beverages, sweetened infusions, pasta, rice, and potatoes. Patients were divided into quartiles based on the volume of high carbohydrate foods or fruits and vegetables or fat rich food intake they consumed, being high intake or quartile 4 = 75–100% intake score versus reference group (0–25%) for example.
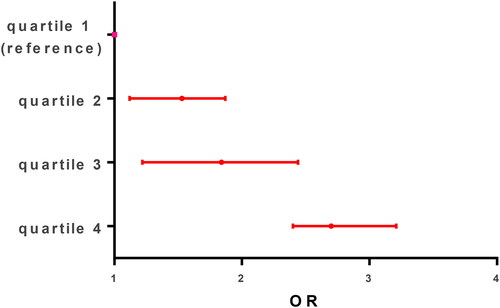
Figure 2. Protein intake and asthma severity. Patients were divided into quartiles based on the volume of protein-rich food groups included red meat, poultry, and fish, being high intake or quartile 4 = 75–100% intake score versus reference group (0–25%) for example.
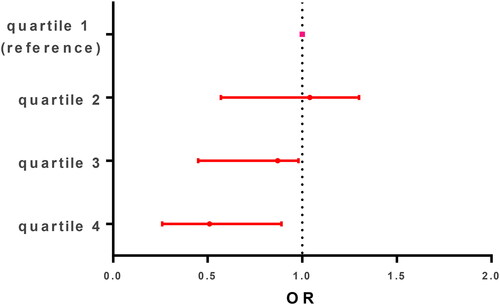
Table 2. Factors independently associated with severity of asthma exacerbation.
Discussion
In this article, we addressed important questions about the influence of macronutrients on the severity of asthma exacerbations in children between 2 and 6 years old. We found a positive association between a high carbohydrate-rich food intake and the severity of asthma exacerbations adjusted by other known risk factors such as atopy, smoking, and reactive C protein. Also, we found a negative association between the severity of the asthma exacerbation and high protein-rich food intake, adjusted for the factors mentioned above.
In previous studies, intake of carbohydrate-rich food has been shown to be associated with a risk of wheeze (Citation8). This diet promotes a pro-inflammatory environment by alteration of microbiota balances (Citation16). This pro-inflammatory environment has a negative effect on general immunity and the airways, favoring rapid progression to severe asthma (Citation17). Previously, evidence in asthmatic children aged 6–7 years showed that intake of fast food three or more times per week was statistically significantly associated with current wheeze (OR: 1.74; 95% CI: 1.30–2.34) (Citation8). Also, a frequent intake of fast food was positively associated with a higher prevalence of eczema and rhino-conjunctivitis in older children (ORs 1.32; 95% CI 1.22, 1.43, and OR 1.32; 95% CI 1.24, 1.40, respectively) in a multicenter study in eleven Latin American countries in children aged 6–7 years (Citation18). A systematic review of 16 observational studies reported that asthma was associated with the consumption of fast foods (OR: 1.34; 95% CI: 1.23–1.46). This review showed that the consumption of fast foods increased the risk of current wheeze in five cross-sectional studies (OR: 1.21; 95% CI: 1.16–1.27) and history of wheeze in one cross-sectional study (OR: 1.65; 95% CI: 1.07–2.52) (Citation10). Our study focused on children less than 6 years old, which usually is a population with more heterogeneity in their risk of asthma than the above-mentioned studies. Despite this heterogeneity, we found an association similar to that reported in studies with older children. The proinflammatory effect of consuming a high-carbohydrate diet is consistent throughout childhood, as shown by the evidence in children under 2 years of age, children over 6 years of age, and our evidence in children between 2 and 6 years of age (Citation9,Citation18). In our study the proportion of patients with obesity were higher in patients with severe exacerbation than moderate or mild exacerbation, but this difference was not statistically significant may be due to small sample size; however we adjust all variables in the multivariate regression with the weight due previous literature on the topic.
A negative association between severity of asthma exacerbations and high protein-rich food intake has been reported previously. Our results are in line with previous evidence. In Colombia, in an ISAAC analysis of children aged 6–7 years, a regular intake of milk was negatively associated with eczema (OR: 0.33; 95% CI: 0.23–0.47) (Citation8). A 2013 systematic review reported negative associations between a Mediterranean diet adherence in children and 3 respiratory outcomes: “current wheeze” (P = 0.02), “current severe wheeze” (P = 0.008), and “history of asthma” (P = 0.06), when compared with a non-Mediterranean diet in adolescents (Citation19). It cannot be ruled out that high carbohydrate and protein intake are per se surrogate variables of a more general variable such as poor health habits. However, as mentioned, such an association was documented in children with bronchiolitis and asthma older than 6 years. Our study closes this gap by providing evidence in children between 1 and 6 years of age.
In our study, we did not find an association between 25-OH vitamin D levels and the severity of asthma exacerbations. The objective of our study was not to evaluate this association. However, our findings may encourage local researchers to answer this question with the advantage of already having some data in our population. A recent systematic review and meta-analysis of randomized controlled trials on prenatal Vitamin D supplementation and child respiratory health did not find evidence of a relationship in six clinical trials between vitamin D intake during pregnancy and occurrence of asthma in the offspring (RR 0.89, 95% CI 0.69–1.15 I2=46%) (Citation20). This contrasts with evidence from observational studies, where a significant inverse correlation was found between serum levels of 25-OH vitamin D3 and pediatric asthma (r = −0.483, p = 0.001) (Citation21,Citation22). Recent meta-analysis of four randomized clinical trials (RTC) shows that Vitamin D supplementation safely reduced the rate of asthma exacerbation (RR 0.70; 95 CI%, 0.53, 0.92; P = 0.001) with very low heterogeneity, I2 = 0.00%) in adult treated with corticosteroids (Citation23),The role of the effect of vitamin D in asthma is not yet elucidated, and more evidence from controlled clinical trials with supplementation in recurrent asthma is needed to be conclusive.
A few important limitations need to be considered: First, as occurs in longitudinal studies, information bias cannot be excluded. However, we included all patients registered in the hospital, and the reported incidence cannot be attributed to bias of selection. Administrative factors such as inefficiencies in administrative procedures or payments with health insurers can sometimes increase the days of hospital stay, and this was not measured. However, the model obtained was plausible with the variables biologically associated with the severity of asthma and it is unlikely that these administrative factors have influenced the results of the final model. Also, we do not include in specific questions for plant-based proteins, exposure to microbial substances, exposure to air pollutants, diet in pregnancy and nutrition during the first year of life or if the children receive prebiotics and/or probiotics were not measured in our study and are beyond the scope of this study. We recognize that they may have some association and declare the limitation of our study by not having measured them and having the respective confounding bias. Our study only performs one measurement of the nutrition over time using FFQ. Other studies should evaluate the impact of nutritional temporal variations in the incidence of asthma, with the advantage of already having initial estimates such as those provided by our study.
In conclusion, high carbohydrate-rich food intake was associated with severity of asthma exacerbation adjusted by other known risk factors such as atopy, smoking, and reactive C protein. Also, we found a negative association between the severity of the asthma exacerbation and a high protein-rich food intake, adjusted by the above-mentioned factors. This evidence should motivate the development of public health policies to control the consumption of sugar-rich products in children under 6 years of age.
Author’s contributions
JAB, RAC, HLT participated in designing the work and the acquisition, analysis, and interpretation of data. All authors approved the version to be published.
Source(s) of support: own funds
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Asher MI, Montefort S, Björkstén B, Lai CKW, Strachan DP, Weiland SK, Williams H; ISAAC Phase Three Study Group. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368(9537):733–743. doi:10.1016/S0140-6736(06)69283-0.
- Han YY, Blatter J, Brehm JM, Forno E, Litonjua AA, Celedon JC. Diet and asthma: vitamins and methyl donors. Lancet Respir Med. 2013;1(10):813–822. doi:10.1016/S2213-2600(13)70126-7.
- Julia V, Macia L, Dombrowicz D. The impact of diet on asthma and allergic diseases. Nat Rev Immunol. 2015;15(5):308–322. doi:10.1038/nri3830.
- Nurmatov U, Devereux G, Sheikh A. Nutrients and foods for the primary prevention of asthma and allergy: systematic review and meta-analysis. J Allergy Clin Immunol. 2011;127(3):724–733. doi:10.1016/j.jaci.2010.11.001.
- Agostoni C, Silano M, Fattore G. Health implications of dietary habits in transition countries-a life course perspective. Pediatr Res. 2018;83(4):754–756. doi:10.1038/pr.2017.319.
- Han Y-Y, Forno E, Brehm JM, Acosta-Pérez E, Alvarez M, Colón-Semidey A, Rivera-Soto W, Campos H, Litonjua AA, Alcorn JF, et al. Diet, interleukin-17, and childhood asthma in Puerto Ricans. Ann Allergy Asthma Immunol. 2015;115(4):288–293 e1. doi:10.1016/j.anai.2015.07.020.
- Han Y-Y, Forno E, Alvarez M, Colón-Semidey A, Acosta-Perez E, Canino G, Celedón JC. Diet, lung function, and asthma exacerbations in Puerto Rican children. Pediatr Allergy Immunol Pulmonol. 2017;30(4):202–209. doi:10.1089/ped.2017.0803.
- Cepeda A, Del Giacco S, Villalba S, Tapias E, Jaller R, Segura A, Reyes G, Potts J, Garcia-Larsen V. A Traditional diet is associated with a reduced risk of eczema and wheeze in colombian children. Nutrients. 2015;7(7):5098–5110. doi:10.3390/nu7075098.
- Ferolla FM, Hijano DR, Acosta PL, Rodríguez A, Dueñas K, Sancilio A, Barboza E, Caría A, Gago GF, Almeida RE, et al. Macronutrients during pregnancy and life-threatening respiratory syncytial virus infections in children. Am J Respir Crit Care Med. 2013;187(9):983–990. doi:10.1164/rccm.201301-0016OC.
- Wang CS, Wang J, Zhang X, Zhang L, Zhang HP, Wang L, Wood LG, Wang G. Is the consumption of fast foods associated with asthma or other allergic diseases? Respirology. 2018;23(10):901–913. doi:10.1111/resp.13339.
- (DANE) DNdE. Archivo nacional de datos 2019. Available from: https://sitios.dane.gov.co/anda-index/.
- Asthma GIf. Global Strategy for Ashtma Management and Prevention 2021. . Available from: https://ginasthma.org/ [last accessed 6 May 2021].
- Esteban-Figuerola P, Jardí C, Canals J, Arija V. Validación de un cuestionario corto de frecuencia de consumo alimentario en niños pequeños. Nutrición Hospitalaria. 2020;37:101–113.
- Cade J, Thompson R, Burley V, Warm D. Development, validation and utilisation of food-frequency questionnaires - a review. Public Health Nutr. 2002;5(4):567–587. doi:10.1079/PHN2001318.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Endocrine Society Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi:10.1210/jc.2011-0385.
- Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat Immunol. 2011;12(1):5–9. doi:10.1038/ni0111-5.
- Relman DA. Microbial genomics and infectious diseases. N Engl J Med. 2011;365(4):347–357. doi:10.1056/NEJMra1003071.
- Cepeda AM, Thawer S, Boyle RJ, Villalba S, Jaller R, Tapias E, Segura AM, Villegas R, Garcia-Larsen V, ISAAC Phase III Latin America Group. Diet and respiratory health in children from 11 Latin American countries: Evidence from ISAAC Phase III. Lung. 2017;195(6):683–692. doi:10.1007/s00408-017-0044-z.
- Alwarith J, Kahleova H, Crosby L, Brooks A, Brandon L, Levin SM, Barnard ND. The role of nutrition in asthma prevention and treatment. Nutr Rev. 2020;78(11):928–938. doi:10.1093/nutrit/nuaa005.
- Tareke AA, Hadgu AA, Ayana AM, Zerfu TA. Prenatal vitamin D supplementation and child respiratory health: A systematic review and meta-analysis of randomized controlled trials. World Allergy Organ J. 2020;13(12):100486. doi:10.1016/j.waojou.2020.100486.
- Mohammadzadeh I, Darvish S, Qujeq D, Hajiahmadi M, Vaghari-Tabari M. Association of serum 25-OH vitamin D(3) with serum IgE and the Pediatric Asthma Severity Score in patients with pediatric asthma. Allergy Asthma Proc. 2020;41(2):126–133. doi:10.2500/aap.2020.41.190025.
- Dogru M, Seren LP. Serum 25-hydroxyvitamin D levels in children with recurrent wheezing and relation to the phenotypes and frequency of wheezing. Eur Ann Allergy Clin Immunol. 2017;49(06):257–262. doi:10.23822/EurAnnACI.1764-1489.14.
- Chen Z, Peng C, Mei J, Zhu L, Kong H. Vitamin D can safely reduce asthma exacerbations among corticosteroid-using children and adults with asthma: a systematic review and meta-analysis of randomized controlled trials. Nutr Res. 2021;92:49–61. doi:10.1016/j.nutres.2021.05.010.
