Abstract
Objective
To assess the cost-effectiveness of benralizumab (benra) vs. mepolizumab (mepo) and dupilumab (dupi) for the treatment of patients with severe uncontrolled asthma from the Spanish Health System perspective.
Methods
Exacerbations avoided, quality-adjusted life years (QALYs) gained and costs in a 5-year period were estimated with a Markov model for a cohort of 1,000 patients in which, based on published evidence, 31% of the patients received biologics + oral corticosteroids (OCS) and 69% received only biologics. Efficacy data (exacerbation reduction and OCS elimination) were derived from a matching-adjusted indirect comparison. Published EQ-5D utilities per health state (biologic alone, biologic + OCS, standard of care + OCS, exacerbations, and post-exacerbations) were used for QALY estimation. Utility decrements associated with exacerbation management [-0.1 (OCS or emergency visits), −0.2 (hospitalization)] derived from the literature were applied. Costs (€, 2022) included drug acquisition (ex-factory price), administration and disease management. An expert panel (2 pneumologists and 1 pharmacist) validated all inputs.
Results
Benra was more effective (52.21 QALYs) than mepo (51.39 QALYs) and dupi (51.30 QALYs). Benra avoided more exacerbations (2.87 exacerbations) compared to mepo (4.70 exacerbations) and dupi (5.11 exacerbations) for the 5-year horizon. Total costs/patient were €56,093.77 (benra), €59,280.45 (mepo) and €62,991.76 (dupi), resulting in benra dominating (more QALYs with lower costs) vs. mepo and dupi.
Conclusions
Benralizumab can be considered as a dominant treatment alternative vs. other biologic drugs for the treatment of uncontrolled severe eosinophilic asthma patients in Spain.
Introduction
Asthma is a chronic inflammatory disease of the airways characterized by a variety of symptoms, such as wheezing, shortness of breath, chest tightness and/or cough, and various airflow limitations (Citation1). This pathology is recognized as a highly prevalent chronic respiratory disease, affecting approximately 30 million people in Europe (Citation2) and 4.9% of the adult population in Spain (Citation3).
Asthma is a complex and heterogeneous disease, where the treatment goal is to achieve and maintain disease control by minimizing the clinical expression of asthma, considering the manifestations of the disease present in the patient’s daily life, and the prevention of future risk, especially in the development of exacerbations and chronic airflow obstruction. These manifestations can potentially be a risk to the patient’s life and burden to both patients and society (Citation1,Citation4).
The occurrence of exacerbations, severity of the disease and frequency of asthmatic episodes depend on patient characteristics and asthma phenotype (Citation4). Severe eosinophilic asthma is a phenotype characterized by eosinophilic inflammation that affects approximately 80% of patients with severe asthma (Citation5). Uncontrolled severe asthma is defined as asthmatic disease that remains poorly controlled despite increasing stepwise treatment to the point of being treated with a combination of high-dose inhaled corticosteroids (ICS) along with long-acting β2-agonist (LABA) for the last year or oral corticosteroids (OCS) for at least six months (Citation4,Citation6). These patients experience symptoms and exacerbations more often and are also at an increased risk of adverse events associated with more intensive drug treatment, leading to a high burden and health care costs due to the consumption of health care resources such as additional drugs, specialist visits, hospitalizations, and management of adverse events (Citation1,Citation7). In relation to the economic impact, a study conducted in Spain estimated that patients with severe asthma incurred an average annual direct cost of 7,472 euros per patient, 85% of which was attributable to pharmaceutical treatment (Citation7). Therefore, severe asthma has a high clinical, humanistic, and economic impact on the overall burden of asthma (Citation8,Citation9).
In relation to pharmaceutical treatment, a regular assessment is recommended to verify whether the patient’s treatment objectives are met and to be able to adjust treatment according to the level of control of the disease. For this, it is necessary to follow an individualized strategy for each patient considering the most effective therapeutic options, the safety and cost of the different alternatives, and the patient preference (Citation4). In recent years, the incorporation of biologic therapies in patients with severe uncontrolled asthma has become more important due to the reduction of exacerbations, hospitalizations, and reduced use of OCS (Citation4,Citation10). One such example is benralizumab, a humanized, anti-interleukin-5 receptor α monoclonal antibody that induces rapid and near-complete depletion of eosinophils through enhanced antibody-dependent cell-mediated cytotoxicity. In 2018, benralizumab was approved by the European Medicines Agency (EMA) as an additional maintenance treatment in adult patients with severe eosinophilic asthma inadequately controlled despite high-dose ICS plus LABA (Citation11).
The objective of the present analysis was to assess the cost-effectiveness of benralizumab in comparison with mepolizumab and dupilumab for the treatment of patients with severe uncontrolled asthma in Spain.
Methods
Model structure
A cost-effectiveness model was developed in Microsoft Excel® to simulate the progression of a hypothetical cohort of 1,000 adult patients with severe uncontrolled eosinophilic asthma on biologic therapy with or without concomitant OCS. The clinical characteristics of this cohort, including average age (55.30 years) and the proportion of men (29.20%), were defined from the population included in the ENEAS study (Citation12), considered to be representative of the Spanish adult population suffering from severe asthma.
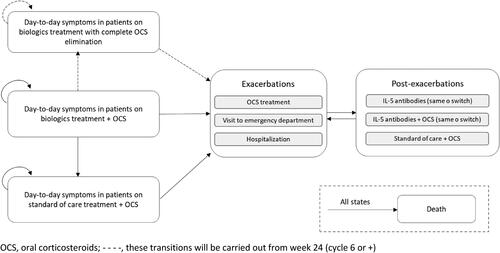
The Markov model used for the present analysis represents, in 4-week cycles, the disease course through 6 main mutually exclusive health states: day-to-day symptoms in patients on biologic therapy with complete OCS elimination, day-to-day symptoms in patients on biologic therapy + OCS, day-to-day symptoms in patients on standard of care (SOC) treatment + OCS, exacerbations, post-exacerbations and death as absorbent state ().
At the initiation of the simulation, based on the opinion of the experts consulted, 30.82% of the patient cohort was considered to receive biologic therapy + OCS and 69.18% received only biologic therapy according to the data available from the REDES study (Citation13). Throughout the simulation, to reflect the potential evolution of the patient in real life, patients could remain in or transition through the health states described previously. However, due to the evidence available, patients from the health state "day-to-day symptoms in patients on biologic therapy + OCS" could transition to the health state "day-to-day symptoms in patients on biologic therapy with complete OCS elimination" only from week 24 onwards. For the health state "exacerbations", three types of events were considered: 1) exacerbations requiring treatment with OCS, 2) exacerbations requiring a visit to the emergency department or 3) exacerbations requiring hospital admission. After experiencing an exacerbation, patients transitioned to the health state "post-exacerbation", where they could be treated with the same biologic or switched to another biologic, with the same biologic + OCS or switched to another biologic + OCS or with SOC + OCS. Finally, patients can transition to the health state "death" from any health state at any moment in the simulation ().
A 5-year time horizon was applied to estimate exacerbations avoided, quality-adjusted life years (QALYs) gained and the total costs of the therapies considered in the model. The perspective of the Spanish National Health System (NHS) was adopted for the analysis. In accordance with the recommendations of the Spanish guidelines for economic evaluations (Citation14), the analysis used a yearly discount rate of 3.0% for both costs and health outcomes.
The model was designed and conceptualized by health economic experts. Subsequently, all input values and the model structure were validated and approved by a panel of 3 clinical experts from different geographical areas with the aim of reflecting Spanish clinical practice. For this, online questionnaires were individually completed by the members of the expert panel, and subsequently, a consensus meeting was conducted to agree on values and assumptions when needed.
Treatment alternatives
The therapeutic alternatives compared in the analysis comprised benralizumab (Fasenra®), mepolizumab (Nucala®) or dupilumab (Dupixent®), as they are part of the current therapeutic approach for patients in Spain. In addition, these alternatives were used in the adjusted indirect comparison identified in the literature (Citation15).
For biologic therapy with or without OCS, the regimens and doses of treatment observed in the clinical trials (Citation16–18) and the summaries of product characteristics (Citation11,Citation19,Citation20) of each alternative were used ().
For SOC + OCS treatment, an average cost identified in the literature was used that considered a pool of the following treatments: ICS, LABA, short-acting beta agonists, anti-leukotriene, theophylline, and prednisolone (Citation21).
Clinical data
To the best of the authors’ knowledge, there are no available head-to-head clinical trials studying the alternatives evaluated. Efficacy data were obtained from published MAIC regarding the efficacy of benralizumab versus mepolizumab and dupilumab (Citation15). The primary efficacy outcomes considered in the analysis were the elimination of OCS use as well as the reduction of exacerbations. Regarding OCS elimination, the MAIC data showed an odds ratio of 1.96 for benralizumab versus mepolizumab and 1.48 versus dupilumab. For the annual exacerbation rate reduction, a relative risk of 0.45 was reported for benralizumab versus mepolizumab and 0.75 versus dupilumab (Citation15).
Transition probabilities between each health status were obtained from the available literature (Citation12,Citation15–18). To estimate the transition probabilities for the health state "day-to-day symptoms in patients on SOC + OCS", an annualized rate of 2 exacerbations/year was considered from the available data from the ENEAS study (Citation12). The transition probabilities included in the analysis are summarized in .
Table 1. Clinical data.
The distribution of the management of exacerbations was obtained from the data reported in the UTILITY study (Citation22), and the distribution of patients according to treatment after suffering an exacerbation was established based on the consensus of the expert panel according to usual clinical practice in Spain ().
Mortality
All-cause mortality data were applied to show the annual probability of death by age and sex, derived from Spanish mortality tables (Citation23). In addition, asthma-related mortality was applied in those patients who experienced an exacerbation requiring hospitalization (Citation23).
Utilities and disutilities
To estimate QALYs, different utility values were considered depending on the health states. The term “utility” refers to the quality of their health status perceived by patients and an assigned value between 0 and 1, where 0 represents death and 1 represents perfect health.
The utility values were taken from the UTILITY study (Citation22) using the EuroQol 5-Dimensions with 5 levels (EQ-5D-5L) questionnaire, which were applied to the different health states. In addition, a decrease in utility value (disutility) was applied for exacerbations requiring treatment with OCS, emergency department visits or hospitalization based on the data available in the literature (Citation24). shows the utility and disutility values considered in the analysis.
Table 2. Utility and disutility values.
Resource consumption and costs
In line with the perspective of the analysis, only direct health care costs were considered, which included pharmaceutical cost, administration cost, exacerbation management cost and disease management cost per health state.
The pharmaceutical costs for each of the drugs assessed were calculated from the ex-factory prices published on the website of the General Council of Official Colleges of Pharmacists (Citation25), applying the national mandatory deductions established by Royal Decree-Law 8/2010 (Citation26) ().
Table 3. Resource consumption and costs.
In relation to the administration cost, it was assumed that 80% of patients receiving biologic therapy self-administered the treatment. For the remaining patients (20%), the administration cost was equivalent to the cost of a nursing consultation. The nursing consultation unit cost was obtained from a health costs database at the national level (Citation27) ().
The management costs of the exacerbations (including OCS treatment, emergency department visit or hospitalization) were obtained from a multicentre observational study developed in Spain (Citation28) ().
Disease management costs were calculated by multiplying the health resource consumption by the corresponding unit costs. The resource use, which referred to specialist visits, hospitalization, medical examinations, and diagnostic tests, was provided by experts with extensive experience and knowledge about the pathology, and the unit costs were obtained from the local national database of health care costs (Citation27) ().
All costs were expressed in euros, 2022 values, and those costs obtained from the literature, when needed, were inflated to 2022 based on the Spanish general consumer price index provided by the Statistic National Institute (Citation29).
Cost-effectiveness analysis
Total costs, exacerbations avoided and QALYs gained were estimated for each of the alternatives considered in the model. The efficiency between total costs and health outcomes is expressed as an incremental cost-effectiveness ratio (ICER) in terms of cost per exacerbation avoided and as an incremental cost-utility ratio (ICUR) in terms of cost per additional QALY.
Sensitivity analyses
Two sensitivity analyses (SA) were performed to evaluate the influence of parameter variation on the model and to confirm the model robustness. First, one-way sensitivity analysis (OWSA) was carried out, modifying the following parameters individually: time horizon (1 year), discount rate (0% and 5%), distribution of patients by exacerbation management (±10%), transition probabilities (no population adjustment), only drug costs were considered, administration costs were modified by €21.62/administration, and exacerbation costs and management costs by health status were varied by ±20%.
In the probabilistic sensitivity analysis (PSA), 1,000 Monte Carlo simulations were performed. The value of each key model parameter was varied within a specific probability distribution assigned to each parameter. Dirichlet distributions were applied for probabilities, beta distributions for utility and disutility values and gamma distributions for costs.
Results
Base case
Over a 5-year time horizon, benralizumab was associated with 2.87 exacerbations and 52.21 QALYs and was the most effective option compared with mepolizumab (4.70 exacerbations and 51.39 QALYs) and dupilumab (5.11 exacerbations and 51.30 QALYs).
The estimated total cost per patient at the end of the 5-year simulation for each of the therapeutic alternatives from the NHS perspective was €56,093.57 for benralizumab, €59,280.45 for mepolizumab and €62,991.76 for dupilumab.
Benralizumab showed cost savings compared to mepolizumab and dupilumab, with incremental costs of €-3,186.88 versus mepolizumab and €-6,898.18 versus dupilumab.
The ICER for benralizumab was €-1,737.53 per exacerbation avoided and €-3,076.84 per exacerbation avoided versus mepolizumab and dupilumab, respectively. In this model, benralizumab was a dominant treatment strategy because it was more effective and less costly than mepolizumab and dupilumab for the treatment of patients with uncontrolled severe eosinophilic asthma in Spain.
The expected 5-year cost, survival measured by exacerbations avoided and QALY gained for the alternatives considered are shown .
Table 4. Base-case results.
Sensitivity analyses
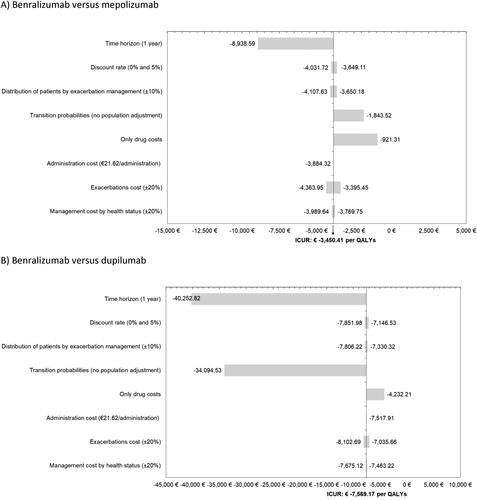
The OWSA results confirmed the robustness of the model in the 8 proposed scenarios. Benralizumab was a dominant strategy over mepolizumab and dupilumab in all deterministic SAs performed ().
Figure 2. One-way sensitivity analyses. A) Benralizumab versus mepolizumab, B) Benralizumab versus dupilumab.
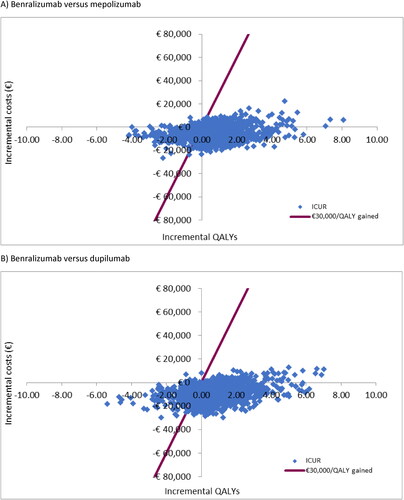
The cost-effectiveness plane allows 4 possible scenarios to be sketched, each corresponding to one of the four quadrants of the coordinate axes. At this point, it is easy to see that only the comparisons in quadrants I and III require the application of a decision rule. In our analysis, the PSA showed that, considering the latest proposed willingness-to-pay threshold of €30 000 per QALY gained (Citation30), benralizumab was cost-effective in 72% and 77% of cases versus mepolizumab and dupilumab, respectively ().
Discussion
Severe asthma, being a chronic respiratory disease, is associated with a significant impact on patients’ quality of life as well as a high burden on the health care system, mainly due to the future costs associated with the evolution of the disease leading to complications such as the development of exacerbations and/or chronic airflow obstruction (Citation1). Current guidelines recommend the use of biologic therapies as treatment for patients with severe uncontrolled asthma, i.e. those patients who, after receiving a combination of high-dose ICS and LABA for the last year or OCS for at least six months, are unable to control their disease (Citation4). However, the guidelines do not include cost-effectiveness analysis as a criterion for treatment selection. This is a relevant issue, especially when budgets are limited, the disease requires long-term treatment and the drugs and costs of the disease are supported by the public health systems.
This analysis carried out a cost-effectiveness model to evaluate the efficiency of biologic therapies in patients with severe uncontrolled asthma in Spain. The results obtained with the present simulation suggest that, in a 5-year period from an NHS perspective, benralizumab would avoid 1.83 and 2.24 exacerbations per patient compared to mepolizumab and dupilumab, respectively. In addition, benralizumab treatment resulted in 0.82 additional QALYs per patient compared to mepolizumab and 0.91 additional QALYs per patient compared to dupilumab. The total cost savings per patient for benralizumab versus mepolizumab and dupilumab would be reduced to €3,186.88 and €6,898.18, respectively. Therefore, the use of benralizumab would result in a percentage cost savings of 5.7% compared to mepolizumab and 6.3% compared to dupilumab. However, it is interesting to mention that dupilumab has been the last biologic approved for this disease, so it is possible that the expert panel had limited experience in clinical practice with this drug.
As a strength of the study, it could be mentioned that a conservative analysis was performed since studies carried out in Spain and the most updated data available in the literature were considered as much as possible. In addition, all parameters included in the analysis were validated by a panel of clinical experts with experience in pathology to confirm that they were adapted to Spanish clinical practice.
On the other hand, the present analysis has some limitations, which are common for this type of pharmacoeconomic model and methodology applied. First, due to the lack of a direct comparison between the alternatives evaluated, the clinical data used in the analysis were extracted from different sources. However, all parameters are based on publications with a high level of clinical evidence and have been validated by a panel of experts with experience in the management of the disease under study. The efficacy variables employed were obtained from an indirect comparison in a meta-analysis (Citation15). However, it should be mentioned that indirect comparisons are a standard method that can provide useful and complementary data on the relative efficacy of alternatives being compared (Citation31). Another possible limitation is related to the transferability of data extracted from the literature of studies conducted in other countries, as for some parameters, no studies were identified, specifically in Spain. This is the case for the utility decrement associated with exacerbation management used in the model, which was derived from a UK study (Citation24). However, these data were validated by the expert panel and considered to be representative of the Spanish asthma population.
Despite the limitations described above, the SA results confirmed the robustness of the model, as the uncertainty associated with the parameters used in the modeling did not represent a relevant deviation from the results obtained in the base case.
In conclusion, from this analysis, it could be highlighted that benralizumab is a valuable option to consider due to its clinical benefit, in terms of reduced exacerbations (1.83 vs. mepolizumab and 2.24 vs. dupilumab) and higher QALYs (0.82 vs. mepolizumab and 0.91 vs. dupilumab), and to its economic benefits, as benralizumab was associated with lower pharmacological cost (1.7% vs. mepolizumab and 8.4% vs. dupilumab), lower exacerbation cost (64.0% vs. mepolizumab and 78.2% vs. dupilumab) and lower disease management cost by health status (6.8% vs. mepolizumab and 7.2% vs. dupilumab). These results suggest that benralizumab could be considered as a dominant treatment alternative (i.e. it is more effective and less costly) compared to mepolizumab and dupilumab for the treatment of patients with severe uncontrolled eosinophilic asthma and could be a valuable alternative for the Spanish NHS.
Declaration of interest
María Mareque and Itziar Oyagüez are currently employed at PORIB, a consultant company specializing in the economic evaluation of health interventions, which received financial support from AstraZeneca for the development of this study. Mónica Climente, Eva Martinez-Moragon and Alicia Padilla have received honouraria from Pharmacoeconomics and Outcomes Research Iberia for advocacy tasks related to this project. Carolina Touron, Covadonga Torres and Anisia Martinez are employees of AstraZeneca Spain.
Additional information
Funding
References
- Global Initiative for Asthma. Global strategy for asthma management and prevention (2021 update); 2021. Available from: https://ginasthma.org/gina-reports/.
- Price D, Fletcher M, van der Molen T. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med. 2014;24(1):1–10.
- Quirce S. Asthma in alergológica-2005. J Investig Allergol Clin Immunol. 2009;19(Suppl 2):14–20.
- Sociedad Española de Neumología y Cirugía Torácica. Guía española para el manejo del asma (GEMA 5.2); 2022. Available from: https://gemasma.com/.
- Heaney LG, Perez de Llano L, Al-Ahmad M, Backer V, Busby J, Canonica GW, Christoff GC, Cosio BG, FitzGerald JM, Heffler E, et al. Eosinophilic and noneosinophilic asthma: an expert consensus framework to characterise phenotypes in a global real-life severe asthma cohort. Chest. 2021;160(3):814–830. doi:10.1016/j.chest.2021.04.013.
- Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2018;52(1):1352020.
- Melero Moreno C, Quirce S, Huerta A, Uría E, Cuesta M. Economic impact of severe asthma in Spain: multicentre observational longitudinal study. J Asthma. 2019;56(8):861–871. doi:10.1080/02770903.2018.1499035.
- Chen S, Golam S, Myers J, Bly C, Smolen H, Xu X. Systematic literature review of the clinical, humanistic, and economic burden associated with asthma uncontrolled by GINA Steps 4 or 5 treatment. Curr Med Res Opin. 2018;34(12):2075–2088. doi:10.1080/03007995.2018.1505352.
- Nagase H, Adachi M, Matsunaga K, Yoshida A, Okoba T, Hayashi N, Emoto K, Tohda Y. Prevalence, disease burden, and treatment reality of patients with severe, uncontrolled asthma in Japan. Allergol Int. 2020;69(1):53–60. doi:10.1016/j.alit.2019.06.003.
- Chiner E, Pulido A, Maestre L. Autoadministración de fármacos biológicos en el asma grave. Rev Asma. 2020;5(1):1–11.
- Agencia Española de Medicamentos y Productos Sanitarios. Ficha técnica de Fasenra® 30 mg solución inyectable [Internet]. Madrid: Agencia Española de Medicamentos y Productos Sanitarios; 2013. Available from: https://cima.aemps.es/cima/pdfs/es/ft/1171252001/FT_1171252001.pdf
- Pérez de Llano L, Martínez-Moragón E, Plaza Moral V, Trisan Alonso A, Sánchez CA, Callejas FJ, Vera E, Soto Campos JG, Martinez Rivera C, Alcázar Navarrete B, et al. Unmet therapeutic goals and potential treatable traits in a population of patients with severe uncontrolled asthma in Spain. ENEAS study. Respir Med. 2019;151:49–54. doi:10.1016/j.rmed.2019.03.006.
- Domingo Ribas C, Carrillo Díaz T, Blanco Aparicio M, Martínez Moragón E, Banas Conejero D, Sánchez Herrero MG, Muñoz M, Cabrerizo H, Valero A, Arismendi E, et al. Real world effectiveness and safety of mepolizumab in a multicentric spanish cohort of asthma patients stratified by eosinophils: the REDES study. Drugs. 2021;81(15):1763–1774. doi:10.1007/s40265-021-01597-9.
- López-Bastida J, Oliva J, Antoñanzas F, García-Altés A, Gisbert R, Mar J, Puig-Junoy J. Spanish recommendations on economic evaluation of health technologies. Eur J Health Econ. 2010;11(5):513–520. doi:10.1007/s10198-010-0244-4.
- Bourdin A, Husereau D, Molinari N, Golam S, Siddiqui MK, Lindner L, Xu X. Matching-adjusted comparison of oral corticosteroid reduction in asthma: systematic review of biologics. Clin Exp Allergy. 2020;50(4):442–452. doi:10.1111/cea.13561.
- Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, Barker P, Sproule S, Ponnarambil S, Goldman M, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376(25):2448–2458. doi:10.1056/NEJMoa1703501.
- Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, Ortega HG, Pavord ID. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–1197. doi:10.1056/NEJMoa1403291.
- Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, Zhu H, Hamilton JD, Swanson BN, Khan A, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378(26):2475–2485. doi:10.1056/NEJMoa1804093.
- Agencia Española de Medicamentos y Productos Sanitarios. Ficha técnica de Nucala® 100 mg solución inyectable [Internet]. Madrid: Agencia Española de Medicamentos y Productos Sanitarios; 2013. Available from: https://cima.aemps.es/cima/pdfs/es/ft/1151043001/FT_1151043001.pdf.
- Agencia Europea de Medicamentos. Ficha técnica de Dupixent® 300 mg solución inyectable [Internet]. Londres: Agencia Europea de Medicamentos; 2011. Available from: https://www.ema.europa.eu/en/documents/product-information/dupixent-epar-product-information_en.pdf.
- González-Barcala FJ, Muñoz-Gall X, Mariscal E, García A, Yang S, van de Wetering G, Izquierdo-Alonso JL. Cost-effectiveness analysis of anti-IL-5 therapies of severe eosinophilic asthma in Spain. J Med Econ. 2021;24(1):874–882. doi:10.1080/13696998.2021.1941065.
- Martínez-Moragon E, Entrenas Costa LM, Sánchez-Covisa Hernández J, Torres González C, de Prado Moncusi A, Monteaguado, Ruiz G. Cuantificación de la medida de utilidad genérica y específica en pacientes con asma grave no controlada en España en vida real. Estudio utility. Arch Bronconeumol. 2022;58(Espec Cong 1):1–369.
- Instituto Nacional de Estadística. Indicadores de mortalidad. Tasas de mortalidad según sexo y edad hasta 100 años y más (desde 1991). Resultados nacionales. En: INEbase. [Internet]. Madrid: Instituto Nacional de Estadística; 2020. Available from: www.ine.es
- Lloyd A, Price D, Brown R. The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Primary Care Respir J. 2007;16(1):22–27. doi:10.3132/pcrj.2007.00002.
- Consejo General de Colegios Oficiales de Farmacéuticos. Base de datos del Conocimiento Sanitario - Bot Plus 2.0. [Internet]. Madrid: Consejo General de Colegios Oficiales de Farmacéuticos; 2021. Available from: https://botplusweb.portalfarma.com/
- Ministerio de Sanidad, Servicios Sociales e Igualdad. Relación informativa de medicamentos afectados por las deducciones establecidas en el Real Decreto Ley 8/2010 de 20 de Mayo por el que se adoptan medidas extraordinarias para la reducción del déficit público. [Internet]. Madrid: Ministerio de Sanidad, Servicios Sociales e Igualdad; 2010. Available from: https://www.sanidad.gob.es/profesionales/farmacia/pdf/Deducciones_Abril_22.pdf.
- Oblikue Consulting. Base de datos de costes sanitarios eSalud. [Internet]. Barcelona: Oblikue Consulting; 2022. Available from: http://www.oblikue.com/bddcostes/
- Quirce S, Melero C, Huerta A, Uría E, Cuesta M. Economic impact of severe asthma exacerbations in Spain: multicentre observational study. J Asthma. 2021;58(2):207–212. doi:10.1080/02770903.2019.1674330.
- Instituto Nacional de Estadísitica. Índice de precios de consume; 2022. Available from: http://www.ine.es.
- Vallejo-Torres L, García-Lorenzo B, Rivero-Arias O, Pinto-Prades JL. The societal monetary value of a QALY associated with EQ-5D- 3 L health gains. Eur J Health Econ. 2020;21(3):363–379. doi:10.1007/s10198-019-01140-4.
- Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ. 2003;326(7387):472. doi:10.1136/bmj.326.7387.472.