Abstract
Objective
To review the evidence for the use of open-inhaler (inhaled corticosteroid [ICS] plus long-acting β2-agonist [LABA] with separate add-on long-acting muscarinic antagonist [LAMA]) versus single-inhaler triple therapy (ICS/LABA/LAMA combination) and the merits of add-on LAMA to ICS/LABA in patients with uncontrolled asthma.
Data Sources
Original research articles were identified from PubMed using the search term “triple therapy asthma.” Information was also retrieved from the ClinicalTrials.gov website.
Study Selections
Articles detailing the use of add-on LAMA to ICS plus LABA (open-inhaler triple therapy), and closed triple therapy compared with ICS plus LABA dual therapy, addressing patient symptoms, exacerbations, and health-related quality of life.
Results
Open-inhaler triple therapy was associated with a significantly reduced incidence of hospitalizations and emergency department visits and a decrease in ICS dose, oral corticosteroids use, and antibiotics use. Exacerbations and acute respiratory events were also reduced. Single-inhaler triple therapy showed a greater improvement in lung function, asthma control, and health status and was noninferior to open-inhaler triple therapy for Asthma Quality of Life Questionnaire scores. Single-inhaler triple therapy may also lead to improved therapy adherence.
Conclusion
Add-on LAMA to ICS plus LABA (open- or single-inhaler triple therapy) improves the response in patients who remain symptomatic and provides a reasonable alternative to ICS dose escalation in treatment-refractory patients.
Introduction
Asthma affects approximately 26 million physician-diagnosed patients in the United States (US) and more than 358 million individuals of all age groups globally. It is predicted that by 2025, >458 million people will be diagnosed with asthma (Citation1–3). The economic burden of asthma is substantial and has increased in recent years, with predictions of an estimated increase to $963 billion over the next 2 decades (Citation2).
Controlling symptoms and reducing the risk of future exacerbations are the primary targets of appropriate asthma management and preservation of lung function (Citation2). Current treatment recommendations follow a stepwise approach, with inhaled corticosteroids (ICSs) plus formoterol (a long-acting β2-agonist [LABA]) being the preferred management options (Citation4). Despite optimal treatment adherence, approximately 30%–50% of patients treated with ICS plus LABA have uncontrolled asthma (Citation5). Uncontrolled asthma has been associated with markedly reduced health-related quality of life (HRQoL) and an increase in exacerbations, use of rescue inhalers and systemic corticosteroids, healthcare resource utilization (HCRU), emergency department visits, hospitalizations, and number of deaths compared with well-controlled asthma (Citation5,Citation6). When asthma control is not achieved, increasing the ICS dosage or adding other bronchodilator treatments, such as a long-acting muscarinic antagonist (LAMA), and biologic agents are recommended options (Citation4,Citation7).
ICS dose escalation may not improve asthma control in all asthma patient phenotypes (e.g. those with low eosinophil levels) (Citation8). LAMA as an add-on to ICS plus LABA (triple therapy) is recommended by both the Global Initiative for Asthma (GINA) strategy document and the National Asthma Education and Prevention Program (NAEPP) guidelines before escalating to biologic agents or oral corticosteroids (OCS) (Citation7,Citation9). Use of open triple therapy (i.e. ICS plus LABA with separate add-on LAMA) is supported by evidence demonstrating a reduced number of exacerbations and improved pulmonary function in patients with poorly controlled asthma (Citation9,Citation10). Single-inhaler triple therapy (ICS/LABA/LAMA given in a combined inhaler, also known as closed-inhaler therapy) has shown consistent efficacy on pulmonary function compared with ICS plus LABA, while benefits on exacerbations were less clear and may be dependent on factors such as ICS dose, blood eosinophil count, and lung function reversibility (Citation9).
In this review, we aim to summarize evidence for the use of open-inhaler versus single-inhaler triple therapy and the value of add-on LAMA to ICS/LABA in patients with uncontrolled asthma. Notably, use of LAMA in some of the studies included in this review is not in accordance with US Food and Drug Administration (FDA)-approved labels. The GINA 2022 report and 2020 NAEPP guidelines do not specify the LAMA and dose to be used for asthma treatment; hence, it is recommended that clinicians follow the approved label for each product when prescribing a LAMA to patients with asthma.
Patient phenotyping and precision medicine
Asthma pathophysiology is often divided into two major pathways: a type 2 high endotype with increased allergic or eosinophilic airway inflammation and a non type 2/type 2 low endotype with either neutrophilic or paucigranulocytic airway inflammation (Citation11). Despite being distinct forms of asthma, allergic and non-allergic asthma phenotypes overlap in the underlying inflammatory process and clinical presentation (Citation11). Regardless of the similarity of clinical symptoms, patients may respond differently to the same treatment (Citation11).
Precision medicine explores specific phenotypes for targeted treatments and consequently better outcomes (Citation12). In asthma patients, there is an urgent need to unveil specific molecular phenotypes (Citation11). Patients with a ≥2% sputum eosinophil level show increases in forced expiratory volume in 1 s (FEV1) with the use of ICS, whereas those with a low (<2%) eosinophil level may not respond to ICS therapy (Citation13). In the Clinical Study in Asthma Patients Receiving Triple Therapy in a Single Inhaler (CAPTAIN), ICS escalation reduced the exacerbation rate and improved lung function in inadequately controlled moderate or severe asthma patients with elevated baseline eosinophil levels (T2 high endotype), while no improvements were registered for patients with low eosinophil levels (T2 low endotype). Conversely, the improvement in lung function observed with the addition of LAMA seemed to be independent of the patient’s blood eosinophil levels (Citation8,Citation14,Citation15). Thus, ICS dose escalation should be considered according to the patient’s T2 phenotype and the possibility of ICS side effects with little efficacy gain (Citation16). These preliminary results indicate that phenotype stratification in asthma patients can help guide proper treatment (Citation8).
Add-on LAMA to ICS plus LABA (open-inhaler triple therapy) versus ICS plus LABA
The 2022 GINA report recommends low-dose ICS-formoterol as the preferred controller treatment for adults and adolescents at steps 1 and 2 and low-, medium-, and high-dose ICS-formoterol as the preferred controller treatments for adults and adolescents at steps 3, 4, and 5, respectively (Citation4). The GINA report has recommended the use of ICS-formoterol since 2019 as the preferred rescue option for any level of asthma severity, except when patients are using ICS plus LABA in combination as a controller (Citation4). Add-on LAMA is currently recommended for patients at step 5 (4). LAMAs are used for the long-term treatment of asthma. The 2020 NAEPP guidelines recommend LAMAs as add-on to ICS plus LABA and as add-on to ICS controller therapy in patients with uncontrolled persistent asthma (Citation17). A significant proportion of patients with severe asthma remain symptomatic despite recommendation-based treatment with ICS plus LABA (Citation5,Citation11,Citation14,Citation18). A high proportion of patients require more than two OCS rescue treatments annually to manage their exacerbations, predisposing these patients toward an increased incidence of adverse events (Citation19). The use of LAMAs in asthma is supported by the synergistic interaction between LAMAs and LABAs (Citation14). LAMAs inhibit the muscarinic receptors on the smooth muscles; inhibition of the M1 and M3 receptors reduces smooth muscle tone and causes bronchodilation, whereas inhibition of the M2 receptors causes bronchoconstriction (Citation20). Depending on their duration of action (DOA), muscarinic antagonists are classified as short acting (e.g. ipratropium; DOA, 4–8 h) and long acting (e.g. tiotropium, glycopyrronium, and umeclidinium; DOA, ≥24 h) (Citation9,Citation21). LAMAs offer an alternative add-on option for the management of asthma with the convenience of a single daily dose regimen (Citation17). Furthermore, LAMAs plus ICS synergistically enhance the relaxation of passively sensitized medium and small bronchi (Citation14).
Tiotropium as an add-on to ICS plus LABA is currently recommended by the NAEPP Expert Panel before escalation to treatment with biologics (Citation18). The NAEPP Expert Panel conditionally recommends adding a LAMA to ICS plus LABA versus continuing the same dose of ICS plus LABA in uncontrolled persistent asthma patients aged ≥12 years (Citation18). The efficacy of tiotropium as an add-on to ICS plus LABA on lung function and exacerbations was assessed in several clinical trials ( and ) (Citation22–25). Tiotropium is currently FDA approved for the long-term, maintenance treatment of asthma in patients ≥6 years of age (Citation26).
Figure 1. Effect of open-inhaler triple therapy versus ICS plus LABA on lung function and symptoms in patients with asthma.
*Results were statistically significant. Lung function (significance defined by 95% confidence interval (Citation22) and P-value (Citation23)); Exacerbations (significance defined by P-value (Citation20)); Asthma control (significance defined by P-value (Citation20)).
FLU, fluticasone; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; OCS, oral corticosteroids; SAL, salmeterol; TIO, tiotropium.
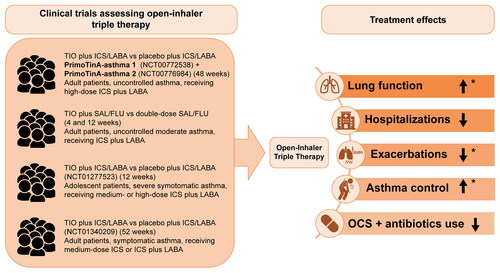
Table 1. Add-on LAMA to ICS plus LABA (open-inhaler triple therapy) vs ICS plus LABA.
Real-world evidence shows that LAMAs have been prescribed by primary care physicians—mostly as add-on therapy in older patients with poorly controlled asthma in the United Kingdom since 2002 (Citation14). Add-on tiotropium, a LAMA, is associated with a significantly reduced incidence of hospitalizations and emergency department visits and a decrease in ICS dose, OCS use, and antibiotics use (Citation14). Exacerbations and acute respiratory events were also reduced across various age groups (Citation14,Citation27). In the two replicate, randomized placebo-controlled trials, (PrimoTinA-asthma® 1 and 2) improvements were observed in peak FEV1 from baseline and predose trough FEV1 from baseline to week 24 of treatment in the tiotropium 5 µg group compared with placebo in patients with poorly controlled asthma (Citation22). In the tiotropium group, improvements in peak FEV1 tended to be higher in patients with a lower FEV1 as a percentage of the predicted value in men and in former smokers with a history of less than 10 pack-years (Citation22). The average daily diurnal peak expiratory flow (PEF) variability improved at 4 and 12 weeks after add-on tiotropium therapy in patients with symptomatic moderate asthma (Citation23), while double-dose ICS demonstrated no significant differences in PEF variability. However, patients receiving double-dose ICS presented with a higher pneumonia risk (Citation23). Similar results were observed with lower tiotropium doses (2.5 µg and 5 µg) at 24 weeks in patients with moderate symptomatic asthma, in ex-smokers or those without a smoking history (Citation28).
Tiotropium therapy (5 µg) was also associated with a 21% reduction in the risk of severe exacerbations in adult patients (Citation22). Conversely, no statistically significant improvement in peak FEV1 (0–3 h) was observed with once-daily tiotropium Respimat® 5 µg add-on to ICS plus one or more controller therapies in adolescents aged 12–17 years with severe symptomatic asthma, despite numerical improvements (Citation25). An increase in Asthma Control Questionnaire-7 (ACQ-7) responder rates was observed for tiotropium (both 5 and 2.5 µg) compared with placebo after 24 weeks of treatment in adults (Citation24,Citation28); however, at 52 weeks, responder rates were similar across treatment groups (Citation24). Although no significant difference was observed in the response of both ACQ-6 and ACQ-7 scores between tiotropium and placebo after 12 weeks of treatment, statistically significant benefits in peak FEV1 (0–3 h) response were seen with tiotropium 2.5 μg compared with placebo (111 mL; P = 0.046) (Citation25).
Although effective, patients using multiple inhalers have suboptimal adherence to medication (Citation29). It should be noted that two different inhaler types requiring different inhaler techniques could contribute to further complications (Citation29). Treatment with a single inhaler increases adherence and reduces the risk of asthma-related emergency department/inpatient events, as well as reduces the use of rescue medication, which might indicate superior symptom control (Citation30–32).
Single-inhaler triple therapy versus open triple therapy and ICS plus LABA
Recently, several triple combinations of ICS plus LABA plus LAMA in a single inhaler providing different dosages and more personalized treatment have become commercially available both in the Europe and the US (Citation16). Single-inhaler triple therapy (fluticasone furoate plus umeclidinium plus vilanterol) is widely approved as a once-daily treatment for chronic obstructive pulmonary disease (Citation8). Results of recent clinical trials also support its use in asthma with approval for this indication received in several countries including the US () (Citation8,Citation33,Citation34). Single-inhaler triple therapy reported in the Triple in Asthma With Uncontrolled Patients on Medium Strength of ICS/LABA (TRIMARAN) and Triple in Asthma High Strength Versus ICS/LABA HS and Tiotropium (TRIGGER) studies reduced the rate of moderate and severe exacerbations and significantly improved predose FEV1 versus ICS plus LABA () (Citation34). In TRIGGER, there was no significant difference in predose FEV1 and rate of moderate and severe exacerbations between the single-inhaler and open-inhaler triple therapy groups (Citation34). Similarly, results of the ARGON study showed that single-inhaler triple therapy was noninferior to open-inhaler triple therapy for Asthma Quality of Life Questionnaire scores but showed greater improvement in lung function, asthma control, and health status (Citation35). In the IRIDIUM study, single-inhaler triple therapy significantly improved trough FEV1 versus ICS plus LABA in patients with inadequately controlled asthma (Citation36). In CAPTAIN, triple therapy significantly improved lung function (change from baseline in trough FEV1 at 24 weeks of treatment) and symptoms (as measured by ACQ-7 and St George’s Respiratory Questionnaire) but did not reduce moderate and/or severe exacerbations versus ICS plus LABA in patients with uncontrolled moderate or severe asthma on ICS plus LABA () (Citation8). This could possibly be explained by the fact that the population under study had a lower risk than assumed in the power calculation; further, the statistical limitations did not allow for exploring the effect of LABA in reducing exacerbations (Citation8). The CAPTAIN study also concluded that single-inhaler triple therapy is an effective treatment option with a favorable risk-benefit profile in this patient population (Citation8). A systematic review analyzing both observational and interventional clinical studies showed that single-inhaler use was associated with decreased HCRU and improved cost-effectiveness versus multiple-inhaler use (Citation30).
Figure 2. Effect of single-inhaler triple therapy versus ICS plus LABA on lung function and symptoms in patients with asthma.
*Results were statistically significant. Lung function (significance defined by 95% CI (Citation8) and P-value (Citation36)); Exacerbations (significance defined by 95% CI (Citation34)); Asthma control (significance defined by P-value (Citation36)).
BDP, beclomethasone dipropionate; CI, confidence interval; FF, fluticasone furoate; FLU, fluticasone; GLY, glycopyrronium; ICS, inhaled corticosteroid; IND, indacaterol; LABA, long-acting β2-agonist; MF, mometasone furoate; SAL, salmeterol; UMEC, umeclidinium; VI, vilanterol.
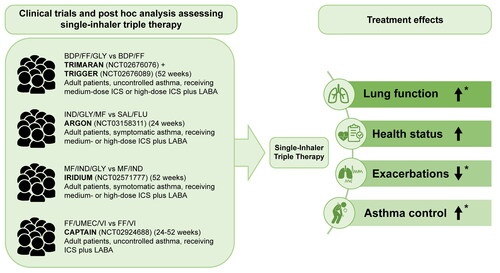
Table 2. Single-inhaler triple therapy vs ICS/LABA.
A recent meta-analysis showed that triple therapy, including high-dose ICS, has greater beneficial impact than medium-dose single-inhaler triple therapy and both medium- and high-dose single-inhaler ICS plus LABA in reducing the risk of moderate-to-severe exacerbations in patients with uncontrolled asthma (Citation37). However, single-inhaler therapies can also be associated with higher costs and reduced flexibility in independent dose adjustments (Citation16).
Type 2 inflammatory asthma and LAMAs
There is currently limited evidence that LAMAs possess anti-inflammatory activity (Citation38). The anti-inflammatory and anti-proliferative (Citation39,Citation40) effects of LAMA in reducing airway remodeling induced by allergens (Citation41) has been demonstrated in animal models; however, these effects in humans have not been definitively demonstrated (Citation42). Pharmacological interactions between muscarinic antagonists, ICS, and/or LABAs support the use of LAMAs in asthma (Citation14). In the CAPTAIN study, the addition of umeclidinium (62.5 μg) to ICS plus LABA as a single-inhaler triple therapy improved FEV1 in patients across a range of blood eosinophil and fractional exhaled nitric oxide levels; however, the effect of LAMAs on inflammatory biomarkers in this study may have been confounded by the concurrent use of ICS (Citation8). As such, without concomitant ICS, LAMA monotherapy is linked to an increased risk of severe asthma exacerbations since it has minimal anti-inflammatory effects and is not advised for treating asthma (Citation43,Citation44).
Unmet needs and future directions
Patients with uncontrolled asthma have fewer treatment options in the context of medium/high doses of ICS plus LABA therapy. The use of LAMA as an add-on therapy to ICS plus LABA has shown significant improvements in asthma control and reduction of airflow obstruction without major safety concerns, with effectiveness being independent of type 2 inflammatory biomarkers (Citation8). Triple therapy can represent a new therapeutic option for these specific asthma patient populations, but further studies confirming the differentiating effect of biomarkers of type 2 inflammation on treatment outcomes and precision medicine for asthma patients are required.
The effects of LAMA add-on therapy and ICS escalation appear to be influenced by patient Th2 levels and choice of outcome measures (e.g. exacerbations versus symptoms), indicating that different clinical symptoms respond to different treatment options. This highlights the need for reliable disease biomarkers that can foster precision medicine and personalized treatments in asthma patients (Citation8). Most of the clinical studies assessing LAMA add-on therapy (i.e. ICS plus LAMA and triple therapy) have included the adult population, leading to a restriction in medication licensing for children and underscoring the need to assess both efficacy and safety in pediatric populations (Citation45).
Asthma severity can change over time, and therapy escalation and/or de-escalation might be needed (Citation45). However, de-escalating from triple to dual bronchodilator therapy may be problematic in some patients who are well-controlled on this regimen and may be reluctant to review/change their medication (Citation46). Consequently, direction and education from bodies including the GINA and NAEPP on the best approach to de-escalate patients who no longer require triple therapy may be helpful (Citation46).
Conclusions
The evidence presented in this review supports the use of triple therapy with LAMAs as an add-on in asthma patients (open-inhaler triple therapy) as well as the inclusion of LAMAs as part of a single-inhaler (closed-inhaler) triple therapy regimen. Open-inhaler triple therapy with add-on LAMA to ICS plus LABA or triple therapy with a single inhaler improves the response in patients who remain symptomatic despite receiving ICS plus LABA therapy. Compared with multiple inhaler use, single inhaler use showed improvement in HCRU. Add-on LAMA provides a reasonable alternative to ICS escalation in patients who do not respond to low- or medium-dose ICS. Single-inhaler therapy may potentially increase treatment compliance and reduce treatment costs, leading to better asthma management and increased patient HRQoL. Triple therapy offers an additional strategy to improve response in patients who are symptomatic despite standard ICS plus LABA therapy; further studies are required to determine whether open- versus closed-inhaler approaches are optimal for the management of uncontrolled asthma and more importantly, for specific subsets of patients.
Open versus Closed Triple Therapy Podcast_V03.mp3
Download MP3 Audio (10.9 MB)Triple Therapy_Audioscript_for blind review.docx
Download MS Word (44 KB)Acknowledgements
Writing, editorial support, and formatting assistance was provided by Sandra Brasil and Shruti Muralidharan of Cactus Life Sciences (part of Cactus Communications), which was contracted and compensated by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI) for these services. BIPI was given the opportunity to review the manuscript for medical and scientific accuracy, as well as intellectual property considerations. To ensure independent interpretation of clinical study results and enable authors to fulfill their role and obligations under the ICMJE criteria, BIPI grants all external authors access to relevant clinical study data. In adherence with the BIPI Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data after publication of the primary manuscript in a peer-reviewed journal, regulatory activities are complete, and other criteria are met. Researchers should use the https://vivli.org/ link to request access to study data and visit https://www.mystudywindow.com/msw/datasharing for further information.
Supplementary information
Additional file 1: A podcast of the review article – Podcast.
Disclosure statement
Dr. Wechsler reports grants and personal fees from Cohero Health, Novartis, and Sanofi; personal fees from Regeneron, Genentech, GlaxoSmithKline plc (GSK), Sentien, Restorbio, Equillium, and Genzyme; grants, personal fees, and nonfinancial support from Teva and AstraZeneca; and personal fees and nonfinancial support from Boehringer Ingelheim Pharmaceuticals, Inc. outside the submitted work. Dr. Wechsler also has received consulting, advisory, or speaking honoraria from Amgen, AstraZeneca, Avalo Therapeutics, Boehringer Ingelheim Pharmaceuticals, Inc., Cerecor, Cohero Health, Cytoreason, Eli Lilly, Equillium, GSK, Incyte, Kinaset, Novartis, Om Pharma, Phylaxis, Pulmatrix, Rapt Therapeutics, Regeneron, Restorbio, Roche/Genentech, Sanofi/Genzyme, Sentien, Sound Biologics, Tetherex Pharmaceuticals, Teva, and Upstream Bio. Dr. Oppenheimer reports to have served on adjudication committees/data- and safety-monitoring boards for AstraZeneca, GSK, Novartis AG, and Sanofi.
Additional information
Funding
References
- Nunes C, Pereira AM, Morais-Almeida M. Asthma costs and social impact. Asthma Res Pract. 2017;3:1. doi:10.1186/s40733-016-0029-3.
- Yaghoubi M, Adibi A, Safari A, FitzGerald JM, Sadatsafavi M. The projected economic and health burden of uncontrolled asthma in the United States. Am J Respir Crit Care Med. 2019;200(9):1102–1112. doi:10.1164/rccm.201901-0016OC.
- Buhl R, Heaney LG, Loefroth E, Larbig M, Kostikas K, Conti V, Cao H. One-year follow up of asthmatic patients newly initiated on treatment with medium- or high-dose inhaled corticosteroid-long-acting β2-agonist in UK primary care settings. Respir Med. 2020;162:105859. doi:10.1016/j.rmed.2019.105859.
- GINA. Global Strategy for Asthma Management and Prevention. 2022. update. Available from: https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf [last accessed 10 June 2022].
- Oppenheimer J, Slade DJ, Hahn BA, Zografos L, Gilsenan A, Richardson D, McSorley D, Lima R, Molfino NA, Averell CM. Real-world evidence: patient views on asthma in respiratory specialist clinics in America. Ann Allergy Asthma Immunol. 2021;126(4):385–393.e2. doi:10.1016/j.anai.2020.12.015.
- Peters SP, Ferguson G, Deniz Y, Reisner C. Uncontrolled asthma: a review of the prevalence, disease burden and options for treatment. Respir Med. 2006;100(7):1139–1151. doi:10.1016/j.rmed.2006.03.031.
- Cloutier MM, Baptist AP, Blake KV, Brooks EG, Bryant-Stephens T, DiMango E, Dixon AE, Elward KS, Hartert T, Krishnan JA, et al. 2020 focused updates to the asthma management guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol. 2020;146(6):1217–1270. doi:10.1016/j.jaci.2020.10.003.
- Lee LA, Bailes Z, Barnes N, Boulet LP, Edwards D, Fowler A, Hanania NA, Kerstjens HAM, Kerwin E, Nathan R, et al. Efficacy and safety of once-daily single-inhaler triple therapy (FF/UMEC/VI) versus FF/VI in patients with inadequately controlled asthma (CAPTAIN): a double-blind, randomised, phase 3A trial. Lancet Respir Med. 2021;9(1):69–84. doi:10.1016/S2213-2600(20)30389-1.
- Papi A, Fabbri LM, Kerstjens HAM, Rogliani P, Watz H, Singh D. Inhaled long-acting muscarinic antagonists in asthma – a narrative review. Eur J Intern Med. 2021;85:14–22. doi:10.1016/j.ejim.2021.01.027.
- Virchow JC. Assessing the benefits of triple versus dual fixed-dose combinations for the treatment of severe asthma. Lancet Respir Med. 2020;8(10):937–939. doi:10.1016/S2213-2600(20)30303-9.
- Luz MI, Aguiar R, Morais-Almeida M. The reality of LAMAs for adult asthmatic patients. Expert Rev Respir Med. 2020;14(11):1087–1094. doi:10.1080/17476348.2020.1794828.
- Seymour CW, Gomez H, Chang CH, Clermont G, Kellum JA, Kennedy J, Yende S, Angus DC. Precision medicine for all? Challenges and opportunities for a precision medicine approach to critical illness. Crit Care. 2017;21(1):257. doi:10.1186/s13054-017-1836-5.
- Lazarus SC, Krishnan JA, King TS, Lang JE, Blake KV, Covar R, Lugogo N, Wenzel S, Chinchilli VM, Mauger DT, et al. Mometasone or tiotropium in mild asthma with a low sputum eosinophil level. N Engl J Med. 2019;380(21):2009–2019. doi:10.1056/NEJMoa1814917.
- Cazzola M, Puxeddu E, Matera MG, Rogliani P. A potential role of triple therapy for asthma patients. Expert Rev Respir Med. 2019;13(11):1079–1085. doi:10.1080/17476348.2019.1657408.
- Casale TB, Bateman ED, Vandewalker M, Virchow JC, Schmidt H, Engel M, Moroni-Zentgraf P, Kerstjens HAM. Tiotropium Respimat add-on is efficacious in symptomatic asthma, independent of T2 phenotype. J Allergy Clin Immunol Pract. 2018;6(3):923–935.e9. doi:10.1016/j.jaip.2017.08.037.
- Agusti A, Fabbri L, Lahousse L, Singh D, Papi A. Single inhaler triple therapy (SITT) in asthma: systematic review and practice implications. Allergy. 2022;77(4):1105–1113. doi:10.1111/all.15076.
- NHLBI, NIH. 2020. Focused Updates to the Asthma Management Guidelines: A Report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. Available from: https://nhlbi.nih.gov/health-topics/asthma-management-guidelines-2020-updates [last accessed 10 October 2021].
- Kerstjens HAM, Moroni-Zentgraf P, Tashkin DP, Dahl R, Paggiaro P, Vandewalker M, Schmidt H, Engel M, Bateman ED. Tiotropium improves lung function, exacerbation rate, and asthma control, independent of baseline characteristics including age, degree of airway obstruction, and allergic status. Respir Med. 2016;117:198–206. doi:10.1016/j.rmed.2016.06.013.
- Chung LP, Upham JW, Bardin PG, Hew M. Rational oral corticosteroid use in adult severe asthma: a narrative review. Respirology. 2020;25(2):161–172. doi:10.1111/resp.13730.
- Aalbers R, Park HS. Positioning of long-acting muscarinic antagonists in the management of asthma. Allergy Asthma Immunol Res. 2017;9(5):386–393. doi:10.4168/aair.2017.9.5.386.
- Gross NJ. Anticholinergic agents in asthma and COPD. Eur J Pharmacol. 2006;533(1-3):36–39. doi:10.1016/j.ejphar.2005.12.072.
- Kerstjens HAM, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, Sigmund R, Seibold W, Moroni-Zentgraf P, Bateman ED. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med. 2012;367(13):1198–1207. doi:10.1056/NEJMoa1208606.
- Wang K, Tian P, Fan Y, Wang Y, Liu C. Assessment of second-line treatments for patients with uncontrolled moderate asthma. Int J Clin Exp Med. 2015;8:19476–19480.
- Ohta K, Ichinose M, Tohda Y, Engel M, Moroni-Zentgraf P, Kunimitsu S, Sakamoto W, Adachi M. Long-term once-daily tiotropium Respimat® is well tolerated and maintains efficacy over 52 weeks in patients with symptomatic asthma in Japan: a randomised, placebo-controlled study. PLoS ONE. 2015;10(4):e0124109. doi:10.1371/journal.pone.0124109.
- Hamelmann E, Bernstein JA, Vandewalker M, Moroni-Zentgraf P, Verri D, Unseld A, Engel M, Boner AL. A randomised controlled trial of tiotropium in adolescents with severe symptomatic asthma. Eur Respir J. 2017;49(1):1601100. doi:10.1183/13993003.01100-2016.
- Boehringer Ingelheim Pharmaceuticals, Inc. SPIRIVA® RESPIMAT® (Tiotropium bromide). Inhalation spray. 2004. Available from: https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Spiriva%20Respimat/spirivarespimat.pdf [last accessed 22 August 2022].
- Kaplan A, Chang K-L. Tiotropium in asthma – perspectives for the primary care physician. Postgrad Med. 2021;133(5):552–564. doi:10.1080/00325481.2020.1816329.
- Kerstjens HAM, Casale TB, Bleecker ER, Meltzer EO, Pizzichini E, Schmidt O, Engel M, Bour L, Verkleij CB, Moroni-Zentgraf P, et al. Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trials. Lancet Respir Med. 2015;3(5):367–376. doi:10.1016/S2213-2600(15)00031-4.
- George M, Bender B. New insights to improve treatment adherence in asthma and COPD. Patient Prefer Adherence. 2019;13:1325–1334. doi:10.2147/PPA.S209532.
- Zhang S, King D, Rosen VM, Ismaila AS. Impact of single combination inhaler versus multiple inhalers to deliver the same medications for patients with asthma or COPD: a systematic literature review. Int J Chron Obstruct Pulmon Dis. 2020;15:417–438. doi:10.2147/COPD.S234823.
- Stanford RH, Averell C, Parker ED, Blauer-Peterson C, Reinsch TK, Buikema AR. Assessment of adherence and asthma medication ratio for a once-daily and twice-daily inhaled corticosteroid/long-acting β2-agonist for asthma. J Allergy Clin Immunol Pract. 2019;7(5):1488–1496.e7. doi:10.1016/j.jaip.2018.12.021.
- Rajesh V, Augustine J, Divya R, Cleetus M. Inhaled formoterol-fluticasone single inhaler therapy in asthma: real-world efficacy, budget impact, and potential to improve adherence. Can Respir J. 2020;2020:1–8. doi:10.1155/2020/8631316.
- Singh D, Virchow JC, Canonica GW, Vele A, Kots M, Georges G, Papi A. Extrafine triple therapy in patients with asthma and persistent airflow limitation. Eur Respir J. 2020;56(3):2000476. doi:10.1183/13993003.00476-2020.
- Virchow JC, Kuna P, Paggiaro P, Papi A, Singh D, Corre S, Zuccaro F, Vele A, Kots M, Georges G, et al. Single inhaler extrafine triple therapy in uncontrolled asthma (TRIMARAN and TRIGGER): two double-blind, parallel-group, randomised, controlled phase 3 trials. Lancet. 2019;394(10210):1737–1749. doi:10.1016/S0140-6736(19)32215-9.
- Gessner C, Kornmann O, Maspero J, van Zyl-Smit R, Krüll M, Salina A, Gupta P, Bostel S, Fucile S, Conde LG, et al. Fixed-dose combination of indacaterol/glycopyrronium/mometasone furoate once-daily versus salmeterol/fluticasone twice-daily plus tiotropium once-daily in patients with uncontrolled asthma: a randomised, phase IIIb, non-inferiority study (ARGON). Respir Med. 2020;170:106021. doi:10.1016/j.rmed.2020.106021.
- Kerstjens HAM, Maspero J, Chapman KR, van Zyl-Smit RN, Hosoe M, Tanase AM, Lavecchia C, Pethe A, Shu X, D’Andrea P. Once-daily, single-inhaler mometasone-indacaterol-glycopyrronium versus mometasone-indacaterol or twice-daily fluticasone-salmeterol in patients with inadequately controlled asthma (IRIDIUM): a randomised, double-blind, controlled phase 3 study. Lancet Respir Med. 2020;8(10):1000–1012. doi:10.1016/S2213-2600(20)30190-9.
- Rogliani P, Ritondo BL, Calzetta L. Triple therapy in uncontrolled asthma: a network meta-analysis of phase III studies. Eur Respir J. 2021;58(3):2004233. doi:10.1183/13993003.04233-2020.
- Gosens R, Gross N. The mode of action of anticholinergics in asthma. Eur Respir J. 2018;52(4):1701247. doi:10.1183/13993003.01247-2017.
- Gosens R, Nelemans SA, Grootte Bromhaar MM, McKay S, Zaagsma J, Meurs H. Muscarinic M3-receptors mediate cholinergic synergism of mitogenesis in airway smooth muscle. Am J Respir Cell Mol Biol. 2003;28(2):257–262. doi:10.1165/rcmb.2002-0128OC.
- Gosens R, Dueck G, Rector E, Nunes RO, Gerthoffer WT, Unruh H, Zaagsma J, Meurs H, Halayko AJ. Cooperative regulation of GSK-3 by muscarinic and PDGF receptors is associated with airway myocyte proliferation. Am J Physiol Lung Cell Mol Physiol. 2007;293(5):L1348–L1358. doi:10.1152/ajplung.00346.2007.
- Bos IS, Gosens R, Zuidhof AB, Schaafsma D, Halayko AJ, Meurs H, Zaagsma J. Inhibition of allergen-induced airway remodelling by tiotropium and budesonide: a comparison. Eur Respir J. 2007;30(4):653–661. doi:10.1183/09031936.00004907.
- Matera MG, Belardo C, Rinaldi M, Rinaldi B, Cazzola M. New perspectives on the role of muscarinic antagonists in asthma therapy. Expert Rev Respir Med. 2020;14(8):817–824. doi:10.1080/17476348.2020.1758069.
- Baan EJ, Hoeve CE, De Ridder M, Demoen L, Lahousse L, Brusselle GG, Verhamme KMC. The ALPACA study: (In)Appropriate LAMA prescribing in asthma: a cohort analysis. Pulm Pharmacol Ther. 2021;71:102074. doi:10.1016/j.pupt.2021.102074.
- Casale TB, Foggs MB, Balkissoon RC. Optimizing asthma management: role of long-acting muscarinic antagonists. J Allergy Clin Immunol. 2022;150(3):557–568. doi:10.1016/j.jaci.2022.06.015.
- Kaplan A, FitzGerald JM, Buhl R, Vogelberg C, Hamelmann E. Comparing LAMA with LABA and LTRA as add-on therapies in primary care asthma management. NPJ Prim Care Respir Med. 2020;30(1):50. doi:10.1038/s41533-020-00205-9.
- Pulmonology Advisor. Treating Severe Uncontrolled Asthma: Can Single-Inhaler, Fixed Triple-Dose Therapy Improve Outcomes? Available from: https://www.pulmonologyadvisor.com/howtotreat/treating-severe-uncontrolled-asthma-can-single-inhaler-fixed-triple-dose-therapy-improve-outcomes/ [last accessed 22 October 2021].
