Abstract
Background
There is a lack of information on house dust mite (HDM) sensitization and phenotype distribution in patients with severe asthma (SA) living permanently at high-altitude (HA) in tropical regions, which may be different.
Objective
The aim of this study was to characterize adults with SA in a tropical high altitude city (2,640 m): Bogotá, Colombia.
Material and Methods
This observational cross-sectional study included severe asthmatic outpatients (n = 129) referred to the ASMAIRE program of the Fundación Neumológica Colombiana in Bogotá (2,640 m). Clinical history, spirometry, total IgE, blood eosinophils, and skin prick test (SPT), including HDM allergens, were performed. Phenotype definitions: Allergic/atopic (AA): IgE ≥100 IU/mL and/or at least one positive SPT; eosinophilic (EOS): blood eosinophils ≥300 cells/µL; type 2-high: AA and/or EOS phenotype; type 2-low: non-AA/non-EOS phenotype (IgE <100 IU/mL, negative SPT, and blood eosinophils <300 cells/µL).
Results
A total of 129 adults with SA were included, 79.8% female. Phenotype distribution: AA: 61.2%; EOS: 37.2%; type 2-high: 72.1%; type 2-low: 27.9%. Among AA patients, HDM sensitization was present in 87% and 34.9% were non-eosinophilic. There was a significant overlap between the phenotypes.
Conclusions
In contrast to non-tropical high-altitude regions, we found a high frequency of HDM sensitization in patients with AA phenotype living in a tropical high-altitude city. We also found a discrete lower frequency of EOS phenotype with no other significant differences in the phenotypic distribution compared to that described at low altitudes. We propose that tropical location may modify the effect of high altitude on HDM concentrations and allergenicity.
Introduction
Severe asthma (SA) is defined by the European Respiratory Society (ERS), the American Thoracic Society (ATS) and by the Global Initiative for Asthma (GINA) as asthma that remains uncontrolled despite optimized treatment with high-dose of inhaled corticosteroids (ICS) in combination with a long-acting beta-agonist (LABA) or that requires such treatment to avoid becoming uncontrolled despite optimal adherence and treatment of contributing factors (Citation1,Citation2). Due to the heterogeneity of asthma, under-diagnosis, and varying definitions, prevalence of SA has been difficult to establish, but has been estimated to be 4–8% (Citation3). More than 50% of patients with SA remain uncontrolled and require long-term or intermittent oral corticosteroids (Citation4), resulting in a significant individual and economic burden (Citation5,Citation6).
There is no information about the characteristics and behavior of patients with SA living permanently at high-altitude (HA), in either non-tropical or tropical regions. However, it has been described that moving to medium or HA is an optional effective therapy for patients with uncontrolled SA in non-tropical temperate countries and it has been named alpine altitude climate treatment (AACT) (Citation7). Although some environmental characteristics at these altitudes in temperate regions, such as the low temperature and humidity, and the increased UV radiation, could cause a reduction in aeroallergens such as house dust mites (HDM) (Citation8,Citation9), the AACT can also improve patients with non-allergic phenotypes suggesting other pathophysiological pathways (Citation7,Citation10). Different from temperate zones, in tropical regions, because of the humidity and temperature, the conditions could favor the presence of HDM and helminth infections, which could cause eosinophilia and variation of the allergic and type-2 immune responses (Citation11–14), and the behavior of SA could be different at HA.
In contrast to most studies conducted in HA in temperate countries, some studies in HA in tropical countries, not focused on adults with SA, have shown high concentrations of HDM (Citation15–17) and a high prevalence of HDM sensitization in asthmatic children (Citation18). However, there is a lack of information on HDM sensitization and phenotype distribution in adult patients with SA living permanently in HA in tropical regions, and these may be different. Describing the behavior of SA in these geographical conditions is essential to look for differences in the diagnostic approach and management and could apply to millions of people living in the same conditions in Latin America, Asia, and Africa. This cross-sectional study aimed to characterize a cohort of adults with SA living in Bogotá (2,640 m), a city located at a high altitude in a tropical country.
Material and methods
Study design and population
An observational cross-sectional study was performed in adults with SA, aged between 18 and 80 years, from the ASMAIRE program (Institutional program for patients with asthma) of the Fundación Neumológica Colombiana in Bogotá (2,640 m above sea level), the capital of Colombia, located very close to the equator (latitude: 4°, tropical location) and the most populated city in the world at HA (>2,500 m). The ATS/ERS criteria were used to confirm the diagnosis of SA (Citation1): patients requiring high doses of inhaled corticosteroids (ICS) in combination with a long-acting beta2-agonist (LABA) who remain uncontrolled or require these doses to maintain asthma control. Patients should have good adherence and inhaler technique and optimal management of comorbidities.
Patients who met the inclusion criteria (confirmed SA diagnosis for at least three years, permanent residence at HA for the last ten years, no asthma exacerbation and stable treatment in the last two months, no biologic therapy) and provided written informed consent were prospectively recruited from January to December 2019. As an accessible reference center for the whole city, it was considered that the SA patients of the institution are representative of SA patients residing in Bogota (HA city), and a convenience sample of at least of one hundred patients was considered appropriate for the proposed objectives. Almost all the patients were born and have lived at high altitudes (Bogota and different cities or villages located above 2,500 m. This vast zone is called: altiplano cundiboyacense). To reduce bias, patients were enrolled in a consecutive manner. Patients with chronic obstructive pulmonary disease or other obstructive diseases other than asthma, significant missing data of key clinical variables, or unreliable information were excluded. The study was submitted to and approved by the Ethics Committee of the Fundación Neumológica Colombiana with approval number 201709-23205.
Procedures and questionnaires
All patients were evaluated with a complete clinical history, asthma questionnaires, spirometry, total immunoglobulin E (IgE), blood eosinophils, and skin prick test (SPT).
Questionnaires
We used the ACQ-5 (Asthma Control Questionnaire) to measure asthma control and the mini-AQLQ (Asthma Quality of Life Questionnaire) to measure the quality of life.
Spirometry
Pre-bronchodilator and post-broncodilator spirometry was performed in each subject according to the recommendations of the ATS/ERS (Citation19). Airflow limitation (obstruction) was defined by a forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC) below the lower limit of the normal (Citation19).
Total serum immunoglobulin E (IgE)
IgE levels were determined by the enzyme-linked immunosorbent assay (ELISA) technique.
Blood eosinophil count
A blood sample was taken from each patient to determine the number of eosinophils by µL (cells/µL).
Skin prick test (SPT)
SPT for common aeroallergens was performed according to international recommendations (Citation20). The test was performed on the anterior forearm of each subject by a trained allergist physician or nurse, using the following standardized allergens: Dermatophagoides pteronyssinus (Der p), Dermatophagoides farinae (Der f), Blomia tropicalis (Blo t), cat and dog epithelium, cockroach, ant, gramineae (grass), rabbit, eucalyptus, cypress, fraxinus, latex, Aspergillus fumigatus, and Alternaria. Saline solution and 10 mg/mL of histamine were used as negative and positive controls, respectively. The allergens tested were grouped as follow: House dust mites (HDM): Der p, Der f, Blo t; Danders: cat, dog, rabbit; Pollen: gramineae (grass), eucalyptus, cypress, fraxinus; Molds: Aspergillus fumigatus, Alternaria; cockroach; ant; and latex. Multisensitization was defined as testing positive to two or more groups.
Definition of phenotypes
Using some summarizing reviews as references (Citation21–23), we defined the following phenotypes: Allergic/atopic phenotype (AA) was defined by an IgE level ≥100 IU/mL and/or at least one positive SPT; eosinophilic phenotype (EOS), if blood eosinophil count was ≥300 cells/µL; non-AA/non-EOS: IgE <100 IU/mL, negative SPT, and blood eosinophils <300 cells/µL. Type 2-High: AA, and/or EOS; type 2-Low: non-AA + non-EOS. A secondary analysis of phenotype distribution was performed using an IgE level ≥ 30 IU/mL and a blood eosinophil count ≥ 150 cells/µL as cutoffs to define AA and EOS phenotypes, respectively.
Statistical analysis
Categorical variables were described by absolute and relative frequencies, and quantitative variables by means and standard deviations or medians and percentiles according to the Kolmogorov–Smirnov normality test. Independent samples t-test or Mann–Whitney U-test was used to evaluate differences between type 2-high and type 2-low phenotypes. The X2 test was used to compare proportions. Two-sided hypotheses were formulated with a significance level of less than 0.05. The SPSS version 20.0 statistical program was used. A Venn proportional diagram was used to show the distribution of asthma phenotypes.
Results
A total of 129 adults with SA were enrolled. Most of the patients were female (79.8%) with a mean age of 58.9 ± 14.6 years and a body mass index (BMI) of 29.2 ± 5.2 kg/m2. A history of atopy was found in 72.9% of the patients, with allergic rhinitis being the most common allergic comorbidity. shows the clinical, functional, and biomarker characteristics of the patients. According to the protocol, 100% of the patients received high-dose of ICS (fluticasone or budesonide) and a LABA (salmeterol or formoterol), 46.5% a leukotriene modifier, 2.3% tiotropium, and 3.1% long-term oral corticosteroids (OCS). Total serum IgE levels were greater than 100 UI/mL in 55 (42.6%) patients and blood eosinophils were greater than 300 cells/µL in 48 (37.2%) of patients with a median absolute eosinophil count of 230 cells/µL (IQR: 120 - 400). Most patients had fewer than two exacerbations in the previous year (86%). Airflow obstruction was present in 24% of patients, with a mean pre-bronchodilator FEV1/FVC % ratio of 64.3 (±14.8).
Table 1. General characteristics of patients (N = 129).
Phenotype distribution
The AA phenotype was present in 79 patients (61.2%) and the EOS phenotype in 48 (37.2%); of the 79 patients with AA, 45 (57%) were non-eosinophilic, representing 34.9% of the total number of SA patients. Type 2-high in 93 (72.1%) and type 2-low in 36 (27.9%) (). Using cutoffs of total IgE in ≥ 30 IU/µL and blood eosinophil count ≥ 150 cells/µL, the proportion of patients with the AA phenotype increased to 74.4% and the EOS phenotype to 69% ().
Figure 1. Phenotype distribution.
AA phenotype: IgE ≥100 IU/mL and/or at least one positive SPT; EOS phenotype: blood eosinophils count ≥ 300 cells/µL; AA/non- EOS phenotype: IgE ≥100 IU/mL and/or at least one positive SPT and blood eosinophils <300 cells/µL; AA and EOS (overlap phenotype): IgE ≥100 IU/mL and/or at least one positive SPT and blood eosinophils count ≥300 cells/µL; EOS/non-AA phenotype: blood eosinophils ≥300 cells/µL with IgE <100 IU/and a negative SPT; Type 2–High phenotype: IgE ≥100 IU/mL and/or at least one positive SPT and/or EOS blood eosinophils ≥300 cells/µL; Type 2-Low (Non-AA/non-EOS) phenotype: IgE <100 IU/mL, negative SPT and blood eosinophils <300 cells/µL.
Data are presented as proportions: N (%).
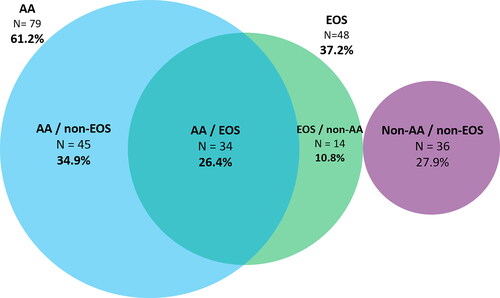
Phenotypic overlap
Of the 79 patients with the AA phenotype, 34 (43%) also had the EOS phenotype (26.4% of the total of 129 SA patients), and among the 48 patients with the EOS phenotype, the same 34 (70.8%) also had the AA phenotype ().
SPT and HDM sensitization
Sixty-six patients (51.2%) had at least one positive SPT; among the positive SPT patients, Dermatophagoides pteronnysinus (80.3%), Dermatophagoides farinae (78.8%), and Blomia tropicalis (68.2%) were the most common positive allergens (). Multisensitization was found in 48/66 (72.7%) with the following groups being the most frequently positive: HDM: 59/66 (89.4%), dander: 36/66 (54.5%), cockroach: 19/66 (28.8%), and pollens: 18/66 (27.3%). Most patients (74.4%) with a positive SPT had a total IgE greater than 30 UI/µL.
Table 2. Tested aeroallergens by SPT (Patients with positive SPT = 66)Table Footnote*.
Differences between type 2-High and type 2-Low phenotypes
Patients with the type 2-high phenotype were younger (p = <0.001), had a lower BMI (p = 0.013), and had more frequently allergic rhinitis (p = 0.006) than type 2-low patients. IgE levels, blood eosinophil counts, and positive SPT were higher in type 2-high patients. No other significant differences were observed ().
Table 3. Differences between Type 2-High and Type 2-Low phenotypes.
Discussion
This is the first cross-sectional study focused on adults with severe asthma living permanently at HA (2,640 m) in a tropical country. Although there is no consensus, HA is usually defined as an altitude above 2,500 m (∼ 8200 feet) (Citation24). Due to the HA, one might have expected a low frequency of HDM sensitization, as it has been described at HA in temperate countries (Citation7–9,Citation25,Citation26), and due to the tropical location, one might have expected a different blood eosinophil count, probably higher, because of the prevalence of helminth infections and the modification of allergy and type-2 immune responses (Citation11–13). However, contrary to expectations, our study showed a high frequency of sensitization to HDM: Dermatophagoides pteronnysinus (80.3%), Dermatophagoides farinae (78.8%) and Blomia tropicalis (68.2%) among our SA patients with positive SPT (atopic) (51.2%), and showed a discrete lower frequency of eosinophilic phenotype, with no other significant differences in phenotypic distribution compared to that described at low altitude. Since most of the patients were born and lived above 2,500 m, sensitization probably occurred at HA. Although Dennis et al. had described 281 patients with asthma symptoms in the past year in the same city (a prevalence of 13.5% [281/2392]), this study did not focus on SA. They found a positive specific IgE for Dermatophagoides pteronnysinus and for Blomia tropicalis of 46.1% and 41%, respectively (Citation17).
It is noteworthy that the overall prevalence of asthma in Colombia, as a tropical country, does not seem to differ from that described in other countries. Two previous population-based studies in this country showed a prevalence of asthma of 12.1% and 9.0% in the general population (Citation17) and in people older than 40 years (Citation27), respectively, which is not significantly different from that described in non-tropical regions. The Andean mountain range crosses the Colombian territory from south to north, so that despite its tropical location, the climate depends largely on the altitude, with many cities and villages located at high altitudes with permanent cold weather (Citation28). About 20% of the country’s population, about 10 million people, live in HA, eight million of them in Bogotá (2,640 m, the most populated city in the world located at HA). The two studies mentioned included cities located at very different altitudes and found no differences in the asthma prevalence according to altitude (Citation17,Citation27), with similar prevalence in Bogotá, in contrast to a recent study done in Mexico that showed a decreasing risk of asthma with increasing altitude (Citation29).
The AA phenotype frequency in our study (61.2%) was similar to that described in the ENFUMOSA study group in Europe (58%) (Citation30) and in some countries such as Hungary (56.6%) (Citation31). However, it was lower than that described in the U-BIOPRED cohort (78.3%) (Citation32), the Italian cohort (55.2%) (Citation23) and the SARP study (71%) (Citation33). As mentioned above, in contrast to what has been described in some studies supporting AACT and showing a decrease in HDM allergen concentration and sensitization in areas higher than 1,500 m above sea level in non-tropical temperate countries (Citation7–9,Citation34), we did not find a lower sensitization to HDM. This has been described in HA in other tropical countries (Citation15,Citation16) and in asthmatic children in Colombia (Citation18), and could be explained by the higher humidity and temperature, independent of altitude, in tropical areas (Citation11,Citation14). Recently, some studies have suggested that the paradigm of lower HDM concentrations in HA in temperate countries may have changed due to global warming and the use of newer construction techniques that alter the internal temperatures of buildings (Citation10,Citation35,Citation36). A study in Germany and Austria in which a total of 122 dust samples were collected from different buildings located at altitudes between 400 and 2,600 m, found no statistically significant difference in allergen concentration between samples collected above or below 1,500 m (Citation35). It is known that an increase in temperature can increase humidity and affect the growth and survival of HDM since mites absorb vital moisture from the air and require high air humidity to prevent excessive water loss (Citation14,Citation35). The Dennis’ study in Colombia (Citation17) showed that the frequency of sensitization to Dermatophagoides pteronnysinus and Blomia tropicalis, as assessed by specific IgE, was significantly higher in asthmatic subjects than in controls, but this difference was not adjusted for altitude and was not assessed in SA. We did not measure humidity and temperature directly. However, as a tropical city, Bogotá does not have seasons, the humidity is high and relatively stable (annual median of 78.2%), and the average temperature was 13° (8 to 19) in 2019. This level of humidity and temperature is higher than the average observed at the same high altitude in non-tropical regions and could favor the presence of HDM and sensitization at HA in this tropical city.
The median total IgE level of our patients (54.3 UI/mL) was lower than those reported by SARP: 301 UI/mL (Citation33), U-BIOPRED: 119.5 UI/mL (Citation32), Al-Jadali et al: 99.5 UI/mL (Citation37), Ricciardolo et al: 125.3 UI/mL (Citation23) and Marques Mello et al: 537 UI/mL (Citation38). There is no clear explanation for this finding; the lower prevalence of the AA phenotype (51.2%) in our study compared to some cohorts (Citation32,Citation33) could partially explain the lower values of IgE levels. Interestingly, the previous study by Dennis et al. in Colombia (Citation17) had similarly shown a higher total IgE than our study, but the result was not adjusted for altitude in the published data. Boonpiyathad et al. demonstrated a reduced expression of chemoattractant receptor homologue expressed on Th2 cells (CRTH2), due to desensitization, with an induced decrease of prostaglandin D2 signaling and also reduced levels of IL-5 and IL-13, in patients with SA moving to higher altitude for AACT (Citation39). Although this may reduce the IgE levels (Citation40), it does not appear to be applicable to our population of long-term HA residents.
The frequency of the EOS phenotype in our study (37.2%), defined as ≥ 300 cells/µL, was lower than that reported in other countries (Sweden, 42.3%; Saudi Arabia, 45%; Australia, 44% and Italy, 41.7%) (Citation3,Citation23,Citation37,Citation41) and in the International Severe Asthma Registry(ISAR) cohort (83.8%) (Citation42). The median absolute eosinophil count in our study was 230 cells/µ but the median was 200 cells/µL in the Swedish cohort, 250 cells/µL in the Saudi cohort, 400 cells/µL in the Australian cohort and 312 cells/µL in the Italian cohort (Citation3,Citation23,Citation37,Citation41). In the ISAR cohort, the median absolute eosinophil count was not reported (Citation42). There is no obvious explanation for this finding. One might have expected a higher frequency of eosinophilia because of the frequency of sensitization, including HDM sensitization; it is increasingly evident that the relationship between sensitization, allergy, and eosinophils is complex and many other mechanisms are involved in the behavior of eosinophils in asthma (Citation43). However, using a lower cutoff for blood eosinophil count (≥ 150 cells/µL), the proportion of patients with the EOS phenotype increased to 69%. The median blood eosinophil count in our patients (230 cells/µL) is similar to that reported in other cohorts (Citation32,Citation37,Citation38,Citation41). Although helminth infections are common in tropical regions and could be associated with eosinophilia and increased allergy and type-2 responses (Citation11–13), our sample of patients resides permanently in Bogota with good hygienic conditions and, although we did not evaluate it, their likelihood of parasitism is lower than in other areas of the country. We could not adjust the blood eosinophils count according to systemic steroid therapy because of the low number of patients on OCS (3.1%) and incomplete information (dose and duration) in several cases. We did not include patients receiving biologic therapies for SA.
As it has been described (Citation44–46), obesity was very common in our patients (41.9%), higher in the type 2-low phenotype (61.1%). Obesity has been associated with paucigranulocytic inflammation, and non-eosinophilic later-onset SA, especially in women with SA (Citation47), and several studies have suggested that obesity could reduce eosinophil count and the fractional exhaled nitric oxide (FeNO) (Citation48). Our results could be similarly influenced by the high frequency of obesity and overweight which could partially explain the relatively lower frequency of the eosinophilic phenotype.
In our study, the percentage of pre-bronchodilator FEV1 (77 ± 19.9%) was better than that reported in low altitudes cohorts such as the SARP cohort (62%) (Citation33), the U-BIOPRED cohort (67.5%) (Citation32), the ENFUMOSA cohort (71.8%) (Citation30), and the Saudi Arabia cohort (67.3%) (Citation37). There is no information about pulmonary function in patients with SA and long-term stay in HA. However, in the Vinnikov’s systematic review and meta-analysis, 93% of patients with asthma who transferred to HA to receive AACT had an improvement in lung function, with baseline FEV1 values ranging from 64.5% to 105.8%. This meta-analysis included patients with asthma and did not differentiate between those with severe and non-severe asthma (Citation7,Citation49).
Our study is innovative in showing that patients with SA living in a tropical city at high-altitude have a high prevalence of HDM sensitization among patients with the AA phenotype, unlike non-tropical temperate regions, and a slightly lower frequency of the eosinophilic phenotype. Small previous studies at high tropical altitudes did not focus on adults with SA (Citation15–18). These findings may apply to the nearly 400 million people worldwide who live permanently above 1,500 meters (Citation50). Although the study was focused on patients living in HA, the results contribute to the scarce information on SA in Latin America (Citation51). As a strength, this study has a significant number of patients who were prospectively recruited and evaluated using standardized procedures.
Some limitations include the lack of a control group residing at low altitude due to the economic and logistical difficulties of assembling it in our country. However, we included a significant number of patients with SA (n = 129) with general characteristics (age, sex, body mass index, clinical and functional variables) similar to those described in most studies conducted at low altitudes in non-tropical areas with which we compared our results. Another limitation of our study is the definition of the allergic/atopic (AA) phenotype as a total IgE level ≥100 IU/mL and/or at least one positive SPT. We did not perform an allergen-specific challenge test, and therefore we recognize that we can’t guarantee the true role of allergy in these patients. However, as one of the most used clinical-epidemiologic definitions of allergic asthma (Citation21), our findings could be globally compared with studies at low altitude using a similar definition. We did not perform HDM measurements in this study.
Although altitude may adversely affect air pollution (Citation52,Citation53), both outdoor and indoor, and there is an association between air pollution and allergic sensitization (Citation14,Citation54,Citation55), we did not include environmental and air pollution variables in our study because our goal was primarily to characterize the population of SA at HA, not to directly compare low altitude and HA. Due to unavailability at the time of the study, we did not include fractional exhaled nitric oxide (FeNO) measurements.
We recognize that the one-time assessment of the patients inherent in cross-sectional studies may not reflect the usual condition of some patients. We rigorously included patients in stable condition with no exacerbation in the previous two months. Our patients came from a single institution (Fundación Neumológica Colombiana) in Bogotá. However, since this institution is a reference center for respiratory diseases and receives patients from all over the city, the patients attending our asthma program and the selected sample are representative of patients with SA living in Bogotá at 2,640 m (high altitude). Another limitation is that we did not measure humidity and temperature. However, as mentioned above, these were relatively stable during the study period.
Conclusion
In conclusion, in these patients with SA permanently living in a high-altitude city in a tropical country, unlike several studies supporting AACT, we found a high prevalence of HDM sensitization. Compared with that described in non-tropical settings at low altitude, we found a discretely lower prevalence of the eosinophilic phenotype, with no other significant differences in the distribution of SA phenotypes. It remains to be determined whether AACT is useful as a therapeutic intervention in SA in tropical countries. Our results call for the need for well-designed studies to evaluate the impact of HA residence in the clinical course of SA, both in tropical and non-tropical regions.
| Abbreviations | ||
| SA | = | Severe asthma |
| ERS | = | European Respiratory Society |
| ATS | = | American Thoracic Society |
| ICS | = | inhaled corticosteroids |
| LABA | = | long-acting beta-agonist |
| HA | = | High altitude |
| AACT | = | alpine altitude climate treatment |
| HDM | = | House dust mites |
| ACQ-5 | = | Asthma Control Questionnaire |
| AQLQ | = | Asthma Quality of Life Questionnaire |
| SPT | = | Skin prick test |
| AA | = | Allergic/atopic phenotype |
| EOS | = | eosinophilic phenotype. |
Declaration of interest
Carlos A. Torres-Duque has received fees as an advisory board participant and/or speaker from AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Novartis, and Sanofi-Aventis; has taken part in clinical trials from AstraZeneca, Novartis, and Sanofi-Aventis; has received unrestricted grants for investigator-initiated studies at Fundación Neumologica Colombiana from AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Grifols and Novartis.
Abraham Alí-Munive has received fees as an advisory board participant and/or speaker from AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Grifols, Novamed, Novartis, and Sanofi-Aventis; has taken part in clinical trials from Grifols, Novartis and Sanofi-Aventis; has received unrestricted grants for investigator-initiated studies at Fundación Neumologica Colombiana from AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Grifols and Novartis.
Diego Severiche-Bueno declares no potential conflicts of interest concerning the research, authorship, and/or publication of this article.
Mauricio Durán-Silva has received fees as an advisory board participant and/or speaker from Boehringer-Ingelheim, Novartis, and Sanofi-Aventis; has taken part in clinical trials from AstraZeneca, Novartis, and Sanofi-Aventis.
Carlos E. Aguirre-Franco has received fees as an advisory board participant and/or speaker from Boehringer-Ingelheim and Sanofi-Aventis; has received unrestricted grants for educational activities at Fundacion Neumologica Colombiana from AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Novartis, and Sanofi-Aventis.
Angélica González-Florez has received fees as an advisory board participant and/or speaker from Janssen and Merck.
María José Pareja-Zabala is employed by Biogen and a former employee of Sanofi-Pasteur; she declares no potential conflicts of interest concerning the research, authorship, and/or publication of this article.
Libardo Jiménez-Maldonado has received fees as an advisory board participant and/or speaker from AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Novartis, and Sanofi-Aventis; has participated in clinical trials for AstraZeneca, Novartis, and GlaxoSmithKline.
Mauricio Gonzalez-Garcia declares no potential conflicts of interest concerning the research, authorship, and/or publication of this article.
Additional information
Funding
References
- Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi:10.1183/09031936.00202013.
- Reddel HK, Bacharier LB, Bateman ED, Brightling CE, Brusselle GG, Buhl R, Cruz AA, Duijts L, Drazen JM, FitzGerald JM, et al. Global initiative for asthma strategy 2021: executive summary and rationale for key changes. Am J Respir Crit Care Med. 2022;205(1):17–35. doi:10.1164/rccm.202109-2205PP.
- Ronnebjerg L, Axelsson M, Kankaanranta H, Backman H, Radinger M, Lundback B, Ekerljung L. Severe asthma in a general population study: prevalence and clinical characteristics. J Asthma Allergy. 2021;14:1105–1115. doi:10.2147/JAA.S327659.
- Wang E, Wechsler ME, Tran TN, Heaney LG, Jones RC, Menzies-Gow AN, Busby J, Jackson DJ, Pfeffer PE, Rhee CK, et al. Characterization of severe asthma worldwide: data from the international severe asthma registry. Chest. 2020;157(4):790–804. doi:10.1016/j.chest.2019.10.053.
- McDonald VM, Hiles SA, Jones KA, Clark VL, Yorke J. Health-related quality of life burden in severe asthma. Med J Aust. 2018;209(S2):S28–s33. doi:10.5694/mja18.00207.
- López-Tiro J, Contreras-Contreras A, Rodríguez-Arellano ME, Costa-Urrutia P. Economic burden of severe asthma treatment: a real-life study. World Allergy Organ J. 2022;15(7):100662. doi:10.1016/j.waojou.2022.100662.
- Fieten KB, Drijver-Messelink MT, Cogo A, Charpin D, Sokolowska M, Agache I, Taborda-Barata LM, Eguiluz-Gracia I, Braunstahl GJ, Seys SF, et al. Alpine altitude climate treatment for severe and uncontrolled asthma: an EAACI position paper. Allergy. 2022;77(7):1991–2024. doi:10.1111/all.15242.
- Vervloet D, Penaud A, Razzouk H, Senft M, Arnaud A, Boutin C, Charpin J. Altitude and house dust mites. J Allergy Clin Immunol. 1982;69(3):290–296. doi:10.1016/s0091-6749(82)80006-7.
- Charpin D, Birnbaum J, Haddi E, Genard G, Lanteaume A, Toumi M, Faraj F, Van der Brempt X, Vervloet D. Altitude and allergy to house-dust mites. A paradigm of the influence of environmental exposure on allergic sensitization. Am Rev Respir Dis. 1991;143(5):983–986. doi:10.1164/ajrccm/143.5_Pt_1.983.
- Rijssenbeek-Nouwens LH, Fieten KB, Bron AO, Hashimoto S, Bel EH, Weersink EJ. High-altitude treatment in atopic and nonatopic patients with severe asthma. Eur Respir J. 2012;40(6):1374–1380. doi:10.1183/09031936.00195211.
- Caraballo L, Zakzuk J, Lee BW, Acevedo N, Soh JY, Sánchez-Borges M, Hossny E, García E, Rosario N, Ansotegui I, et al. Particularities of allergy in the tropics. World Allergy Organ J. 2016;9:20. doi:10.1186/s40413-016-0110-7.
- Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118(4):1311–1321. doi:10.1172/JCI34261.
- Alcântara-Neves NM, de S G Britto G, Veiga RV, Figueiredo CA, Fiaccone RL, da Conceição JS, Cruz ÁA, Rodrigues LC, Cooper PJ, Pontes-de-Carvalho LC, et al. Effects of helminth co-infections on atopy, asthma and cytokine production in children living in a poor urban area in Latin America. BMC Res Notes. 2014;7(1):817. doi:10.1186/1756-0500-7-817.
- Acevedo N, Zakzuk J, Caraballo L. House dust mite allergy under changing environments. Allergy Asthma Immunol Res. 2019;11(4):450–469. doi:10.4168/aair.2019.11.4.450.
- Valdivieso R, Iraola V, Estupinan M, Fernandez-Caldas E. Sensitization and exposure to house dust and storage mites in high-altitude areas of ecuador. Ann Allergy Asthma Immunol. 2006;97(4):532–538. doi:10.1016/S1081-1206(10)60946-5.
- Valdivieso R, Iraola V, Pinto H. Presence of domestic mites at an extremely high altitude (4800 m) in Andean Ecuador. J Invest Allergol Clin Immunol. 2009;19:323–324.
- Dennis RJ, Caraballo L, Garcia E, Rojas MX, Rondon MA, Perez A, Aristizabal G, Penaranda A, Barragan AM, Ahumada V, et al. Prevalence of asthma and other allergic conditions in Colombia 2009-2010: a cross-sectional study. BMC Pulm Med. 2012;12(1):17. doi:10.1186/1471-2466-12-17.
- Duenas-Meza E, Torres-Duque CA, Correa-Vera E, Suárez M, Vásquez C, Jurado J, Del Socorro Medina M, Barón O, Pareja-Zabala MJ, Giraldo-Cadavid LF. High prevalence of house dust mite sensitization in children with severe asthma living at high altitude in a tropical country. Pediatr Pulmonol. 2018;53(10):1356–1361. doi:10.1002/ppul.24079.
- Stanojevic S, Kaminsky DA, Miller M, Thompson B, Aliverti A, Barjaktarevic I, Cooper BG, Culver B, Derom E, Hall GL, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J. 2022;60(1):2101499. doi:10.1183/13993003.01499-2021.
- Dreborg S, Frew A. Position paper: allergen standardization and skin tests. Allergy. 1993;48:48–82.
- Akar-Ghibril N, Casale T, Custovic A, Phipatanakul W. Allergic endotypes and phenotypes of asthma. J Allergy Clin Immunol Pract. 2020;8(2):429–440. doi:10.1016/j.jaip.2019.11.008.
- Carr TF, Zeki AA, Kraft M. Eosinophilic and noneosinophilic asthma. Am J Respir Crit Care Med. 2018;197(1):22–37. doi:10.1164/rccm.201611-2232PP.
- Ricciardolo FLM, Sprio AE, Baroso A, Gallo F, Riccardi E, Bertolini F, Carriero V, Arrigo E, Ciprandi G. Characterization of T2-low and T2-high asthma phenotypes in real-life. Biomedicines. 2021;9(11):1684. doi:10.3390/biomedicines9111684.
- Barry PW, Pollard AJ. Altitude illness. BMJ. 2003;326(7395):915–919. doi:10.1136/bmj.326.7395.915.
- van Velzen E, van den Bos JW, Benckhuijsen JA, van Essel T, de Bruijn R, Aalbers R. Effect of allergen avoidance at high altitude on direct and indirect bronchial hyperresponsiveness and markers of inflammation in children with allergic asthma. Thorax. 1996;51(6):582–584. doi:10.1136/thx.51.6.582.
- Spieksma FT, Zuidema P, Leupen MJ. High altitude and house-dust mites. Br Med J. 1971;1(5740):82–84. doi:10.1136/bmj.1.5740.82.
- Gonzalez-Garcia M, Caballero A, Jaramillo C, Maldonado D, Torres-Duque CA. Prevalence, risk factors and underdiagnosis of asthma and wheezing in adults 40 years and older: a population-based study. J Asthma. 2015;52(8):823–830. doi:10.3109/02770903.2015.1010733.
- Torres-Duque CA. Letter from Colombia. Respirology. 2019;24(11):1115–1116. doi:10.1111/resp.13658.
- Vargas MH, Becerril-Ángeles M, Medina-Reyes IS, Rascón-Pacheco RA. Altitude above 1500 m is a major determinant of asthma incidence. An ecological study. Respir Med. 2018;135:1–7. doi:10.1016/j.rmed.2017.12.010.
- ENFUMOSA Study Group. The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. Eur Respir J. 2003;22:470–477.
- Csoma Z, Gál Z, Gézsi A, Herjavecz I, Szalai C. Prevalence and characterization of severe asthma in Hungary. Sci Rep. 2020;10(1):9274. doi:10.1038/s41598-020-66445-4.
- Shaw DE, Sousa AR, Fowler SJ, Fleming LJ, Roberts G, Corfield J, Pandis I, Bansal AT, Bel EH, Auffray C, et al. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur Respir J. 2015;46(5):1308–1321. doi:10.1183/13993003.00779-2015.
- Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, Calhoun WJ, Castro M, Chung KF, Clark MP, et al. Characterization of the severe asthma phenotype by the national heart, lung, and blood institute’s severe asthma research program. J Allergy Clin Immunol. 2007;119(2):405–413. doi:10.1016/j.jaci.2006.11.639.
- Charpin D, Kleisbauer JP, Lanteaume A, Razzouk H, Vervloet D, Toumi M, Faraj F, Charpin J. Asthma and allergy to house-dust mites in populations living in high altitudes. Chest. 1988;93(4):758–761. doi:10.1378/chest.93.4.758.
- Grafetstatter C, Prossegger J, Braunschmid H, Sanovic R, Hahne P, Pichler C, Thalhamer J, Hartl A. No concentration decrease of house dust mite allergens with rising altitude in Alpine Regions. Allergy Asthma Immunol Res. 2016;8(4):312–318. doi:10.4168/aair.2016.8.4.312.
- Charpin D. High altitude and asthma: beyond house dust mites. Eur Respir J. 2012;40(6):1320–1321. doi:10.1183/09031936.00096712.
- Al-Jahdali H, Wali S, Albanna AS, Allehebi R, Al-Matar H, Fattouh M, Beekman M. Prevalence of eosinophilic, atopic, and overlap phenotypes among patients with severe asthma in Saudi Arabia: a cross-sectional study. BMC Pulm Med. 2022;22(1):67. doi:10.1186/s12890-022-01856-9.
- Marques Mello L, Viana KP, Moraes Dos Santos F, Saturnino LTM, Kormann ML, Lazaridis E, Torreão CD, Soares CR, Abreu GA, Lima VB, et al. Severe asthma and eligibility for biologics in a Brazilian cohort. J Asthma. 2021;58(7):958–966. doi:10.1080/02770903.2020.1748049.
- Boonpiyathad T, Capova G, Duchna HW, Croxford AL, Farine H, Dreher A, Clozel M, Schreiber J, Kubena P, Lunjani N, et al. Impact of high-altitude therapy on type-2 immune responses in asthma patients. Allergy. 2020;75(1):84–94. doi:10.1111/all.13967.
- Hammad H, Lambrecht BN. The basic immunology of asthma. Cell. 2021;184(6):1469–1485. doi:10.1016/j.cell.2021.02.016.
- Hiles SA, Gibson PG, McDonald VM. Disease burden of eosinophilic airway disease: comparing severe asthma, COPD and asthma-COPD overlap. Respirology. 2021;26(1):52–61. doi:10.1111/resp.13841.
- Heaney LG, Perez de Llano L, Al-Ahmad M, Backer V, Busby J, Canonica GW, Christoff GC, Cosio BG, FitzGerald JM, Heffler E, et al. Eosinophilic and noneosinophilic asthma: an expert consensus framework to characterize phenotypes in a global real-life severe asthma cohort. Chest. 2021;160(3):814–830. doi:10.1016/j.chest.2021.04.013.
- Matucci A, Vultaggio A, Maggi E, Kasujee I. Is IgE or eosinophils the key player in allergic asthma pathogenesis? Are we asking the right question? Respir Res. 2018;19(1):113. doi:10.1186/s12931-018-0813-0.
- Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol. 2018;141(4):1169–1179. doi:10.1016/j.jaci.2018.02.004.
- Tashiro H, Shore SA. Obesity and severe asthma. Allergol Int. 2019;68(2):135–142. doi:10.1016/j.alit.2018.10.004.
- Schatz M, Zeiger RS, Yang S-J, Chen W, Sajjan S, Allen-Ramey F, Camargo CA. Prospective study on the relationship of obesity to asthma impairment and risk. J Allergy Clin Immunol Pract. 2015;3(4):560–565.e561. doi:10.1016/j.jaip.2015.03.017.
- Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, Wardlaw AJ, Green RH. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178(3):218–224. doi:10.1164/rccm.200711-1754OC.
- Sharma V, Ricketts HC, Steffensen F, Goodfellow A, Cowan DC. Obesity affects type 2 biomarker levels in asthma. J Asthma. 2023;60(2):385–392. doi:10.1080/02770903.2022.2051548.
- Vinnikov D, Khafagy A, Blanc PD, Brimkulov N, Steinmaus C. High-altitude alpine therapy and lung function in asthma: systematic review and meta-analysis. ERJ Open Res. 2016;2(2):00097–2015. doi:10.1183/23120541.00097-2015.
- Burtscher M. Effects of living at higher altitudes on mortality: a narrative review. Aging Dis. 2014;5(4):274–280. doi:10.14336/AD.2014.0500274.
- Neffen H, Moraes F, Viana K, Di Boscio V, Levy G, Vieira C, Abreu G, Soares C. Asthma severity in four countries of Latin America. BMC Pulm Med. 2019;19(1):123. doi:10.1186/s12890-019-0871-1.
- Bravo Alvarez H, Sosa Echeverria R, Sanchez Alvarez P, Krupa S. Air quality standards for particulate matter (PM) at high altitude cities. Environ Pollut. 2013;173:255–256. doi:10.1016/j.envpol.2012.09.025.
- Brakema EA, Tabyshova A, Kasteleyn MJ, Molendijk E, van der Kleij RM, van Boven JF, Emilov B, Akmatalieva M, Mademilov M, Numans ME, et al. High COPD prevalence at high altitude: does household air pollution play a role? Eur Respir J. 2019;53(2):1801193. doi:10.1183/13993003.01193-2018.
- Chatkin J, Correa L, Santos U. External environmental pollution as a risk factor for asthma. Clin Rev Allergy Immunol. 2022;62(1):72–89. doi:10.1007/s12016-020-08830-5.
- Xing Z, Yang T, Shi S, Meng X, Chen R, Long H, Hu Y, Chai D, Liu W, Tong Y, et al. Ambient particulate matter associates with asthma in high altitude region: a population-based study. World Allergy Organ J. 2023;16(5):100774. doi:10.1016/j.waojou.2023.100774.
