?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Omalizumab is a humanized monoclonal antibody that specifically binds to free human immunoglobulin E. The introduction of this drug raises concerns about economic impact in scenarios with constrained. This study aimed to estimate the cost utility of omalizumab in adults with severe asthma uncontrolled in Colombia.
Methods
We used a Markov state-transition model to estimate the cost and QALYs associated with omalizumab compared to standard of care; from a third payer perspective over a lifetime horizon. This model used local costs while utilities were derived from international literature. Cost and transition probabilities were obtained from a mixture of Colombian-specific and internationally published data.
Results
The mean incremental cost of omalizumab versus standard of care is US$3 481. The mean incremental benefit of omalizumab versus standard of care 0.094 QALY. The incremental expected cost per unit of benefit is estimated at US$36846 per QALY. There is only a probability of 0.032 that Omalizumab is more cost-effective than standard of care at a threshold of US$5180 per QALY.
Conclusion
Omalizumab is not cost-effective in adults with severe asthma uncontrolled in Colombia. If the cost of Omalizumab is reduced by 83%, this treatment would be cost-effective in our country. Our study provides evidence that should be used by decision-makers to improve clinical practice guidelines and should be replicated to validate their results in other middle-income countries.
Keywords:
Background
Asthma is a common chronic disease with an estimated 300 million patients worldwide (Citation1). Omalizumab is a humanized monoclonal antibody that specifically binds to free human immunoglobulin E (IgE) and inhibits its interaction with the IgE receptor (Citation2). Many clinical trials and real-life studies have shown that omalizumab reduces exacerbation risk and improves quality of life (Citation3,Citation4). Omalizumab, a monoclonal antibody used for the treatment of severe asthma, has been studied extensively in terms of its cost-effectiveness (Citation5). Several economic evaluations have been conducted to assess the cost-effectiveness of omalizumab in comparison to standard therapies for severe asthma (Citation6). The results of these studies have been mixed, with some indicating that omalizumab is a cost-effective option for severe asthma, while others have shown less favorable results. The cost-effectiveness of omalizumab can vary depending on factors such as the specific patient population, healthcare setting, and the willingness-to-pay threshold considered (Citation6,Citation7). The cost-effectiveness can differ across countries and healthcare systems due to variations in treatment costs, resource availability, and reimbursement policies. In Colombia, omalizumab was approved for the treatment of severe asthma in 2017. With the introduction concerns with respect to if the extra benefit offered by this drug outweighs the additional cost compared to standard of care in severe asthma. This question is even more relevant in developing countries with an increasing prevalence of asthma and constrained healthcare. An economic evaluation of these new drugs could provide evidence to optimize the efficiency of using economic resources in these countries. This study aimed to estimate the cost utility of omalizumab plus standard of care vs standard of care alone in adults with severe asthma uncontrolled.
Materials and methods
We conducted a cost-utility analysis from third payer perspective In this cost-utility study, the benefits of omalizumab were expressed as quality-adjusted life-years (QALYs) gained. Standard of care (SOC) refers to treatments recommended for severe asthma by international guidelines (GINA 2022); typically defined as inhaled corticosteroids in combination with long-acting beta-agonist (LABA) with or without an additional controller therapy (Citation5). Omalizumab was considered cost-effective if the incremental cost-utility ratio per QALY gained was less than $5180, or if the net monetary benefit (NMB) of this strategy surpassed that of the strategy without omalizumab (Citation8).
Model development
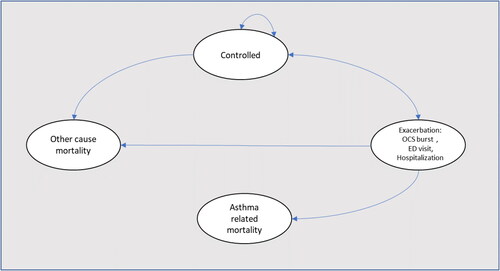
We used a Markov state-transition model to estimate the cost and QALYs associated with omalizumab plus SOC compared to SOC alone. In this mathematical model, patients could transition between three mutually exclusive health states (asthma on omalizumab and SOC, asthma on SOC alone, and death). During each cycle, patients in non-death health states could transit to any of three levels of asthma exacerbations: OCS burst (was defined as relatively major symptoms during the week and need of use of oral corticosteroids to achieve the control of symptoms), emergency department (patient that request treatment with systemic corticosteroids) and hospitalization. Asthma-related mortality following an exacerbation or all-cause mortality could also occur (). We made this analysis from third payer perspective over a lifetime horizon (only including direct costs), using a lifetime horizon and a cycle length of 4 wk. Half-cycle correction and an annual discounting rate of 5% were applied to both costs and QALYs.
Clinical data
Data of relative risk (RR) of omalizumab and transition probabilities were obtained from systematic review of clinical trials and real-world studies of omalizumab in severe asthma (Citation4,Citation9–12); while data of utilities were extracted from a systematic reviews of utilities in asthma (Citation13,Citation14), . Since these data do not come from the Colombian population, they were subjected to probabilistic sensitivity analysis as detailed below as recommended by Consolidated Health Economic Evaluation Reporting Standards (CHEERS) Statement. In this sensitivity analysis, to build the range of RR to be used in this analysis, we use data published by clinical trials and in real-life studies. In the case of utilities, the upper and lower ranges were estimated by adding or subtracting 25% of the value from the central value defined for the base case. The risk of mortality from other causes was estimated using age- and gender-specific Colombian life tables for all-cause mortality over 5 years (2016 to 2020) (Citation15).
Table 1. Base case.
We assumed that 22.6% of patients discontinued the treatment after 16 wk of treatment (Citation11). Patients who discontinued (non-adherent) treatment had the same costs and clinical outcome values as adherent patients for the first 16 wk. But these input values changed to those of the placebo group for all transition cycles after 16 wk. Similarly, we assumed a 60.5% response rate after 16 wk of treatment (Citation12). In this case, the utilities, exacerbation rates, and costs for the non-responders were the same as the inputs of the placebo group. Sensitivity analysis of percentage of non-adherents and response rates were made by estimating the upper and lower range of each value by adding or subtracting 25% of the value defined previously. All costs for each health state defined in the model were extracted from a previously Colombian health care cost and resource utilization study (Citation16). Briefly, this study identified the asthma-related direct cost of 20410 patients with asthma from January 1, 2004, through December 31, 2014, in Colombia. Unit costs for omalizumab were taken from the National Drug Price Information System (SISMED, 2020). All cost costs were transformed to 2020 costs using official inflation data in Colombia (Citation17). The incremental cost-effectiveness ratio (ICER) was calculated using the following formulae:
Also, we estimated the net monetary benefit (NMB). NMB represents the value of an intervention in monetary terms (Citation18). NMB was calculated using the following formula:
where incremental NMB measures the difference in NMB between alternative interventions, a positive incremental NMB indicates that the intervention is cost-effective compared with the alternative at the given willingness-to-pay threshold.
Sensitivity analysis
We conducted a one-way sensitivity analysis, presenting the results in the form of a tornado diagram showing the incremental cost-effectiveness ratio. We performed price threshold analysis. This analysis allows us to identify the maximum price where its treatment would be considered cost-effective. In other words, what is the maximum price for a product where the ICER for the new strategy would be equal to the WTP threshold. Additionally, we performed a probabilistic sensitivity analysis by randomly sampling from the parameter distributions. The relative risk and utilities were modeled using the beta distribution, while the costs followed a gamma distribution (refer to for details). For each treatment strategy, we calculated the expected costs and Quality-Adjusted Life Years (QALYs) by considering all combinations of parameter values in the model. This calculation involved a second-order Monte Carlo simulation with 10,000 replications for each parameter, resulting in the expected cost-utility for each treatment strategy. To account for decision uncertainty, we plotted the cost-effectiveness and acceptability frontiers. Microsoft Excel ® was used in all analyses. Ethics approval was not required as the data used in this study came from publicly available data.
Results
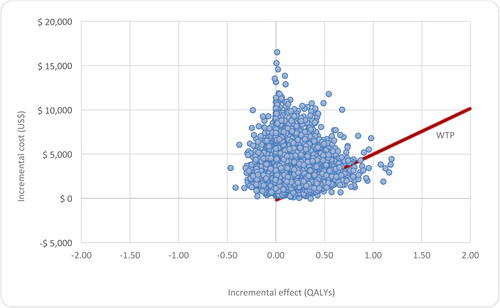
Cost-effectiveness plane
The mean incremental cost of omalizumab versus no omalizumab is US$3481. This suggests that omalizumab is more costly. The incremental cost is uncertain because the model parameters are uncertain. The 95% credible interval for the incremental cost ranges from US$906 to US$7689. The probability that omalizumab is cost saving compared to no omalizumab is 0.0004.
The mean incremental benefit of omalizumab versus no omalizumab is 0.094 QALY. This suggests that omalizumab is more beneficial. Again, there is uncertainty in the incremental benefit due to uncertainty in the model parameters. The 95% credible interval for the incremental benefit ranges from 0.078 QALYs to 0.47 QALYs, . The probability that Omalizumab is more beneficial than no omalizumab is 0.877.
Table 2. Result of cost effectiveness analysis.
The incremental expected cost per unit of benefit is estimated at US$36846 per QALY. There is a probability of 0.032 that Omalizumab is more cost-effective than no omalizumab at a threshold of US$5180 per QALY
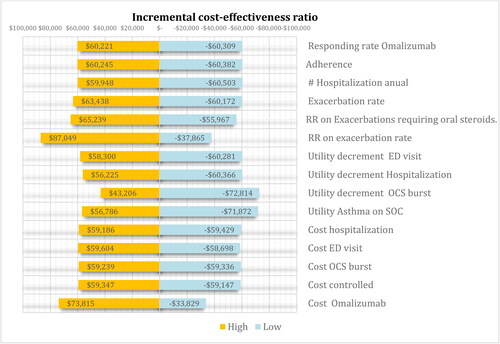
Sensitivity analysis
Our model was robust, and the ICER had no significant changes in one-way sensitivity analysis, . We performed price threshold analysis. This analysis allows us to identify the maximum price for one 150-mg vial of omalizumab where its would be considered cost-effective would be of US$ 52; this is a reduction of 83% of the current price.
The cost-effectiveness plane shows the standardized cost-effectiveness plane per person based on 10000 model runs in which uncertain model parameters are varied simultaneously in a probabilistic sensitivity analysis. The willingness-to-pay threshold is shown as a degree line, . The results of probabilistic sensitivity analysis are graphically represented in the cost-effectiveness plane, where 12% of simulations were graphed in quadrant 4 (higher cost, lower QALYs), and 88% in quadrant 1 of this plane (high cost, high QALYs), .
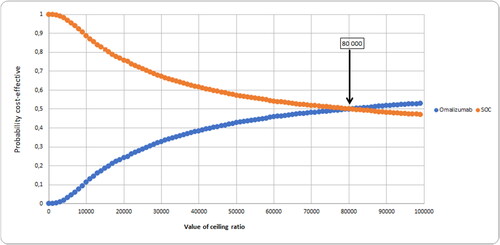
Cost-effectiveness acceptability curve
This graph shows the cost-effectiveness acceptability curve for the comparison of strategies. The results show that at a threshold value for cost-effectiveness of US$5180 per QALY the strategy with the highest probability of being most cost-effective is Control., with a probability of 0.96, .
Net benefit
Net benefit is a calculation that puts discounted lifetime costs (US$) and discounted Lifetime QALYs onto the same scale. The strategy with the highest expected net benefit is no omalizumab, with an expected net benefit of US$23108 (CI 95% 10828 to 31909) while the expected Net Benefit of omalizumab was US$ 20116 (CI 95% 7186 to 29797)
Expected value of perfect information
The overall EVPI per person affected by the decision is estimated to be 29797, or US$23427 by each 1000 patients in Colombia. If the decision relevance horizon is 5 years, then the overall expected value of removing decision uncertainty for Colombia would be US$49.24. When thinking about the overall expected value of removing decision uncertainty, one needs to consider how long the current comparison will remain relevant. If the decision relevance horizon is 10 years, then the overall expected value of removing decision uncertainty for Colombia would be US$234273. Research or data collection exercises costing more than this amount would not be considered an efficient use of resources. This is because the return on investment from the research – as measured by the health gain and cost savings resulting from enabling the decision maker to better identify the best decision option – is expected to be no higher than US$234273.
Discussion
In our study, omalizumab was not cost-effective in Colombia. This result is relevant due high financial burden implied by biological drugs in Colombia. In Colombia, approximately 36% ($1.25 billion) of the total pharmaceutical market corresponded to biological drugs, cost paid largely (80% to 95%) with public funds (Citation19,Citation20). Our study provides direct evidence for decision-making at the national level regarding the inclusion of this drug in health benefit plans for public and private entities in our country.
Our model based in a developing country, yields similar results to other economic analysis in high-income countries. A previously systematic review identified 19 studies from 2000 to 2018 that analyzed the cost-effectiveness of omalizumab in patients with moderate to severe allergic asthma (Citation21). The incremental cost-effectiveness ratio ranged from $287,200 per QALY gained (2008 dollars) to $821,000 per QALY gained (2005 dollars) during a lifetime horizon. Across these studies, 10 found that omalizumab was cost-effective in base-case scenarios; 5 found that it was not cost-effective, and the remaining found that omalizumab was only cost-effective when targeted to specific severe subgroups. In this review, reported than in 11 model-based evaluations using efficacy and effectiveness inputs and eight trial-based evaluations from real world clinical practice. This is a central point because previously economic evaluation, based only on data from clinical trials, has limited external validity as they do not include in their models’ essential variables that impact the cost and consequences, such as adherence or responding rate. In clinical trials drug adherence is higher than in real-world clinical practice, and economic models based relying on clinical trial evidence may overestimate the benefits of these drugs, which in turn can overestimate its impact on quality of life. In our model we not only included estimation of adherence or responding rate using data from real-world studies; we also built the ranges of relative risk considering the values published also in these studies.
We in a previously economic evaluation, assessed the cost-effectiveness of tiotropium versus omalizumab as add-on therapies to ICS + LABA for patients with uncontrolled allergic asthma (Citation22). Using a Markov model, we showed that tiotropium was associated with lower cost than standard therapy and omalizumab (US$5,590 vs. US$5,693 vs. U$18,154 average annual cost per patient), and higher QALYs (11.8 vs. 11.3 vs. 11.9) average per patient), showing dominance respect to standard therapy. Rodriguez-Martinez in an economic evaluation of omalizumab in Colombian children showed that compared with the standard treatment strategy, the omalizumab strategy involved higher costs (US$72,142.3 vs. $20,243.4 average cost per patient) and greater gain in QALYs (0.8718 vs. 0.8222 QALYs on average per patient) (Citation23). Compared with standard treatment, omalizumab was not a cost-effective strategy for treating pediatric patients with uncontrolled severe allergic asthma, with an incremental cost-utility ratio of omalizumab compared with standard treatment of US$82,748.1 per QALY.
In our model, if the cost of Omalizumab is reduced by 83%, this treatment would be cost-effective in our country. This value constitutes a suggested starting point between the government and the pharmaceutical industry for its regular market price. The results of the probabilistic sensitivity analyses confirm the robustness of the model results. Since transition probabilities and utilities do not come from the Colombian population, they were subjected to probabilistic sensitivity analysis as detailed below as recommended by Consolidated Health Economic Evaluation Reporting Standards (CHEERS) Statement (Citation24).
Knowledge of the pathogenesis of asthma has allowed the entry of new biological therapies in the last 20 years, including omalizumab. The introduction of new therapies has therefore, an increase in the cost of health services and especially biological ones, which are usually classified as high-cost therapies even in high-income countries. The latest version of the Global Initiative for Asthma (GINA 2020) gives some recommendations to choose between the different biologics available only in patients with severe asthma (Citation5). These recommendations reduce the unnecessary use of these therapies, improving the relationship between cost and benefit obtained (Citation25). Economic evaluations provide valuable evidence for the rational use of drugs, in addition to controlled clinical trials. We hope that our study encourages decision-making at the government level and serves as an input for price control strategies for this drug. Additionally, the adjusted prices of medicines according to the real possibilities of a country facilitate their access to a greater number of patients, thus improving their impact at the Societal level and at the same time offsetting the possible economic losses of the laboratory that produces them.
Our study has some limitations. The cost data were collected retrospectively. Nevertheless, asthma treatment and the costs in question, including hospital prices, did not markedly change to our days. Furthermore, our country has been characterized by having minimum price variation in the last ten years, especially in health services (Citation17). Additionally, we use utilities extracted from the literature and not estimated directly from our population. As was mentioned previously, the reliability and robustness of the results were evaluated by sensitivity analysis. Our result only refers to patients with severe asthma uncontrolled by medium-dosage to high-dosage inhaled corticosteroids plus long-acting β2-agonists and cannot be extrapolated to patients with use of oral daily corticosteroids.
In conclusion, omalizumab is not cost-effective in adults with severe asthma uncontrolled in Colombia. If the cost of Omalizumab is reduced by 83%, this treatment would be cost-effective in our country. Our study provides evidence that should be used by decision-makers to improve clinical practice guidelines and should be replicated to validate their results in other middle-income countries.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of University of Antioquia (2015-4690).
Declaration of interest
No potential conflict of interest was reported by the author(s).
Disclosure statement
All authors declare that they do not have any conflict of interest in this publication.
Data availability statement
BD CU Omalizumab [Data set]. Zenodo. http://doi.org/10.5281/zenodo.4700232.
Additional information
Funding
References
- To T, Stanojevic S, Moores G, Gershon AS, Bateman ED, Cruz AA, Boulet L-P. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health. 2012;12(1):204. doi:10.1186/1471-2458-12-204.
- McKeage K. Omalizumab: a review of its use in patients with severe persistent allergic asthma. Drugs. 2013;73(11):1197–1212. doi:10.1007/s40265-013-0085-4.
- Abraham I, Alhossan A, Lee CS, Kutbi H, MacDonald K. Real-life’ effectiveness studies of omalizumab in adult patients with severe allergic asthma: systematic review. Allergy. 2016;71(5):593–610. doi:10.1111/all.12815.
- Agache I, Beltran J, Akdis C, Akdis M, Canelo-Aybar C, Canonica GW, Casale T, Chivato T, Corren J, Del Giacco S, et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines - recommendations on the use of biologicals in severe asthma. Allergy. 2020;75(5):1023–1042. doi:10.1111/all.14221.
- Global Iniciative for Asthma Management and Prevention 2020. Available from: www.ginaasthma.org.
- Veettil SK, Vincent V, Shufelt T, Behan E, Syeed MS, Thakkinstian A, Young DC, Chaiyakunapruk N. Incremental net monetary benefit of biologic therapies in moderate to severe asthma: a systematic review and meta-analysis of economic evaluation studies. J Asthma. 2023;60(9):1702–1714.
- Tugay D, Top M, Aydin Ö, Bavbek S, Damadoğlu E, Öner Erkekol F, Koca Kalkan I, Kalyoncu AF, Karakaya G, Oğuzülgen IK, et al. Real-world patient-level cost-effectiveness analysis of omalizumab in patients with severe allergic asthma treated in four major medical centers in Turkey. J Med Econ. 2023;26(1):720–730. doi:10.1080/13696998.2023.2209417.
- Espinosa O, Rodríguez-Lesmes P, Orozco L, Ávila D, Enríquez H, Romano G, Ceballos M. Estimating cost-effectiveness thresholds under a managed healthcare system: experiences from Colombia. Health Policy Plan. 2022;37(3):359–368. doi:10.1093/heapol/czab146.
- Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;(1):CD003559. doi:10.1002/14651858.CD003559.pub4.
- Humbert M, Beasley R, Ayres J, Slavin R, Hébert J, Bousquet J, Beeh K-M, Ramos S, Canonica GW, Hedgecock S, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309–316. doi:10.1111/j.1398-9995.2004.00772.x.
- Casale TB, Luskin AT, Busse W, Zeiger RS, Trzaskoma B, Yang M, Griffin NM, Chipps BE. Omalizumab Effectiveness by Biomarker Status in Patients with Asthma: evidence From PROSPERO, A Prospective Real-World Study. J Allergy Clin Immunol Pract. 2019;7(1):156–164 e1. doi:10.1016/j.jaip.2018.04.043.
- Bousquet J, Rabe K, Humbert M, Chung KF, Berger W, Fox H, Ayre G, Chen H, Thomas K, Blogg M, et al. Predicting and evaluating response to omalizumab in patients with severe allergic asthma. Respir Med. 2007;101(7):1483–1492. doi:10.1016/j.rmed.2007.01.011.
- Einarson TR, Bereza BG, Nielsen TA, Hemels ME. Utilities for asthma and COPD according to category of severity: a comprehensive literature review. J Med Econ. 2015;18(7):550–563. doi:10.3111/13696998.2015.1025793.
- Lloyd A, Price D, Brown R. The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Prim Care Respir J. 2007;16(1):22–27. doi:10.3132/pcrj.2007.00002.
- Departamento, Nacional, (DANE). DE. Archivo Nacional de Datos 2019. Available from https://sitios.dane.gov.co/anda-index/.
- Florez-Tanus A, Parra D, Zakzuk J, Caraballo L, Alvis-Guzman N. Health care costs and resource utilization for different asthma severity stages in Colombia: a claims data analysis. World Allergy Organ J. 2018;11(1):26. doi:10.1186/s40413-018-0205-4.
- Estadisticas DAN. Índice de Precios al Consumidor – IPC. 2020. Available from: https://www.dane.gov.co/index.php/estadisticas-por-tema/precios-y-costos/indice-de-precios-al-consumidor-ipc.
- Consortium YHE. Net Monetary Benefit. 2016. Available from: https://yhec.co.uk/glossary/net-monetary-benefit/.
- Gaviria A, Gonzalez CP, Munoz CG, Morales AA. The debate on regulating biotechnology drugs: Colombia in the international context. Rev Panam Salud Publica. 2016;40(1):40–47.
- Ramires MA, Guzman G, Delgado CA. Revision del uso de los medicamentos biosimilares vs biologicos implicaciones para la salud en Colombia. 2015. Available from: https://repository.urosario.edu.co/bitstream/handle/10336/12196/REVISI%C3%93N%20DEL%20USO%20DE%20LOS%20MEDICAMENTOS%20BIOSIMILARES%20VS.%20BIOL%C3%93GICOS%20%20IMPLICACIONES%20PARA%20LA%20SALUD%20EN%20COLOMBIA.pdf?sequence=1&isAllowed=y.
- Sullivan PW, Li Q, Bilir SP, Dang J, Kavati A, Yang M, Rajput Y. Cost-effectiveness of omalizumab for the treatment of moderate-to-severe uncontrolled allergic asthma in the United States. Curr Med Res Opin. 2020;36(1):23–32. doi:10.1080/03007995.2019.1660539.
- Jefferson Antonio B, Patino DG, Cossio-Giraldo YE. Cost-effectiveness of tiotropium versus omalizumab for uncontrolled allergic asthma. J Asthma. 2022;59(10):2016–2023.
- Rodriguez-Martinez CE, Sossa-Briceno MP, Castro-Rodriguez JA. Cost-utility of omalizumab for the treatment of uncontrolled moderate-to-severe persistent pediatric allergic asthma in a middle-income country. Pediatr Pulmonol. 2021;56(9):2987–2996. doi:10.1002/ppul.25541.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, Augustovski F, Briggs AH, Mauskopf J, Loder E, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)–explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231–250. doi:10.1016/j.jval.2013.02.002.
- Sánchez J, Morales E, Santamaria L-C, Acevedo A-M, Calle A, Olivares M, Gomez C, Amaya D, Cardona R. IgE, blood eosinophils and FeNO are not enough for choosing a monoclonal therapy among the approved options in patients with type 2 severe asthma. World Allergy Organ J. 2021;14(3):100520. doi:10.1016/j.waojou.2021.100520.