Abstract
Objective
This scoping review investigated the existing literature and identified the evidence gaps related to diagnosis and management in children aged 2–18 years presenting to hospitals with a co-diagnosis of asthma and community-acquired pneumonia.
Data sources
We designed a scoping review following Arksey and O’Malley’s scoping review framework and PRISMA extension for a scoping review. We searched literature using five electronic databases: PubMed, CINAHL, Scopus, Web of Science, and Embase from 2003 to June 2023.
Results
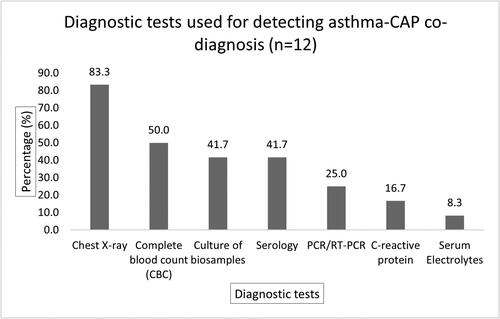
A total of 1599 abstracts with titles were screened and 12 abstracts were selected for full review. Separate guidelines including Modified Global Initiative for Asthma (GINA) guidelines; modified Integrated Management of Childhood Illness (IMCI) guidelines; and a consensus guideline developed by the Pediatric Infectious Diseases Society (PIDS) and Infectious Diseases Society of America (IDSA) were used for diagnosing asthma and CAP individually. Chest X-rays were used in 83.3% (10/12) of studies to establish the co-diagnosis of asthma-CAP in children. Variations were observed in using different laboratory investigations across the studies. Infectious etiologies were detected in five (41.7%) studies. In 75% (9/12) of studies, children with asthma-CAP co-diagnosis were treated with antimicrobials, however, bacterial etiology was not reported in 44.4% (4/9) of the studies.
Conclusions
Our scoping review suggests that chest X-rays are commonly used to establish the co-diagnosis of asthma-CAP and antibiotics are often used without laboratory confirmation of a bacterial etiology. Clinical practice guidelines for the management of asthma and pneumonia in children who present with co-diagnosis may standardize clinical care and reduce variation.
Introduction
Childhood asthma and community-acquired pneumonia (CAP) cause significant morbidity and are the leading causes of health encounters all over the world, both in communities and hospital settings (Citation1–5). In the United States of America, asthma and pneumonia result in more than 1 million emergency department visits combined; 0.2 million pediatric hospitalizations; and the total direct cost is ∼ US$ 6 billion per year for disease management (Citation4,Citation6–8). Pneumonia in early childhood is associated with abnormal lung function and increases the risk of developing asthma at a later period (Citation9). Similarly, children with asthma have a higher risk of developing recurrent pneumonia (Citation10). Asthma is usually characterized as a chronic airway inflammation and defined by the history of respiratory symptoms, such as difficulty in breathing, wheeze, chest tightness, and cough, that changes over time and in intensity with variable expiratory airflow limitation (Citation11). Chest X-rays and antimicrobials are not routinely advised for asthma exacerbation diagnosis and treatment (Citation11). Pneumonia is an acute respiratory infection of the lungs presenting with cough and/or difficulty in breathing, with or without fever, with either fast breathing or lower chest wall indrawing, and antimicrobials are used to treat infections (Citation12). These two lung diseases can show some overlying clinical features and can occur concurrently. Asthma-CAP co-diagnosis has been described as a syndrome where “patients have a documented past history of asthma, documented wheezing on current visit, use of bronchodilators, and a new focal infiltrate on chest radiograph” (Citation13). The concomitant presentation of asthma and CAP in a patient can aggravate the symptoms of both conditions, increase the risk of hospitalization, and incur a substantial burden on the health systems and families through hospitalization; transfer to a higher level of care; prolonged length of stay; readmission to the hospital within 30 days of discharge; antimicrobial use; and treatment cost (Citation14,Citation15).
The Global Initiative for Asthma (GINA) has developed guidelines for asthma management, while the Integrated Management of Childhood Illness (IMCI) guidelines developed by the World Health Organization (WHO) provides guidance on the management of CAP. Nevertheless, there are some challenges and limitations in using current guidelines for a child with both asthma and pneumonia. For example, chest X-ray is considered as a first-line investigation in suspected pneumonia (Citation16) and suggested by guidelines to exclude other lung diseases (Citation17). On the contrary, the routine use of chest radiographs for asthma in acute care facilities is not recommended by GINA guidelines (Citation18). Despite this, chest X-rays are often performed in acute asthma exacerbation management (Citation19), and in many circumstances, they may demonstrate focal changes due to mucus plugging mimicking infective pneumonia (Citation20). There are also challenges in treating and classifying the severity of pneumonia, using the IMCI guideline (2013 version) for the management of childhood pneumonia in hospitals, particularly when asthma is also present. In the IMCI guidelines (2013 version) the sign of lower chest wall indrawing was shifted downwards to become a sign of non-severe pneumonia and be treated with oral antibiotics at home (Citation21). The indrawing of lower chest is not considered as a sign of severe pneumonia but is considered as a sign of severe asthma (Citation22).
Patients with asthma are more susceptible to respiratory infection due to impaired immune responses (Citation23–25). There is a paucity of data on infectious etiologies of pneumonia in children with a concurrent asthma exacerbation presenting to hospitals which is important to guide clinical management. Respiratory infections including human rhinovirus type C (HRV-C), adenovirus (AdV), bocavirus (BoV), RSV, etc. are the main triggers of asthma exacerbation in school going children (Citation26). Therefore, treatment of asthma exacerbation with antibiotics is not recommended unless there is a strong evidence of bacterial lung infection (Citation18). Respiratory viruses are also the primary etiologies for CAP in children. However, the use of laboratory investigations to confirm infectious etiology in children with overlapping symptoms of asthma and pneumonia is not well established. Antimicrobials are not commonly recommended in children with an asthma attack but are recommended in children with CAP highlighting the complexity of antimicrobial use in children who present with both asthma and pneumonia. Antimicrobial use without laboratory confirmation of a bacterial etiology can lead to inappropriate use of antimicrobials in children with asthma-CAP which may contribute to emergence of antimicrobial resistance. Therefore, the aims of the scoping review were to systematically scope the existing literature on asthma-CAP co-diagnosis in children regarding diagnosis, diagnostic criteria, diagnostic tools, infectious etiologies, and antimicrobial use, and to identify evidence gaps in the current research related to asthma-CAP co-diagnosis among children aged 2–18 years.
Methodology
A standard protocol was developed following the framework developed by Arksey and O’Malley (Citation27,Citation28) outlining the five stages of research as follows: (1) establishing the research question, (2) identifying relevant studies, (3) selecting studies, (4) data charting, and (5) collating, recapitulating, reporting results. The PRISMA checklist including the extension for scoping reviews (Citation29) was followed during the scoping review.
Eligibility criteria for the scoping review
The study focused on globally available data on children aged 2–18 years (as asthma diagnosis is challenging in children younger than 2 years) who visited hospital with asthma-CAP co-diagnosis. Randomized controls trials, cross-sectional studies, and longitudinal studies were included in the scoping review. We looked for open access articles that reported data on at least one of the following aspects of asthma-CAP co-diagnosis; etiology; diagnosis; and management. Review articles, systematic reviews, meta-analyses were excluded from the study. Moreover, studies reported in vitro or at cellular level or in non-humans or animal models, mathematical modeling not grounded on observational data in humans, studies focusing on genomics, and publications not in English were excluded from the review.
Search strategy
We performed search for open access research articles between 2003 and 2023 (22 June 2023) using five electronic databases: PubMed, CINAHL, Scopus, Web of Science, and Embase (Supplement 1). Keywords, such as asthma, child, adolescent, pediatric, paediatric, juvenile, symptoms flare up, exacerbate, exaggerate, acute, attack, pneumonia, and infections were applied. Following the search, all the references were incorporated into Endnote to detect and reduce the duplicates. After deleting the duplicates, all references were uploaded to an online review platform “Rayyan” (https://www.rayyan.ai/) (Citation30) for facilitating an independent review process. Two reviewers (MR and SA) screened the titles and abstracts on the online platform. Articles that passed the screening of titles and abstracts were recovered for full text review. If only abstracts were available, authors were emailed for the full article. Disagreements were corrected by the consensus between two reviewers following discussions on the eligibility criteria. A third reviewer (NH) was involved if any discrepancies persisted. Studies were excluded when they failed to meet the inclusion criteria. We also checked reference list of the selected articles and performed keyword search for more eligible articles.
Data extraction
According to the study objectives, the following information was extracted from each study: study title; author; year of publication; year of data collection; type of publication (full article/conference paper); participant’s number; participants’ age; location/country; study design; definitions of asthma, pneumonia, and asthma pneumonia co-diagnosis, diagnostic criteria for asthma and CAP and diagnostic tests for asthma and CAP used; infectious etiologies; and antimicrobials used.
Synthesis of results
The extracted data were summarized by charting and tallying according to different outcomes and reported through tabular form and narrative description of the overall findings. The current study did not perform any quality assessment or assessing the risk of bias in the articles (Citation31–33).
Results
Study identification
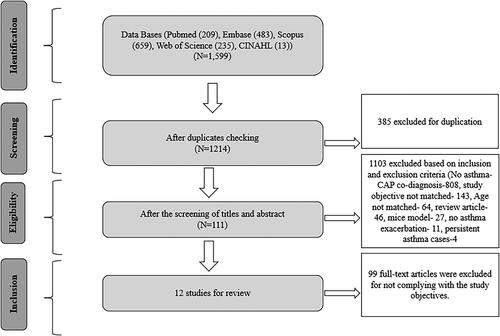
A total of 1,599 records were identified through the systematic searches (). After checking for duplications, 385 records were excluded. During title and abstract screening, 1,103 records were excluded, and the remaining 111 records were kept for full text review. Finally, twelve studies were included in the final analysis (). Among the selected studies, there were eight research articles, three conference papers, and one research letter.
Description of selected studies in the scoping review
The selected studies were published between January 2003 to 22 June 2023. Seven studies were cross-sectional (Citation15,Citation34–39), and five were retrospective cohort studies (Citation13,Citation14,Citation40–42). Four studies were conducted in the USA (Citation13–15,Citation41); one in Ukraine (Citation39); one in the USA and Pakistan combined (Citation42); one in Indonesia (Citation40); one in Macedonia (Citation34); one in Thailand (Citation35); one in Uganda (Citation36); one in Columbia (Citation37); and one in Argentina (Citation38) (). In total, 70,130 children were included in the selected studies and their age ranged from 24 days to 18 years (). Five studies (41.7%) included children 2–18 years age group and 6 studies (50%) included children <2 years age groups. One study did not specify the age group and the mean age of the study population was 7 years (Citation34). The studies analyzed data on clinical characteristics (Citation13,Citation15), adherence to guidelines (Citation14), infectious etiologies (Citation34,Citation35,Citation37–39), and prevalence of respiratory infections (Citation34,Citation35,Citation37–39), in children with asthma-CAP co-diagnosis. Among the five retrospective cohort studies, three used routinely collected hospital administrative records including Pediatric Health Information System (Citation14), Electronic Health Records (Citation41), and Premier Healthcare Database (Citation15) for identifying children with asthma, CAP, and asthma-CAP co-diagnosis. Two other studies used retrospective chart review (Citation13,Citation42). The rest seven studies used cross-sectional study design. Participants were enrolled prospectively from in-patient department in six studies (Citation13–15,Citation38,Citation40,Citation42); emergency department in three studies (Citation36,Citation37,Citation41); out-patient and in-patient department in one study (Citation35); and the clinical setting was not specified in two studies (Citation34,Citation39). In addition, seven studies were conducted at tertiary level hospitals (Citation13,Citation14,Citation35–41), and the rest were in a general hospital, small community hospitals, free-standing hospitals, non-free-standing hospitals, university and non-university hospitals (Citation15,Citation42). In one study, hospital or study site was not specified (Citation34).
Figure 2. Geographical distribution of the countries where the studies included in the final scoping review were conducted.
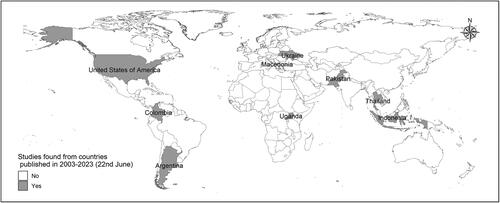
Table 1. Characteristics of studies included in the scoping review (N = 12).
Table 2. Bio-sample collection, diagnostic test, infectious etiologies, and antimicrobials used for asthma-CAP co-diagnosis.
Guidelines and International Classification of Diseases (ICD) codes used to detect asthma-CAP co-diagnosis
In three out of 12 studies (25%), asthma was diagnosed using modified GINA guidelines for detecting asthma, and pneumonia was diagnosed clinically and radiologically. One study used the modified version of asthma definition derived from GINA guidelines by excluding chest tightness and use of peak flowmeter or spirometry (Citation36). Maffey et al. (Citation38) defined asthma among <3 years age group using the criteria described in Castro-Rodríguez et al. (Citation43) and among children >3 years using the GINA guidelines. In the study conducted by Castro-Rodríguez et al. (Citation43), two predictive indices for diagnosis of asthma in children age <3 years were described based on the presence of two major criteria (medically diagnosed asthma in parents and medically diagnosed eczema in the child) and three minor criteria (medically diagnosed allergic rhinitis, wheezing apart from colds or eosinophilia). For pneumonia diagnosis, one study (8.3%) used IMCI guidelines with modification (Citation36), and two studies (16.7%) used a consensus guideline published by the Pediatric Infectious Diseases Society (PIDS) and Infectious Diseases Society of America (IDSA) combined. In addition, asthma-CAP co-diagnosis was diagnosed clinically, spirometrically, and radiologically in one study (Citation39); clinically and radiologically in one study (Citation34); and clinically in one study (Citation35).
Out of 12 studies, six (50%) used International Classification of Diseases and Related Health Problems (ICD) codes related to asthma and pneumonia separately to record asthma-CAP co-diagnosis in children who presented with both asthma and CAP symptoms (Citation13–15,Citation40–42). Among the six studies using the ICD codes, four studies applied ICD-9 codes including 481 (pneumococcal pneumonia), 482 (other bacterial pneumonia), 485 (bronchopneumonia, organism unspecified), 486 (pneumonia, organism unspecified), and 493 (asthma with clinical modification diagnosis codes for asthma). The other two studies applied ICD-10 codes including J11–J18 (influenza and pneumonia subclassifications) and J45 (asthma). Among the six studies, three studies (Citation14,Citation40,Citation42) used pneumonia as primary diagnosis, two studies (Citation15,Citation41) used exacerbation of asthma as primary diagnosis and one study (Citation13) used asthma and pneumonia both as primary diagnosis.
Laboratory investigations used in children with asthma-CAP co-diagnosis
Chest radiograph was used in 10 studies (83.3%) to establish asthma-CAP co-diagnosis (). Less than half of the studies (41.7%; five studies) used blood culture to identify infectious etiology of pneumonia in asthma-CAP co-diagnosis. Serology [such as ELISA, pneumoslide IF IgM, Immunofluorescence Assay (IFA), direct fluorescent antibody test (dFA)] was used in five studies (41.7%) to detect specific antibodies for infectious etiology. Three studies (25%) used polymerase chain reaction (PCR) or reverse transcription-polymerase chain reaction (RT-PCR). In total, three studies (25%) used culture, serology test, and chest radiograph; two studies (16.7%) used culture and chest x-ray; and one study (8.3%) used PCR/RT-PCR, serology, and chest radiograph for confirming infectious etiology in asthma-CAP co-diagnosis.
Infectious etiology associated with pneumonia in asthma-CAP co-diagnosis
Out of 12 studies, five studies (41.7%) reported infectious etiologies related to pneumonia co-diagnosis in children with asthma exacerbations. Chlamydophila pneumoniae and Mycoplasma pneumoniae were identified as the infectious etiology for pneumonia in one study (Citation39). Boskovska et al. (Citation34), showed predominance of M. pneumoniae among the patients with the co-diagnosis. The remaining three out of five studies reported viral infections and co-infections (RSV-human corona virus co-infections and RSV-bacterial co-infections) (Citation35–37). One study reported viral-bacterial (virus+ M. pneumoniae) co-infections, although the specific viral etiology was not reported (Citation37).
Use of antimicrobials in asthma-CAP co-diagnosis
Nine (75%) out of twelve studies reported antimicrobial use in children with asthma-CAP co-diagnosis. Macrolides were used for treating asthma-CAP co-diagnosis in six studies (50%). One study mentioned aminopenicillin, cephalosporin, and the use of more than one antibiotic for treating asthma-CAP co-diagnosis. Four studies (33.3%) mentioned the use of antibiotics, but the type of antibiotics was not reported. In four out of nine studies (44.4%) where antibiotics were used, there was no report of infectious organisms. No studies reported the use of antiviral therapies.
Discussion
Our scoping review has identified some of the variations regarding clinical management of asthma-CAP co-diagnosis in children. This included the use of separate guidelines for diagnosing asthma and pneumonia co-diagnosis in children and we did not identify the use of one common guideline accommodating the needs of this co-diagnosis. In addition, about half of the studies in our scoping review enrolled children <2 years, an age group where asthma diagnosis is challenging, and often children present with viral induced wheeze which can be misdiagnosed as asthma (Citation13). There is no specific entity for asthma-CAP co-diagnosis in current ICD coding system (Citation44,Citation45) which was reflected in our findings. The included studies used ICD codes for asthma and CAP to detect asthma-CAP co-diagnosis from health records of hospitals. There was a difference in the use of different diagnostic tests or laboratory investigations for the detection of asthma-CAP co-diagnosis. Moreover, patients with asthma-CAP co-diagnosis were treated with antimicrobials without proper microbiological assessment.
The scoping review revealed that 41.7% of the studies (Citation34–37,Citation39) reported the presence of an infectious etiology in children with asthma-CAP co-diagnosis. In addition, different laboratory investigations were used to confirm pneumonia with chest x-ray being the most frequently used diagnostic investigation. Studies in the scoping review suggest that pneumonia in children with asthma is often associated with respiratory viruses. In this regard, PCR is an efficient minimally invasive laboratory investigation that can be done rapidly, even at point of care, and detect respiratory viruses with high sensitivity and specificity (Citation46). However, PCR technology was only used in 25% of the identified studies. Guidelines recommending PCR as the first line laboratory investigation in children presenting with concomitant symptoms of asthma and pneumonia may reduce unwarranted exposure to radiation of X-ray and improve identification of infectious etiology.
We have identified that antimicrobials were used for treating asthma-CAP co-diagnosis in 75% of studies, despite 45% of these studies not confirming an infectious etiology through laboratory investigation. The lack of antimicrobial guidelines for asthma-CAP co-diagnosis may increase the irrational use of antibiotics (Citation47,Citation48). Fever and hypoxia might be a strongest predictors a pneumonia (Citation49), but, fever associated cough and/or wheeze does not necessarily represent bacterial pneumonia among patients with asthma-CAP co-diagnosis (Citation50). In addition, a portion of the asthma-CAP co-diagnosis cases were caused by respiratory viral infections where the use of antibiotics is not required. In a study included in the scoping review, Parikh et al. (Citation42) used evidence-based tools to raise awareness of asthma-CAP co-diagnosis and rational use of antibiotics among physicians in emergency and inpatient departments. The study demonstrated a decrease in asthma-CAP co-diagnosis by physicians and increase in justified use of narrow spectrum antibiotics and macrolides for asthma-CAP co-diagnosis treatment (Citation42). The findings can be used as a framework to develop antimicrobial and clinical guidelines for asthma-CAP co-diagnosis management in hospitals.
Limitations
There are some limitations in this scoping review. During the systematic search, we limited our search to English language. This approach could have resulted in the exclusion of high-quality pediatric studies conducted in other languages. We considered only open access articles which might limit our search to non-open access articles. The scoping review was limited to studies published since 2003 which might have excluded findings from older studies. However, we were interested in exploring research and practice on asthma-CAP co-diagnosis in the recent years. We did not perform any quality assessment for the scoping review as it is usually not assessed in the scoping review.
Conclusions
In conclusion, our scoping review identified that asthma and pneumonia co-diagnosis in children is established using separate guidelines for asthma and pneumonia highlighting the need for one Clinical Practice Guideline (CPG) for the management of asthma-CAP co-diagnosis. This may minimize variation in clinical care, improve use of optimal laboratory investigations, promote proper detection of infectious etiology, and reduce inappropriate use of antibiotics in children presenting with asthma-CAP co-diagnosis in hospitals.
Supplemental Material
Download MS Word (30 KB)Acknowledgements
We acknowledge the contribution of Dr. AYM Alamgir Kabir for providing assistance with the preparation of .
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, Symington P, O’Toole S, Myint SH, Tyrrell DA, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ 1995;310(6989):1225–1229. doi:10.1136/bmj.310.6989.1225.
- Averell CM, Laliberté F, Germain G, Slade DJ, Duh MS, Spahn J. Disease burden and treatment adherence among children and adolescent patients with asthma. J Asthma 2022;59(8):1687–1696. doi:10.1080/02770903.2021.1955377.
- Mengstie LA. Prevalence of pneumonia and associated factors among children aged 6–59 months in Angolela Tera district, North Shoa, Ethiopia, 2021, community-based cross-sectional study. Bull Natl Res Cent 2022;46(1):1–8. doi:10.1186/s42269-022-00921-x.
- Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med 2016;11(11):743–749. doi:10.1002/jhm.2624.
- Madhi SA, De Wals P, Grijalva CG, Grimwood K, Grossman R, Ishiwada N, Lee P-I, Nascimento-Carvalho C, Nohynek H, O’Brien KL, et al. The burden of childhood pneumonia in the developed world: a review of the literature. Pediatr Infect Dis J 2013;32(3):e119–e127. doi:10.1097/INF.0b013e3182784b26.
- Perry R, Braileanu G, Palmer T, Stevens P. The economic burden of pediatric asthma in the United States: literature review of current evidence. Pharmacoeconomics 2019;37(2):155–167. doi:10.1007/s40273-018-0726-2.
- Chamberlain JK, Cull WL, Melgar T, Kaelber DC, Kan BD. The effect of dual training in internal medicine and pediatrics on the career path and job search experience of pediatric graduates. J Pediatr 2007;151(4):419–424. doi:10.1016/j.jpeds.2007.04.064.
- McDermott KW, Stocks C, Freeman WJ. Overview of Pediatric Emergency Department Visits, 2015. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2006. Statistical Brief #242.
- Collaro AJ, Chang AB, Marchant JM, Chatfield MD, Vicendese D, Blake TL, McElrea MS, Dharmage SC. Early childhood pneumonia is associated with reduced lung function and asthma in first Nations Australian Children and Young Adults. J Clin Med 2021;10(24):5727. doi:10.3390/jcm10245727.
- Lodha R, Puranik M, Natchu U, Kabra S. Recurrent pneumonia in children: clinical profile and underlying causes. Acta Pædiatrica 2007;91(11):1170–1173. doi:10.1111/j.1651-2227.2002.tb00123.x.
- Global Initiative for Asthma Global Strategy for Asthma Management and Prevention (updated 2023); 2023.
- World Health Organization. Pneumonia in children; 2022. Available from: https://www.who.int/news-room/fact-sheets/detail/pneumonia
- Wasser CD, Grushevsky A, Johnson ST, Smith SR. Asthmonia: a clinical definition of a commonly used colloquial term. J Asthma 2018;55(11):1237–1241. doi:10.1080/02770903.2017.1409235.
- Wilson KM, Torok MR, Localio R, McLeod L, Srivastava R, Luan X, Mohamad Z, Shah SS, Pediatric Research in Inpatient Settings (PRIS) Network. Hospitalization for community-acquired pneumonia in children: effect of an asthma codiagnosis. Hosp Pediatr 2015;5(8):415–422. doi:10.1542/hpeds.2015-0007.
- Leyenaar JK, Shieh MS, Pekow PS, Lindenauer PK. Variation in pediatric asthmonia diagnosis and outcomes among hospitalized children. Ann Am Thorac Soc 2021;18(9):1514–1522. doi:10.1513/AnnalsATS.202009-1146OC.
- Niederman MS, Mandell LA, Anzueto A, Bass JB, Broughton WA, Campbell GD, Dean N, File T, Fine MJ, Gross PA, et al. Guidelines for the management of adults with community-acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med 2001;163(7):1730–1754. doi:10.1164/ajrccm.163.7.at1010.
- World Health Organization. Pocket book of hospital care for children. 2nd ed. Geneva, Switzerland: WHO Press; 2013.
- Global Initiative for Asthma Global Strategy for Asthma Management and Prevention (2022 update); 2022.
- Al-Shamrani A, Al-Harbi AS, Bagais K, Alenazi A, Alqwaiee M. Management of asthma exacerbation in the emergency departments. Int J Pediatr Adolesc Med 2019;6(2):61–67. doi:10.1016/j.ijpam.2019.02.001.
- Hogg JC, Chu FSF, Tan WC, Sin DD, Patel SA, Pare PD, Martinez FJ, Rogers RM, Make BJ, Criner GJ, et al. Survival after lung volume reduction in chronic obstructive pulmonary disease: insights from small airway pathology. Am J Respir Crit Care Med 2007;176(5):454–459. doi:10.1164/rccm.200612-1772OC.
- Mulholland K. Problems with the WHO guidelines for management of childhood pneumonia. Lancet Glob Health 2018;6(1):e8–e9. doi:10.1016/S2214-109X(17)30468-0.
- World Health Organization. Integrated management of childhood illness. Chart Booklet. Geneva, Switzerland: WHO Press; 2014.
- Henderson J, Hilliard TN, Sherriff A, Stalker D, Shammari NA, Thomas HM, The ALSPAC Study Team. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatr Allergy Immunol 2005;16(5):386–392. doi:10.1111/j.1399-3038.2005.00298.x.
- Carroll KN, Wu P, Gebretsadik T, Griffin MR, Dupont WD, Mitchel EF, Hartert TV. The severity-dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. J Allergy Clin Immunol 2009;123(5):1055–1061.e1. doi:10.1016/j.jaci.2009.02.021.
- Smyth RL, Openshaw PJ. Bronchiolitis. Lancet 2006;368(9532):312–322. doi:10.1016/S0140-6736(06)69077-6.
- Papadopoulos NG, Christodoulou I, Rohde G, Agache I, Almqvist C, Bruno A, Bonini S, Bont L, Bossios A, Bousquet J, et al. Viruses and bacteria in acute asthma exacerbations–a GA2 LEN-DARE systematic review. Allergy 2011;66(4):458–468. doi:10.1111/j.1398-9995.2010.02505.x.
- Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005;8(1):19–32. doi:10.1080/1364557032000119616.
- Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implementation Sci 2010;5(1):1–9. doi:10.1186/1748-5908-5-69.
- Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, Moher D, Peters MD, Horsley T, Weeks L, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169(7):467–473. doi:10.7326/M18-0850.
- Rayyan.ai. Available from: https://rayyan.qcri.org/
- Rumrill PD, Fitzgerald SM, Merchant WR. Using scoping literature reviews as a means of understanding and interpreting existing literature. Work 2010;35(3):399–404. doi:10.3233/WOR-2010-0998.
- Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J 2009;26(2):91–108. doi:10.1111/j.1471-1842.2009.00848.x.
- Brien SE, Lorenzetti DL, Lewis S, Kennedy J, Ghali WA. Overview of a formal scoping review on health system report cards. Implement Sci 2010;5(1):2. doi:10.1186/1748-5908-5-2.
- Boskovska K, Maneva M, Petrusevska-Kolekevska L, Ilievska T, Popova G. Mycoplasma pneumoniae infection and asthma exacerbation in children. Allergy 2011;94:156.
- Theamboonlers A, Samransamruajkit R, Thongme C, Amonsin A, Chongsrisawat V, Poovorawan Y. Human coronavirus infection among children with acute lower respiratory tract infection in Thailand. Intervirology 2007;50(2):71–77. doi:10.1159/000097392.
- Nantanda R, Tumwine JK, Ndeezi G, Ostergaard MS. Asthma and pneumonia among children less than five years with acute respiratory symptoms in Mulago Hospital, Uganda: evidence of under-diagnosis of asthma. PLOS One 2013;8(11):e81562. doi:10.1371/journal.pone.0081562.
- Duenas Meza E, Jaramillo CA, Correa E, Torres-Duque CA, García C, González M, Rojas D, Hernández A, Páez AM, Delgado MDP, et al. Virus and Mycoplasma pneumoniae prevalence in a selected pediatric population with acute asthma exacerbation. J Asthma 2016;53(3):253–260. doi:10.3109/02770903.2015.1075548.
- Maffey AF, Barrero PR, Venialgo C, Fernández F, Fuse VA, Saia M, Villalba A, Fermepin MR, Teper AM, Mistchenko AS, et al. Viruses and atypical bacteria associated with asthma exacerbations in hospitalized children. Pediatr Pulmonol 2010;45(6):619–625. doi:10.1002/ppul.21236.
- Nesterenko Z, Ivanina O, editors. Asthma in children with community-acquired pneumonia caused by atypical pathogens. Allergy. Hoboken (NJ): Wiley-Blackwell; 2010.
- Wulandari DA, Kartasasmita C. Risk factor asthma in pediatric pneumonia patients. World Allergy Organ J 2016;9(SUPPL.1):204.
- Florin TA, Carron H, Huang G, Shah SS, Ruddy R, Ambroggio L. Pneumonia in children presenting to the emergency department with an asthma exacerbation. JAMA Pediatr 2016;170(8):803–805. doi:10.1001/jamapediatrics.2016.0310.
- Parikh K, Biondi E, Nazif J, Wasif F, Williams DJ, Nichols E, Ralston S, Value in Inpatient Pediatrics Network Quality Collaborative For Improving Care In Community Acquired Pneumonia. A multicenter collaborative to improve care of community acquired pneumonia in hospitalized children. Pediatrics 2017;139(3):e1–e8. doi:10.1542/peds.2016-1411.
- Castro-Rodríguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med 2000;162(4 Pt 1):1403–1406. doi:10.1164/ajrccm.162.4.9912111.
- World Health Organization. ICD-10 Version:2019; 2019. Available from: https://icd.who.int/browse10/2019/en#/X
- World Health Organization. ICD-11 Coding Tool Mortality and Morbidity Statistics (MMS); 2023. Available from: ICD-11 Coding Tool Mortality and Morbidity Statistics (MMS) 2023–01.
- Clark TW, Lindsley K, Wigmosta TB, Bhagat A, Hemmert RB, Uyei J, Timbrook TT. Rapid multiplex PCR for respiratory viruses reduces time to result and improves clinical care: results of a systematic review and meta-analysis. J Infect 2023;86(5):462–475. doi:10.1016/j.jinf.2023.03.005.
- World Health Organization. Antibiotic resistance; 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance
- Trivedi KK, Dumartin C, Gilchrist M, Wade P, Howard P. Identifying best practices across three countries: hospital antimicrobial stewardship in the United Kingdom, France, and the United States. Clin Infect Dis 2014;59 Suppl 3(suppl_3):S170–S178. doi:10.1093/cid/ciu538.
- Mathews B, Shah S, Cleveland RH, Lee EY, Bachur RG, Neuman MI. Clinical predictors of pneumonia among children with wheezing. Pediatrics 2009;124(1):e29–e36. doi:10.1542/peds.2008-2062.
- Pavia AT. Viral infections of the lower respiratory tract: old viruses, new viruses, and the role of diagnosis. Clin Infect Dis 2011;52 Suppl 4(Suppl 4):S284–S289. doi:10.1093/cid/cir043.