Abstract
Objective
The study aimed to reach a consensus on the most relevant patient-reported outcomes (PROs), the corresponding measures (PROMs), and measurement frequency during severe asthma patient follow-up.
Methods
Two Delphi rounds were conducted. The questionnaire was developed based on a systematic literature review, a focus group with patients, and a nominal group with experts. It assessed PROs’ relevance and the appropriateness (A) and feasibility (F) of PROMs using a Likert scale (1=totally agree; 9=totally disagree). The consensus was established when ≥75% of participants agreed (1-3) or disagreed (7-9).
Results
Sixty-three professionals (25 hospital pharmacists, 14 allergists, 13 pulmonologists, and 11 nurses) and 5 patients answered the Delphi questionnaire. A consensus was reached on all PROs regarding their relevance. Experts agreed on the use of ACT (A:95.24%; F:95.24%), mini AQLQ (A:93.65; F:79.37%), mMRC dyspnea scale (A:85.71%; F:85.71%), TAI (A:92.06%; F:85.71%), MMAS (A:75.40%; F:82%), and the dispensing register (A:96.83%; F:92.06%). Also considered suitable were: SNOT-22 (A:90.48%; F:73.80%), PSQI (A:82.54; F:63.90%), HADS (A:82.54; F:64%), WPAI (A:77.78%; F:49.20%), TSQM-9 (A:79.37; F:70.50%) and knowledge of asthma questionnaire (A:77%; F:68.80%); however, their use in clinical practice was considered unfeasible. Panelists also agreed on the appropriateness of EQ-5D, which was finally included despite being considered unfeasible (A: 84.13%; F:67.20%) in clinical practice. Agreement was reached on using ACT, TAI, mMRC, and a dispensing register every three months; mini-AQLQ and MMAS every six months; and EQ-5D every twelve months.
Conclusion
This consensus paves the way toward patient-centered care, promoting the development of strategies supporting routine assessment of PROs in severe asthma management.
Introduction
Asthma is a serious global health problem, affecting 339 million people worldwide (Citation1). It is a chronic inflammatory disease of the airways, usually associated with bronchial hyperresponsiveness and a variable degree of airflow limitation. Its severity can vary from intermittent to severe persistent, depending on the intensity of the process and response to treatment (Citation2,Citation3). Therefore, disease management requires a multidisciplinary and individualized approach, which must be periodically adjusted to minimize symptoms and prevent exacerbations (Citation4).
Severe asthma is characterized by intense symptomatology, frequent exacerbations, multiple comorbidities, and the need for high-intensity treatment (Citation3,Citation5–7). It affects between 3% and 10% of the asthmatic population (Citation2,Citation8) and has a major impact on their health-related quality of life (HRQoL) (Citation7–9). In fact, despite high-intensity treatment, patients with severe disease are usually refractory to therapy, and poor symptom control affects their social, working, physical and mental wellbeing (Citation2,Citation6,Citation8,Citation10). In addition, this condition is associated with higher direct and indirect medical costs compared to mild or moderate asthma (Citation11,Citation12).
Given the significant impact of severe asthma on HRQoL, disease management has moved toward a patient-centered approach. As a result, pharmacologic and behavioral interventions have become more widespread, targeting disease control, assessing and treating related risk factors and comorbidities, and improving patients’ HRQoL (Citation4). Additionally, recent studies have recommended using asthma-specific HRQoL measures in clinical trials for moderate-to-severe asthma, in order to assess the long-term response to treatments (Citation7,Citation13–15).
In this context, patient-reported outcomes (PROs) play an increasingly important role when assessing the impact of severe asthma healthcare interventions (Citation16–18). PROs are reported directly by the patient regarding their health condition status associated with the health care or treatment (Citation16). Therefore, their implementation in clinical practice is essential to improve shared decision-making, symptom monitoring and management, patient satisfaction, and HRQoL (Citation19).
Patient-reported outcome measures (PROMs) are the instruments used to report PROs. These tools must be carefully defined and accurately used to capture relevant patient-reported information and compare it with other measurements (Citation20). PROMs can be generic or disease-specific; they assess symptoms, functional and health status, and social and psychological wellbeing (Citation8,Citation15). Multiple PROMs have been developed and validated in the context of airway diseases such as severe asthma (Citation18). However, the increasing implementation of PROMs in clinical practice (Citation16) makes it necessary to standardize their use.
This project aims to reach a consensus on the most relevant PROs, PROMs, and their measurement frequency during severe asthma patient follow-up, taking into account both the patients’ and healthcare professionals’ (HCP) perspectives, within the Spanish National Health System framework.
Material and methods
The project was led and coordinated by a scientific committee consisting of healthcare professionals who are experts in the management of severe asthma: a pulmonologist (EM), an allergologist (IA), and a hospital pharmacist (MM).
The project comprised five phases: 1) literature review; 2) focus group with patients; 3) first scientific committee meeting; 4) two nominal groups with healthcare professionals; 5) Delphi consultation with patients and healthcare professionals, and 6) final scientific committee meeting ().
Literature review
To identify PROs, PROMs, and frequency of measurement used during severe asthma patient follow-up, a systematic literature review according to Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Citation21) was performed by consulting the international PubMed/Medline database.
Observational studies, randomized clinical trials, and systematic reviews published in English or Spanish between 03/01/2016 and 03/01/2020 were selected and reviewed. Search terms and strategy are shown in Supplementary Table S1. In addition, Clinical Practice Guidelines (CPGs) for severe asthma with information on PROs and PROMs were reviewed.
Focus groups with patients
The focus group is a variant of the group interview in which participants describe their perceptions, opinions, beliefs, and attitudes toward a specific topic (Citation22). An online focus group with severe asthma patients was conducted to explore the most relevant PROs and how they were assessed during medical visits from the patients’ perspective.
Patients were contacted and invited to participate in the focus group by the Spanish patient advocacy group (Federación Española de Asociaciones de Pacientes Alérgicos y con Enfermedades Respiratorias, FENAER).
During the focus group, PROs identified in the literature were presented, and various questions were asked for discussion: the impact of the disease and its treatment on their daily life, the disease-related symptomatology, and their perception of the assessment of PROs during medical visits.
First scientific committee meeting
The first online meeting with the scientific committee was organized to present the literature review results and the focus group conclusions. The objective of this meeting was to establish the most relevant PROs and PROMs to be presented in the nominal group meetings with healthcare professionals.
Nominal groups with healthcare professionals
The nominal group technique is a qualitative research methodology based on a semi-structured group discussion that guarantees all participants have the opportunity to express their ideas, ensuring that their participation is balanced (Citation23). Two online multidisciplinary nominal group meetings with healthcare professionals were held to reach a preliminary consensus on the most relevant PROs, PROMs, and their frequency of measurement, for their inclusion in the Delphi consultation.
Members of the nominal groups were contacted and invited to participate in the nominal group meetings by the coordinating team (on behalf of the scientific committee). They were selected based on their experience in severe asthma management, PRO measurement, implementing strategies to standardize health outcomes, and their availability and interest in the project.
The PROs and PROMs selected by the scientific committee were presented to the nominal groups. A selection of PROs and PROMs was established according to their relevance for severe asthma patient follow-up and availability in the current clinical setting. A consensus was reached when at least 70% of the healthcare professionals agreed on the inclusion/exclusion of the PRO and PROM.
Delphi consultation
The Delphi technique is a widely used consensus method implemented in research to achieve a general agreement on a particular topic, preserving participants’ anonymity (Citation24). It is typically conducted over consecutive rounds, answered by a panel of participants with relevant expertise in the research field (Citation24,Citation25). The survey rounds iteratively ask the panelists to rate different statements or questions, providing controlled feedback on the previous round’s group results (Citation26). Participants may then adjust their initial ratings based on feedback from the overall group in several subsequent iterations (Citation27).
Two-round Delphi consultations with patients and healthcare professionals were conducted between November 2021 and January 2022. Participants were given two weeks to respond to the questionnaire for each round. In addition, three reminders were sent to non-respondents during each period.
Two versions of the electronic questionnaire were developed, one for healthcare professionals and the other for patients, to make it easier for the latter to understand the wording. Furthermore, patients were not asked directly about PROMs due to their lack of knowledge on the existing PROMs and the difficulty in deciding on their suitability and feasibility of use in clinical practice.
Panelists rated their responses on a nine-point scale (1 = Strongly disagree; 2= Disagree; 3 = Moderately disagree; 4 = Somewhat disagree; 5 = Neither agree nor disagree; 6 = Somewhat agree; 7 = Moderately agree; 8 = Agree; 9 = Strongly agree).
The questionnaire of the first round consisted of four parts.
Baseline characteristics. Sociodemographic characteristics of healthcare professionals (age, gender, specialty, region, and years of experience) and patients (age, gender, region, and age at diagnosis) were collected.
PROs. Panelists (healthcare professionals and patients) were asked to rate the relevance (R) of including each PRO (classified according to the nominal groups’ proposal) in severe asthma patient follow-up.
PROMs. Panelists (healthcare professionals only) were requested to rate the appropriateness (A) and feasibility (F) of the predefined PROMs for each PRO (classified according to nominal groups’ proposal).
Frequency of measurement. Panelists (healthcare professionals and patients) were asked to rate the appropriateness (A) and feasibility (F) of two PROMs frequencies of measurement proposals. In the case of the questionnaire for patients, the frequency was related to PROs (PROMs were not presented to them). See an example in Supplementary Material 1.
In the second-round, an individualized reminder of the option that most participants had indicated accompanied the questions, and the panelists had to decide whether to maintain their assessment or to modify it. Moreover, reasons for disagreement were explored through some additional questions, and panelists were asked to prioritize the PROMs to be employed during severe asthma patient follow-up.
Panelists
Panelists were identified and invited to participate in the Delphi consultation by the scientific committee members in collaboration with patient advocacy groups (FENAER). Healthcare professionals were selected based on their experience managing severe asthma and their knowledge of PROs and PROMs. Panelists received the link to the Delphi questionnaire, username, and password (exclusive for each participant) by e-mail.
Consensus definition
The consensus was defined a priori as agreement on relevance (R), appropriateness (A), and feasibility (F) (scores 7-9) in a statement or question by 75% or more of the panel members.
Data Analysis
The percentage of panelists who selected each option and percentile distributions (25, 50, and 75) were calculated using STATA statistical software, V.14. The percentages described in the text refer to the final scores (score of the round in which consensus was achieved).
Final scientific committee meeting
A final meeting with the scientific committee was conducted to review the results obtained in the two rounds of Delphi consultation and to define the final standard set of PROs and PROMs for severe asthma patient follow-up.
Results
Literature review
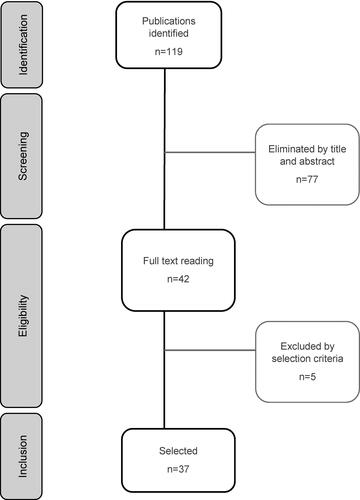
The literature search yielded 119 potentially relevant references, of which 37 were considered eligible for inclusion (). In addition, six CPGs with information on PROs and PROMs were reviewed (Supplementary Table S2).
Thirty-two PROs and 21 PROMs were identified in the reviewed publications. PROs were categorized into symptom-related PROs (n = 24) and global PROs (n = 8). For the collection of PROs, validated PROMs and other assessment methods such as informal direct questions or a symptom diary were identified ().
Table 1. PROs and PROMs identified in the literature review.
Focus group
Four patients on treatment for severe asthma (gender: 75% females; age range: 22-62 years; time from diagnosis range: 3-6 years) participated in the focus group.
Most of the symptom-related PROs identified in the literature review had been experienced by the patients, except for urticaria, eye symptoms (conjunctivitis), and taste/smell disorders. Patients selected chest tightness, dyspnea, feeling of suffocation, sleep disorders, and fatigue as the five most frequently occurring symptoms. In addition, fatigue, dyspnea, feeling of suffocation, sleep disorders, anxiety, and concentration difficulties were the symptoms with the most significant impact on daily routine, regardless of their frequency of occurrence. Finally, patients proposed gastroesophageal reflux, dental and oral problems (yellow teeth, oral candidiasis), corticosteroid-induced tremors, menstrual alterations (dysmenorrhea and menorrhagia), and migraine as other relevant symptom-related PROs, additional to those identified in the literature.
Regarding the assessment of PROs in clinical practice, patients indicated that during follow-up of severe asthma, they were asked how the symptoms of the disease and its treatment affected their daily life. This assessment was done using open response and Likert scale questionnaires, Visual Analog Scales (VAS), and informal questions (asked by both the specialist and the nurse) during the medical visits, but not on a standardized basis.
First scientific committee meeting
The scientific committee considered as relevant 20 symptom-related PROs out of the 24 previously identified in the literature review and by the focus group. Apnea, localized pain, ear symptoms, and urticaria were not considered for inclusion. In addition, they decided to add five new symptom-related PROs: dental and oral problems, tremors, blocked nose, rhinitis, and sneezing. All global PROs were deemed relevant for presentation to the nominal groups. Furthermore, the scientific committee proposed one new global PRO (work productivity and activity impairment).
Regarding PROMs, 8 of 21 PROMs identified in the literature were considered relevant: Mini Asthma Quality of Life Questionnaire (Mini-AQLQ) (Citation28); Asthma Control TestTM (ACT) (Citation29); Leicester Cough Questionnaire (LCQ) (Citation30); Sino-Nasal Outcome Test (SNOT-22) (Citation31); Insomnia Severity Index (ISI) (Citation32); European Quality of Life-5 Dimensions (EQ-5D) (Citation33); Treatment Satisfaction Questionnaire for Medication-9 items (TSQM-9) (Citation34); and Hospital Anxiety and Depression Scale (HADS) (Citation35). The Asthma Quality of Life Questionnaire, Adult Carer Quality of Life Questionnaire, Asthma Control Questionnaire, Saint George Respiratory Questionnaire, Dyspnea-12 questionnaire, STOP-BANG questionnaire, Medical Outcomes Study Sleep Scale, 36-item Short Form Survey, Beck Depression Inventory-II, symptom diaries, self-reports, questions during medical visits, and electronic registration were not considered for inclusion. The scientific committee also proposed including 8 additional PROMs: the modified Medical Research Council dyspnea scale (mMRC) (Citation36); Nijmegen Questionnaire (NQ) (Citation37); Pittsburg Sleep Quality Index (PSQI) (Citation38); Morisky-Green Medication Adherence Scale (MMAS) (Citation39); Test of Adherence to Inhalers (TAI) (Citation40); Work Productivity and Activity Impairment questionnaire (WPAI) (Citation41); a visual analogical scale (VAS) for treatment satisfaction; and the medication dispensing register.
Thus, the scientific committee selected 25 symptom-related PROs, nine global PROs, and 16 PROMs for presentation and evaluation during the nominal group meetings (Supplementary Table S3).
Nominal groups of healthcare professionals
Nineteen experts on severe asthma from different specialties (n = 9 hospital pharmacists, n = 4 pulmonologists, n = 4 allergologists, and n = 2 nurses) and various geographical areas of Spain participated in two nominal group meetings.
Healthcare professionals assessed the relevance of symptom-related PROs and global PROs (Supplementary Tables S4, S5). Additionally, the most adequate and feasible PROMs and measurement frequency in clinical practice were also discussed by the nominal groups.
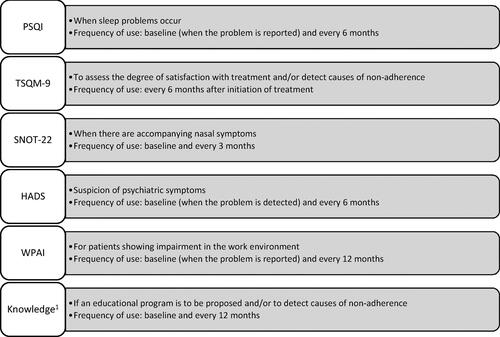
Consensus was reached for 16 PROs and 12 measurement instruments, proposed by the scientific committee during the two nominal group meetings. In addition, it was agreed to include a questionnaire to assess the patient’s knowledge of the disease. The members of the nominal groups also reached an agreement on a new classification of PROs (symptom-related, comorbidity-related, and other) and PROMs (core and complementary) (). The core group of PROMs included all those considered essential for any patient with severe asthma (ACT, mini-AQLQ, EQ-5D, mMRC, TAI, and MMAS), along with the pharmacological dispensing register. Complementary PROMs included those to be used in certain circumstances or interventions (SNOT-22: when there are accompanying nasal symptoms; PSQI: when sleep problems are reported; HADS: when psychiatric symptoms are suspected; WPAI: in patients with work/academic impairment; TQSM-9: to assess satisfaction with treatment; patient’s knowledge questionnaire: when an educational program is proposed).
Table 2. PROs and PROMs classification proposed by nominal groups.
A minimum frequency of 3 months (at each follow-up visit) was established for measuring the core PROMs. In addition, the frequency of evaluation with complementary PROMs was set as follows: SNOT-22 (every 3 months); PSQI: (every 6 months); HADS (every 6 months); WPAI (every 6 months); TSQM-9 (every 6 months); Patient’s knowledge about the disease (every 12 months).
Delphi consultation
A total of 63 healthcare professionals involved in the management of severe asthma [n = 25 hospital pharmacists, n = 13 pulmonologists, n = 14 allergologists, and n = 11 nurses; mean time in specialty 15.32 (SD: 10.3) years)] and 5 patients (different from those of the focus group) with severe asthma [mean age: 47.2 (range: 23-62) years; mean age at diagnosis: 38 years (range: 14-59) years] participated in the first round of the Delphi consultation. Consensus was reached on all items included in the patients’ questionnaire in the first round; therefore, healthcare professionals (HCP) participated in the second round. The response rate of the second round was 96.8% (n = 61).
Results of the Delphi consultation are shown in Supplementary Tables S6–S8. A consensus was reached regarding the relevance of all PROs presented in the Delphi questionnaire for severe asthma patient follow-up, namely: symptom-related [wheezing (97.06%), dyspnea (97.06%), coughing (97.06%), sleep disorders (97.06%), and chest tightness (88.24%)]; comorbidity-related [nocturnal awakening (98.53%), anxiety (94.12%), depression (91.18%), fatigue (88.24%), blocked nose (82.35%), and taste/smell disorders (77.94%)]; other PROs [treatment adherence (98.53%), treatment satisfaction (97.06%), knowledge about asthma (97.06%), HRQoL (95.59%), and work productivity impairment (95.59%)] (Table S6).
During the first round, panelists proposed other PROs, such as allergic clinical pyrosis (n = 2), reflux (n = 1), plugged ears (n = 1), sore throat (n = 1), collection of side effects (n = 2), drug allergies (n = 1), and patient knowledge about their treatment (n = 3). However, the number of participants who proposed their inclusion was not sufficiently representative to be added to the second-round questionnaire.
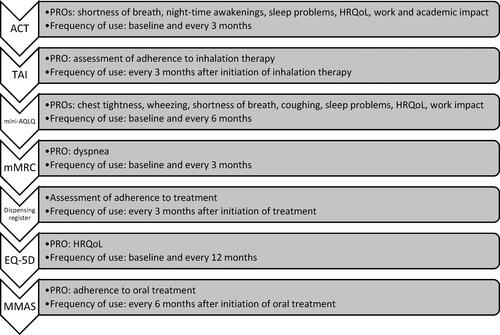
Consensus was reached for ACT (A:95.24%; F: 95.24%) for collecting data on dyspnea, nocturnal awakening, sleep disorders, HRQoL, and work productivity impairment (Table S8). Mini AQLQ (A: 93.65; F: 79.37%) was considered appropriate and feasible for assessing dyspnea, chest tightness, wheezing, coughing, sleep disorders, anxiety, depression, and HRQoL. The mMRC dyspnea scale (A: 85.71%; F: 85.71%) was chosen for assessing dyspnea. To assess adherence, panelists agreed to use TAI (A:92.06%; F: 85.71%), MMAS (A:75.40%; F:82%), and the dispensing register (A: 96.83%; F: 92.06%). Although panelists agreed on the appropriateness of EQ-5D (A: 84.13%; F: 67.20%) for HRQoL, anxiety, and depression assessment, this PROM was not considered feasible for implementation in clinical practice. A brief description of these questionnaires is included in Supplementary Table S9. See for the PROs evaluated by each PROM.
Regarding the frequency of measurement, panelists reached a consensus on the use of core PROMs at (1) diagnosis and (2) every three months (≥75%), except for MMAS (68.90%) and EQ-5D (65.60%). The alternative frequency proposed for MMAS was every six months (26.3%; n = 5) and every six or twelve months for EQ-5D (40.9%, n = 9). Mini-AQLQ frequency of measurement was not considered feasible in the second-round consultation (72.10%).
The complementary PROMs SNOT-22 (A:90.48%; F:73.80%), PSQI (A:82.54; F:63.90%), HADS (A:82.54; F:64%), WPAI (A:77.78%; F:49.20%), TSQM-9 (A:79.37; F:70.50%) and knowledge of asthma questionnaire (A:77%; F:68.80%) were considered adequate to assess the proposed symptomatology or condition, but a consensus was not reached on their feasibility of use in clinical practice (Table S8). Nonetheless, there was consensus on the appropriateness of frequency of measurement for complementary PROMs (≥75%), except for WPAI (73.70%).
Reasons for disagreement on PROMs feasibility (use and frequency of measurement) in clinical practice were explored. The main barriers identified were the lack of time and support staff, limited information about PROMs, and other administrative burdens, such as the lack of digital tools allowing systematic and automatic PROMs compilation.
Finally, when panelists were asked to prioritize the PROMs to be employed during severe asthma patient follow-up, the following core PROMs order of priority was established: 1st) ACT; 2nd) TAI; 3r) Mini-AQLQ, 4th) mMRC, 5th) dispensing register 6th) EQ-5D; 7th) MMAS.
Final scientific committee meeting
The scientific committee reviewed the results obtained in the two Delphi rounds and defined the final standard set of PROs and PROMs for the follow-up of any patient with severe asthma. Although a consensus was not reached on the EQ-5D questionnaire regarding its feasibility, the scientific committee did recommend its use at least once a year. Moreover, the measurement frequency of Mini-AQLQ and MMAS was extended to six months. shows the core group of PROMs, according to the order of priority, indicating the PROs considered relevant for severe asthma assessed and the recommended frequency of measurement.
Figure 3. Core group of measurement instruments in order of priority, PROs considered relevant for assessment of severe asthma, and frequency of measurement.
Complementary PROMs (SNOT-22, PSQI, HADS, WPAI TSQM-9, and knowledge of asthma questionnaire) were not included in the final standard set selection. However, the scientific committee agreed to recommend their use in specific cases with the proposed frequency of measurement, extending the frequency of the WPAI to once a year ().
Discussion
A systematic and standardized collection of PROs during follow-up of patients with severe asthma is crucial in order to move toward more effective and efficient patient-centered care. The development of a core set of outcomes to be measured in drug clinical trials in patients with moderate-to-severe asthma trpough a multistakeholder Delphi consensus has been previously published (Citation42). However, to the best of our knowledge, this is the first study defining an approach to severe asthma patient follow-up in Spain with the standardized integration of patients’ perspectives into clinical practice.
The present study provides a standard set of 16 PROs and 6 PROMs jointly selected by severe asthma patients and healthcare professionals, which serve as a guide for the development of a structured PRO measurement system for severe asthma care and related research. The PROs included in the standard set represent commonly reported symptoms in severe asthma, such as chest tightness, wheezing, dyspnea, coughing, and nocturnal awakening (Citation43). As more than one disease is usual in severe asthma patients, the standard set of PROs also included comorbidity-related symptoms (blocked nose, sleep disorders, fatigue, taste/smell disorders, anxiety, and depression) (Citation44). In addition, assessment of other PROs such as HRQoL, treatment satisfaction, treatment adherence, work productivity impairment, and knowledge about asthma may provide a more holistic view of patients’ care, being crucial for optimizing disease management. These results are in line with current GINA guidelines for the management of severe asthma (Citation45), which also give importance to the patient’s perspective and systematic consideration of factors that might contribute to uncontrolled symptoms and, consequently, a poorer quality of life (e.g. incorrect inhaler technique, suboptimal adherence, comorbidities, anxiety, depression and social and economic problems, etc.), recommend the provision of self-management education and an assessment of interventions after 3-6 months.
The PROMs were selected based on their appropriateness and feasibility in clinical practice. Some of them, such as ACT and Mini-AQLQ, are asthma-specific questionnaires that have been increasingly used to measure the impact of severe asthma treatment on HRQoL (Citation18,Citation46). ACT is one of the tools experts recommend to measure changes in asthma control in clinical trials, and mini-AQLQ to measure asthma-specific quality of life (Citation42). Other questionnaires (mMRC, TAI, EQ-5D, or MMAS) are generic PROMs, commonly used in conjunction with asthma-specific instruments. However, most asthma-specific questionnaires have been developed in the research context rather than in clinical settings, and real-life studies on biological therapies assessing PRO are scarce (Citation46). Therefore, further work is needed to evaluate their suitability for use in clinical practice with individual patients (Citation47). Regarding mMRC, this is a widely recommended scale to assess dyspnea, although it is not very accurate in detecting changes in severity, as has been pointed out by some researchers who also refer to the importance of validating the content of PROMs (Citation48). Differences in the mMRC descriptors may not be detected quarterly, but this is the consensually agreed monitoring frequency for evaluating severe asthma treatment (Citation3,Citation49).
This standard set for severe asthma marks a starting point; however, during the Delphi consultation several barriers were identified that should be addressed in order to promote and ensure the collection of PROs in clinical practice: 1) barriers related to the health system itself; 2) barriers associated with healthcare professionals; 3) barriers related to patients. For example, healthcare professionals pointed out the lack of resources (i.e. electronic records, time in consultation, and professional staff). In addition, HCP’s limited information about PROMs, or the assumption that information from PROMs is irrelevant for clinical decision-making, were also identified as barriers to implementing the set of PROs and PROMs defined previously (Citation50). Likewise, the availability of electronic tools and appropriate technology is necessary to support the collection of PROMs in clinical practice and reduce the burden on the consultation schedule (Citation50,Citation51). Other barriers identified in the literature are the lack of patient perspectives on PROMs, or the lack of knowledge of how to analyze and interpret PROM data (Citation47).
Despite the many barriers, it is indisputable that adopting PROMs in clinical practice can help healthcare professionals make decisions for individual patients (Citation50–52). By measuring health issues that are important to patients, PROMs can inform clinicians about health management and treatment plans (Citation52). Patient-centered care may be pivotal in improving health outcomes for patients with asthma. There is enough evidence to support incorporating PROs into disease management to improve results and patient health (Citation53). PROMs allow standardization of health assessment from the patient’s perspective and complement the information gathered with clinical markers. Routine use of PROMs can help identify the issues severe asthma patients face that they would like to see improved by treatment, thus contributing to the implementation of a value-based healthcare model (Citation54).
This project has several limitations that may imply some uncertainty in synthesizing the results. First, this set of PROs and PROMs reflects the opinion of a multidisciplinary group of 63 HCP and five severe asthma patients. Although no significant differences are expected, different participants could have reached a consensus on other PROs and PROMs. Patient representation may be considered low, but these were expert patients belonging to a patient advocacy group who, together with the patients who participated in the focus group, showed their full agreement on the relevance of all PROs and the need to be evaluated in routine clinical practice, so it was not considered necessary to involve more expert patients. Second, the PROs and PROMs were selected to reflect current therapeutic strategies. As treatments for severe asthma continue to be developed, the symptoms’ nature and impact on patients’ HRQoL may also vary with time. With the incorporation of new biologic therapies developed for treating severe asthma (Citation14,Citation46), regular updates on the settings defined are recommendable. Third, a final consultation with patients was not carried out following the consensus reached on the core group of PROMs, and future studies should assess patient opinion or satisfaction with the regular use of these questionnaires. Finally, study participants (HCP and severe asthma patients) were confined to Spain; therefore, some of the selected PROs may only be relevant for Spanish patients, and extrapolations to other healthcare settings should be made with caution.
Despite these limitations, this work highlights the value of including PROs and PROMs in research and clinical practice to fully understand the impact of interventions, therapies, and services on severe asthma patients. Consequently, a holistic and multidisciplinary approach must move toward an effective and efficient patient-centered healthcare system, ensuring the best quality care and evaluating evidence from clinical outcomes and PROs.
Conclusion
This study provides a standard set of PROs and PROMs selected by patients and healthcare professionals, which may be incorporated into severe asthma care and research. The results obtain may be used to develop implementation strategies that support the routine inclusion of PRO reporting in severe asthma care settings, thereby improving care quality.
Supplemental Material
Download MS Word (84.2 KB)Acknowledgments
The authors would like to thank the Spanish patient advocacy group FENAER (Federación Española de Asociaciones de Pacientes Alérgicos y con Enfermedades Respiratorias) and the following Spanish Scientific Societies for endorsing the project: Sociedad Española de Neumología y Cirugía Torácica (SEPAR), Sociedad Española de Alergología e Inmunología Clínica (SEAIC), and Sociedad Española de Farmacia Hospitalaria (SEFH) (declaración de interés científico sanitario). The authors would also like to thank all participants in the Delphi consultation, both patients and healthcare professionals from hospital pharmacy (Montserrat Alonso, Milagros Álvarez, María Blanco, Germán Blanco, Mònica Climente, Inés Fernández, Araceli Fernández, Jenifer González, Mónica Granero, Victoria Lerma, Larraitz Leunda, Pilar Ana López, Belén López, Hilario Martínez, Ma Teresa Martínez, Jose Javier Martínez, Herminia Navarro, Inmaculada Plasencia, Jesus Prada, Isabel Puertolas, Esther Ramírez, Julia Sánchez, Isabel Sánchez, Elena Villamañán, Ma Dolores Zamora), allergology (Mónica Antón, David González de Olano, Darío Antolín, Luis Ángel Navarro, Javier Domínguez, Marta Viñas, Antonio Valero, Regina Paulaskas, Irán Sánchez, Soledad Jiménez, Ernesto Enrique, José Vicente Castelló, Joan Bartra, Almudena Testera), pulmonology (Alfredo de Diego, Alicia Padilla, Carolina Cisneros, Marina Blanco, Andrea Trisan, Rocío Díaz, Cleofé Fernández, Elena Curto, Julia García de Pedro, Carlos Almonacid, Mónica Vidal, Guillermo Bentabol, Sandra Vañes), and nursery (Elisa Lillo, Sonia Giraldós, Milagros Figueroa, Marisa Escobar, Pilar de la Calle, Pilar García, Ma Teresa Rodríguez, Ma José Pla, Ma Auxiliadora Guerrero, Ma del Carmen Hernández, Ma Carmen Sánchez). Finally, the authors would like to thank Laura Benedito-Palos, who works for an independent scientific and strategic consultancy (Outcomes’10, S.L.U.), for her contribution to developing and coordinating the project and writing this manuscript. Carla Prego was the person in charge of overseeing the study from GSK.
Disclosure statement
The authors have no conflict with the funding source. IAE declares relationships with GSK, AstraZeneca, and Sanofi. Among the participants in the nominal groups, ECV reports personal fees or non-financial support from AstraZeneca, GSK, Novartis, Sanofi, Chiesi, Boehringer Ingelheim, and Linde; IEG reports consulting fees from Sanofi, AstraZeneca, GSK, Novartis, Chiesi, Leti Pharma, Allergy Therapeutics and Viatrix and payment of honoraria from AstraZeneca, GSK, Novartis, Chiesi, Leti Pharma, and Diater; AHM reports personal fees or non-financial support from AstraZeneca, GSK, Novartis, Sanofi, Chiesi, ALK-Abelló, and Bioproject, outside the submitted work. The other authors declare no conflicts of interest.
Additional information
Funding
References
- The global asthma report 2018. Global asthma network; 2018. Available from: http://globalasthmareport.org.
- Hekking PW, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896–902. doi:10.1016/j.jaci.2014.08.042.
- GEMA 5.1. Guía Española para el Manejo del Asma: Sociedad Española de Neumología y Cirugía Torácica. 2021.
- Reddel HK, Bacharier LB, Bateman ED, Brightling CE, Brusselle GG, Buhl R, Cruz AA, Duijts L, Drazen JM, FitzGerald JM, et al. Global Initiative for Asthma Strategy 2021: Executive summary and rationale for key changes. Eur Respir J. 2022;59(1):2102730. doi:10.1183/13993003.02730-2021.
- Global Initiative for Asthma (GINA) 2021. Available from: https://www.ginasthma.com/.
- Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi:10.1183/09031936.00202013.
- Upham JW, Le Lievre C, Jackson DJ, Masoli M, Wechsler ME, Price DB, Delphi panel defining a severe asthma super-responder: findings from a Delphi process. J Allergy Clin Immunol Pract. 2021;9(11):3997–4004. doi:10.1016/j.jaip.2021.06.041.
- McDonald VM, Hiles SA, Jones KA, Clark VL, Yorke J. Health-related quality of life burden in severe asthma. Med J Aust. 2018;209(S2):S28–S33. doi:10.5694/mja18.00207.
- Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald JM, Gibson P, Ohta K, O’Byrne P, Pedersen SE, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31(1):143–178. doi:10.1183/09031936.00138707.
- Santino TA, Monteiro KS, de Paiva Azevedo M, Patino CM, Ahmed S, de Mendonça K. Patient- and proxy-reported outcome measures instruments for the assessment of asthma control among adult and pediatric population: A protocol for systematic review. Medicine (Baltimore). 2020;99(19):e20078. doi:10.1097/MD.0000000000020078.
- Song HJ, Blake KV, Wilson DL, Winterstein AG, Park H. Medical costs and productivity loss due to mild, moderate, and severe asthma in the United States. J Asthma Allergy. 2020;13:545–555. doi:10.2147/JAA.S272681.
- Martínez-Moragón E, Serra-Batllés J, De Diego A, Palop M, Casan P, Rubio-Terrés C, Pellicer C; por el Grupo de Investigadores del estudio AsmaCost [Economic cost of treating the patient with asthma in Spain: The AsmaCost study]. Arch Bronconeumol. 2009;45(10):481–486. doi:10.1016/j.arbres.2009.04.006.
- Kroes JA, Zielhuis SW, van der Meer AN, de Jong K, Ten Brinke A, van Roon EN. Patient-reported outcome measures after 8 weeks of mepolizumab treatment and long-term outcomes in patients with severe asthma: An observational study. Int J Clin Pharm. 2021;44(2):570–574. doi:10.1007/s11096-021-01362-8.
- Corren J, Garcia Gil E, Griffiths JM, Parnes JR, van der Merwe R, Sałapa K, O’Quinn S. Tezepelumab improves patient-reported outcomes in patients with severe, uncontrolled asthma in PATHWAY. Ann Allergy Asthma Immunol. 2021;126(2):187–193. doi:10.1016/j.anai.2020.10.008.
- Chen J, Ou L, Hollis SJ. A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv Res. 2013;13(1):211. doi:10.1186/1472-6963-13-211.
- Basch E, Barbera L, Kerrigan CL, Velikova G. Implementation of patient-reported outcomes in routine medical care. Am Soc Clin Oncol Educ Book. 2018;38:122–134. doi:10.1200/EDBK_200383.
- Braido F, Baiardini I, Canonica GW. Patient-reported outcomes in asthma clinical trials. Curr Opin Pulm Med. 2018;24(1):70–77. doi:10.1097/MCP.0000000000000440.
- Worth A, Hammersley V, Knibb R, Flokstra-de-Blok B, DunnGalvin A, Walker S, et al. Patient-reported outcome measures for asthma: A systematic review. NPJ Prim Care Respir Med. 2014;24:14020.
- Hyland ME, Lanario JW, Menzies-Gow A, Mansur AH, Dodd JW, Fowler SJ, Hayes G, Jones RC, Masoli M. Comparison of the sensitivity of patient-reported outcomes for detecting the benefit of biologics in severe asthma. Chron Respir Dis. 2021;18:14799731211043530. doi:10.1177/14799731211043530.
- Kocks JWH, Seys SF, van Duin TS, Diamant Z, Tsiligianni IG. Assessing patient-reported outcomes in asthma and COPD patients: Which can be recommended in clinical practice? Curr Opin Pulm Med. 2018;24(1):18–23. doi:10.1097/MCP.0000000000000447.
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097.
- Glaser BG, Strauss AL. The discovery of grounded theory: Strategies for qualitative research. New York: Aldine Publishing Company; 1967.
- Gallagher M, Hares T, Spencer J, Bradshaw C, Webb I. The nominal group technique: A research tool for general practice? Fam Pract. 1993;10(1):76–81. doi:10.1093/fampra/10.1.76.
- McMillan SS, King M, Tully MP. How to use the nominal group and Delphi techniques. Int J Clin Pharm. 2016;38(3):655–662. doi:10.1007/s11096-016-0257-x.
- Sinha IP, Smyth RL, Williamson PR. Using the Delphi technique to determine which outcomes to measure in clinical trials: Recommendations for the future based on a systematic review of existing studies. PLoS Med. 2011;8(1):e1000393. doi:10.1371/journal.pmed.1000393.
- Birko S, Dove ES, Özdemir V. Evaluation of nine consensus indices in delphi foresight research and their dependency on Delphi survey characteristics: A simulation study and debate on Delphi design and interpretation. PLoS One. 2015;10(8):e0135162. doi:10.1371/journal.pone.0135162.
- Diamond IR, Grant RC, Feldman BM, Pencharz PB, Ling SC, Moore AM, Wales PW. Defining consensus: A systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014;67(4):401–409. doi:10.1016/j.jclinepi.2013.12.002.
- Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14(1):32–38. doi:10.1034/j.1399-3003.1999.14a08.x.
- Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, Murray JJ, Pendergraft TB. Development of the asthma control test: A survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi:10.1016/j.jaci.2003.09.008.
- Birring SS, Prudon B, Carr AJ, Singh SJ, Morgan MD, Pavord ID. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax. 2003;58(4):339–343. doi:10.1136/thorax.58.4.339.
- de los Santos G, Reyes P, del Castillo R, Fragola C, Royuela A. Cross-cultural adaptation and validation of the sino-nasal outcome test (SNOT-22) for Spanish-speaking patients. Eur Arch Otorhinolaryngol. 2015;272(11):3335–3340. doi:10.1007/s00405-014-3437-0.
- Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi:10.1093/sleep/34.5.601.
- EuroQol: EQ-5D instruments. Available from: https://euroqol.org/eq-5d-instruments/.
- Bharmal M, Payne K, Atkinson MJ, Desrosiers MP, Morisky DE, Gemmen E. Validation of an abbreviated Treatment Satisfaction Questionnaire for Medication (TSQM-9) among patients on antihypertensive medications. Health Qual Life Outcomes. 2009;7(1):36. doi:10.1186/1477-7525-7-36.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi:10.1111/j.1600-0447.1983.tb09716.x.
- mMRC (Modified Medical Research Council). Dyspnea scale. Available from: https://www.mdcalc.com/mmrc-modified-medical-research-council-dyspnea-scale#:∼:text=The%20mMRC%20Dyspnea%20Scale%20quantifies,obstructive%20pulmonary%20disease%20(COPD).
- Grammatopoulou EP, Skordilis EK, Georgoudis G, Haniotou A, Evangelodimou A, Fildissis G, Katsoulas T, Kalagiakos P. Hyperventilation in asthma: A validation study of the Nijmegen Questionnaire–NQ. J Asthma. 2014;51(8):839–846. doi:10.3109/02770903.2014.922190.
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi:10.1016/0165-1781(89)90047-4.
- Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi:10.1097/00005650-198601000-00007.
- Plaza V, López-Viña A, Cosio BG; en representación del Comité Científico del Proyecto TAI. Test of adherence to inhalers. Arch Bronconeumol. 2017;53(7):360–361. doi:10.1016/j.arbres.2016.08.006.
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–365. doi:10.2165/00019053-199304050-00006.
- Tejwani V, Chang H-Y, Tran AP, Naber JA, Gutzwiller FS, Winders TA, Khurana S, Sumino K, Mosnaim G, Moloney RM, et al. A multistakeholder Delphi consensus core outcome set for clinical trials in moderate-to-severe asthma (coreASTHMA). Ann Allergy Asthma Immunol. 2021;127(1):116–122.e7. doi:10.1016/j.anai.2021.03.022.
- Osman LM, McKenzie L, Cairns J, Friend JA, Godden DJ, Legge JS, Douglas JG. Patient weighting of importance of asthma symptoms. Thorax. 2001;56(2):138–142. doi:10.1136/thorax.56.2.138.
- Porsbjerg C, Menzies-Gow A. Co-morbidities in severe asthma: Clinical impact and management. Respirology. 2017;22(4):651–661. doi:10.1111/resp.13026.
- Global Initiative for Asthma (GINA) 2023. Available from: https://ginasthma.org/severeasthma/.
- Di Bona D, Minenna E, Albanesi M, Nettis E, Caiaffa MF, Macchia L. Benralizumab improves patient reported outcomes and functional parameters in difficult-to-treat patients with severe asthma: data from a real-life cohort. Pulm Pharmacol Ther. 2020;64:101974. doi:10.1016/j.pupt.2020.101974.
- Worth A, Hammersley VS, Nurmatov U, Sheikh A. Systematic literature review and evaluation of patient reported outcome measures (PROMs) for asthma and related allergic diseases. Prim Care Respir J. 2012;21(4):455–458. doi:10.4104/pcrj.2012.00084.
- Sunjaya A, Poulos L, Reddel H, Jenkins C. Qualitative validation of the modified Medical Research Council (mMRC) dyspnoea scale as a patient-reported measure of breathlessness severity. Respir Med. 2022;203:106984. doi:10.1016/j.rmed.2022.106984.
- Alvarez-Gutiérrez FJ, Blanco-Aparicio M, Casas-Maldonado F, Plaza V, González-Barcala FJ, Carretero-Gracia JÁ, Castilla-Martínez M, Cisneros C, Diaz-Pérez D, Domingo-Ribas C, et al. Consensus document for severe asthma in adults. 2022 update. Open Respir Arch. 2022;4(3):100192. doi:10.1016/j.opresp.2022.100192.
- Foster A, Croot L, Brazier J, Harris J, O’Cathain A. The facilitators and barriers to implementing patient reported outcome measures in organisations delivering health related services: A systematic review of reviews. J Patient Rep Outcomes. 2018;2(1):46. doi:10.1186/s41687-018-0072-3.
- Philpot LM, Barnes SA, Brown RM, Austin JA, James CS, Stanford RH, Ebbert JO. Barriers and benefits to the use of patient-reported outcome measures in routine clinical care: A qualitative study. Am J Med Qual. 2018;33(4):359–364. doi:10.1177/1062860617745986.
- Field J, Holmes MM, Newell D. PROMs data: Can it be used to make decisions for individual patients? A narrative review. Patient Relat Outcome Meas. 2019;10:233–241. doi:10.2147/PROM.S156291.
- Qamar N, Pappalardo AA, Arora VM, Press VG. Patient-centered care and its effect on outcomes in the treatment of asthma. Patient Relat Outcome Meas. 2011;2:81–109. doi:10.2147/PROM.S12634.
- Clark VL, Gibson PG, McDonald VM. What matters to people with severe asthma? Exploring add-on asthma medication and outcomes of importance. ERJ Open Res. 2021;7(1):00497–2020. doi:10.1183/23120541.00497-2020.