Abstract
Objective
This study aimed to analyze the sensitization rate of different aeroallergens in children of different age, sex, and disease groups, describe the changing trend of different aeroallergens in different ages, and analyze the sensitization risk factors for asthma.
Methods
Children (<18 years old) with suspected atopic diseases who visited the Department of Allergy of Children’s Hospital Affiliated to Capital Institute of Pediatrics and underwent a skin prick test (SPT) were retrospectively enrolled from January 2019 to November 2021.
Results
A total of 5465 patients (3514 boys, 1951 girls; mean age, 7 ± 3 years) were enrolled. Of them, 3703 patients (67.8%) were sensitized to at least one aeroallergen. Before 4 years of age, mold was the most prevalent aeroallergen (103/380 [27.1%]), whereas after 4 years of age, weed pollen was the most prevalent aeroallergen. After 6 years of age, tree pollen became the second most prevalent aeroallergen. After 12 years of age, the sensitization rate of indoor aeroallergens was lower than that of outdoor aeroallergens. Logistic regression showed that sensitization to mold (odds ratio [OR]:1.4, 95% confidence interval (CI): 1.2–1.7, p < 0.001), animal dander (OR: 1.6, 95% CI: 1.4–1.9, p < 0.001), and polysensitization (OR: 1.4, 95% CI: 1.0–1.8, p = 0.038) were potential sensitization risk factors for asthma.
Conclusions
Mold is an important allergen in early life. Different kinds of allergens affect different age groups. Patients who are sensitized to mold or animal dander or experience polysensitization should be carefully monitored for asthma risk.
Introduction
Allergic diseases are becoming increasingly more prevalent worldwide. For example, allergic rhinitis (AR) affects approximately 40% of the global population, with an increasing prevalence over the past 20 years (Citation1,Citation2). The International Study of Asthma and Allergies in Childhood (ISAAC) showed that the prevalence of AR was 8.5% in children 6–7 years of age and 14.6% in children 13–14 years of age according to a multinational cross-sectional questionnaire survey (Citation3). A phase III of the ISAAC study showed that the global prevalence of wheezing ranged from 0.8% to 32.6% in different countries in children of 13–14 years of age and from 2.4% to 37.6% in different countries in children of 6–7 years of age (Citation4). In China, the prevalence of AR in children was 7.8–20.4% in different cities in 2009, while the prevalence of asthma in children was 2.1–7.5% (Citation5). An investigation of asthma across 33 cities in China showed that the prevalence of asthma increased from 1.59% in 2000 to 2.11% in 2010 (Citation6). Patients with allergic diseases may have impaired sleep and learning difficulties, which may lower their quality of life (Citation1). Identifying allergen sensitization patterns is important, as it will provide clinicians with evidence of the most important indoor and outdoor aeroallergens (including dust mites, animal dander, mold, tree pollen, and weed pollen) for each age group when referring a patient for an allergy evaluation. Allergen identification is vital to diagnosis, and is the basis for the treatment and management of allergic diseases. Previous cross-sectional studies of children in China revealed a 77.8% and 56.1–73.0% sensitization rate to aeroallergens, respectively (Citation7,Citation8). The number and proportion of young children under six years old was smaller than that in this study. Children fewer than 6 years old were not divided into further age groups, and the changing trend of aeroallergens among different age groups was not described.
The skin prick test (SPT) is an important method for the identification of allergens. The SPT has a high sensitivity and good specificity when performed and interpreted correctly. In fact, it is the most frequently used method for allergen testing because of its rapidity, simplicity, and low cost (Citation9). Accordingly, SPT can be widely used, especially in primary care clinics. The main limitations of SPT are the allergic reaction risk, and the need for eligible pulmonary function test and discontinuation of influencing drugs; therefore, it should be performed under the supervision of professionals.
Knowledge of sensitization patterns among children, especially young children, is limited. This study aimed to analyze the sensitization rate of different aeroallergens in children, provide more information on the sensitization profiles of different age, sex, and disease groups, describe the changing trend of different aeroallergens in different ages, and analyze the risk factors of asthma by different allergens.
Methods
Patients
This was a retrospective cross-sectional single-center study. Children (<18 years old) with suspected atopic diseases who visited the Department of Allergy of Children’s Hospital Affiliated to Capital Institute of Pediatrics and underwent an SPT were retrospectively enrolled and their data were collected from January 2019 to November 2021. Patients for whom incomplete information regarding demographic data, diagnosis, and SPT results were available were excluded. Demographic data, diagnosis, and SPT results were collected. This study was approved by the Institutional Review Board of Children’s Hospital Affiliated to Capital Institute of Pediatrics (SHERLL2021011), in accordance with approved published guidelines. The requirement for informed consent was waived by the ethics committee as this was a retrospective and observational (non-interventional) study.
Skin prick test
Allergen extracts (Allergen Products Research and Development Center, Peking Union Medical College Hospital) of dust mites, cockroaches, cat dander, dog dander, tree pollen (Salix, Fraxinus pennsylvanica, Juniperus chinensis, Betula, Platanus acerifolia, and Populus), weed pollen (Artemisia sieversiana, Humulus scandens, Ambrosia artemisiifolia, and Chenopodium album), and molds (Alternaria alternate, Cladosporium cladosporioides, and Aspergillus niger) were used in the SPT performed on the volar surface of the forearm of all patients. The concentration of allergen extracts was 1:20 to 1:50 wt/vol. The SPT was performed using a practical guide as a standard method (Citation10). Histamine (10 mg/mL) and physiological saline solution were used as the positive and negative controls, respectively. A wheal that was 3 mm greater in diameter than the negative control at 15 min was considered positive. Medications that might have influenced the SPT results were withheld before the testing. Sensitization to aeroallergens was defined as a positive SPT result. Polysensitization was defined as a positive reaction to two or more classes of the following allergens (dust mites, animal dander, mold, tree pollen, and weed pollen). Dust mites, mold, animal dander, and cockroaches were considered indoor allergens. Tree and grass pollens were considered outdoor allergens (Citation11).
Statistical analysis
The prevalence rates of sensitization to each aeroallergen in different age groups, sex, and diseases were calculated. Categorical data were expressed as percentages. Prevalence rates among the different groups were compared using Pearson’s χ2 test (when the sample size was > 40 and each theoretical frequency was ≥ 5) or Fisher’s exact probability test (when the sample size was < 40 or any theoretical frequency was < 1). A univariate logistic regression analysis was used to evaluate candidate predictors of asthma, and variables with values of p < 0.1 were further analyzed in the multivariate analysis. The variables were selected as categorical variables. A multivariate stepwise analysis was used to identify the risk factors for asthma. Statistical significance was set at a two-sided p value <0.05. All statistical analyses were performed using Statistical Package for the Social Sciences version 22.0 (SPSS, Chicago, IL).
Results
Sensitization and polysensitization rate of aeroallergens in different age groups
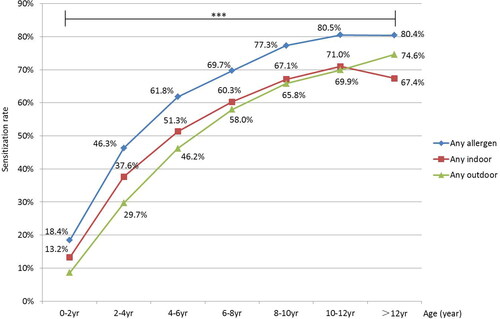
A total of 5465 patients were enrolled, including 3514 boys and 1951 girls, with an average age of 7 ± 3 years. A total of 3703 patients (3,703/5,465, 67.8%) were sensitized to at least one aeroallergen. The patients were divided into seven age groups: 0–2, 2–4, 4–6, 6–8, 8–10, 10–12, and >12 years (). The total sensitization rate to aeroallergens increased before 12 years of age (from 28/152 [18.4%] to 458/569 [80.5%]), while it remained stable in the >12 years group (358/445 [80.4%]) (). Before 4 years of age, mold was the most prevalent aeroallergen (103/380 [27.1%]), whereas after 4 years of age, weed pollen exceeded mold and became the most prevalent aeroallergen. After 6 years of age, tree pollen exceeded mold and became the second most prevalent aeroallergen. Sensitization to dust mites and cockroaches was always lower than that of mold, weed pollen, and tree pollen in all age groups. After 12 years of age, the sensitization rate to animal dander exceeded that to mold (, Supplemental Table 1). The polysensitization rate of aeroallergens increased with age from 10/152 (6.6%) in the 0–2-years group to 316/445 (71.0%) in the > 12-years group ().
Figure 1. Sensitization rates of different kinds of aeroallergens in different age groups. ***Differences in sensitization rates to tree pollen, weed pollen, mold, animal, dust mite, and cockroaches among the seven age groups were all statistically significant (p < 0.001).
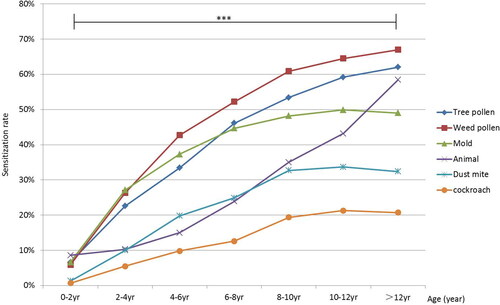
Figure 2. Polysensitization rates of aeroallergens in different age groups. ***Differences in polysensitization rates among the seven age groups were statistically significant (p < 0.001).
Table 1. Prevalence of sensitization to at least one aeroallergen.
The youngest age at which a patient was sensitized to a certain indoor aeroallergen was 4 months (cat dander), followed by 13 (mold and dust mites) and 21 months (dogs and cockroaches). The youngest age at which the patient was sensitized to a certain outdoor aeroallergen was 13 (weed pollen) and 23 months (tree pollen). The sensitization rate to each tested allergen is shown in Supplemental Table 2.
Sensitization rate of indoor and outdoor aeroallergens
Totally 3022/3703 (81.6%) of children were sensitized to outdoor aeroallergens, and 3157/3703 (85.3%) of children were sensitized to indoor aeroallergens. The sensitization rate of outdoor aeroallergens increased with age (from 13/152 [8.6%] to 332/445 [74.6%]). The sensitization rate of indoor aeroallergens increased with age before 12 years (from 20/152 [13.2%] to 404/569 [71.0%]) and decreased after 12 years (300/445 [67.4%]). Before 12 years of age, the sensitization rate of indoor aeroallergens was higher than that of outdoor aeroallergens. In contrast, after 12 years of age, the sensitization rate of indoor aeroallergens was lower than that of outdoor aeroallergens ().
Sensitization rate of aeroallergens of different sex
Overall, the sensitization rate of any aeroallergen in boys was higher than that in girls (2477/3514 [70.5%] vs. 1226/1951 [62.8%], p < 0.001). The sensitization rate of outdoor aeroallergens in boys was higher than that in girls (2010/3514 [57.2%] vs. 1012/1951 [51.9%], p < 0.001), which was the same as that of indoor aeroallergens in boys (2148/3514 [61.1%] vs. 1011/1951 [51.8%], p < 0.001). For the age group of 0–2, 2–4, 6–8, 8–10, and >12 years, the sensitization rate of aeroallergens was higher in boys than that in girls (p < 0.05) ().
Table 2. Sensitization rate of aeroallergens of different genders in different age groups.
Sensitization rate of aeroallergens in different disease groups
The sensitization rate of tree pollen, weed pollen, mold, animal dander, and dust mites in patients with AR was lower than that in patients with asthma (p < 0.001). The polysensitization rate in patients with AR was lower than that in patients with asthma (711/1565 [45.4%] vs. 1920/3262 [58.9%], p < 0.001) (). The above factors were included in the logistic regression analysis, which showed that sensitization to mold (odds ratio [OR]: 1.4, 95% confidence interval (CI): 1.2–1.7, p < 0.001), animal dander (OR: 1.6, 95% CI: 1.4–1.9, p < 0.001), and polysensitization (OR: 1.4, 95% CI: 1.0–1.8, p = 0.038) were potential risk factors for asthma according to different allergens ().
Figure 4. Risk factors of different aeroallergens of asthma. OR: odds ratio, CI: confidence interval.
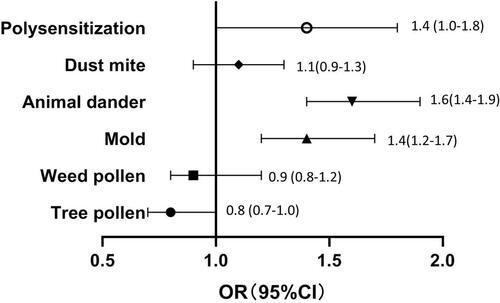
Table 3. Sensitization rate of aeroallergens in different disease groups.
Discussion
The SPT is a convenient method for the identification of allergens. However, aeroallergen sensitization rates in children, especially young children, are limited. In this study, we analyzed the sensitization rates of different types of aeroallergens in a large number of children, including young children. The total sensitization rate of aeroallergens increased with age before 12 years, while it remained stable in the group older than 12 years. After 12 years of age, the sensitization rate of indoor aeroallergens was lower than that of outdoor aeroallergens. The polysensitization rate increased with age. This study provided the sensitization profile of children and information about risk factors for asthma according to different allergens.
In this study, the general sensitization rate in children was 67.8% (3703/5465), and the rate remained stable after 12 years of age. A cross-sectional survey of children from seven cities in China revealed a 77.8% sensitization rate to 11 aeroallergens (based on serum specific immunoglobulin E [sIgE] levels) (Citation7). The children included in that study were with self-reported AR, and not with asthma. Children under 6 years old were only 333, and were not divided into further age groups. The changing trend of aeroallergens among different age groups was not described. More children with younger age were focused in our study. We divided the age group every two years to better observe the changing trend of aeroallergens in different ages. And included cities were mainly within western and southern China. These differences might lead to the higher sensitization rate than that found here. The positivity rate of inhaled allergens (based on sIgE levels) was 80.05% in another retrospective study in Changzhou, China (Citation12), the participants of which were mainly aged 8–14 years. Changzhou city is in the southeast of China, located near the Yangtze River. While the study site of this study, Beijing, is located in the north of China. China is a country with a large geography and diverse climates (Citation13), the differences in the geographic position might have led to the differences of sensitization rate to different aeroallergens.
In our study, the sensitization rate to outdoor aeroallergens, which mainly included pollen, exceeded that to indoor aeroallergens in children 12 years of age. In another birth cohort study in Sweden, the analysis of sIgE-antibodies revealed that the sensitization rate of timothy and birch pollen also increased after 12 years of age (Citation14). These data are consistent with previous recommendations encouraging indoor allergen tests in younger children and outdoor allergen tests in older children (Citation15). Children tend to participate in more outdoor activities as they grow up, including attending kindergarten after 3 years of age and attending school after 6 years of age. Therefore, they will be exposed to more outdoor allergens. In our study, weed pollen became the most highly sensitized allergen in children over 4 years of age. In another study on the aeroallergen sensitization rate (based on SPT results) in children in Boston, sensitization rates to dust mites and trees were the highest in all ages above 4 years (Citation16). This reflects the differences in sensitization profiles among regions, which may due to differences in environment and climate. Another study on the pollen sensitization features (based on SPT results) of seasonal AR in Northern Chinese children and adolescents showed that weed pollen was the most common allergen (Citation17). A study of the characteristics and changes of aeroallergens (based on sIgE-antibody levels) in children from 2015 to 2020 showed that the sensitization rate to weed and tree pollen increased with time (Citation18). This change might be explained by the increased global green area (an increase of 5% from 2000 to 2017), among which China alone accounts for 25% of the global net increase in leaf area, and 42% of the greening in China is from the forests (Citation19). In recent years, the increase in the amount of pollen in the Beijing area might also have contributed to the increased pollen sensitization rate. A larger amount of pollen in the pollen seasons leads to a stronger sensitizing effect and more patients with allergies (Citation20). Early childhood traffic-related air pollution exposure may also be related to asthma and allergic diseases (Citation21). Changes in climate and correlated global warming affect respiratory health, which contributes to the development of allergic respiratory diseases such as AR and asthma (Citation22). A previous study found that approximately half of the patients with weed pollen AR developed seasonal asthma within 9 years (Citation23). This sensitization profile reminded pediatricians or physicians of the most considerate allergen in children of different ages, and weed pollen should be especially considered in Northern China. This study showed that the polysensitization rate increased with age. The polysensitization rate in school children was 54.6%. Another cross-sectional study of allergic sensitization prevalence based on SPT results in Greece showed that 59.7% of schoolchildren were polysensitized, which reminded us to appreciate polysensitization in schoolchildren as above one-half would have two or more kinds of aeroallergens (Citation24). In this study, we found that the youngest age sensitized to indoor aeroallergens was 4–21 months, and the youngest age sensitized to outdoor aeroallergens was 13–23 months. The age at which they were sensitized to indoor aeroallergens was lower than that of outdoor aeroallergens. Other studies also suggested that children could be sensitized to aeroallergens (based on sIgE levels) at an early age, and 65% of children sensitized by 1 year of age had asthma at the age of 13 years (Citation25–27). Children who are sensitized to aeroallergens at a very early age should be regularly followed up to determine whether asthma develops.
The sensitization rates of all allergens and outdoor and indoor allergens in boys were higher than those in girls. Another study of children and adolescents in Poland using SPT to evaluate sensitization showed a significant difference in sensitization between boys and girls (Citation28). A Swedish population-based birth cohort study also showed that the male sex is associated with immunoglobulin E sensitization to aeroallergens at all ages (Citation14). This probably could not only be explained by general differences in allergen exposure; whether there are primarily genetic, hormonal, or other environmental factors related to sex differences remains to be further studied (Citation14).
The sensitization rate of any allergen and the polysensitization rate in children with asthma were all higher than those in children with AR. In this study, polysensitization, mold, and animal dander were the risk factors for asthma. Another cross-sectional study of Korean children 6–7 years of age also showed that the number of allergens sensitized (based on SPT results) increased the risk of wheezing attacks (one allergen: adjusted OR [aOR] 2.22, four or more allergens: aOR 9.39) and nasal symptom severity (Citation29). Another study showed that polysensitization was significantly associated with some quality of life issues (Citation30). As the particles of mold and animal dander allergens are relatively small and can easily enter small airways, children allergic to mold and animal dander may be more likely to have asthma. Among the perennial allergens, the sensitization rates of mold and animal dander were higher than those of dust mites before 4 years of age. A German multicenter allergy study investigated children’s asthma and measured perennial allergen IgE levels and showed that sensitization to perennial allergens developing in the first 3 years of life was associated with lung function loss at school age and exposure to high levels of perennial allergens in early life aggravated this process (Citation31). Sensitization of mold and animal dander in early life is important in clinical practice.
This study had some limitations. First, this was a retrospective cross-sectional study, and the age distribution of aeroallergen sensitization should be confirmed in a multicenter prospective cohort study. Second, the sample sizes of some age groups were relatively small, which might have led to bias. Third, our data included only SPT results; thus, our findings require confirmation with sIgE results. And finally, this was a single-center study whose participants were mainly derived from Northern China; therefore, our findings may not be representative of patients in other countries or regions.
Conclusions
In conclusion, this study demonstrated the sensitization spectrum of aeroallergens in Northern China based on a large number of children, including young children. It provided information about the most important aeroallergens for each age group that will aid clinicians who are considering referring a young patient for an allergy evaluation. Mold was an important allergen in early life. After 4 years of age, weed pollen became the most prevalent aeroallergen, while after 6 years of age, tree pollen surpassed mold and became the second prevalent aeroallergen. Children with polysensitization or sensitization to mold and animal dander should be carefully monitored for asthma development.
Supplemental Material
Download MS Word (19.8 KB)Acknowledgements
We thank Editage for English language editing.
Declaration of interest
The author declared no conflicts of interest.
Data availability statement
All the available data are included in the manuscript.
Additional information
Funding
References
- Drazdauskaitė G, Layhadi JA, Shamji MH. Mechanisms of allergen immunotherapy in allergic rhinitis. Curr Allergy Asthma Rep 2020;21(1):2. doi:10.1007/s11882-020-00977-7.
- Patil VK, Kurukulaaratchy RJ, Venter C, Grundy J, Roberts G, Dean T, Arshad SH. Changing prevalence of wheeze, rhinitis and allergic sensitisation in late childhood: findings from 2 Isle of Wight birth cohorts 12 years apart. Clin Exp Allergy 2015;45(9):1430–1438. doi:10.1111/cea.12534.
- Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, Williams H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006;368(9537):733–743. doi:10.1016/s0140-6736(06)69283-0.
- Lai CK, Beasley R, Crane J, Foliaki S, Shah J, Weiland S. Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2009;64(6):476–483. doi:10.1136/thx.2008.106609.
- Zhao J, Bai J, Shen K, Xiang L, Huang S, Chen A, Huang Y, Wang J, Ye R. Self-reported prevalence of childhood allergic diseases in three cities of China: a multicenter study. BMC Public Health 2010;10(1):551. doi:10.1186/1471-2458-10-551.
- Sha L, Shao M, Liu C, Li S, Li Z, Luo Y, Wang Q, Xu C, Zhao J, Ma Y, et al. The prevalence of asthma in children: a comparison between the year of 2010 and 2000 in urban China. Zhonghua Jie He He Hu Xi Za Zhi 2015;38(9):664–668. doi:10.3760/cma.j.issn.1001-0939.2015.09.009.
- Wu L, Luo W, Hu H, Zheng X, Cheng Z, Huang D, Huang X, Zhang H, Liu Y, Zhang R, et al. A multicenter study assessing risk factors and aeroallergens sensitization characteristics in children with self-reported allergic rhinitis in China. J Asthma Allergy 2021;14:1453–1462. doi:10.2147/JAA.S342495.
- Guan K, Zhu W, Sha L, Liu C, Zhao J, Yin J, Chen Y. Prevalence of sensitization to aeroallergens in greater Beijing region children with respiratory allergy. Front Pediatr 2022;10:848357. doi:10.3389/fped.2022.848357.
- Ansotegui IJ, Melioli G, Canonica GW, Caraballo L, Villa E, Ebisawa M, Passalacqua G, Savi E, Ebo D, Gómez RM, et al. IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper. World Allergy Organ J 2020;13(2):100080. doi:10.1016/j.waojou.2019.100080.
- Bousquet J, Heinzerling L, Bachert C, Papadopoulos NG, Bousquet PJ, Burney PG, Canonica GW, Carlsen KH, Cox L, Haahtela T, et al. Practical guide to skin prick tests in allergy to aeroallergens. Allergy 2012;67(1):18–24. doi:10.1111/j.1398-9995.2011.02728.x.
- Singh M, Hays A. Indoor and outdoor allergies. Prim Care 2016;43(3):451–463. doi:10.1016/j.pop.2016.04.013.
- Hu Z, Xue J, Pan M, Bao Y, Zou W, Wang C, Ma J. Prevalence of allergen sensitization among children with allergic rhinitis in Changzhou, China: a retrospective observational study. BMC Pediatr 2023;23(1):466. doi:10.1186/s12887-023-04291-9.
- Li Q, Zhang X, Feng Q, Zhou H, Ma C, Lin C, Wang D, Yin J. Common allergens and immune responses associated with allergic rhinitis in China. J Asthma Allergy 2023;16:851–861. doi:10.2147/JAA.S420328.
- Melén E, Bergström A, Kull I, Almqvist C, Andersson N, Asarnoj A, Borres MP, Georgellis A, Pershagen G, Westman M, et al. Male sex is strongly associated with IgE-sensitization to airborne but not food allergens: results up to age 24 years from the BAMSE birth cohort. Clin Transl Allergy 2020;10(1):15. doi:10.1186/s13601-020-00319-w.
- Phipatanakul W. Allergic rhinoconjunctivitis: epidemiology. Immunol Allergy Clin North Am 2005;25(2):263–281, vi. doi:10.1016/j.iac.2005.03.001.
- Sheehan WJ, Rangsithienchai PA, Baxi SN, Gardynski A, Bharmanee A, Israel E, Phipatanakul W. Age-specific prevalence of outdoor and indoor aeroallergen sensitization in Boston. Clin Pediatr 2010;49(6):579–585. doi:10.1177/0009922809354326.
- Wang X, Guo M, Wang H, Wang X. [Pollen allergen sensitization feature of seasonal allergic rhinitis in children and adolescents in northern China]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2020;34(11):1005–1010. doi:10.13201/j.issn.2096-7993.2020.11.011.
- Pang C, Bian SN, Liu CH, Guo LL, Cui Y, Lin F, Yin X, Liu C, Guan K. [The characteristics and change of aeroallergens in children from 2015 to 2020 in a hospital of pediatric in Beijing]. Zhonghua Yu Fang Yi Xue Za Zhi 2021;55(7):840–846. doi:10.3760/cma.j.cn112150-20210506-00441.
- Chen C, Park T, Wang X, Piao S, Xu B, Chaturvedi RK, Fuchs R, Brovkin V, Ciais P, Fensholt R, et al. China and India lead in greening of the world through land-use management. Nat Sustain 2019;2(2):122–129. doi:10.1038/s41893-019-0220-7.
- Li Z, Heng H, Sun G, Tang R, Gu J, Sun J. Allergy-related airborne pollen in urban area of Beijing. Basic Clin Med 2015;35:734–738.
- Bowatte G, Lodge C, Lowe AJ, Erbas B, Perret J, Abramson MJ, Matheson M, Dharmage SC. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: a systematic review and a meta-analysis of birth cohort studies. Allergy 2015;70(3):245–256. doi:10.1111/all.12561.
- D’Amato G, Chong-Neto HJ, Monge Ortega OP, Vitale C, Ansotegui I, Rosario N, Haahtela T, Galan C, Pawankar R, Murrieta-Aguttes M, et al. The effects of climate change on respiratory allergy and asthma induced by pollen and mold allergens. Allergy 2020;75(9):2219–2228. doi:10.1111/all.14476.
- Yin J, Yue FM, Wang LL, He HJ, Xu T, [Li H, Wen LP, Sun JL, Gu JQ, Han SM, et al. Natural course from rhinitis to asthma in the patients with autumnal pollinosis: a clinical study of 1096 patients. Zhonghua Yi Xue Za Zhi 2006;86:1628–1632. doi:10.3760/j:issn:0376-2491.2006.23.011.
- Katotomichelakis M, Danielides G, Iliou T, Anastassopoulos G, Nikolaidis C, Kirodymos E, Giotakis E, Constantinidis TC. Allergic sensitization prevalence in a children and adolescent population of northeastern Greece region. Int J Pediatr Otorhinolaryngol 2016;89:33–37. doi:10.1016/j.ijporl.2016.07.027.
- Kato M, Yamada Y, Maruyama K, Hayashi Y. Age at onset of asthma and allergen sensitization early in life. Allergol Int 2014;63:23–28. doi:10.2332/allergolint.13-OA-0631.
- Rubner FJ, Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Pappas TE, Gern JE, Lemanske RF. Early life rhinovirus wheezing, allergic sensitization, and asthma risk at adolescence. J Allergy Clin Immunol 2017;139(2):501–507. doi:10.1016/j.jaci.2016.03.049.
- Ibekwe PU, Otu TI, Ekop EE, Bassi PU. Food and aeroallergen sensitization, eosinophils levels and risk of atopic dermatitis in abuja. West Afr J Med 2023;40(11):1216–1222.
- Kaczmarski M, Citko D, Wasilewska J. IgE-dependent sensitization to tropho- and aeroallergens with regard to age, sex and birth season of children and adolescents living in the north-eastern region of Poland. Postepy Dermatol Alergol 2020;37(6):981–985. doi:10.5114/ada.2020.102120.
- Ha EK, Baek JH, Lee SY, Park YM, Kim WK, Sheen YH, Lee SJ, Bae Y, Kim J, Lee KJ, et al. Association of polysensitization, allergic multimorbidity, and allergy severity: a cross-sectional study of school children. Int Arch Allergy Immunol 2016;171(3–4):251–260. doi:10.1159/000453034.
- Ciprandi G, Alesina R, Ariano R, Aurnia P, Borrelli P, Cadario G, Capristo A, Carosso A, Casino G, Castiglioni G, et al. Characteristics of patients with allergic polysensitization: the POLISMAIL study. Eur Ann Allergy Clin Immunol 2008;40:77–83.
- Illi S, Mutius E, Lau S, Niggemann B, Grüber C, Wahn U. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet 2006;368(9537):763–770. doi:10.1016/S0140-6736(06)69286-6.