Abstract
Objective
This study investigated the utilization of nebulized budesonide for acute asthma and COPD exacerbations as well as for maintenance therapy in adults.
Data Sources
We conducted a search on PubMed for nebulized budesonide treatment.
Selected Studies
Selecting all English-language papers that utilize Mesh phrases "asthma," "COPD," "budesonide," "nebulized," "adult," "exacerbation," and "maintenance" without temporal restrictions, and narrowing down to clinical research such as RCTs, observational studies, and real-world studies.
Results
Analysis of 25 studies was conducted to assess the effectiveness of nebulized budesonide in asthma (n = 10) and COPD (n = 15). The panel in Thailand recommended incorporating nebulized budesonide as an additional or alternative treatment option to the standard of care and systemic corticosteroids (SCS) based on the findings.
Conclusion
Nebulized budesonide is effective and well-tolerated in treating asthma and COPD, with less systemic adverse effects compared to systemic corticosteroids. High-dose nebulized budesonide can enhance clinical outcomes for severe and mild exacerbations with slow systemic corticosteroid response. Nebulized budesonide can substitute systemic corticosteroids in some situations.
Introduction
Asthma and chronic obstructive pulmonary disease (COPD) are common, chronic respiratory diseases. (Citation1) Asthma is a heterogeneous clinical syndrome characterized by wheezing, chest tightness, shortness of breath, variable airflow limitation, and airway hyperresponsiveness.(Citation2) Airway inflammation is central to the pathophysiology of asthma and susceptibility to adverse risks, including exacerbations.(Citation3) The Global Initiative for Asthma (GINA) define asthma exacerbations as episodes characterized by a progressive increase in symptoms of shortness of breath, cough, wheezing or chest tightness and a progressive decrease in lung function.(Citation4) Exacerbations are associated with a progressive loss of lung function (Citation5), and patients with a history of exacerbations are at risk of future exacerbations.(Citation6–8) Patients experience exacerbations of varying severity, ranging from mildly increased symptoms to severe attacks, which can lead to respiratory failure and even death.(Citation3,Citation9) Exacerbations may occur at random with no obvious precipitating factors but may also be caused by poor adherence to therapy or triggered by exposure to aeroallergens (e.g. house dust mites, animal fur or pollen), tobacco smoke, air pollution, or viral infection such as rhinovirus and influenza.(Citation10)
COPD is characterized by persistent respiratory symptoms (breathlessness, cough, sputum production) and airflow limitation due to airway and/or alveolar abnormalities caused by significant exposure to noxious particles or gases.(Citation11) COPD is most commonly associated with a history of exposure to tobacco smoke; however, an estimated 25–45% of patients with COPD have never smoked.(Citation12) A substantial number of patients with COPD suffer from an acute exacerbation (AECOPD) which has been defined as an acute worsening of respiratory symptoms that results in the need for additional therapy.(Citation11) Notably, Celli et al. have proposed a new definition of AECOPD as: ‘an event characterized by dyspnea and/or cough and sputum that worsen over ≤14 days, which may be accompanied by tachypnea and/or tachycardia and is often associated with increased local and systemic inflammation caused by airway infection, pollution, or other insult to the airways’.(Citation13) Most AECOPDs are associated with airway infection, with respiratory viruses (most commonly rhinovirus) and alterations in the airway bacterial microbiome – ‘dysbiosis’.(Citation14)
Corticosteroids are by far the most effective therapy for asthma, as they inhibit airway inflammation.(Citation15,Citation16) Asthma guidelines recommend a stepwise approach to achieve disease control according to disease severity. This involves the commencement of an inhaled corticosteroid (ICS) such as budesonide, followed by an increased ICS dose if asthma is not well controlled or the use of ICS in combination with a long-acting ß2-agonist (LABA; typically formoterol) as maintenance and reliever therapy.(Citation4) This approach has been shown to be effective in reducing the annual rate of severe exacerbations in patients with mild asthma. (Citation17) During an acute exacerbation, several guidelines recommend the initiation of systemic corticosteroids (SCS) simultaneously with intermittent dosing of short-acting bronchodilators (short-acting ß2-agonist [SABA]), such as salbutamol with or without short-acting antimuscarinic antagonists [SAMA]. (Citation4) However, although SCS represent the standard of care for asthma exacerbations, there is the fundamental dilemma over SCS in the context of slow onset of anti-inflammatory effects, inevitably resulting in the delayed improvement of pulmonary function that takes considerable time (6–24 h) to emerge produce measurable effects on pulmonary function.(Citation16,Citation18,Citation19) Alternative treatment options for acute asthma in adults, particularly ICS, is of particular interest.(Citation16,Citation18–21) A systematic review of the effectiveness of ICS in treating acute asthma in the emergency department (ED) concluded that there is insufficient evidence that ICS therapy results in clinically important changes in pulmonary function or clinical scores when used in acute asthma in addition to SCS.(Citation22)
The mainstay of the pharmacological approach to reducing AECOPD risk is long-acting bronchodilator therapy, composed of combination LABA and long-acting muscarinic antagonists (LAMA), such as tiotropium, aclidinium, umeclidinium, and glycopyrrolate. Several guidelines indicate that mild AECOPD episodes can be managed with repeated dosing of SABA. However, for AECOPD that fails to respond to SABA treatment, short-course SCS should be introduced.(Citation11,Citation14,Citation23) Extending the duration of SCS use does not provide any additional benefit (Citation14), and conversely increases the risk of adverse effects, including osteoporosis and hyperglycemia, as well as steroid myopathy (Citation24), which can contribute to muscle weakness, decreased functionality, and respiratory failure in patients with severe COPD. (Citation25) In contrast to asthma, the use of ICS in patients with COPD should be restricted to those who are most likely to benefit, in part because ICS is associated with an increased risk of pneumonia.(Citation14) In a study of 123 Spanish patients with AECOPD, domiciliary ICS (budesonide or fluticasone) did not influence the exacerbated airway inflammation or the clinical presentation of hospitalized AECOPD patients.(Citation26) However, these results could not exclude the possibility that ICS may be used as an alternative treatment to SCS for AECOPD patients, for whom the benefits would far outweigh the risks of adverse events.
The rising asthma prevalence and high asthma-related mortality rates in developing countries suggest that improvement in asthma diagnosis and care in these countries represent unmet needs. (Citation27) In Thailand, the prevalence of asthma in adults has been estimated at 3–7%. (Citation28,Citation29) Moreover, a survey of asthma management found that 36% of patients with asthma (>12 years) in Thailand had experienced an exacerbation in the previous year. (Citation30) Similarly, the prevalence of COPD was estimated to be 5.5% within a rural community in central Thailand. (Citation31) Additionally, Pothirat et al. (Citation32) reported that 28.7% of patients with COPD in Thailand had a history of a severe acute exacerbation at least once within the past year in primary care practice; however, only 23.7% of these patients had received regular long-acting bronchodilator therapy in accordance with clinical guidelines. (Citation11)
Nebulized inhalation therapy provides patients with asthma and COPD an alternative administration route that offers ease of use without requirements for forceful inspiration, manual dexterity, or complex hand–breath coordination. With the recent advent of more sophisticated nebulizer devices, treatment via nebulization could be a valuable alternative to handheld inhalation devices, particularly for patients with cognitive, neuromuscular, or ventilatory impairments.(Citation33) However, although there are a number of published articles describing the use of nebulized inhalation therapy in chronic respiratory disease, practical guidance on the use of nebulized ICS for the management of asthma and COPD in clinical practice in Thailand remains scarce. Based on this unmet clinical need, an expert panel was convened to review the literature and develop evidence-based, consensus recommendations on the use of nebulized budesonide for the management of asthma exacerbations and AECOPD in adults in Thailand.
Methods
Expert panel
The expert panel consisted of 13 respiratory physicians (nine pulmonologists, one allergist, and three emergency department [ED] consultants) who were treating patients with asthma and COPD in tertiary medical centers in Thailand. Panel members were invited based on their expertise in research and clinical practice in asthma and COPD.
Literature search strategy and inclusion criteria
The PubMed database (https://pubmed.ncbi.nlm.nih.gov/) was used to identify published studies that evaluated nebulized budesonide in adults with asthma or COPD. Searches included the keywords “asthma,” “COPD,” “budesonide,” “nebulized,” “adult,” “exacerbation,” and “maintenance”, and were carried out without any time filters. Included studies were restricted to clinical studies, including randomized controlled trials (RCTs), observational studies, and real-world studies, that were published in English, and that assessed the efficacy or effectiveness of nebulized budesonide either as maintenance therapy or in the management of acute exacerbations in adults with asthma or COPD. Case studies and review articles were excluded from the search results.
Expert panel consensus-based recommendations
The results of the literature searches were considered independently by each member of the expert panel. A panel meeting was conducted to discuss the published evidence and develop expert recommendations for clinical practice. Committee members were jointly selected by AstraZeneca and a group of respiratory advisors who had worked collaboratively in the Medical Association in Thailand as guideline committee members and invited based on a relevant publication history, their clinical experience and expertise in asthma and COPD. A narrative review of published studies on nebulized budesonide in adults with asthma and COPD were undertaken to evaluate the evidence and develop expert recommendations through panel discussions for implementation in clinical practice through consensus statement. Consensus-based recommendations were developed during two rounds of panel meetings held on 15 December, 2021 and 27 April, 2022. At both panel meetings, an informed facilitator encouraged the sharing and discussion of reasons for the recommendations made by panel members. A consensus recommendation was defined a priori as a recommendation agreed by the majority (≥7 of 13) of panel members. Opinions and responses from panelists were collated and developed into practical treatment recommendations pertaining to the use of nebulized budesonide for the management of asthma exacerbations and AECOPD in adults. Recommendations were classified as strong or conditional in accordance with Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology. A strong recommendation was one for which the panel had a high level of confidence that the beneficial effects of adherence to the recommendation outweighed the potential detrimental effects. A conditional recommendation was one for which the panel concluded that the beneficial effects probably outweigh the potential detrimental effects, but the panel did not feel confident about making such a recommendation. Reasons for this lack of confidence included the absence of high-quality evidence and small sample sizes. (Citation34,Citation35)
Results
Overview of published studies on the use of nebulized budesonide for the management of asthma in adults
A total of 25 studies were identified by the search, of which 10 met the inclusion criteria. (, ). Otulana et al. (Citation36) conducted a small, proof-of-principle, open-label trial of nebulized budesonide in 18 patients with chronic steroid-dependent asthma over 12–18 months. After administration of a daily dose of 4–8 mg nebulized budesonide, 14 patients successfully stopped SCS and the SCS dose was reduced in three patients; one patient failed to respond. Overall, there was an increase in mean forced expiratory volume in 1 s (FEV1) and mean peak expiratory flow (PEFR), and a significant decrease in the mean annual number of hospital admissions. Ediger et al. (Citation37) compared the effects of nebulized budesonide and SCS on pulmonary function and respiratory symptom scores in 30 adult patients with acute asthma exacerbations during the study period of 7-days. Patients were randomized into three groups: 4 mg/day budesonide (group I), 1 mg/kg/day methylprednisolone (group II), and budesonide plus methylprednisolone (group III). Spirometry significantly improved in all groups at day 7, with no difference observed between the groups. FEV1% levels increased significantly at day 1 in the budesonide groups (group I and III) (p < 0.05) but did not change until day 5 in group II. Mean symptom scores decreased significantly by day 2 in group I (p < 0.05), and at day 4 in the other two groups. In conclusion, this study suggested that FEV1% and asthma symptoms improved at a faster rate with budesonide therapy than with SCS. Shah et al. (Citation38) used a gamma camera and radiolabelled aerosol to examine the effects of nebulized budesonide versus SCS on mucociliary clearance in outpatients with asthma. In this small study (n = 13), budesonide treatment did not affect mucociliary clearance or FEV1 whereas SCS treatment was associated with a significant improvement in both parameters. Murphy et al. (Citation39) conducted a large (n = 758), 12-week RCT to compare the efficacy of four different doses of nebulized budesonide (0.5 mg once daily, 1 mg once daily, 1 mg twice daily and 2 mg twice daily) in adolescents and adults with persistent asthma who transitioned from ICS delivered via a dry powder inhaler (DPI) or a pressurized metered-dose inhaler (pMDI). Budesonide 400 µg twice daily administered via a DPI was the active control arm. There was no significant difference in mean change from baseline in FEV1 between the nebulized budesonide 0.5 mg once daily and 2 mg twice daily groups. All treatments were well tolerated. Overall, all nebulized budesonide dosages elicited similar responses for variables associated with asthma control. Chian et al. (Citation40) retrospectively evaluated the efficacy of a five-day course of budesonide inhalation suspension compared with SCS (oral prednisolone) in adults with mild to severe acute asthma exacerbations in Taiwan. Improvements in PEFR, FEV1, and daytime symptom scores were similar in response to the two different treatments, indicating that budesonide inhalation suspension is an alternative treatment of acute asthma exacerbation in adults. Vogelmeier et al. (Citation41) demonstrated that nebulized budesonide as add-on therapy to GINA step 5 had steroid-sparing effects in oral steroid-dependent patients with asthma while improving pulmonary function and maintaining exacerbation control. Sheikh-Motahar-Vahedi et al. (Citation42) reported the results of a small, placebo-controlled clinical trial designed to evaluate the effectiveness of nebulized budesonide versus standard treatment in the management of moderate-to-severe acute asthma attacks in 41 ED patients in Iran. Heart rate, respiratory rate and O2 saturation in the budesonide group were significantly improved in comparison with the standard treatment group, indicating that nebulized budesonide might be effective in reducing respiratory distress in adults suffering with acute asthma attack. In a retrospective study of 98 patients admitted to hospital with severe asthma exacerbations, Ito et al. (Citation43) reported that treatment with nebulized budesonide plus LABA (procaterol) resulted in shorter length of stay, faster recovery from symptoms, and reduced hospitalization costs versus LABA alone. Marghli et al. (Citation44) performed a RCT to assess the effect of high and repeated doses of nebulized budesonide combined with standard treatment (intravenous hydrocortisone) in 50 adult patients with acute asthma in the ED setting. Overall, it suggested that the addition of nebulized budesonide to systemic corticosteroids had no additive benefit over treatment with hydrocortisone alone for adult asthmatic attacks. Nevertheless, the conclusion of this study was drawn on the basis of its limited power.
Figure 1. PRISMA diagrams summarizing the literature search process for asthma (A) and COPD (B) studies. DPI: dry powder inhaler; pMDI: pressurized metered dose inhaler; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
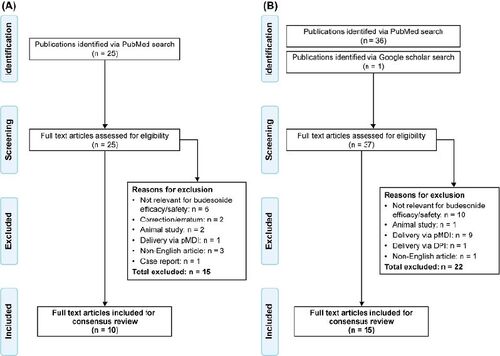
Table 1. Clinical studies assessing the efficacy and/or safety of nebulized budesonide in adults with asthma.
Overview of published studies on the use of nebulized budesonide for the management of AECOPD in adults
A total of 36 studies were identified, of which 15 met the inclusion criteria (, ). Morice et al. (Citation45) had conducted a small randomized parallel-group trial of 19 adults with severe AECOPD designed to compare the efficacy and safety between nebulized budesonide (2 mg twice daily) and oral prednisolone (30 mg once daily). Over the five days of the study, improvement in baseline FEV1 was comparable in both groups. However, biochemical markers associated with SCS-induced osteoporosis (serum osteocalcin, urinary calcium to creatinine ratio) were significantly lower in patients treated with budesonide than in those who received prednisolone. Maltais et al. (Citation46) conducted RCT and reported the efficacy of nebulized budesonide, oral prednisolone, and placebo in hospitalized 199 patients with acute severe exacerbations of COPD. There was no significant differences in FEV1 improvement between budesonide and prednisolone, and both treatments improved airflow limitation to a similar degree compared with placebo. In addition, it found no different onset of action between nebulized budesonide and oral prednisolone. In another RCT comparing the efficacy and safety of nebulized budesonide with SCS (n = 40), Mirici et al. (Citation47) showed that there were no significant differences between both treatment groups in terms of PEFR, arterial partial pressures of O2 and CO2, pH, and oxygen saturation. Gunen et al. (Citation48) reported the efficacy of three different treatment strategies in hospitalized patients with AECOPD (n = 159) who were randomly assigned to three groups: group 1 received standard bronchodilator treatment (SBDT) only; group 2 received SCS plus SBDT; and group 3 received nebulized budesonide plus SBDT. They found that arterial blood gases and spirograms improved faster in groups 2 and 3, whereas blood glucose levels exhibited an upward trend only in group 2. Ucar et al. (Citation49) compared the efficacy and safety of nebulized budesonide (4 mg and 8 mg) with SCS in 86 hospitalized patients suffering from AECOPD in Turkey. There were no significant differences between the groups for all parameters assessed. In addition, the overall occurrence of adverse events was not statistically significant between the groups. It indicated that nebulized budesonide in both doses were as effective as SCS in the management of AECOPD. Sun et al. (Citation50) compared the efficacy of inhaled budesonide and SCS on systemic inflammation in 30 AECOPD patients in China. Improvements in symptoms, pulmonary function, and blood gases in both treatment groups were similar; however, the incidence of adverse events was lower in the budesonide group. Ding et al. (Citation51) reported the consistent results in 410 AECOPD patients in China in the context of the equivalent efficacy but higher safety profile of nebulized budesonide treatment. Jiang et al. (Citation52) investigated the combined effects of ipratropium bromide (a short-acting muscarinic antagonist) plus budesonide suspension delivered via ventilator mask atomization inhalation versus placebo on circulating levels of inflammatory factors and pulmonary function in 86 patients with AECOPD. It demonstrated that the combination treatment with ipratropium bromide and budesonide suspension significantly reduced serum levels of inflammatory markers, improved lung function and abnormal arterial blood gases, and shortened total treatment time in patients with AECOPD. Hashemian et al. (Citation53) investigated the effect of nebulized budesonide on the release of pro-inflammatory cytokines and time-to-wean off mechanical ventilation in COPD stage 4 patients. Budesonide significantly reduced the number of days on mechanical ventilation, and decreased bronchoalveolar lavage CXCL-8 and IL-6 levels versus placebo. Dynamic compliance was also significantly improved in the budesonide group versus placebo. Zheng et al. (Citation54) conducted post-hoc analysis of a subset of data collected from a large, retrospective non-interventional study (n = 3121) to demonstrate clinical outcomes of AECOPD patients treated with nebulized budesonide or SCS. Initial treatment of AECOPD with either nebulized budesonide or SCS resulted in similar outcomes and improvements in FEV1 and arterial blood gases. The authors concluded that nebulized budesonide can be used for initial treatment of AECOPD in certain patients who had lower initial PaCO2. Zhang et al. (Citation55) compared the efficacy and safety of different doses of nebulized budesonide (4 mg budesonide/day [1 mg every 6 h]; 8 mg budesonide/day [2 mg every 6 h]; 8 mg budesonide/day [4 mg every 12 h]) in the management of AECOPD. Overall, high dose budesonide (8 mg/day) improved pulmonary function and symptoms more effectively than the conventional dose of budesonide (4 mg/day) in the early treatment of moderate-to-severe AECOPD, especially when given as 4 mg twice daily. However, no significant differences in adverse effects, hospital stay length, or frequency of exacerbations within 3 months of discharge were observed. A real-world study conducted by Chen et al. (Citation56) was designed to compare the effectiveness of nebulized budesonide, SCS, and nebulized budesonide plus SCS in Chinese patients with AECOPD. Following propensity score matching, budesonide plus SCS was associated with a greater length of hospital stay and greater hospitalization costs than the other two treatments in patients without respiratory failure. This study demonstrated that nebulized budesonide provided the same effectiveness as SCS, indicating that nebulized budesonide may be used as an alternative therapeutic option for the treatment of AECOPD. The study by Aghili et al. (Citation57) aimed to compare the effectiveness of nebulized budesonide versus oral prednisolone in increasing PEFR in 84 AECOPD patients in the ED setting in Iran. The data showed that nebulized budesonide was more effective than oral prednisolone in improving PEFR in patients with AECOPD during the initial 24 h of treatment. Nguyen et al. (Citation58) evaluated clinical outcomes and costs of nebulized budesonide plus SCS combination therapy versus SCS monotherapy for the treatment of AECOPD. Daily SCS dose increases, progression in ventilatory support, in-hospital mortality, and COPD 30-day readmissions were all similar in the two groups. However, patients in the combination therapy arm had significantly longer lengths of hospital stay. This study showed no significant differences in clinical outcomes between the combined treatment with nebulized budesonide and SCS, and SCS monotherapy, suggesting that the latter treatment may be more cost-effective. Gu et al. (Citation59) assessed the cost-effectiveness of nebulized budesonide and intravenous methylprednisolone in the treatment of AECOPD in China. The authors found no statistically significant difference between the budesonide and methylprednisolone groups in clinical efficacy rates (94.6% vs 93.7%, respectively). However, the higher costs associated with nebulized budesonide indicated that it may not a cost-effective strategy for AECOPD patients in China.
Table 2. Clinical studies assessing the efficacy and/or safety of nebulized budesonide in adults with COPD.
Expert panel consensus recommendations
Asthma exacerbations in hospital
High-dose nebulized budesonide (1 mg every 20 minutes within first hour) plus SABA bronchodilator. This can be added on to standard therapy for the treatment of asthma exacerbations under the following circumstances:
I. Mild exacerbations not responding to initial treatment with SCS (conditional recommendation, low quality evidence)
II. Moderate-to-severe exacerbations with impending respiratory failure (conditional recommendation, low quality evidence)
Patient response to budesonide should be reviewed after 2–3 hours or earlier, depending on clinical urgency (strong recommendation, low quality evidence)
Asthma exacerbations at home
Nebulized budesonide can be co-administrated with SABA bronchodilators in one nebulization session. If symptoms persist after 3 sessions of nebulized budesonide plus bronchodilator, patients must go to the hospital for appropriate management of their worsening symptoms. However, if their symptoms improve, the patient’s current asthma treatment should be continued together with oral corticosteroids (conditional recommendation, low quality evidence).
Stable asthma
Nebulization provides an alternative drug delivery method for patients who are unable to correctly use a DPI or pMDI due to impaired cognitive function, movement disorders or manual dexterity problems. For these patients, the recommended starting dose of nebulized budesonide is 0.25 mg twice daily or 0.5 mg once daily (can be titrated up to a maximum dose of 4 mg/day) (conditional recommendation, low quality evidence)
AECOPD in hospital
In patients with severe AECOPD with impending respiratory failure, high-dose nebulized budesonide (4–8 mg daily [2–4 administrations per day]) may be added to SCS until clinical improvement (no longer than 10 days) (conditional recommendation, high quality evidence).
In patients with mild AECOPD who have a slow response to SCS, the recommended dose of nebulized budesonide is 1–2 mg every 12 hours for 3–7 days (conditional recommendation, low quality evidence)
High-dose nebulized budesonide may be considered as an alternative to SCS in hospitalized patients with AECOPD under the following circumstances:
Patients at high risk for adverse effects of SCS, such as hyperglycemia or serious infection, including pneumonia or sepsis (conditional recommendation, high quality evidence)
Patients with an uncertain diagnosis of AECOPD, especially when occult infection is a possible diagnosis (conditional recommendation, low quality evidence)
Patients with hyperglycemia or serious infection, including pneumonia or sepsis (strong recommendation, high quality evidence)
Patients with comorbidities such as bronchiectasis, osteoporosis or poorly controlled diabetes, cardiovascular disease, and uncontrolled infection are suitable for nebulized budesonide as an alternative treatment to SCS in AECOPD patients (strong recommendation, high quality evidence).
Patient response to budesonide should be reviewed after 2–3 hours or earlier, depending on clinical urgency (strong recommendation, low quality evidence)
AECOPD at home
COPD patients with worsening symptoms can be caused by many possible differential diagnoses, including AECOPD. In addition, triple therapy (ICS + LAMA + LABA) has usually been prescribed in these patients. These patients should go directly to hospital for appropriate management of their worsening symptoms rather than self-management at home (conditional recommendation, low quality evidence).
Stable COPD
Nebulization provides an alternative method for drug delivery in COPD patients who require treatment with ICS. This includes, but is not limited to, patients with frequent exacerbations, and those unable to correctly use a DPI or pMDI. The recommended nebulized budesonide dose for these patients is 0.5–1 mg/day plus add-on dual SABA/LAMA bronchodilator therapy (conditional recommendation, low quality evidence)
Asthma and COPD patients with corona virus disease 2019 (COVID-19)
Asthma and COPD patients who may benefit from treatment with nebulization may continue to use this technique as long as standard COVID-19 safety measures are in force.
In the outpatient setting, including the ED, nebulization should be performed in a negative pressure room/tent or a zone separated by a distance of at least 2 meters from adjacent areas (strong recommendation, high quality evidence).
In the inpatient setting, including an intensive care unit, nebulization should be performed in patients who have a negative polymerase chain reaction test result for COVID-19. In the case of patients who test positive for COVID-19, collaborative discussion among healthcare practitioners regarding the risks and benefits of nebulization for each patient is recommended. Standard COVID-19 safety measures should be applied during nebulization sessions (strong recommendation, high quality evidence).
In the home setting, nebulization should be performed in a separated zone, by an educated caregiver to ensure maximal nebulization benefits whilst maintaining standard infection control precautions (strong recommendation, high quality evidence).
Discussion
Herein, we have developed evidence-based, consensus recommendations on the use of nebulized budesonide for the management of adults with asthma and COPD during exacerbations and stable disease in Thailand. Nebulized budesonide is not a new treatment: the first approval of nebulized budesonide for use in asthma occurred over 30 years ago in 1992. (Citation60) Budesonide was the first topical corticosteroid selected for improved local selectivity, and its rapid uptake into tissue made it a good prospect for inhalation therapy, both of which were the basis for its development for asthma and ultimate use in the treatment of COPD. (Citation60) Budesonide’s unique pharmacokinetic/pharmacodynamic properties promote a better balance between efficacy and safety than many of the newer ICSs, such as fluticasone, ciclesonide, and mometasone. (Citation60) Moreover, budesonide is less lipophilic, and therefore more water soluble, than most of the other ICSs (Citation61,Citation62), resulting in a shorter dissolution time in human bronchial fluid (Citation62,Citation63). This allows budesonide to enter lung tissues quickly, thereby minimizing removal from the airways by mucociliary clearance or phagocytosis. (Citation62,Citation64,Citation65) In addition, a shorter dissolution time may reduce bronchial immunosuppression, which could lower the risk of pneumonia compared with other ICSs, given the impaired mucociliary clearance and altered lung microbiome commonly observed in COPD. (Citation66,Citation67) Budesonide’s moderate lipophilicity is associated with a shorter elimination half-life (Citation68) and a smaller volume of distribution than that of other ICSs, contributing to lower systemic exposure and a reduced risk of adverse events. (Citation63,Citation69,Citation70)
After absorption into airway tissues, a proportion of budesonide undergoes intracellular esterification to produce inactive, highly lipophilic fatty acid esters (mainly budesonide-21-oleate). (Citation71) These budesonide esters are hydrolyzed by lipases as intracellular levels of free budesonide fall, releasing the active drug to interact with the glucocorticoid receptor in a ‘sustained-release’ mechanism within airway tissues that prolongs drug residence time at the intracellular target level. (Citation72,Citation73)
The first paper on the efficacy of inhaled budesonide in the treatment of asthma was published in 1980 by Ellul-Micallef et al. (Citation74) This was a small study of 12 patients with chronic asthma in which inhaled budesonide produced a statistically significant increase in PEF 2 h after being administered. Since that study, an abundance of data on the efficacy and safety of budesonide in asthma have been published. A subsequent keynote study by Haahtela et al., published in the New England Journal of Medicine in 1991, compared budesonide 600 μg twice daily with SABA terbutaline 375 μg twice daily over 2 years in patients whose asthma had appeared within the previous year. Results from this study demonstrated that FEV1 PEF, and asthma symptoms all showed greater improvement with budesonide compared with terbutaline; the budesonide group also required less ‘rescue’ therapy with terbutaline. These results were important early evidence in supporting the use of budesonide in newly detected asthma. (Citation75) Another important study, published in 1998, investigated the dose-dependent nature of the clinical efficacy of budesonide in a 12-week study of patients with moderate-to-severe asthma. Budesonide was administered via DPI twice daily at a total dose of 200, 400, 800, or 1600 μg. The effects on PEF and FEV1 were significantly greater with the 1600 μg dose than with the 200-μg dose; however, results with the 1600 μg dose were not significantly greater than those of the 400 μg and 800 μg doses. (Citation76) A subsequent meta-analysis of the dose-response relationship of inhaled budesonide in adult asthma concluded that in adolescents and adults with mild-to-moderate asthma, most of the therapeutic benefit of budesonide (delivered by DPI or pMDI) is achieved with a dose of approximately 400 μg per day and the maximum effect is achieved at approximately 1000 μg per day. (Citation77)
In the current study, published studies on the use of nebulized budesonide for the management of asthma indicate that nebulized budesonide provides similar benefits to SCS and is therefore an effective alternative to SCS for the treatment of acute asthma exacerbations in adults. For stable asthma, the recommended starting dose of nebulized budesonide is 0.25 mg twice daily or 0.5 mg once daily (which can be titrated up to a maximum dose of 4 mg/day). For hospital treatment of asthma exacerbations, we recommend high-dose nebulized budesonide (1 mg every 20 min within first hour) plus SABA bronchodilator therapy adjunctive to standard therapy.
The first paper on the efficacy of inhaled budesonide in the treatment of COPD was published in 1995 by Weiner et al. who reported that treatment with high-dose inhaled budesonide (800 μg per day) improved spirometry data and inhaled SABA consumption in approximately 25% of patients with stable COPD; this rate increased to around 75% in patients who were responders to beta 2-agonist inhalation. (Citation78) Two separate meta-analyses of the comparative efficacy of nebulized budesonide and SCS in the treatment of AECOPD both reported that nebulized budesonide (4–8 mg per day) was noninferior to SCS in the treatment of ACOPD. (Citation79,Citation80) With regard to safety outcomes, the Pleasants et al. meta-analysis (Citation79) found that the incidence of hyperglycemia was significantly less frequent with high-dose nebulized budesonide than with SCS (p = 0.002). These results supported guidelines that recommend nebulized budesonide therapy as an effective alternative to SCS for the management of AECOPD. (Citation11)
The current literature review, which identified 15 AECOPD studies, is consistent with the above meta-analyses. Overall, these studies indicate that nebulized budesonide is an effective and safe alternative to SCS in the management of AECOPD. For stable COPD, the recommended nebulized budesonide dose is 0.5–1 mg/day plus add-on dual SABA/LAMA bronchodilator therapy. For severe AECOPD, high-dose nebulized budesonide (4–8 mg daily [2–4 administrations per day] should be administered until clinical improvement for no longer than 10 days. In patients with mild AECOPD who have a slow response to SCS, the recommended dose of nebulized budesonide is 1–2 mg every 12 h for 3–7 days.
Budesonide like other ICS, while beneficial for therapy, can lead to significant local side effects such as throat irritation, oropharyngeal candidiasis, and hoarseness/dysphonia.(Citation81,Citation82) Oropharyngeal candidiasis is mostly caused by the deposition of inhaled corticosteroids in the upper respiratory tract, resulting in symptoms such as sore throat and hoarseness. (Citation81) Proper inhalation methods and rinsing after using ICS can help decrease the amount of ICS deposited in the upper airways, therefore lowering the risk of oropharyngeal candidiasis. Systemic exposure to ICS, albeit minimal, may provide potential concerns for aberrant growth and osteoporosis with increased dosage. (Citation81,Citation83) The growth retardation induced by ICS has a time-dependent pattern, with a greater degree observed at the onset of treatment and diminishing as treatment progresses. (Citation84) Nonetheless, adult height was unaffected by long-term budesonide treatment, in contrast to poorly managed asthma. (Citation84,Citation85)
Nebulization inhalation therapy improves drug distribution to smaller airways but adherence to usage precautions is necessary based on patient and nebulization inhalation device features. (Citation86) The efficacy of nebulizer utilization in inhalation treatment is contingent upon the patient’s cognitive and cooperative skills. Appropriate inhalation performance including taking non-coercive long breaths in and holding can enhance the deposition of aerosol particles in the lower airway. (Citation86) If the patient is physically able, they should hold the nebulizer upright. If unable to lie flat, one can try lying on their side and elevating the head of the bed by 30° to help lower the diaphragm, improve chest expansion, and boost the treatment’s effectiveness. Before starting nebulization inhalation therapy, it is crucial to effectively remove sputum and atelectasis by promoting expectoration in order to improve the distribution of aerosol to the lower respiratory tract. Patients may experience adverse effects during nebulization inhalation, including dry mouth, nausea, dizziness, hand numbness, severe coughing, chest tightness, shortness of breath, palpitations, dyspnea, decreased blood oxygen saturation (especially in patients with CO2 retention), and skin and mucous membrane damage, such as at the corners of the mouth due to nebulizer chafing abrasion. (Citation86) Adverse responses may occur due to the drug’s direct impact or be linked to hyperventilation, requiring assessment and care. After use, nebulizers, breathing tubes, and nebulizing masks should be cleaned promptly according to the manufacturer’s instructions. Each set should be assigned to a specific patient to avoid aerosol-related infections. (Citation86)
In light of the ongoing coronavirus (COVID-19) pandemic, there is an urgent need to understand the risk of viral aerosol transmission during nebulizer treatment of asthma/COPD patients infected with respiratory viruses. Goldstein et al. (Citation87) performed a systematic literature search with the aim of assessing the risk of transmitting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), severe acute respiratory syndrome (SARS), Middle East Respiratory Syndrome (MERS), and influenza by administration of drugs via a nebulizer. Overall, no specific evidence was identified that exposure to nebulizer treatment increases transmission of coronaviruses similar to COVID-19. The authors concluded that clinical treatment decisions that utilize nebulizers should be driven by the most appropriate type of nebulizer for a given patient and the safest mode of medication delivery for exposed healthcare workers and patient caregivers. Additional studies quantifying viral aerosolization from a variety of nebulizer delivery devices are clearly warranted.
Conclusions
Nebulized inhalation therapy provides patients with asthma and COPD an alternative administration route that offers ease of use with no requirements for forceful inspiration, manual dexterity, or complex hand–breath coordination. Nebulized budesonide is an effective and well-tolerated treatment option for the management of asthma and COPD, in accordance with GINA and GOLD guidelines, with considerations for adverse reactions and usage precautions. The addition of high-dose nebulized budesonide to systemic corticosteroids can improve clinical outcomes both in patients with severe exacerbation with impending respiratory failure and in patients with mild exacerbations who have a slow response to initial SCS therapy.
Acknowledgments
Medical writing and editorial support was provided by Fernando Gibson, PhD, of Cactus Life Sciences, part of Cactus Communications, Mumbai, India, in accordance with Good Publication Practice guidelines (https://www.ismpp.org/gpp-2022), and was fully funded by AstraZeneca.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data-sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli could be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca/.
Additional information
Funding
References
- World Health Organization. Chronic respiratory diseases. https://www.who.int/health-topics/chronic-respiratory-diseases#tab=tab_1
- Holgate ST, Wenzel S, Postma DS, Weiss ST, Renz H, Sly PD. Asthma. Nat Rev Dis Primers. 2015;1(1):15025. doi:10.1038/nrdp.2015.25.
- McIntyre A, Busse WW. Asthma exacerbations: the Achilles heel of asthma care. Trends Mol Med. 2022;28(12):1112–1127. doi:10.1016/j.molmed.2022.09.001.
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention. 2023. https://ginasthma.org/wp-content/uploads/2023/05/GINA-2023-Full-Report-2023-WMS.pdf.
- Soremekun S, Heaney LG, Skinner D, Bulathsinhala L, Carter V, Chaudhry I, Hosseini N, Eleangovan N, Murray R, Tran TN, et al. Asthma exacerbations are associated with a decline in lung function: a longitudinal population-based study. Thorax. 2023;78(7):643–652. doi:10.1136/thorax-2021-217032.
- Ansari SF, Memon M, Kumar R, Rizwan A. Risk factors associated with frequent acute exacerbations of asthma. Cureus. 2020;12(10):e11090. doi:10.7759/cureus.11090.
- DiMango E, Rogers L, Reibman J, Gerald LB, Brown M, Sugar EA, Henderson R, Holbrook JT. Risk factors for asthma exacerbation and treatment failure in adults and adolescents with well-controlled asthma during continuation and step-down therapy. Ann Am Thorac Soc. 2018;15(8):955–961. doi:10.1513/AnnalsATS.201711-886OC.
- Sims EJ, Price D, Haughney J, Ryan D, Thomas M. Current control and future risk in asthma management. Allergy Asthma Immunol Res. 2011;3(4):217–225. doi:10.4168/aair.2011.3.4.217.
- Bourdin A, Bjermer L, Brightling C, Brusselle GG, Chanez P, Chung KF, Custovic A, Diamant Z, Diver S, Djukanovic R, et al. ERS/EAACI statement on severe exacerbations in asthma in adults: facts, priorities and key research questions. Eur Respir J. 2019;54(3):1900900. doi:10.1183/13993003.00900-2019.
- Elsey L, Allen D. Management of acute exacerbations of airways disease: advice for the non-respiratory physician. Clin Med. 2021;21(6):e567–e70. doi:10.7861/clinmed.2021-0649.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for Prevention, Diagnosis and Management of COPD. 2024. https://goldcopd.org/2024-gold-report/.
- Ruvuna L, Sood A. Epidemiology of Chronic Obstructive Pulmonary Disease. Clin Chest Med. 2020;41(3):315–327. doi:10.1016/j.ccm.2020.05.002.
- Celli BR, Fabbri LM, Aaron SD, Agusti A, Brook R, Criner GJ, Franssen FME, Humbert M, Hurst JR, O’Donnell D, et al. An updated definition and severity classification of chronic obstructive pulmonary disease exacerbations: the rome proposal. Am J Respir Crit Care Med. 2021;204(11):1251–1258. doi:10.1164/rccm.202108-1819PP.
- Jeyachandran V, Hurst JR. Advances in chronic obstructive pulmonary disease: management of exacerbations. Br J Hosp Med (Lond). 2022;83(7):1–7. doi:10.12968/hmed.2022.0275.
- Barnes PJ. How corticosteroids control inflammation: quintiles Prize Lecture 2005. Br J Pharmacol. 2006;148(3):245–254. doi:10.1038/sj.bjp.0706736.
- Barnes PJ, Adcock IM. How do corticosteroids work in asthma? Ann Intern Med. 2003;139(5 Pt 1):359–370. doi:10.7326/0003-4819-139-5_part_1-200309020-00012.
- O’Byrne PM, FitzGerald JM, Bateman ED, Barnes PJ, Zhong N, Keen C, Jorup C, Lamarca R, Ivanov S, Reddel HK. Inhaled combined budesonide-formoterol as needed in mild asthma. N Engl J Med. 2018;378(20):1865–1876. doi:10.1056/NEJMoa1715274.
- Rodrigo G, Rodrigo C. Corticosteroids in the emergency department therapy of acute adult asthma: an evidence-based evaluation. Chest. 1999;116(2):285–295. doi:10.1378/chest.116.2.285.
- Rowe BH, Spooner C, Ducharme FM, Bretzlaff JA, Bota GW. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2001;(1):CD002178. doi:10.1002/14651858.CD002178.
- Afilalo M, Guttman A, Colacone A, Dankoff J, Tselios C, Stern E, Wolkove N, Kreisman H. Efficacy of inhaled steroids (beclomethasone dipropionate) for treatment of mild to moderately severe asthma in the emergency department: a randomized clinical trial. Ann Emerg Med. 1999;33(3):304–309. doi:10.1016/s0196-0644(99)70367-7.
- Rodrigo G, Rodrigo C. Inhaled flunisolide for acute severe asthma. Am J Respir Crit Care Med. 1998;157(3 Pt 1):698–703. doi:10.1164/ajrccm.157.3.9704022.
- Edmonds ML, Milan SJ, Camargo CA, Jr., Pollack CV, Rowe BH. Early use of inhaled corticosteroids in the emergency department treatment of acute asthma. Cochrane Database Syst Rev. 2012;12(12):CD002308. doi:10.1002/14651858.CD002308.pub2.
- National Institute for Health and Care Excellence (NICE). Chronic obstructive pulmonary disease in over 16s: diagnosis and management. https://www.nice.org.uk/guidance/ng115.
- Stanbury RM, Graham EM. Systemic corticosteroid therapy–side effects and their management. Br J Ophthalmol. 1998;82(6):704–708. doi:10.1136/bjo.82.6.704.
- Decramer M, Lacquet LM, Fagard R, Rogiers P. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med. 1994;150(1):11–16. doi:10.1164/ajrccm.150.1.8025735.
- Crisafulli E, Guerrero M, Menéndez R, Huerta A, Martinez R, Gimeno A, Soler N, Torres A. Inhaled corticosteroids do not influence the early inflammatory response and clinical presentation of hospitalized subjects with COPD exacerbation. Respir Care. 2014;59(10):1550–1559. doi:10.4187/respcare.03036.
- Trikamjee T, Comberiati P, Peter J. Pediatric asthma in developing countries: challenges and future directions. Curr Opin Allergy Clin Immunol. 2022;22(2):80–85. doi:10.1097/ACI.0000000000000806.
- Boonsawat W, Charoenphan P, Kiatboonsri S, Wongtim S, Viriyachaiyo V, Pothirat C, Thanomsieng N. Survey of asthma control in Thailand. Respirology. 2004;9(3):373–378. doi:10.1111/j.1440-1843.2004.00584.x.
- Dejsomritrutai W, Nana A, Chierakul N, Tscheikuna J, Sompradeekul S, Ruttanaumpawan P, Charoenratanakul S. Prevalence of bronchial hyperresponsiveness and asthma in the adult population in Thailand. Chest. 2006;129(3):602–609. doi:10.1378/chest.129.3.602.
- Boonsawat W, Thompson PJ, Zaeoui U, Samosorn C, Acar G, Faruqi R, Poonnoi P. Survey of asthma management in Thailand - the asthma insight and management study. Asian Pac J Allergy Immunol. 2015;33(1):14–20. doi:10.12932/AP0473.33.1.2015.
- Kitjakrancharoensin P, Yasan K, Hongyantarachai K, Ratanachokthorani K, Thammasarn J, Kuwuttiwai D, Ekanaprach T, Jittakarm R, Nuntapravechpun R, Hotarapavanon S, et al. Prevalence and risk factors of chronic obstructive pulmonary disease among agriculturists in a rural community, Central Thailand. Int J Chron Obstruct Pulmon Dis. 2020;15:2189–2198. doi:10.2147/COPD.S262050.
- Pothirat C, Phetsuk N, Deesomchok A, Theerakittikul T, Bumroongkit C, Liwsrisakun C, Inchai J. Clinical characteristics, management in real world practice and long-term survival among COPD patients of Northern Thailand COPD club members. J Med Assoc Thai. 2007;90(4):653–662. Cited: in:: PMID: 17487118.
- Barjaktarevic IZ, Milstone AP. Nebulized therapies in COPD: past, present, and the future. Int J Chron Obstruct Pulmon Dis. 2020;15:1665–1677. doi:10.2147/COPD.S252435.
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, et al. GRADE guidelines: 3. rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi:10.1016/j.jclinepi.2010.07.015.
- Andrews JC, Schünemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, Rind D, Montori VM, Brito JP, Norris S, et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation’s direction and strength. J Clin Epidemiol. 2013;66(7):726–735. doi:10.1016/j.jclinepi.2013.02.003.
- Otulana BA, Varma N, Bullock A, Higenbottam T. High dose nebulized steroid in the treatment of chronic steroid-dependent asthma. Respir Med. 1992;86(2):105–108. doi:10.1016/s0954-6111(06)80224-6.
- Ediger D, Coşkun F, Kunt Uzaslan E, Gürdal Yüksel E, Karadağ M, Ege E, Gözü O. Clinical effectiveness of nebulised budesonide in the treatment of acute asthma attacks. Tuberk Toraks. 2006;54(2):128–136. Cited: in:: PMID: 16924568.
- Shah RV, Amin M, Sangwan S, Smaldone GC. Steroid effects on mucociliary clearance in outpatient asthma. J Aerosol Med. 2006;19(2):208–220. doi:10.1089/jam.2006.19.208.
- Murphy K, Noonan M, Silkoff PE, Uryniak T. A 12-week, multicenter, randomized, partially blinded, active-controlled, parallel-group study of budesonide inhalation suspension in adolescents and adults with moderate to severe persistent asthma previously receiving inhaled corticosteroids with a metered-dose or dry powder inhaler. Clin Ther. 2007;29(6):1013–1026. doi:10.1016/j.clinthera.2007.06.005.
- Chian CF, Tsai CL, Wu CP, Chiang CH, Su WL, Chen CW, Perng WC. Five-day course of budesonide inhalation suspension is as effective as oral prednisolone in the treatment of mild to severe acute asthma exacerbations in adults. Pulm Pharmacol Ther. 2011;24(2):256–260. doi:10.1016/j.pupt.2010.07.001.
- Vogelmeier C, Kardos P, Hofmann T, Canisius S, Scheuch G, Muellinger B, Nocker K, Menz G, Rabe KF. Nebulised budesonide using a novel device in patients with oral steroid-dependent asthma. Eur Respir J. 2015;45(5):1273–1282. doi:10.1183/09031936.00152014.
- Sheikh-Motahar-Vahedi H, Habibi-Samadi M, Vahidi E, Saeedi M, Momeni M. Nebulized Budesonide vs. Placebo in Adults with Asthma Attack; a Double Blind Randomized Placebo-Controlled Clinical Trial. Adv J Emerg Med. 2019;3(1):e4. doi:10.22114/AJEM.v0i0.112.
- Ito K, Kanemitsu Y, Fukumitsu K, Inoue Y, Nishiyama H, Yamamoto S, Kitamura Y, Kurokawa R, Takeda N, Fukuda S, et al. The impact of budesonide inhalation suspension for asthma hospitalization: in terms of length of stay, recovery time from symptoms, and hospitalization costs. Allergol Int. 2020;69(4):571–577. doi:10.1016/j.alit.2020.04.003.
- Marghli S, Bouhamed C, Sghaier A, Chebbi N, Dlala I, Bettout S, Belkacem A, Kbaier S, Jerbi N, Bellou A. Nebulized budesonide combined with systemic corticosteroid vs systemic corticosteroid alone in acute severe asthma managed in the emergency department: a randomized controlled trial. BMC Emerg Med. 2022;22(1):134. doi:10.1186/s12873-022-00691-9.
- Morice AH, Morris D, Lawson-Matthew P. A comparison of nebulized budesonide with oral prednisolone in the treatment of exacerbations of obstructive pulmonary disease. Clin Pharmacol Ther. 1996;60(6):675–678. doi:10.1016/S0009-9236(96)90216-7.
- Maltais F, Ostinelli J, Bourbeau J, Tonnel AB, Jacquemet N, Haddon J, Rouleau M, Boukhana M, Martinot JB, Duroux P. Comparison of nebulized budesonide and oral prednisolone with placebo in the treatment of acute exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med. 2002;165(5):698–703. doi:10.1164/ajrccm.165.5.2109093.
- Mirici A, Meral M, Akgun M. Comparison of the efficacy of nebulised budesonide with parenteral corticosteroids in the treatment of acute exacerbations of chronic obstructive pulmonary disease. Clin Drug Investig. 2003;23(1):55–62. doi:10.2165/00044011-200323010-00007.
- Gunen H, Hacievliyagil SS, Yetkin O, Gulbas G, Mutlu LC, In E. The role of nebulised budesonide in the treatment of exacerbations of COPD. Eur Respir J. 2007;29(4):660–667. doi:10.1183/09031936.00073506.
- Yilmazel Ucar E, Araz O, Meral M, Sonkaya E, Saglam L, Kaynar H, Gorguner AM, Akgun M. Two different dosages of nebulized steroid versus parenteral steroid in the management of COPD exacerbations: a randomized control trial. Med Sci Monit. 2014;20:513–520. doi:10.12659/MSM.890210.
- Sun X, He Z, Zhang J, Deng J, Bai J, Li M, Zhong X. Compare the efficacy of inhaled budesonide and systemic methylprednisolone on systemic inflammation of AECOPD. Pulm Pharmacol Ther. 2015;31:111–116. doi:10.1016/j.pupt.2014.09.004.
- Ding Z, Li X, Lu Y, Rong G, Yang R, Zhang R, Wang G, Wei X, Ye Y, Qian Z, et al. A randomized, controlled multicentric study of inhaled budesonide and intravenous methylprednisolone in the treatment on acute exacerbation of chronic obstructive pulmonary disease. Respir Med. 2016;121:39–47. doi:10.1016/j.rmed.2016.10.013.
- Jiang DH, Wang X, Liu LS, Ji DD, Zhang N. The effect of ventilator mask atomization inhalation of ipratropium bromide and budesonide suspension liquid in the treatment of COPD in acute exacerbation period on circulating levels of inflammation and prognosis. Eur Rev Med Pharmacol Sci. 2017;21(22):5211–5216. doi:10.26355/eurrev_201711_13843.
- Hashemian SM, Mortaz E, Jamaati H, Bagheri L, Mohajerani SA, Garssen J, Movassaghi M, Barnes PJ, Hill NS, Adcock IM. Budesonide facilitates weaning from mechanical ventilation in difficult-to-wean very severe COPD patients: association with inflammatory mediators and cells. J Crit Care. 2018;44:161–167. doi:10.1016/j.jcrc.2017.10.045.
- Zheng JP, Zhang J, Ma LJ, Chen P, Huang M, Ou XM, Zhao ZW, Jiang SJ, Cao J, Yao W. Clinical outcomes of using nebulized budesonide as the initial treatment for acute exacerbations of chronic obstructive pulmonary disease: a post-hoc analysis. Int J Chron Obstruct Pulmon Dis. 2019;14:2725–2731. doi:10.2147/COPD.S196615.
- Zhang R, Zhu J, Liu Y, Li Y, Liu W, Zhang M, Chen B, Zhu S. Optimization of nebulized budesonide in the treatment of acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2020;15:409–415. doi:10.2147/COPD.S235125.
- Chen Y, Liu Y, Zhang J, Yao W, Yang J, Li F, Lu L, Zheng J, Han X, Xu JF. Comparison of the clinical outcomes between nebulized and systemic corticosteroids in the treatment of acute exacerbation of COPD in China (CONTAIN Study): a post hoc analysis. Int J Chron Obstruct Pulmon Dis. 2020;15:2343–2353. doi:10.2147/COPD.S255475.
- Aghili M, Vahidi E, Mohammadrezaei N, Mirrajei T, Abedini A. Effectivness of nebulized budesonide for COPD exacerbation management in emergency department; a randomized clinical trial. Arch Acad Emerg Med. 2020;8(1):e85.
- Nguyen D, Larson T, Leinbach H, Guthrie E. Systemic steroid and nebulized budesonide combination therapy versus systemic steroid monotherapy in patients with acute exacerbation of chronic obstructive pulmonary disease in a community hospital: a retrospective cohort study. Hosp Pharm. 2021;56(6):786–791. doi:10.1177/0018578720965417.
- Gu YL, Sun ZX, Sun Y, Wen Y, Guan X, Jiang DL, Cheng C, Gu H. A real-world cost-effectiveness analysis of nebulized budesonide and intravenous methylprednisolone in acute exacerbation of chronic obstructive pulmonary disease. Front Pharmacol. 2022;13:892526. doi:10.3389/fphar.2022.892526.
- Tashkin DP, Lipworth B, Brattsand R. Benefit: risk Profile of Budesonide in Obstructive Airways Disease. Drugs. 2019;79(16):1757–1775. doi:10.1007/s40265-019-01198-7.
- Daley-Yates PT. Inhaled corticosteroids: potency, dose equivalence and therapeutic index. Br J Clin Pharmacol. 2015;80(3):372–380. doi:10.1111/bcp.12637.
- Edsbäcker S, Wollmer P, Selroos O, Borgström L, Olsson B, Ingelf J. Do airway clearance mechanisms influence the local and systemic effects of inhaled corticosteroids? Pulm Pharmacol Ther. 2008;21(2):247–258. doi:10.1016/j.pupt.2007.08.005.
- Derendorf H, Meltzer EO. Molecular and clinical pharmacology of intranasal corticosteroids: clinical and therapeutic implications. Allergy. 2008;63(10):1292–1300. doi:10.1111/j.1398-9995.2008.01750.x.
- Dalby C, Polanowski T, Larsson T, Borgström L, Edsbäcker S, Harrison TW. The bioavailability and airway clearance of the steroid component of budesonide/formoterol and salmeterol/fluticasone after inhaled administration in patients with COPD and healthy subjects: a randomized controlled trial. Respir Res. 2009;10(1):104. doi:10.1186/1465-9921-10-104.
- Ruge CA, Kirch J, Lehr CM. Pulmonary drug delivery: from generating aerosols to overcoming biological barriers-therapeutic possibilities and technological challenges. Lancet Respir Med. 2013;1(5):402–413. doi:10.1016/S2213-2600(13)70072-9.
- Janson C, Stratelis G, Miller-Larsson A, Harrison TW, Larsson K. Scientific rationale for the possible inhaled corticosteroid intraclass difference in the risk of pneumonia in COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:3055–3064. doi:10.2147/COPD.S143656.
- Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013;68(11):1029–1036. doi:10.1136/thoraxjnl-2012-202872.
- Derendorf H, Nave R, Drollmann A, Cerasoli F, Wurst W. Relevance of pharmacokinetics and pharmacodynamics of inhaled corticosteroids to asthma. Eur Respir J. 2006;28(5):1042–1050. doi:10.1183/09031936.00074905.
- Lipworth BJ, Jackson CM. Safety of inhaled and intranasal corticosteroids: lessons for the new millennium. Drug Saf. 2000;23(1):11–33. doi:10.2165/00002018-200023010-00002.
- Pedersen S, O’Byrne P. A comparison of the efficacy and safety of inhaled corticosteroids in asthma. Allergy. 1997;52(39 Suppl):1–34. doi:10.1111/j.1398-9995.1997.tb05047.x.
- van den Brink KIM, Boorsma M, Staal-van den Brekel AJ, Edsbäcker S, Wouters EF, Thorsson L. Evidence of the in vivo esterification of budesonide in human airways. Br J Clin Pharmacol. 2008;66(1):27–35. doi:10.1111/j.1365-2125.2008.03164.x.
- Hochhaus G. New developments in corticosteroids. Proc Am Thorac Soc. 2004;1(3):269–274. doi:10.1513/pats.200402-007MS.
- Miller-Larsson A, Mattsson H, Hjertberg E, Dahlbäck M, Tunek A, Brattsand R. Reversible fatty acid conjugation of budesonide. Novel mechanism for prolonged retention of topically applied steroid in airway tissue. Drug Metab Dispos. 1998;26(7):623–630.
- Ellul-Micallef R, Hansson E, Johansson SA. Budesonide: a new corticosteroid in bronchial asthma. Eur J Respir Dis. 1980;61(3):167–173.
- Haahtela T, Järvinen M, Kava T, Kiviranta K, Koskinen S, Lehtonen K, Nikander K, Persson T, Reinikainen K, Selroos O. Comparison of a beta 2-agonist, terbutaline, with an inhaled corticosteroid, budesonide, in newly detected asthma. N Engl J Med. 1991;325(6):388–392. doi:10.1056/NEJM199108083250603.
- Busse WW, Chervinsky P, Condemi J, Lumry WR, Petty TL, Rennard S, Townley RG. Budesonide delivered by Turbuhaler is effective in a dose-dependent fashion when used in the treatment of adult patients with chronic asthma. J Allergy Clin Immunol. 1998;101(4 Pt 1):457–463. doi:10.1016/S0091-6749(98)70353-7.
- Masoli M, Holt S, Weatherall M, Beasley R. Dose-response relationship of inhaled budesonide in adult asthma: a meta-analysis. Eur Respir J. 2004;23(4):552–558. doi:10.1183/09031936.04.00076604.
- Weiner P, Weiner M, Azgad Y, Zamir D. Inhaled budesonide therapy for patients with stable COPD. Chest. 1995;108(6):1568–1571. doi:10.1378/chest.108.6.1568.
- Pleasants RA, Wang T, Xu X, Beiko T, Bei H, Zhai S, Drummond MB. Nebulized corticosteroids in the treatment of COPD exacerbations: systematic review, meta-analysis, and clinical perspective. Respir Care. 2018;63(10):1302–1310. doi:10.4187/respcare.06384.
- Gu YL, Pang J, Sun ZX, Hu J, Sun Y, Wu XW, Guo JJ, Yang GS. Comparative efficacies of nebulized budesonide and systemic corticosteroids in the treatment of exacerbations of chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Clin Pharm Ther. 2020;45(3):419–429. doi:10.1111/jcpt.13095.
- Shang W, Wang G, Wang Y, Han D. The safety of long-term use of inhaled corticosteroids in patients with asthma: A systematic review and meta-analysis. Clin Immunol. 2022;236:108960. doi:10.1016/j.clim.2022.108960.
- Spantideas N, Drosou E, Bougea A, Assimakopoulos D. Inhaled Corticosteroids and Voice Problems. What Is New? J Voice. 2017;31(3):384 e1–e7. doi:10.1016/j.jvoice.2016.09.002.
- Chalitsios CV, Shaw DE, McKeever TM. Corticosteroids and bone health in people with asthma: A systematic review and meta-analysis. Respir Med. 2021;181:106374. doi:10.1016/j.rmed.2021.106374.
- Pedersen S. Do inhaled corticosteroids inhibit growth in children? Am J Respir Crit Care Med. 2001;164(4):521–535. doi:10.1164/ajrccm.164.4.2101050.
- Kwda A, Gldc P, Baui B, Kasr K, Us H, S W, Kantha L, Ksh S. Effect of long term inhaled corticosteroid therapy on adrenal suppression, growth and bone health in children with asthma. BMC Pediatr. 2019;19(1):411. doi:10.1186/s12887-019-1760-8.
- Chinese College of Emergency Physicians (CCEP), Emergency Committee of PLA, Beijing Society for Emergency Medicine, and Chinese Emergency Medicine . Expert consensus on nebulization therapy in pre-hospital and in-hospital emergency care. Ann Transl Med. 2019;7:487. doi:10.21037/atm.2019.09.44.
- Goldstein KM, Ghadimi K, Mystakelis H, Kong Y, Meng T, Cantrell S, Von Isenburg M, Gordon A, Ear B, Gierisch JM, et al. Risk of transmitting coronavirus disease 2019 during nebulizer treatment: a systematic review. J Aerosol Med Pulm Drug Deliv. 2021;34(3):155–170. doi:10.1089/jamp.2020.1659.
- Nematollahi AV, Motamed H, Masoumi K, Forouzan A, Nobakht E. Efficacy evaluation of budesonide nebulizer as an adjunctive medication in post-rain asthma acute phase attack. Adv Respir Med. 2022;90(1):37–48. doi:10.5603/ARM.a2022.0007.
- Zhang J, Zheng J, Huang K, Chen Y, Yang J, Yao W. Use of glucocorticoids in patients with COPD exacerbations in China: a retrospective observational study. Ther Adv Respir Dis. 2018;12:1753466618769514. doi:10.1177/1753466618769514.
