Abstract
Background
This meta-analysis aimed to evaluate the effectiveness and adverse effects of specific immunotherapy (SIT) in the management of respiratory allergens, including allergic asthma, rhinitis, and related disorders, based on a review of current literature up to November 8, 2022.
Methods
We conducted a search of databases, including PubMed, Embase, Cochrane, and Web of Science, to identify relevant randomized controlled trials (RCTs) assessing respiratory allergy-specific immunotherapy. We employed the Consolidated Standards of Reporting Trials (CONSORT) Statement to select RCTs that adhered to rigorous reporting standards. Specifically, we focused on double-blind placebo-controlled (DBPC) trials and open studies involving both adults and children, considering factors such as dosage, inclusion criteria, allergens, and primary outcome measurements.
Results
A total of 25 meta-analyses were included in this study. Among them, 14 evaluated sublingual-specific allergen immunotherapy (SLIT), 4 assessed subcutaneous allergen immunotherapy (SCIT), 4 explored both sublingual and subcutaneous immunotherapy, and 3 investigated intralymphatic immunotherapy. The outcomes of these meta-analyses indicated a reduction in medication scores in 20 cases and a decrease in symptom scores in 23 cases. Additionally, six studies reported on changes in IgE levels, seven studies focused on IgG4, four studies examined FEV1 (forced expiratory volume in 1 s), and eight studies reported on symptom and medication scores. Furthermore, 11 studies reported on differences in adverse reactions.
Conclusion
The results of our meta-analysis suggest that specific immunotherapy, while associated with some adverse effects, effectively reduces the symptoms of asthma and rhinitis. Therefore, we recommend its use in the treatment of respiratory allergies.
1. Introduction
It is now believed that allergic diseases are caused by a breakdown in our immunological tolerance to exposure to allergens in the environment, which is most likely T-regulated. A person receiving specific immunotherapy (SIT) receives doses of an allergen repeatedly to develop a specific clinical and immunologic tolerance (Citation1). The symptoms will clinically improve as a result, and the disease’s natural course may be changed.
Long a topic of debate, the value of allergen-specific immunotherapy (AIT) for treating allergic asthma is now widely acknowledged. All asthma patients should have a medical history related to allergies, and allergy testing should be done on a regular basis to confirm allergen avoidance measures and, when necessary, as a treatment option. Most patients with allergic asthma also experience comorbid conditions such as atopic dermatitis, food allergies, and allergic rhinitis concurrently. AIT is the only evidence-based treatment option available for causing tolerance against specific allergens in people with allergic rhinitis. AIT alters the fundamental processes of allergic disease in these people, produces long-lasting therapeutic effects, and stops the progression of sensitization and the emergence of secondary illnesses. The only action of all other approved medications used to treat inhalant allergies is antisymptomatic. Regardless of the severity, patient age, or related comorbidities, AIT is effective. It also has the benefit of being regarded as either an adjuvant or sole treatment, based on the individual patient.
Researchers are learning more about the SIT’s working mechanics. Subcutaneous allergen immunotherapy (SCIT) enhances the synthesis of specific IgG, a “blocking” antibody, while decreasing the production of allergen specific IgE. Through a reduction in inflammatory cell recruitment, activation, and mediator release (prostaglandin D2, eosinophil cationic protein, and histamine), SCIT also lowers allergen-specific inflammation (Citation2). All of these effects aid in immunological tolerance and long-lasting immune system modifications even after the treatment is stopped. The mechanisms of action of sublingual specific allergen immunotherapy (SLIT), which specifically involves mucosal dendritic cells, are not fully understood but appear to be comparable to those of SCIT (Citation3).
The majority of clinical trials conducted on individuals with allergic rhinitis have demonstrated the effectiveness of AIT, and certain studies have also shown its impact on co-occurring allergic asthma. AIT is an ICS-sparing medication because SCIT and SLIT alter the dose of inhaled corticosteroids (ICS) required for controlling asthma. In a proof-of-concept study, SLIT significantly decreased the frequency of severe asthma episodes following ICS withdrawal when compared to a placebo. These days, real-world evidence (RWE) studies and randomized clinical trials (RCTs) supporting the efficiency of various allergen extracts provide the basis for the usefulness of AIT in treating patients with allergic asthma. Regardless of the severity, patient age, or related comorbidities, AIT is effective. It also has the benefit of being regarded as either an adjuvant or sole treatment, based on the individual patient.
SIT has remained the only etiology-based treatment for allergic disorders since Noon and Freeman (Citation4) in 1911 and Lowdermilk in 1914 developed allergen therapy for the treatment of seasonal allergic rhinitis to grass pollen (also known as “hay fever”). Its widespread use is primarily supported by data demonstrating its clinical efficacy. However, due to the significant heterogeneity of studies, the specific key aspects must be considered in the clinical evaluation of SIT’s effectiveness.
A statistical method known as meta-analysis integrates the findings of numerous separate research and pools them together to offer a quantitative estimate of treatment effects. It also has the potential to explain and quantify the heterogeneity in the findings of different studies (Citation5). In this study, we examine the available meta-analyses of the effectiveness of SIT (include SCIT and SLIT) to assist medical professionals in adopting a neutral viewpoint toward this therapy method.
2. Methods
2.1. Search strategy
The available information from meta-analyses is gathered, reevaluated, and summarized in this systematic review without data extraction and statistical data merging to generate a summary conclusion. To find meta-analyses that evaluated the effects of SIT in allergic rhinitis and allergic asthma and were published up to November 8, 2022, we conducted a thorough search of PubMed, Embase, Cochrane, and Web of Science.
2.2. Study selection and data extraction
Only meta-analyses of RCT that adhered to the CONSORT (Consolidated Standards of Reporting Trials) Statement were taken into consideration (Citation6). Double-blind placebo-controlled (DBPC) trials and open studies in adults and children employing doses, inclusion criteria, allergens, and primary outcome measurement were the targets of the metaanalyses that were taken into consideration. Just the most current iteration of a meta-analysis was kept in case it was repeated and revised. Where two or more reviews reviewed the same topic, we chose the one with the most studies.
Studies were assessed for methodological quality in accordance with the Cochrane Collaboration recommendations and QUOROM (Quality of Reporting of Meta-Analyses) (Citation7), including characteristics of included trials, well-defined rationale, studied population, outcomes, details of the intervention, information sources and restrictions, inclusion and exclusion criteria, methods of validity evaluation, clinical heterogeneity, statistical testing, and summarizing flow-charts. Because they are highly reliant on the degree of heterogeneity among studies, we also concentrated on the statistical models and measurements that were used. One analytical strategy in meta-analyses evaluating continuous outcomes when heterogeneity cannot be easily explained is to include it in a random-effects model under the presumption that individual studies are estimating various treatment effects. However, if heterogeneity is not related, a fixed-effects model can be used, assuming that the variations in study results are purely the consequence of chance. Both models include the option of using the standardized mean difference (SMD) or weighted mean difference (SMD). The size of the intervention effect in each trial, as compared to the variability found in that study, is expressed by this summary statistic.
2.3. Statistical analysis
The Cochran’s Q and I2 tests were used to determine the degree of heterogeneity between trials. For I2 > 50% and the associated P value <0.05, a random effect model was used to determine the hazard ratios (HRs), odds ratios (ORs), and their 95% confidence intervals (CIs). Otherwise, the pooled findings were estimated using a fixed effect model. Sensitivity analyses were used to estimate each individual study on the overall impact of the stability of the pooled results. To detect publication bias, Begg’s funnel plot and the Egger test were used. Using the Stata 11 program, all the meta-analysis calculated results were produced. P values < .05 were consistently considered statistically significant.
3. Results
3.1. Literature search
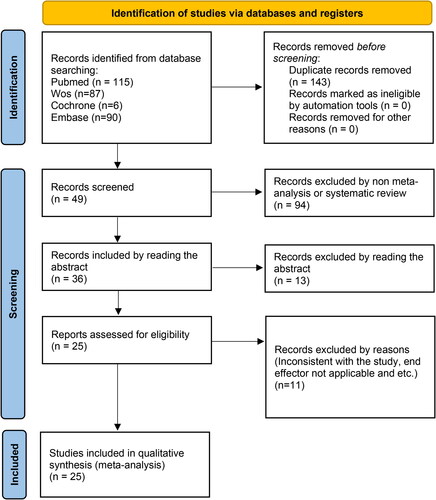
Two hundred and ninety-eight records were obtained. There were 143 records identified through database searching after duplicates were removed. We screened the records, reviewed 49 in full-text. All 25 of the included articles were carefully chosen for the study, as shown in , of which 14 investigated SLIT, 4 tested SCIT, 4 analyzed both SLIT and SCIT, and 3 studied intralymphatic immunotherapy. provides a summary of each trial’s features.
Table 1. Characteristics of included study.
3.2. Meta-analysis of symptom scores
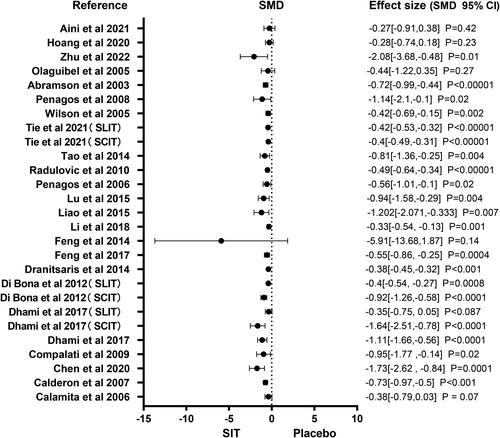
A total of 23 studies reported specific symptom scores. shows a forest plot of the symptom score. Among the different specific immune treatments including SLIT, SCIT and ILIT, the SIT is superior to other pharmacological therapies in reducing symptom score, according to the analysis results of all meta studies. There were enough studies for subgroup analysis for symptom scores. Figure S1 shows the subgroup forest plot of the symptom score. Among the different specific immune treatments including SLIT, SCIT and ILIT, specific immunotherapy is better than other drug treatments in reducing the symptom score.
3.3. Meta-analysis of medication scores
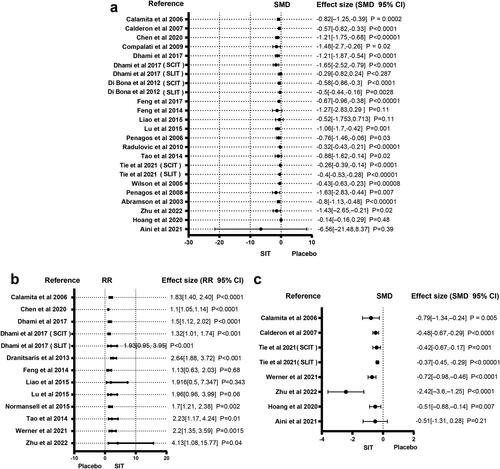
Twenty studies reported medication score. The forest plot of medication score is presented in . SIT is superior to conventional medication treatments in lowering the medication scores among the many specific immunological treatments. There were enough studies for subgroup analysis for medication score. Figure S2 shows the subgroup forest plot of the medication score. Among the different specific immune treatments including SLIT, SCIT and ILIT, specific immunotherapy is better than other drug treatments in reducing the drug score.
3.4. Meta-analysis of adverse events
Eleven included studies reported the incidence of AEs. is a forest plot for adverse reactions. Basically, in the analysis results of meta studies, SIT is more likely to cause adverse reactions than other medication treatments. There were enough studies for subgroup analysis for adverse reactions. Figure S3 shows the subgroup forest plot of the adverse reactions. Among the different specific immune treatments including SLIT, SCIT and ILIT, specific immunotherapy is more likely to cause adverse reactions than other drug treatments.
3.5. Meta-analysis of symptoms plus medication
Eight studies reported the results of the symptoms plus medication. The forest plot of the symptoms plus medication is presented in . In the analysis results of all meta studies, SIT is better than other medication treatments in reducing the symptoms plus medication score.
3.6. Meta-analysis of IgE levels
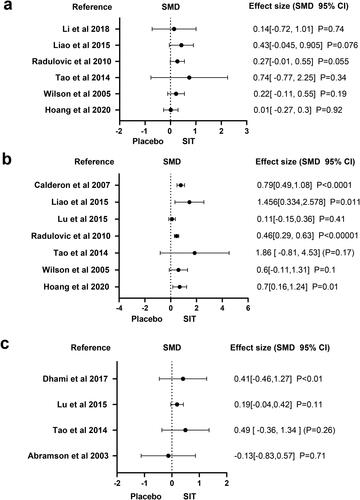
Six studies reported the results of IgE. The forest plot displayed in shows the pooled estimate of the IgE level. The SIT is superior to other medication treatments in increasing IgE levels.
3.7. Meta-analysis of IgG4 levels
Seven studies reported the results of serum IgG4 levels. is a forest plot for the level of IgG4 levels. For raising IgG4 levels, SIT is superior to other medication treatments.
3.8. Meta-analysis of FEV1
Four studies reported the results of FEV1. shows a forest plot of the level of FEV1. SIT outperforms other medication therapies in improving the level of FEV1, according to the analysis results of three meta-studies, whereas 1 study is the inverse.
4. Discussion
The upper and lower airways are thought to function as a single, unified morphological unit, and there has long been evidence of this relationship in both health and illness. An integrated understanding of rhinitis and asthma as a single, united airway disease is supported by the compelling epidemiologic, pathophysiologic, and clinical data. Both asthma and rhinitis are long-term inflammatory illnesses of the upper and lower respiratory tracts that can have several phenotypes and be brought on by allergic or nonallergic repeatable processes. It is necessary to treat asthma and rhinitis together in order to better control both conditions.
Prof. Glenis Scadding (United Kingdom) and other experts back the use of allergen immunotherapy, or AIT, for patients who are not responding to medication and are deemed sensitive to a specific allergen after being exposed to it. The only recognized treatment option for persons with allergic disorders is immunotherapy. AIT’s modification properties allow it to be placed relatively early in the therapy process. According to experts, AIT is a preventive measure that stops symptoms from getting worse and from being more sensitive to allergens other than the ones that first triggered the illness (Citation32–34).
Despite being developed more than a century ago, allergen-specific immunotherapy (SIT) did not gain widespread acceptance until its effectiveness in treating allergic asthma and allergic rhinoconjunctivitis was proven in the proper double-blind, placebo-controlled studies and until its mechanism of action was well understood. In consensus publications, the indications for SIT have been outlined. Patients with severe upper and mild to moderate lower allergic respiratory illnesses that are poorly managed by pharmacologic therapies or who are unable or unwilling to employ this treatment are the main beneficiaries of SIT.
The position of SIT in the overall therapeutic management of respiratory allergies has been strengthened by a number of recent developments, including: (a) rational prescribing practices; (b) the introduction of sublingual tablets and their stringent registration as pharmaceutical therapies by regulatory agencies; (c) an increase in allergen extract quality as a result of standardization; and (d) a better understanding of SIT’s mechanism of action. There is a need for more well planned and carried out randomized trials in children and adults with allergic asthma and rhinitis, with an emphasis on the best possible patient selection, course of treatment, and dosage.
Numerous questions about the suitability of diagnostic, prognostic, and therapeutic methods constantly come up in clinical practice and are typically answered by the ongoing advancement of clinical research. Keeping abreast of this advancement is a tremendous challenge for doctors. In this situation, doctors run the risk of missing the big picture regarding the appropriateness, safety, and specific efficacy of medical therapies in their specific situations.
There are already a number of meta-analyses covering various demographics and disorders (such as rhinitis and asthma) about allergen immunotherapy, SLIT and SCIT (Citation35). These meta-analyses’ findings show that SIT is beneficial at reducing symptom severity and the need for medication. Yet, a number of concerns have been leveled, particularly against SLIT meta-analyses (Citation36–38). Meta-analyses have been said to be impacted by a publication bias that favors the publication of positive research. Negative research might have been done but not published, and as a result, not taken into account in systematic reviews. This issue is actual, but it will eventually be resolved with the registration of clinical trials. The heterogeneity of the studies included in the available meta-analyses, with their varied designs, outcomes, and allergens, is another issue. In particular, the majority of the meta-analyses were made with the goal of desensitizing patients using single-allergen SIT. In this sense, meta-analyses are designed to provide an understanding of a phenomenon through the pooled analysis of individual investigations.
The purpose of this overview was not to offer new knowledge about immunotherapy; rather, it aimed to emphasize and summarize the most trustworthy sources of data that are already available in the literature on this treatment, assisting experts and general practitioners in their everyday work. In the meta-analysis, we found a total of 25 studies, of which 14 investigated SLIT, 4 tested SCIT, 4 evaluated both SCIT and SLIT, and 3 evaluated intralymphatic immunotherapy. In order to achieve a more thorough interpretation, we also conducted subgroup analyses of the symptom score, medication score, and adverse events. While 23 meta-analyses revealed reduced symptom scores, 20 meta-analyses reported lower medication ratings. IgE was offered by six research, IgG4 was reported by seven studies, FEV1 was displayed by four studies, and symptom and medication scores were reported by eight studies. Different adverse effects were documented in eleven meta-analyses.
We conducted both meta-analysis and subgroup analysis of all available variants, some weren’t analyzed before, such as adverse events, IgE levels, IgG4 levels, and FEV1, and evaluated allergen-specific immunotherapy for respiratory allergy based on the currently published data. Meanwhile, some studies during the past 10 years have partly reviewed the impact of allergen immunotherapy in allergic asthma (Citation12,Citation39–41), which are consistent with our research. Our results demonstrate that, despite some somewhat undesirable adverse effects, the specific immunotherapy can effectively lessen the signs and symptoms of asthma and rhinitis. It is advised for the treatment of respiratory allergies as a result. These findings highlight the importance of standardizing symptom and medication scores to make future research easier to interpret. As a result, not all meta-analyses followed strict criteria to include studies. However, due in large part to the robust statistical methods and measurements used as well as the consistency of the sub analysis results, we do not think that this heterogeneity makes it impossible to meaningfully combine the research or invalidates the conclusions.
Author contributions
Xue Li: Conceptualization (Lead), Formal analysis (Equal), Methodology (Equal), Software (Equal), Visualization (Equal), Writing – original draft (Equal); Juju Shang: Conceptualization (Supporting), Formal analysis (Equal), Methodology (Equal), Software (Equal), Visualization (Equal), Writing – original draft (Equal); Jian Liu: Formal analysis (Equal), Visualization (Equal), Writing – original draft (Equal); Yong Zhu: Conceptualization (Equal), Project administration (Equal), Supervision (Equal), Writing – review & editing (Equal).
Ethics statements
PubMed, Embase, Cochrane, and Web of Science belong to public databases. The patients involved in the database have obtained ethical approval. Users can download relevant data for free for research and publish relevant articles. Our study is based on open source data, so there are no ethical issues and other conflicts of interest.
Supplemental Material
Download MS Word (1.3 MB)Acknowledgments
We acknowledge PubMed, Embase, Cochrane, and Web of Science for providing their platforms and contributors for uploading their meaningful data.
Disclosure statement
The authors have no conflicts of interest to declare in this work.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Yacoub M-R, Incorvaia C, Caminati M, Colombo G. Immune mechanisms of allergen-specific immunotherapy. TOALLJ. 2012;5(1):47–52. doi:10.2174/1874838401205010047.
- James LK, Durham S. Update on mechanisms of allergen injection immunotherapy. Clin Exp Allergy. 2008;38(7):1074–1088. doi:10.1111/j.1365-2222.2008.02976.x.
- Scadding G, Durham S. Mechanisms of sublingual immunotherapy. J Asthma. 2009;46(4):322–334. doi:10.1080/02770900902785729.
- Noon L. Prophylactic inoculation against hay fever. Lancet. 1911;177(4580):1572–1573. doi:10.1016/S0140-6736(00)78276-6.
- Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997;315(7121):1533–1537. doi:10.1136/bmj.315.7121.1533.
- Tramèr MR. CONSORT, QUOROM, and structured abstracts–new rules for authors, new tools for readers. Eur J Anaesthesiol. 2001;18(1):1–2.
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Lancet. 1999;354(9193):1896–1900. doi:10.1016/s0140-6736(99)04149-5.
- Calamita Z, Saconato H, Pelá AB, Atallah AN. Efficacy of sublingual immunotherapy in asthma: systematic review of randomized‐clinical trials using the Cochrane Collaboration method. Allergy. 2006;61(10):1162–1172. doi:10.1111/j.1398-9995.2006.01205.x.
- Calderon MA, Carr VA, Jacobson M, Sheikh A, Durham S, Cochrane ENT Group Allergen injection immunotherapy for perennial allergic rhinitis. Cochrane Database Syst Rev. 2019;2019(1):99–110. doi:10.1002/14651858.CD007163.pub2.
- Chen L, Lei L, Cai Y, Li T. Specific sublingual immunotherapy in children with perennial rhinitis: a systemic review and meta‐analysis. Int Forum Allergy Rhinol. 2020;10(11):1226–1235. doi:10.1002/alr.22589.
- Penagos M, Compalati E, Tarantini F, Baena-Cagnani R, Huerta J, Passalacqua G, Canonica GW. Efficacy of sublingual immunotherapy in the treatment of allergic rhinitis in pediatric patients 3 to 18 years of age: a meta-analysis of randomized, placebo-controlled, double-blind trials. Ann Allergy Asthma Immunol. 2006;97(2):141–148. doi:10.1016/S1081-1206(10)60004-X.
- Dhami S, Kakourou A, Asamoah F, Agache I, Lau S, Jutel M, Muraro A, Roberts G, Akdis CA, Bonini M, et al. Allergen immunotherapy for allergic asthma: a systematic review and meta‐analysis. Allergy. 2017;72(12):1825–1848. doi:10.1111/all.13208.
- Di Bona D, Plaia A, Leto-Barone MS, La Piana S, Di Lorenzo G. Efficacy of subcutaneous and sublingual immunotherapy with grass allergens for seasonal allergic rhinitis: a meta-analysis–based comparison. J Allergy Clin Immunol. 2012;130(5):1097–1107. e2. doi:10.1016/j.jaci.2012.08.012.
- Dranitsaris G, Ellis AK. Sublingual or subcutaneous immunotherapy for seasonal allergic rhinitis: an indirect analysis of efficacy, safety and cost. J Eval Clin Pract. 2014;20(3):225–238. doi:10.1111/jep.12112.
- Feng B, Wu J, Chen B, Xiang H, Chen R, Li B, Chen S. Efficacy and safety of sublingual immunotherapy for allergic rhinitis in pediatric patients: a meta-analysis of randomized controlled trials. Am J Rhinol Allergy. 2017;31(1):27–35. doi:10.2500/ajra.2017.31.4382.
- Feng S, Xu Y, Ma R, Sun Y, Luo X, Li H. Cluster subcutaneous allergen specific immunotherapy for the treatment of allergic rhinitis: a systematic review and meta-analysis. PLoS One. 2014;9(1):e86529. doi:10.1371/journal.pone.0086529.
- Li Y, Yu S-Y, Tang R, Zhao Z-T, Sun J-L. Sublingual immunotherapy tablets relieve symptoms in adults with allergic rhinitis: a meta-analysis of randomized clinical trials. Chin Med J (Engl). 2018;131(21):2583–2588. doi:10.4103/0366-6999.244108.
- Liao W, Hu Q, Shen L-L, Hu Y, Tao H-F, Li H-F, Fan W-T. Sublingual immunotherapy for asthmatic children sensitized to house dust mite: a meta-analysis. Medicine (Baltimore). 2015;94(24):e701. doi:10.1097/MD.0000000000000701.
- Yang L, Zhu R. Immunotherapy of house dust mite allergy. Hum Vaccin Immunother. 2017;13(10):2390–2396. doi:10.1080/21645515.2017.1364823.
- Fortescue R, Kew KM, Leung MST. Sublingual immunotherapy for asthma. Cochrane Database Syst Rev. 2015;8(9):CD011293. doi:10.1002/14651858.CD011293.pub2.
- Radulovic S, Calderon MA, Wilson D, Durham S, Group CE, Cochrane ENT Group Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev. 2010;2011(2):1105–1110. doi:10.1002/14651858.CD002893.pub2.
- Tao L, Shi B, Shi G, Wan H. Efficacy of sublingual immunotherapy for allergic asthma: retrospective meta‐analysis of randomized, double‐blind and placebo‐controlled trials. Clin Respir J. 2014;8(2):192–205. doi:10.1111/crj.12058.
- Tie K, Miller C, Zanation AM, Ebert CSJr. Subcutaneous versus sublingual immunotherapy for adults with allergic rhinitis: a systematic review with meta‐analyses. Laryngoscope. 2022;132(3):499–508. doi:10.1002/lary.29586.
- Wilson D, Torres Lima M, Durham S. Sublingual immunotherapy for allergic rhinitis: systematic review and meta‐analysis. Allergy. 2005;60(1):4–12. doi:10.1111/j.1398-9995.2005.00699.x.
- Penagos M, Passalacqua G, Compalati E, Baena-Cagnani CE, Orozco S, Pedroza A, Canonica GW. Metaanalysis of the efficacy of sublingual immunotherapy in the treatment of allergic asthma in pediatric patients, 3 to 18 years of age. Chest. 2008;133(3):599–609. doi:10.1378/chest.06-1425.
- Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8;(8):CD001186. doi:10.1002/14651858.CD001186.pub2.
- Olaguíbel JM, Alvarez Puebla MJ. Efficacy of sublingual allergen vaccination for respiratory allergy in children. Conclusions from one meta-analysis. J Investig Allergol Clin Immunol. 2005;15(1):9–16.
- Werner MT, Bosso JV. Intralymphatic immunotherapy for allergic rhinitis: a systematic review and meta-analysis. Allergy Asthma Proc. 2021;42(4):283–292.
- Zhu W, Gao P, Zhang Q, Chen J. Efficacy and safety of subcutaneous immunotherapy for local allergic rhinitis: a meta-analysis of randomized controlled trials. Am J Rhinol Allergy. 2022;36(2):245–252. doi:10.1177/19458924211050547.
- Hoang MP, Seresirikachorn K, Chitsuthipakorn W, Snidvongs K. Intralymphatic immunotherapy for allergic rhinoconjunctivitis: a systematic review and meta-analysis. Rhinology. 2021;59(3):236–244. doi:10.4193/Rhin20.572.
- Aini NR, Mohd Noor N, Md Daud MK, Wise SK, Abdullah B. Efficacy and safety of intralymphatic immunotherapy in allergic rhinitis: a systematic review and meta‐analysis. Clin Transl Allergy. 2021;11(6):e12055. doi:10.1002/clt2.12055.
- Scadding GK. Grand challenges in rhinology. Front Allergy. 2020;1:584518. doi:10.3389/falgy.2020.584518.
- Scadding GK, Hellings PW, Bachert C, Bjermer L, Diamant Z, Gevaert P, Kjeldsen A, Kleine-Tebbe J, Klimek L, Muraro A, et al. Allergic respiratory disease care in the COVID-19 era: a EUFOREA statement. World Allergy Organ J. 2020;13(5):100124.
- Sedaghat AR, Kuan EC, Scadding GK. Epidemiology of chronic rhinosinusitis: prevalence and risk factors. J Allergy Clin Immunol Pract. 2022;10(6):1395–1403. doi:10.1016/j.jaip.2022.01.016.
- Passalacqua G, Durham SR, Global Allergy and Asthma European Network Allergic rhinitis and its impact on asthma update: allergen immunotherapy. J Allergy Clin Immunol. 2007;119(4):881–891. doi:10.1016/j.jaci.2007.01.045.
- Nelson HS. Allergen immunotherapy: where is it now? J Allergy Clin Immunol. 2007;119(4):769–779. doi:10.1016/j.jaci.2007.01.036.
- Larenas-Linnemann D, Cox LS. Sublingual immunotherapy for asthma: need for high quality meta-analyses to prove the concept. Allergy. 2007;62(6):704–705. doi:10.1111/j.1398-9995.2007.01335.x.
- Sopo SM, Macchiaiolo M, Zorzi G, Tripodi S. Sublingual immunotherapy in asthma and rhinoconjunctivitis; systematic review of paediatric literature. Arch Dis Child. 2004;89(7):620–624. doi:10.1136/adc.2003.030411.
- Nagata M, Nakagome K. Allergen immunotherapy in asthma: current status and future perspectives. Allergol Int. 2010;59(1):15–19. doi:10.2332/allergolint.09-RAI-0150.
- Calderón MA, Casale TB, Togias A, Bousquet J, Durham SR, Demoly P. Allergen-specific immunotherapy for respiratory allergies: from meta-analysis to registration and beyond. J Allergy Clin Immunol. 2011;127(1):30–38. doi:10.1016/j.jaci.2010.08.024.
- Novakova P, Tiotiu A, Baiardini I, Krusheva B, Chong-Neto H, Novakova S. Allergen immunotherapy in asthma: current evidence. J Asthma. 2021;58(2):223–230. doi:10.1080/02770903.2019.1684517.