Abstract
Objective
Inappropriate use of short-acting beta2-agonists (SABA) in asthma has been associated with undesired outcomes. This national expert consensus was developed to increase awareness of SABA overuse and provide recommendations on the ways to eliminate SABA overprescription and overreliance in Malaysia.
Data sources
This expert consensus was developed by searching the PubMed database, using index terms to identify SABA overuse-related burden and recommendations made in asthma guidelines. Consensus recommendations were made via the Delphi method, involving a Malaysian expert committee comprising 13 healthcare professionals (five pulmonologists, four family medicine specialists, two emergency medicine physicians and two pharmacists).
Study selections
The articles reviewed include randomized controlled trials, systematic reviews, meta-analyses, observational studies, guidelines, and surveys, with abstracts in English and published up until June 2023. Relevant recommendations were also sourced from verified websites of medical organizations and societies.
Results
Eleven consensus statements were developed, each statement achieving a priori agreement level of at least 70%. The statements reflect SABA overreliance in asthma care, as well as recommendations to eliminate SABA overprescription and overreliance in Malaysia. Supporting evidence in the literature as well as expert committee discussions leading to the development of the finalized statements were elaborated.
Conclusion
This national expert consensus discussed the burden of SABA overreliance and made specific recommendations to eliminate SABA overprescription and overreliance in the Malaysian context. This consensus document is anticipated to impart better awareness among Malaysian healthcare providers and contribute to the continuous improvement of asthma care in the country.
Introduction
Inappropriate use of short-acting beta2-agonists (SABA) in asthma has been associated with undesired outcomes, such as an increased risk of exacerbation, asthma-related admissions, emergency department (ED) visits, and mortality (Citation1–3). The Global Initiative for Asthma (GINA) no longer recommends SABA monotherapy for mild asthma since 2019 and instead recommends inhaled corticosteroid (ICS)-based treatments (Citation4). However, adoption of the latest guidelines recommendation remains lacking in Malaysia, as evidenced by a high proportion of patients (47.4%) overprescribed with SABA (Citation5). The issue of SABA overprescription is prevalent globally, affecting 26.7% patients in Asian countries (Citation6). The prevalence varies widely in other regions, with rates reported at 14.6% in the United States (Citation7), 25% in the Netherlands (Citation8), 30% in Sweden (Citation1), and 70.1% in Australia (Citation9).
Malaysia, located in Southeast Asia, is a diverse nation with a population of 33.7 million (as of 2023) (Citation10). Asthma was found to affect an estimated 6.4% of adults in Malaysia, based on the National Health Morbidity Survey 2011, with the highest prevalence among those above 75 years at 10.7% (Citation11). In the same survey, no significant differences in the incidence of asthma were observed across marital status, location, income level and occupation (Citation11). The public healthcare system receives substantial government subsidies, while access to private insurance is restricted to individuals who can afford it (Citation12). Healthcare service utilization is generally skewed toward the public healthcare system, with 75.3% of patients admitted to public hospitals (Citation13). Private healthcare facilities are more concentrated in urban areas, and are more commonly utilized by patients from more affluent backgrounds (Citation13). Given the high proportion of patients overusing SABA in local settings (Citation5,Citation14–16), this national expert consensus was developed to provide recognition of SABA overreliance and recommendations on the practical approaches to eliminate SABA overreliance in Malaysia.
Methodology
Expert selection
This consensus was developed by an expert committee comprising 13 healthcare professionals (five pulmonologists, four family medicine specialists, two emergency medicine physicians and two pharmacists) from academic centers, primary care centers, and specialty healthcare centers in Malaysia. The experts were selected based on their experience with asthma management, with each expert having a minimum of 15 years’ experience in asthma care in various healthcare settings within the country.
Consensus development
The expert consensus was developed via the Rand Corporation (RAND)/University of California, Los Angeles (UCLA) modified Delphi panel method () (Citation17,Citation18). A search of the literature in PubMed database was conducted, using index terms to identify SABA overuse-related burdens and recommendations based on GINA guidelines. The studies selected include randomized controlled trials, systematic reviews, meta-analyses, observational studies, guidelines, and surveys, with abstracts in English and published up until June 2023. Relevant recommendations were also sourced from the verified websites of medical organizations and societies (Citation4,Citation19–29). Upon reviewing the results from the literature review, ten preliminary consensus statements were drafted.
Figure 1. Flow diagram on the development process of the expert consensus on SABA overreliance in Malaysia.
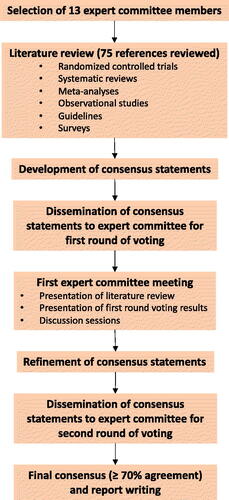
These statements were disseminated to the expert committee, who rated their level of agreement with each statement (“Agree”, “Maybe”, or “Disagree”) via an anonymous virtual voting system (the first voting round) hosted on SurveyMonkey. On the voting platform, the expert panel also anonymously provided their reasons should they not fully agree with a particular statement. The statements were then discussed between the expert panel at an in-person meeting, where any areas of disagreement were debated. Following the meeting, the statements were revised as needed and with the addition of one new statement; these eleven statements were then rated in the second round of anonymous virtual voting. A priori agreement level of 70% and above indicated consensus. This was followed by the development of this report summarizing the areas of agreement and the discussions which took place.
Outcomes
Ten consensus statements were distributed to the expert panel at the first voting round (Appendix 1). Following the discussions which took place at the in-person meeting between the expert panel, several statements were refined, and one new statement was developed. A total of eleven consensus statements were then distributed to the expert panel for a second round of voting. The eleven statements and agreement percentage for each statement are shown in . Eight of the statements achieved unanimous agreement (100%) among the expert panel. Nonetheless, the remaining three statements (Statements 5-7) achieved an agreement rating of at least 85%, well above the a priori consensus threshold of 70%. The supporting evidence in literature as well as discussions leading to the development of the finalized statements are elaborated below.
Table 1. Consensus statements on SABA overreliance in Malaysia, and the distribution of second-round agreement ratings.
Burden of SABA overreliance in Malaysia
Statement 1: SABA overreliance is highly prevalent in Malaysian asthma care across various healthcare settings, including, but not limited to, primary and specialty care, emergency department, as well as community pharmacy.
Statement 2: SABA overreliance will lead to poor asthma control and increased risks of asthma exacerbations, hospitalization, and mortality.
Statement 3: SABA overreliance is a major healthcare issue in asthma care, which warrants proactive, urgent, and systematic intervention in the national health systems.
Several studies have reported elevated numbers of oral and inhaled SABA prescriptions in Malaysia (Citation5,Citation15,Citation16). SABA overprescription is prevalent in various healthcare settings in the country, including primary care, specialty care, ED, and community pharmacy (Citation5,Citation15,Citation16). In addition to SABA inhalers, overuse of SABA tablets is also prevalent (Citation20). In a national survey of primary care encounters (n = 325 818), the rate of oral SABA, oral steroids, inhaled SABA, and ICS prescriptions were 33, 33, 50 and 23 per 100 asthma encounters, respectively (Citation15). Acknowledging that the Malaysian Clinical Practice Guideline for Asthma recommends avoiding oral SABA due to its slower onset of action and higher risk of adverse reactions compared to inhaled SABA (Citation20), this consensus will focus on addressing the overuse of SABA inhalers in Malaysia.
In the Malaysian cohort of the SABINA III study, around half of the patients (47.4%) with asthma in Malaysia were overprescribed with SABA (≥ 3 SABA prescriptions/year); 51.8% for patients with mild asthma, and 44.5% for patients with moderate-to-severe asthma (Citation5). Despite giving background ICS-containing therapy, SABA overprescription persists as evidenced in both specialty care (46.7%) and primary care settings (28.8%) (Citation5). In addition, 9% of patients had purchased SABA without a prescription; of these, 43.9% purchased more than three inhalers (Citation5). Similarly, SABA overuse has also been reported in a retrospective study performed at two tertiary centers in Kuala Lumpur, with over 70% of patients admitted for asthma exacerbation using their SABA inhalers more than three times per week (Citation16). The elevated numbers of SABA prescription may potentially be attributed to the provision of substantial government subsidies for asthma medications (including ICS and SABA) in public healthcare settings (Citation5).
SABA overprescription has been associated with worsened clinical outcome among Malaysian patients. Patients overprescribed with SABA were less likely to achieve at least partially controlled asthma, and the risk of severe asthma exacerbation doubled (Citation5). Furthermore, the ASCOPE study showed an association between high SABA use with poor asthma control in the Malaysian primary care setting (Citation30). The Klang Asthma Cohort Study has also identified SABA use as a major risk factor for poorly controlled asthma (Citation31). Furthermore, SABA overusers were generally less likely to describe their health status and asthma control as ‘excellent’ (Citation32).
Most SABA overusers were unaware that frequent SABA usage could worsen their asthma control (Citation32,Citation33). SABA overusers have also exhibited dependence toward SABA, due to the immediate symptom relief provided from SABA inhalers (Citation32,Citation33). There is a need to identify patients whose perceptions of SABA might place them at risk of SABA overreliance to facilitate early clinical intervention, which includes educating patients on the dangers of SABA overuse and facilitate behavioral change among patients (Citation34).
The frequent overuse of SABA among patients with asthma in Malaysia, as well as the known risk of worsened clinical outcomes associated with SABA overuse demonstrated by multiple studies, suggest that the causation between SABA overuse and poor health outcomes cannot be ruled out. As such, it is important for clinicians to be aware of and recognize SABA overreliance and take proactive measures to address this issue.
Eliminating SABA overprescription and overreliance
Statement 4: Patients with asthma on SABA treatment should receive an assessment of risk of SABA overreliance.
Statement 5: The number of SABA canisters utilized by patients with asthma should be controlled and monitored, whereby it should not exceed 2 canisters/year.
SABA overreliance has been associated with an increased risk of exacerbation, asthma-related admissions, ED visits, as well as mortality (Citation1–3). Therefore, SABA overreliance in patients must be identified and eliminated to improve patient outcomes. The risk of SABA overreliance may be assessed using several tools. An example of this is the Reliever Reliance Test (RRT), which is a five-item self-assessment tool to assess patient perceptions of SABA that lead to overreliance and overuse of SABA (Citation29). The RRT was developed from the validated SABA Reliance Questionnaire (SRQ) (Citation34). Alternatively, the Canadian Thoracic Society’s simplified evaluation of SABA overreliance may be utilized to evaluate asthma control, before using the RRT to obtain a better understanding of the drivers behind SABA overreliance (Citation35). However, these questionnaires were not validated in the local population at the point of preparing this manuscript.
The SENTINEL project introduced a series of ‘gold standard’ asthma prescribing practices to improve adult asthma care in the primary and secondary care settings (Citation36). A reduction in SABA overuse was observed in patients with asthma 12 months after implementing the SENTINEL project in Hull, UK (Citation37). The Malaysian asthma care is anticipated to benefit from similar best practices to monitor and control SABA use.
Currently, controlling SABA utilization may be challenging as SABA inhalers may be provided through multiple sources, including upon discharge from ED, at public health clinics or purchased from retail pharmacies. Hence, the dispensing of SABA should be controlled and monitored so that patients are prescribed no more than two SABA canisters per year (Citation1,Citation19). The threshold for SABA overuse was calculated assuming 200 doses per canister, corresponding to an average use of more than once daily (Citation19). Regardless, exceptions to this threshold might be considered for patients with severe asthma. In addition, SABA should also not be prescribed alongside maintenance and reliever therapy (MART), nor should it be provided as a repeat prescription. Patients who require additional SABA should be referred for consultation with their clinician for further assessment.
Understanding patient habits surrounding SABA use may also help eliminate SABA overreliance. Some SABA overusers perceive that their asthma is well-controlled on SABA alone, while unaware that excessive SABA use worsens asthma control without addressing the underlying inflammation (Citation32,Citation33). As indicated by the expert panel, some patients use inhalers past their expiration date, while others tend to accumulate prescribed SABA canisters and store unused medication at home. Questioning patients about stored SABA at home may help detect instances where they have received excess SABA from multiple sources. Implementing measures to limit the maximum number of SABA canisters dispensed to each patient annually would also improve patient awareness and curb SABA overuse.
Before considering a regimen with a SABA reliever, clinicians should ensure that the patient is likely to adhere to daily controller treatment, and that they are not overreliant on SABA inhalers. Additional strategies that may help reduce SABA overreliance include placing warning labels on SABA canisters and packages, limiting SABA dispensation without prescription, and establishing SABA-free treatment centers. Following an exacerbation, the patient’s asthma history (including SABA use) must be thoroughly examined. Upon discharge from ED, patients should not be discharged with SABA inhaler alone (Citation19). Clinicians are recommended to review SABA inhaler use during clinic visits and provide patient education about asthma control, emphasizing the appropriate use of SABA medications.
Two aspects contribute to SABA overuse in patients and must be addressed: overprescription by clinicians and overreliance by patients. Active efforts to monitor the prescription of SABA inhalers, as well as identifying patients at risk of SABA overreliance, are anticipated to help eliminate SABA overuse.
Eliminating SABA monotherapy from the ED setting
Statement 6: Patients with asthma discharged from hospital/clinic/emergency department following an acute exacerbation should receive ICS-containing treatment and not SABA monotherapy.
The goals for asthma treatment in the ED setting are to reduce the need for hospitalization and mechanical ventilation, decrease the frequency of relapse after discharge, improve patient quality of life, minimize adverse drug events, and maximize patient safety (Citation38). However, in the expert committee’s experience, approximately one-third of patients present recurrently to the ED with severe or life-threatening asthma exacerbations following discharge. This observation is substantiated by a local study reporting a high rate of SABA use of 70.9%, which found that 49.4% of patients with asthma experienced at least one exacerbation in the previous year, requiring an average of at least two admissions per year (Citation16). This may be attributed to inappropriate use of SABA, which has been linked to asthma-related hospitalization, ED visits and increased asthma-related costs (Citation3). The GINA 2024 recommends discharging patients with a regular maintenance ICS-containing treatment; SABA monotherapy is not recommended (Citation19). Other international guidelines such as the New Zealand Adolescent & Adult Asthma Guidelines (2020) (Citation21), Australian Asthma Handbook Version 2.2 (2022) (Citation22) and the Emirates Thoracic Society (2021) (Citation39) have also made similar recommendations when discharging patients.
Eliminating SABA monotherapy involves providing ICS-containing treatment and not SABA monotherapy upon discharge. Conversely, dispensing both ICS and SABA upon ED discharge may result in non-adherence to follow-up appointments. Therefore, patients should receive a thorough review and asthma education at discharge, especially highlighting the importance of attending their follow-up appointments and adhering to their medication. Patients may be provided with a written asthma action plan and education on inhaler technique at discharge, empowering them to continue their treatment properly upon leaving the hospital.
Controlling SABA dispensation
Statement 7: SABA should only be dispensed with prescription.
The dispensation of SABA inhalers without a prescription accounts for a large proportion of SABA inhalers dispensed in Malaysia, with 3.3 million dispensed in 2019 (Citation40). The availability of SABA without a prescription has been linked to the undertreatment of asthma in an Australian study, with nearly 70% of undertreated patients had obtained SABA without prescription, compared to only 50% of adequately treated patients (p = 0.03) (Citation41). Similarly, an Australian community pharmacy-based survey demonstrated an increased proportion of patients with uncontrolled asthma (59.0% vs. 15.4%, p < 0.001), increased use of oral corticosteroids to manage symptoms (26.2% vs. 13.5%, p < 0.01), and increased doctor visits over the past one year (74.5% vs. 62.5%, p < 0.01) among patients who purchased SABA without a prescription and those who overused SABA, compared with SABA non-overusers (Citation9). A systematic review also reported that SABA inhaler purchase without a prescription and SABA overuse often lead to uncontrolled asthma and increased healthcare utilization (Citation42).
To circumvent this issue, a Global Policy Steering Group on Asthma recommends availing SABAs only to patients with a valid prescription or those having a clinical emergency, such as an exacerbation, and that SABA should not be dispensed as a standalone treatment. Moreover, reassessment should be performed if three or more SABA inhalers were used within a year (Citation43). The United States, South Korea and Spain have also implemented similar government policies and/or practices to restrict the dispensation of SABA inhalers without a prescription (Citation23,Citation44,Citation45).
Given that the dispensation of SABA inhalers has been linked to the undertreatment of asthma, patients in the local settings would benefit from SABA inhalers dispensed only with a valid prescription, but this proposition might be met with several challenges. This includes addressing prescriber habits and educating the prescriber to curb SABA overprescription. Improved awareness among clinicians and pharmacists also plays an integral role in limiting SABA dispensation to prevent potential overuse of SABA.
Implementation of GINA treatment strategy
Statement 8: Where appropriate, Global Initiative for Asthma (GINA) Track 1 practice should be implemented in the management of asthma across all severities.
The recent GINA recommendations provide an option of two treatment tracks for asthma: the ‘preferred’ Track 1 with ICS/formoterol as an anti-inflammatory reliever (AIR) (Steps 1-2), and an ‘alternative’ Track 2 either using ICS as needed (Step 1) or daily low-dose ICS (Step 2) and as-required SABA (Citation19). A Cochrane systematic review and meta-analysis has shown that as-required fixed-dose combination inhalers (Track 1) in mild asthma reduced asthma exacerbations requiring systemic steroids (p < 0.00001), annual exacerbation rate (p < 0.00001), and the odds of an asthma-related hospital admission or ED or urgent care visit compared with as-required SABA alone (p = 0.0001) (Citation46). In addition, as-required fixed-dose combination inhalers did not lead to a significant difference in the rate of severe asthma exacerbations requiring systemic steroids but instead reduced the odds of an asthma-related hospital admission or ED or urgent care visit compared with regular maintenance ICS (p = 0.01) (Citation46).
In a systematic review of 16 randomized clinical trials (15 of which evaluated MART as a combination therapy with budesonide and formoterol in a dry-powder inhaler [DPI]), Track 1 MART regimen was associated with a lower risk of asthma exacerbations compared with the same dose or higher dose of ICS and long-acting beta2-agonist (LABA) controller therapy (Track 2 regimen) (Citation47). Furthermore, switching patients with uncontrolled asthma at GINA steps 3-4 to Track 1 was also shown to be more beneficial compared with maintaining or intensifying Track 2 therapy (Citation48). Switching to MART was associated with longer time to first severe asthma exacerbation as well as 30% lower risk of severe asthma exacerbation compared with remaining at the same treatment step (Citation48). Similarly, the SENTINEL project also reported that a higher proportion of patients who transitioned to MART were exacerbation-free following the implementation of SENTINEL quality improvement program (Citation37).
Furthermore, a post-hoc analysis has shown that beta-agonist use within 14 days prior to hospital attendance with a severe asthma exacerbation in GINA Track 2 was more likely to exceed the daily threshold requiring medical review compared with GINA Track 1 approach (Citation49). This may be due to the increasing asthma symptoms nearing asthma exacerbation, which resulted in increased as-needed reliever use (Citation50). Starting patients on a treatment regimen that improves their asthma control would avoid reliance on SABA relievers.
Retrospective studies in the United Kingdom, China and Malaysia have demonstrated a low adherence rate to ICS among patients with asthma (Citation16,Citation51–53), which suggest that the implementation of GINA Track 2 in clinical practice could be challenging. Furthermore, utilizing ICS/formoterol as a reliever in GINA Track 1 also makes it a simpler regimen. The use of ICS/formoterol may also play an important role in replacing SABA monotherapy and, subsequently, reducing severe exacerbations.
Some centers have successfully implemented a SABA-free policy. The Asthma Center at the G Baigorria Hospital in Argentina, has implemented an ICS-containing reliever strategy successfully since 2014 by administering budesonide/formoterol via a single inhaler device for all asthma severities, thus eliminating SABA therapy. The MART/AIR regimen in the SABA-free center showed a reduction in the rate of asthma hospitalizations (p = 0.031) (Citation54).
Another consideration for the selection of asthma treatment is the environmental impact of the respective treatment device used. There has been growing interest in mitigating the environmental impact of asthma; the choice of inhaler should consider the life cycle of the device from manufacturing to recycling, as well as the type of propellants used in pressurized metered dose inhalers (pMDIs) (Citation19). Selection of an effective treatment regimen that enables adequate asthma control not only improves patient outcomes, but also reduces the carbon footprint associated with asthma care (e.g. inhaler overuse, clinic visits due to uncontrolled asthma, hospital or intensive care unit admissions, patient travel). GINA Track 1, due to its potential for better asthma control as well as its use of DPI, may be advantageous in this sense (Citation37,Citation46,Citation48,Citation49,Citation55,Citation56). Regardless, besides the environmental implications of an inhaler, it is essential that treatment selection considers the patients’ ability to use their devices correctly to achieve good asthma control (Citation56).
GINA Track 1 has been considered the preferred approach as it demonstrates efficacy in reducing exacerbations and provides simplicity by utilizing a single device. The local adoption of GINA Track 1 practice could be accelerated by updating national guidelines to align with the latest GINA recommendations. Specific programs and initiatives facilitating the adoption of GINA Track 1 from other countries (e.g. the SENTINEL respiratory quality improvement program) (Citation37) or SABA-free asthma centers (Citation54)) may also be considered for implementation in Malaysia. Implementation of GINA Track 1 could also address the environmental impact of asthma care by reducing the carbon footprint resulting from poorly controlled asthma and SABA overuse (Citation55). Broad access to ICS/formoterol in public primary care settings is essential to enable successful implementation of the GINA Track 1 regimen. While GINA Track 1 is preferred in the management of asthma across all severities, the Track 2 approach may be prescribed for carefully selected patients.
Self-management education improves treatment outcomes
Statement 9: Inhaler technique and medication adherence should be optimized in all patients with asthma while empowering them with self-management education.
Incorrect usage of inhaler device is associated with worse disease outcomes, including suboptimal asthma control and increased exacerbations, creating a considerable impact on the burden of asthma (Citation57,Citation58). Multiple systematic reviews have associated inhaler techniques with treatment efficacy. One such systematic review associated improved inhalation technique with better asthma outcomes across multiple devices (Citation59). Another systematic review has also linked poor inhaler technique, number of inhalation procedure steps and time elapsed since intervention (all p < 0.05) with the effectiveness of the intervention (Citation60). Educational interventions to improve inhaler technique were considered effective in the short-term, and periodical intervention reinforcement was recommended to improve treatment efficacy (Citation60). Aside from inhaler technique, medication adherence also plays a role in influencing treatment outcomes; pharmacist-led interventions on medication adherence and inhalation technique in adult patients with asthma were shown to improve inhalation technique in these patients (Citation61). Good medication adherence was also associated with a lower risk of severe asthma exacerbations in several high-quality studies (Citation62). The Test of Adherence to Inhalers (TAI) is a questionnaire that assesses adherence to asthma inhalers through patient self-report (Citation63), which had been translated into Bahasa Malaysia and validated for use in the Malaysian population (Citation64).
Empowering patients in self-management of asthma has long been recommended by various guidelines (Citation19,Citation28,Citation65). A systematic review and meta-analysis had been performed to compare the impact of three different self-management models (multidisciplinary case management, regularly supported self-management, and minimally supported self-management) on reducing healthcare use and improving quality of life against usual care and education (Citation66). For healthcare use, multidisciplinary case management and regularly supported self-management were shown to perform better than usual care (Citation66). In terms of quality of life, only regularly supported self-management showed benefit compared with usual care (Citation66). Asthma self-management has also been linked with reduced hospitalizations, ED visitations and unscheduled consultations, while also improving markers of control and quality of life for people with asthma (Citation65). Self-management was deemed the most effective when integrated into proactive long-term condition management (Citation65). Therefore, patients should be empowered to take charge of their asthma treatment by optimizing their inhaler technique and medication adherence, as well as providing self-management education to improve treatment outcomes.
Role of pharmacist-led interventions
Statement 10: Increased healthcare resources should be allocated to expand Respiratory Medication Therapy Adherence Clinic (RMTAC) pharmacist-led interventions in improving patients’ inhaler technique and treatment adherence, as well as overall mitigation of the risk of SABA overreliance.
Multiple asthma clinical trials (e.g. FACET, COSMOS, START, and SYGMA 1 and 2 trials) have reported encouraging efficacy of various treatment regimens in reducing exacerbation (Citation67–70). However, this contrasts with real-life scenarios, where a substantial number of patients continue to experience suboptimal asthma outcomes despite treatment. This disparity is evident in Malaysian health clinics, where only 34.1% of adult patients achieved well-controlled asthma (Citation71), which may be attributed to poor treatment adherence and inhaler technique (Citation72).
Pharmacists play an integral role in providing long-term follow-up care for effective management of chronic diseases, including asthma. This is because pharmacists are well-positioned to provide patient education on asthma, treatments and side effects, inhaler technique, as well as to emphasize adherence to therapy (Citation72).
The RMTAC was introduced by the Ministry of Health Malaysia to foster collaboration between pharmacists and other healthcare professionals to effectively improve asthma care through patient education, self-management engagement, asthma trigger avoidance, medication adherence review, inhaler technique training, disease monitoring, while proactively identifying and resolving drug-related problems (Citation27,Citation73). Follow-ups with RMTAC over six months have been shown effective in improving patients’ asthma control (52% with RMTAC vs. 21% in standard care), GINA score, and inhaler technique when compared with standard care (Citation74), while improving cost-efficiency of asthma care (Citation73).
In a randomized controlled trial, patients receiving pharmacist-led intervention had better asthma control after 6 months; the mean Asthma Control Questionnaire (ACQ) scores were significantly improved (p < 0.001) and more patients had controlled asthma (p = 0.028) compared with the control group (Citation75). Furthermore, patients receiving pharmacist-led intervention had seen improved inhaler technique and medication adherence (Citation75). Pharmacist-led interventions can also support the reduction of excessive SABA use among patients; an intervention group providing training on inhaler technique and treatment adherence over a six-month period reduced the need for rescue medicine compared with usual care (p = 0.012) (Citation76). Furthermore, a pharmacist-led intervention aimed at reducing SABA overdispensing by requesting the prescriber to limit SABA inhaler dispensing to less than one canister per month when deemed appropriate, has been successful in reducing SABA prescriptions (Citation77).
Pharmacist-led interventions have shown success in reducing SABA overuse among patients, as well as overprescription by HCPs. Allocating healthcare resources to expand RMTAC services to more healthcare settings in Malaysia would improve patients’ inhaler technique and treatment adherence, while also reducing SABA overreliance.
Research activities to improve asthma care
Statement 11: Research on asthma-related exacerbation and mortality should be a national healthcare priority.
Limited health system surveillance data for asthma hinders the planning of healthcare resources (Citation78,Citation79). Unfortunately, there has long been a mismatch globally between the rising burden of respiratory diseases and research funding made available (Citation79).
Among the suggested research priorities to enhance asthma care in low-to-middle income countries are investigating the local determinants of asthma-related morbidity and mortality, developing feasible and scalable models for long-term asthma care—including access to regular clinical review and education on the use of ICS medications—and implementing pragmatic use of GINA recommendations for as-required ICS/formoterol for Steps 1 and 2 of asthma treatment (Citation78).
Successful research activities play an important role in determining underlying factors that may be associated with asthma hospitalization and mortality. For example, the National Review of Asthma Deaths (NRAD) in the United Kingdom had successfully identified that a considerable proportion of patients had been prescribed more than 12 SABA inhalers in the year before experiencing death due to asthma, with low numbers of preventer medication prescriptions during the same period (Citation80). In addition, the NRAD also reported that 46% of asthma deaths were deemed avoidable if clinicians had implemented recommendations from the British Thoracic Society/Scottish Intercollegiate Guidelines Network Asthma Guidelines (Citation80). Aside from the NRAD, the Swedish National Airway Register, with almost 300 000 individuals registered, provides a unique insight on asthma care in Sweden in evaluating the impact of and adherence to local, national and international guidelines (Citation24).
Recent research efforts on asthma in Malaysia include the RESPIRE research unit, a national audit on asthma management in public health clinics, and the National Health & Morbidity Survey 2023 (Citation25,Citation26,Citation81). However, availing data on asthma exacerbation and mortality burden at the national level remains an area that requires further investigation. Furthermore, the launch of an asthma registry in Malaysia allows collection of data which may be valuable in accessing the gaps in asthma care. Therefore, we propose that research on asthma-related exacerbation and mortality should be made a national healthcare priority.
Conclusion
Inappropriate use of SABA in asthma has been associated with several poor patient outcomes. Increased awareness of SABA overreliance is essential to improve asthma management. This national expert consensus discussed the burden of SABA overreliance and provided recommendations to eliminate SABA overprescription and overreliance in the Malaysian context, and is anticipated to increase the awareness of SABA overreliance among Malaysian healthcare providers and improve asthma care in the country.
Declaration of interest
JM declares receipt of speaker fees from AstraZeneca, GSK, Boehringer Ingelheim, Novartis, Menarini and Orient EuroPharma, and AA declares receipt of speaker fee from AstraZeneca. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Nwaru BI, Ekström M, Hasvold P, Wiklund F, Telg G, Janson C. Overuse of short-acting β(2)-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J. 2020;55(4):1901872. doi:10.1183/13993003.01872-2019.
- Wang CY, Lai CC, Wang YH, Wang HC. The prevalence and outcome of short-acting β2-agonists overuse in asthma patients in Taiwan. NPJ Prim Care Respir Med. 2021;31(1):19. doi:10.1038/s41533-021-00231-1.
- FitzGerald JM, Tavakoli H, Lynd LD, Al Efraij K, Sadatsafavi M. The impact of inappropriate use of short acting beta agonists in asthma. Respir Med. 2017;131:135–140. doi:10.1016/j.rmed.2017.08.014.
- Global Initiative for Asthma - GINA. Global strategy for asthma management and prevention (2019 update) [assessed 15 May 2024]. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf.
- Ban AY-L, Vengadasalam P, Taher SW, Mohd Zim MA, Sirol Aflah SS, Daut UN, Hyder Ali IA, Pereirasamy L, Omar A, Ibrahim A, et al. Short-acting β(2)-agonist prescription patterns and clinical outcomes in Malaysia: a nationwide cohort of the SABINA III study. Malays Fam Physician. 2023;18:32. doi:10.51866/oa.258.
- Wang HC, Djajalaksana S, Sharma L, Theerakittikul T, Lim HF, Yoo KH, et al. Evaluation of short-acting Beta-2-agonist prescriptions and associated clinical outcomes: findings from the SABA use IN Asthma (SABINA) study in Asia. World Allergy Organ J. 2023;16(10):100823.
- Slejko JF, Ghushchyan VH, Sucher B, Globe DR, Lin S-L, Globe G, Sullivan PW. Asthma control in the United States, 2008–2010: indicators of poor asthma control. J Allergy Clin Immunol. 2014;133(6):1579–1587. doi:10.1016/j.jaci.2013.10.028.
- den Akker IL, Werkhoven A, Verheij T. Over-prescription of short-acting beta agonists in the treatment of asthma. Fam Pract. 2021;38(5):612–616. doi:10.1093/fampra/cmab013.
- Azzi EA, Kritikos V, Peters MJ, Price DB, Srour P, Cvetkovski B, Bosnic-Anticevich S. Understanding reliever overuse in patients purchasing over-the-counter short-acting beta(2) agonists: an Australian community pharmacy-based survey. BMJ Open. 2019;9(8):e028995. doi:10.1136/bmjopen-2019-028995.
- Department of Statistics Malaysia. Malaysia at a glance [assessed 3 April 2024. https://open.dosm.gov.my/.
- Ministry of Health Malaysia. National Health and Morbidity Survey 2011. Volume III: healthcare demand and out-of-pocket health expenditure [assessed 15 May 2024] https://iku.moh.gov.my/images/IKU/Document/REPORT/NHMS2011-VolumeIII.pdf.
- Thomas S, Beh L, Nordin RB. Health care delivery in Malaysia: changes, challenges and champions. J Public Health Afr. 2011;2(2):e23. doi:10.4081/jphia.2011.e23.
- Ministry of Health Malaysia. National Health and Morbidity Survey 2019. Non-communicable diseases, healthcare demand, and health literacy: key findings. [assessed 15 May 2024] https://iku.gov.my/images/IKU/Document/REPORT/NHMS2019/Infographic_Booklet_NHMS_2019-English.pdf.
- Bateman ED, Price DB, Wang H-C, Khattab A, Schonffeldt P, Catanzariti A, van der Valk RJP, Beekman MJHI. Short-acting β(2)-agonist prescriptions are associated with poor clinical outcomes of asthma: the multi-country, cross-sectional SABINA III study. Eur Respir J. 2022;59(5):2101402. doi:10.1183/13993003.01402-2021.
- Chin MC, Sivasampu S, Khoo EM. Prescription of oral short-acting beta 2-agonist for asthma in non-resource poor settings: a national study in Malaysia. PLOS One. 2017;12(6):e0180443. doi:10.1371/journal.pone.0180443.
- Poh ME, Ampikaipakan S, Liam CK, Chai CS, Ramanaidoo D, Haja Mydin H. Management of asthma exacerbations in Southeast Asian tertiary care. J Asthma Allergy. 2021;14:629–640. doi:10.2147/JAA.S309143.
- Fink A, Kosecoff J, Chassin M, Brook RH. Consensus methods: characteristics and guidelines for use. Am J Public Health. 1984;74(9):979–983. doi:10.2105/ajph.74.9.979.
- Broder MS, Gibbs SN, Yermilov I. An adaptation of the RAND/UCLA modified Delphi panel method in the time of COVID-19. J Healthc Leadersh. 2022;14:63–70.
- Global Initiative for Asthma - GINA. Global strategy for asthma management and prevention 2024. update) [assessed 15 May 2024]. https://ginasthma.org/wp-content/uploads/2024/05/GINA-2024-Main-Report-WMS-1.pdf.
- Ministry of Health Malaysia. Malaysian CPG for Management of Asthma in Adult 2017 assessed 26 September 2024. https://www.moh.gov.my/moh/resources/Penerbitan/CPG/Respiratory/CPG%20Management%20of%20Asthma%20in%20Adults.pdf.
- Asthma Respiratory Foundation NZ. New Zealand adolescent & adult asthma guidelines 2020. [assessed 26 September 2023]. https://www.nzrespiratoryguidelines.co.nz/uploads/8/3/0/1/83014052/arfnz_adolescent_and_adult_asthma_guidelines_.pdf.
- National Asthma Council Australia. Australian asthma handbook assessed 26 September 2023. https://www.asthmahandbook.org.au/acute-asthma/clinical/post-acute-care.
- International Primary Care Respiratory Group. Asthma right care case study [assessed 27 September 2023. https://www.ipcrg.org/sites/ipcrg/files/content/attachments/2021-09-13/SABA%20OTC%20Case%20Study%20-%20FINAL.pdf.
- The Swedish National Airway Register assessed 28 September 2023. https://lvr.registercentrum.se/in-english/the-swedish-national-airway-register/p/HJAjrgGPD.
- NIHR Global Health Research Unit on Respiratory Health (RESPIRE) UoE. What is RESPIRE? assessed 28 September 2023. https://www.ed.ac.uk/usher/respire/about/what-is-respire.
- Ministry of Health Malaysia. National Health & Morbidity Survey 2023. [accessed 28 September 2023]. Available from: https://iku.gov.my/nhms-2023.
- Ministry of Health Malaysia. Respiratory medication therapy adherence clinic protocol—asthma/COPD (adult & pediatric), 2nd edition [accessed 2 October 2023. https://www.pharmacy.gov.my/v2/sites/default/files/document-upload/book.-protocol-respiratory-2.6-ver2_0.pdf.
- British Thoracic Society. BTS/SIGN Guideline for the management of asthma 2019 [accessed 3 October 2023]. https://www.brit-thoracic.org.uk/document-library/guidelines/asthma/bts-sign-guideline-for-the-management-of-asthma-2019/.
- International Primary Care Respiratory Group. Reliever Reliance Test - English [accessed 2 November 2023. https://www.ipcrg.org/resources/search-resources/reliever-reliance-test-english.
- Mohd Isa NA, Cheng CL, Nasir NH, Naidu V, Gopal VR, Alexander AK. Asthma control and asthma treatment adherence in primary care: results from the prospective, multicentre, non-interventional, observational cohort ASCOPE study in Malaysia. Med J Malaysia. 2020;75(4):331–337.
- Hussein N, Ramli R, Ishak I, Ho BK, Mohamad Isa S, Aman Z, Nordin A, Tan SF, Harun H, Liew SM, et al. Assessment of asthma control and associated risk factors: findings from the Klang Asthma Cohort Study in Malaysia. Eur Respir J. 2021;58(suppl 65):PA1001. doi:10.1183/13993003.congress-2021.PA1001.
- Loh ZC, Hussain R, Balan S, Saini B, Muneswarao J, Ong SC, Babar Z-U-D. Perceptions, attitudes, and behaviors of asthma patients towards the use of short-acting β2-agonists: a systematic review. PLOS One. 2023;18(4):e0283876. doi:10.1371/journal.pone.0283876.
- Blakeston S, Harper G, Zabala Mancebo J. Identifying the drivers of patients’ reliance on short-acting β2-agonists in asthma. J Asthma. 2021;58(8):1094–1101. doi:10.1080/02770903.2020.1761382.
- Chan AHY, Katzer CB, Horne R, Haughney J, Correia de Sousa J, Williams S, et al. SABA Reliance Questionnaire (SRQ): identifying patient beliefs underpinning reliever overreliance in asthma. J Allergy Clin Immunol Pract. 2020;8(10):3482–3489.
- Ellis AK, Foran V, Kaplan A, Mitchell PD. Clarifying SABA overuse: translating Canadian Thoracic Society guidelines into clinical practice. Allergy Asthma Clin Immunol. 2022;18(1):48. doi:10.1186/s13223-022-00690-2.
- Crowther L, Pearson M, Cummings H, Crooks MG. Towards codesign in respiratory care: development of an implementation-ready intervention to improve guideline-adherent adult asthma care across primary and secondary care settings (The SENTINEL Project). BMJ Open Respir Res. 2022;9(1):e001155. doi:10.1136/bmjresp-2021-001155.
- Crooks MG, Crowther L, Cummings H, Cohen J, Huang C, Pitel L, Pearson M, Morice A, Turgoose J, Faruqi S, et al. Improving asthma care through implementation of the SENTINEL programme: findings from the pilot site. ERJ Open Res. 2023;9(3):00685–2022. doi:10.1183/23120541.00685-2022.
- Albertson TE, Sutter ME, Chan AL. The acute management of asthma. Clin Rev Allergy Immunol. 2015;48(1):114–125. doi:10.1007/s12016-014-8448-5.
- Emirates Thoracic Society. Algorithm for the management of adult asthma in the emergency department. 2021.
- IQVIA IMS. SABA inhalers and tablets dispensed in Malaysia. 2019.
- Gibson P, Henry D, Francis L, Cruickshank D, Dupen F, Higginbotham N, Henry R, Sutherland D. Association between availability of non-prescription b2 agonist inhalers and undertreatment of asthma. BMJ. 1993;306(6891):1514–1518. doi:10.1136/bmj.306.6891.1514.
- Loh ZC, Hussain R, Ong SC, Saini B, Muneswarao J, Ur-Rehman A, et al. Over-the-counter use of short-acting beta-2 agonists: a systematic review. J Pharm Policy Pract. 2023;16(1):119.
- Kaplan AG, Correia-de-Sousa J, McIvor A, Global Policy Steering Group on Improving Asthma Outcomes Global quality statements on reliever use in asthma in adults and children older than 5 years of age. Adv Ther. 2021;38(3):1382–1396. doi:10.1007/s12325-021-01621-0.
- Drug Topics. OTC update: asthma medications [accessed 27 September 2023. https://www.drugtopics.com/view/otc-update-asthma-medications.
- Lee H, Ryu J, Chung SJ, Yeo Y, Park TS, Park DW, Moon J-Y, Kim T-H, Sohn JW, Yoon HJ, et al. Short-acting beta2-agonist use in asthma in Korea: a 10-Year population-based study. Allergy Asthma Immunol Res. 2021;13(6):945–953. doi:10.4168/aair.2021.13.6.945.
- Crossingham I, Turner S, Ramakrishnan S, Fries A, Gowell M, Yasmin F, et al. Combination fixed-dose beta agonist and steroid inhaler as required for adults or children with mild asthma. Cochrane Database Syst Rev. 2021;5(5):Cd013518.
- Sobieraj DM, Weeda ER, Nguyen E, Coleman CI, White CM, Lazarus SC, Blake KV, Lang JE, Baker WL. Association of inhaled corticosteroids and long-acting β-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma: a systematic review and meta-analysis. JAMA. 2018;319(14):1485–1496. doi:10.1001/jama.2018.2769.
- Beasley R, Harrison T, Peterson S, Gustafson P, Hamblin A, Bengtsson T, Fagerås M. Evaluation of budesonide-formoterol for maintenance and reliever therapy among patients with poorly controlled asthma: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(3):e220615. doi:10.1001/jamanetworkopen.2022.0615.
- Patel M, Pilcher J, Hancox RJ, Sheahan D, Pritchard A, Braithwaite I, et al. The use of β2-agonist therapy before hospital attendance for severe asthma exacerbations: a post-hoc analysis. NPJ Prim Care Respir Med. 2015;25:14099.
- Tattersfield AE, Postma DS, Barnes PJ, Svensson K, Bauer CA, O’Byrne PM, Löfdahl CG, Pauwels RA, Ullman A. Exacerbations of asthma: a descriptive study of 425 severe exacerbations. The FACET International Study Group. Am J Respir Crit Care Med. 1999;160(2):594–599. doi:10.1164/ajrccm.160.2.9811100.
- Dhruve H, d’Ancona G, Nanzer-Kelly A, Jackson DJ. Adherence to inhaled corticosteroids (ICS) according to demographic characteristics in asthma (Abstract P115). Thorax. 2021;76: a 1–A256.
- Wang J, Zhai C, Wang Q, Shi W, Fang W, Yan X, Zhang Q, Xie X, Li S, Li M, et al. Determinants of ICS therapy adherence in patients with asthma. Am J Manag Care. 2021;27(2):e36–e41. doi:10.37765/ajmc.2021.88587.
- Teng SY, Yusoff AM, Foong SY, Aziz H, Mohamad Pakarul Razy NH, Hashim NI, et al. A cross sectional study on adult asthma patients’ adherence to inhaled corticosteroids (ICS) in Hospital Putrajaya (P55). Med J Malaysia. 2021;76:33.
- Nannini LJ, Neumayer NS, Brandan N, Fernández OM, Flores DM. Asthma-related hospitalizations after implementing SABA-free asthma management with a maintenance and anti-inflammatory reliever regimen. Eur Clin Respir J. 2022;9(1):2110706. doi:10.1080/20018525.2022.2110706.
- Kponee-Shovein K, Marvel J, Ishikawa R, Choubey A, Kaur H, Thokala P, et al. Carbon footprint and associated costs of asthma exacerbation care among UK adults. J Med Econ. 2022;25(1):524–531.
- Levy ML, Bateman ED, Allan K, Bacharier LB, Bonini M, Boulet LP, et al. Global access and patient safety in the transition to environmentally friendly respiratory inhalers: the Global Initiative for Asthma perspective. Lancet. 2023;402(10406):1012–1016.
- Kocks JWH, Chrystyn H, van der Palen J, Thomas M, Yates L, Landis SH, et al. Systematic review of association between critical errors in inhalation and health outcomes in asthma and COPD. NPJ Prim Care Respir Med. 2018;28(1):43.
- Price DB, Román-Rodríguez M, McQueen RB, Bosnic-Anticevich S, Carter V, Gruffydd-Jones K, et al. Inhaler errors in the CRITIKAL Study: type, frequency, and association with asthma outcomes. J Allergy Clin Immunol Pract. 2017;5(4):1071–1081.
- Roche N, Aggarwal B, Boucot I, Mittal L, Martin A, Chrystyn H. The impact of inhaler technique on clinical outcomes in adolescents and adults with asthma: a systematic review. Respir Med. 2022;202:106949. doi:10.1016/j.rmed.2022.106949.
- Klijn SL, Hiligsmann M, Evers S, Román-Rodríguez M, van der Molen T, van Boven JFM. Effectiveness and success factors of educational inhaler technique interventions in asthma & COPD patients: a systematic review. NPJ Prim Care Respir Med. 2017;27(1):24. doi:10.1038/s41533-017-0022-1.
- Jia X, Zhou S, Luo D, Zhao X, Zhou Y, Cui YM. Effect of pharmacist-led interventions on medication adherence and inhalation technique in adult patients with asthma or COPD: a systematic review and meta-analysis. J Clin Pharm Ther. 2020;45(5):904–917. doi:10.1111/jcpt.13126.
- Engelkes M, Janssens HM, de Jongste JC, Sturkenboom MC, Verhamme KM. Medication adherence and the risk of severe asthma exacerbations: a systematic review. Eur Respir J. 2015;45(2):396–407. doi:10.1183/09031936.00075614.
- Plaza V, Fernández-Rodríguez C, Melero C, Cosío BG, Entrenas LM, de Llano LP, et al. Validation of the ‘test of the adherence to inhalers’ (TAI) for asthma and COPD Patients. J Aerosol Med Pulm Drug Deliv. 2016;29(2):142–152.
- Muneswarao J, Hassali MA, Ibrahim B, Saini B, Naqvi AA, Hyder Ali IA, et al. Translation and validation of the test of adherence to inhalers (TAI) questionnaire among adult patients with asthma in Malaysia. J Asthma. 2021;58(9):1229–1236.
- Pinnock H, Parke HL, Panagioti M, Daines L, Pearce G, Epiphaniou E, Bower P, Sheikh A, Griffiths CJ, Taylor SJC, et al. Systematic meta-review of supported self-management for asthma: a healthcare perspective. BMC Med. 2017;15(1):64. doi:10.1186/s12916-017-0823-7.
- Hodkinson A, Bower P, Grigoroglou C, Zghebi SS, Pinnock H, Kontopantelis E, Panagioti M. Self-management interventions to reduce healthcare use and improve quality of life among patients with asthma: systematic review and network meta-analysis. BMJ. 2020;370:m2521. doi:10.1136/bmj.m2521.
- Vogelmeier C, Naya I, Ekelund J. Budesonide/formoterol maintenance and reliever therapy in Asian patients (aged ≥16 years) with asthma: a sub-analysis of the COSMOS study. Clin Drug Investig. 2012;32(7):439–449. doi:10.2165/11598840-000000000-00000.
- Beasley R, Holliday M, Reddel HK, Braithwaite I, Ebmeier S, Hancox RJ, Harrison T, Houghton C, Oldfield K, Papi A, et al. Controlled trial of budesonide-formoterol as needed for mild asthma. N Engl J Med. 2019;380(21):2020–2030. doi:10.1056/NEJMoa1901963.
- O’Byrne PM, FitzGerald JM, Bateman ED, Barnes PJ, Zhong N, Keen C, Jorup C, Lamarca R, Ivanov S, Reddel HK, et al. Inhaled combined budesonide-formoterol as needed in mild asthma. N Engl J Med. 2018;378(20):1865–1876. doi:10.1056/NEJMoa1715274.
- Bateman ED, Reddel HK, O’Byrne PM, Barnes PJ, Zhong N, Keen C, Jorup C, Lamarca R, Siwek-Posluszna A, FitzGerald JM, et al. As-needed budesonide-formoterol versus maintenance budesonide in mild asthma. N Engl J Med. 2018;378(20):1877–1887. doi:10.1056/NEJMoa1715275.
- Hussein N, Liew SM, Hanafi NS, Lee PY, Cheong AT, Ghazali SS, et al. Asthma control and care among six public health clinic attenders in Malaysia: a cross-sectional study. Health Sci Rep. 2023;6(5):e1021.
- Bridgeman MB, Wilken LA. Essential role of pharmacists in asthma care and management. J Pharm Pract. 2021;34(1):149–162. doi:10.1177/0897190020927274.
- Yong YV, Shafie AA. Using a dynamic adherence Markov model to assess the efficiency of respiratory medication therapy adherence clinic (RMTAC) on asthma patients in Malaysia. Cost Eff Resour Alloc. 2018;16(1):36. doi:10.1186/s12962-018-0156-1.
- Chiew SY, Ghazali NH, Choon CJ, Chang PN, Lee MY, Yap LS, et al. Cost-effectiveness of pharmacist-managed respiratory medication therapy adherence clinic (RMTAC) on asthma patients: a prospective multicentre study. Pharm Res Rep. 2022;5(2):18–27.
- García-Cárdenas V, Sabater-Hernández D, Kenny P, Martínez-Martínez F, Faus MJ, Benrimoj SI. Effect of a pharmacist intervention on asthma control. A cluster randomised trial. Respir Med. 2013;107(9):1346–1355. doi:10.1016/j.rmed.2013.05.014.
- Mehuys E, Van Bortel L, De Bolle L, Van Tongelen I, Annemans L, Remon JP, et al. Effectiveness of pharmacist intervention for asthma control improvement. Eur Respir J. 2008;31(4):790–799.
- Wong MD, Manley RT, Stettin G, Chen W, Salmun LM. Intervention to reduce unnecessary dispensing of short-acting beta-agonists in patients with asthma. Ann Pharmacother. 2010;44(4):623–629. doi:10.1345/aph.1M697.
- Meghji J, Mortimer K, Agusti A, Allwood BW, Asher I, Bateman ED, et al. Improving lung health in low-income and middle-income countries: from challenges to solutions. Lancet. 2021;397(10277):928–940.
- Williams S, Sheikh A, Campbell H, Fitch N, Griffiths C, Heyderman RS, et al. Respiratory research funding is inadequate, inequitable, and a missed opportunity. Lancet Respir Med. 2020;8(8):e67–e68.
- Levy ML. The national review of asthma deaths: what did we learn and what needs to change? Breathe . 2015;11(1):14–24. doi:10.1183/20734735.008914.
- Hussein N, Ramli R, Liew SM, Hanafi NS, Lee PY, Cheong AT, Sazlina S-G, Mohd Ahad A, Patel J, Schwarze J, et al. Healthcare resources, organisational support and practice in asthma in six public health clinics in Malaysia. NPJ Prim Care Respir Med. 2023;33(1):13. doi:10.1038/s41533-023-00337-8.
