Abstract
Objectives
To determine whether Opto-Electronic Plethysmography (OEP) can distinguish Exercise-Induced Bronchoconstriction (EIB) breathing patterns by comparing individuals with and without EIB, and between broncho-constriction and recovery. Breathing pattern was quantified in terms of regional contribution, breathing timing, and the phase between chest sub-compartments which indicates the synchronization in movement of the different sub-compartments.
Methods
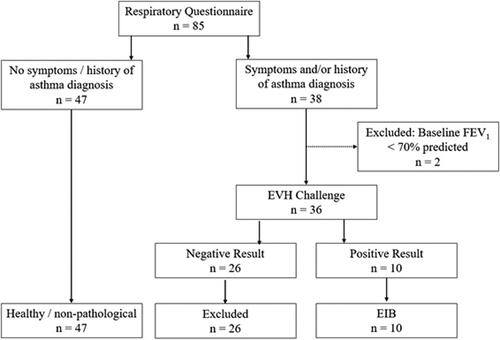
Individuals (n = 47) reporting no respiratory symptoms and no history of any respiratory disease or disorder were assumed to have a healthy breathing pattern. Of 38 participants reporting respiratory symptoms during exercise, and/or a previous diagnosis of asthma or EIB, 10 participants had a positive result to the Eucapnic Voluntary Hyperpnea test, defined as a fall of at least 10% in FEV1 from baseline at two consecutive time points and were classified into the EIB group. OEP data was obtained from 89 markers and an 11-camera motion capture system operating at 100 Hz as follows: pre- and post-EVH challenge, and post-inhaler in participants who experienced a bronchoconstriction, and 2) for the healthy group during tidal breathing.
Results
RCpRCa-Phase (upper versus lower ribcage), RCaS-Phase (lower ribcage versus shoulders), and RCpS-Phase (upper ribcage versus shoulders) differed between bronchoconstriction and rest in athletes with EIB and rest in healthy participants (p < 0.05), in all cases indicating greater asynchrony post-bronchoconstriction, and later movement of the abdominal ribcage (RCa) post-bronchoconstriction. RCpS-Phase was different (p < 0.05) between all conditions (rest, post-bronchoconstriction, and post-inhaler) in EIB.
Conclusions
OEP can characterize and distinguish EIB-associated breathing patterns compared to rest and individuals without EIB at rest.
Introduction
Exercise-induced bronchoconstriction (EIB) is a term which describes the temporary and reversible narrowing of the airways that occurs during or after exercise, limiting expiration (Citation1). EIB is reversible spontaneously post-exercise or with the inhalation of β2 agonists. The prevalence of EIB in athletic individuals generally has been reported to be approximately 20% (Citation2), though prevalence in elite swimmers is up to 69% (Citation3,Citation4). There is no gold standard diagnostic test for EIB, but diagnosis should involve a systematic airway assessment that includes objective airway assessments and questionnaires (Citation5). The eucapnic voluntary hyperpnea (EVH) test is a sensitive method for diagnosing EIB in athletes, and is a surrogate for exercise (Citation6,Citation7). However, athletes may only demonstrate a positive response to one of the provocation tests (Citation8), therefore, more than one test may be required for EIB diagnosis in athletes.
Despite the limitations of EIB diagnosis (Citation9), indirect provocation challenges are recommended as the most appropriate method to obtain a secure diagnosis of EIB (Citation6). However, using these methods it is unknown whether individuals with EIB change their breathing patterns when they experience bronchoconstriction. Better detailed understanding of breathing patterns during bronchoconstriction may help to better understand optimized treatment and help identify co-morbidity of other conditions such as breathing pattern disorders in athletes with and without EIB.
Optoelectronic plethysmography (OEP) has previously been used to investigate the effects of histamine-induced bronchoconstriction (Citation10) and methacholine induced bronchoconstriction (Citation11) on breathing pattern in asthmatics. These studies showed alterations in end expiratory volume during bronchoprovocation and attributed changes to the ribcage compartment. Feitosa and colleagues (Citation12) constructed a receiver operating characteristic curve for OEP-derived end-expiratory volume of the chest wall for schoolchildren with exercise induced asthma to show OEP can accurately be used to identify this condition in children. Whereas these studies focussed on chest compartment volume, an important and unique benefit of OEP is the ability to measure thoracoabdominal asynchrony during movement and exercise. For example, the breathing pattern of individuals with mild asthma but without EIB has been successfully investigated during mild exercise using OEP (Citation13). Our research group have- previously demonstrated that OEP can identify differences in breathing pattern between a breathing pattern disorder (BPD) group and a healthy asymptomatic group (Citation14). In particular, Smyth et al. (Citation14) showed that novel phase angle metrics quantifying the synchrony between the shoulder compartment and the pulmonary and abdominal rib cage distinguished between these groups during exercise. These studies demonstrated OEP provides detailed information of breathing pattern mechanics that cannot be obtained from spirometry. Identifying changes in breathing patterns specifically when individuals are experiencing bronchoconstriction may improve management of athletes with EIB. In particular, would better understand the expected breathing patterns during EIB and whether inhaler therapy will enable athletes with EIB to achieve an efficient breathing pattern.
Therefore, the primary aim of this study was to investigate how OEP-derived breathing pattern parameters including (a) chest compartment contribution to total breath volume and (b) chest compartment synchrony alter during bronchoconstriction induced by EVH in physically active adults with EIB. A second aim was to establish if these breathing parameters differ between physically active adults with and without EIB.
Materials and methods
Participants
Eighty-five active participants gave informed written consent to participate in this study. This study was approved by the University of Kent’s Research Ethics Advisory Group (Prop 21_208_19). Participants were recruited via word of mouth and advertisement to local sports clubs. Participants were required to have a consistent training regime with a minimum of 5 h of physical activity per week. Each participant completed a questionnaire to report previous history of asthma diagnosis, current respiratory symptoms during or post-exercise and in different environmental conditions. Participants were asked to report on the following symptoms: coughing, wheezing breathing in and/or out, chest tightness breathing in and/or out, dyspnea, and excess mucus production during or post-exercise. Individuals who reported no respiratory symptoms and had no history of any respiratory disease/disorder were assumed to have a healthy breathing pattern. Individuals who reported respiratory symptoms and/or had a previous/current diagnosis of asthma/EIB performed an Eucapnic Voluntary Hyperpnea challenge. Participants who demonstrated a positive EVH result were considered to have EIB. summarizes the participant characteristics for these two groups. Individuals were excluded if they had abnormal lung function at rest (FEV1 <70% predicted value). Individuals were excluded if they reported chest infection within 4 wk. Participants were required to be free from musculoskeletal injury for at least 6 wk. Participants were free from cardiovascular and metabolic disease.
Table 1. Participant characteristics (mean ± SD).
Protocol
Participants currently using asthma medication were required to withhold use prior to the EVH challenge; Montelukast’s for 7 days prior, inhaled corticosteroid and combination inhalers for at least 3 days prior, long acting β2-agonists for 2 days prior, and short acting β2-agonists for 1-day prior testing (Citation6). Forced exhaled nitric oxide (FeNO) was measured using nitric oxide monitor (NIOX VERO, Circassia) following guidelines from the American Thoracic Society (Citation15).
OEP data was collected pre- and post-EVH challenge, and post-inhaler in participants who experienced a bronchoconstriction post EVH in a standing position. OEP data was recorded for the healthy group during tidal breathing. The study protocol is summarized in .
Optoelectronic plethysmography
OEP data was collected using 11 Qualisys cameras (Oqus 3, Qualisys AB, Goteborg, Sweden) sampling at 100 Hz. Wand and L-frame calibration was completed prior to data collection and accepted if average residuals for each camera were below 1.0 mm. A marker set of 89 (37 posterior, 10 lateral, 42 anterior) reflective markers were placed on the participants torso in a grid-like pattern (Citation16). The method outlined by Masseroni et al. (Citation16) was used to compute compartment volumes for each frame and this volume-time history was used to obtain the various breathing pattern measures. Briefly, grid-like pattern allowed for the torso to be split into three compartments namely, the abdomen (Ab), abdominal ribcage (RCa), and the pulmonary ribcage (RCp). A further subdivision representing the shoulder compartment was computed from the top two rings of markers (Citation14) (). In order to compute the volume in each of these compartments, groups of 3 or 4 markers on the chest surface are used to form prisms and tetrahedral geometric shapes. The volume of these shapes can be straightforwardly calculated, and the shapes making up each compartment subdivision are summed to give a compartment volume for each frame of data (Citation16).
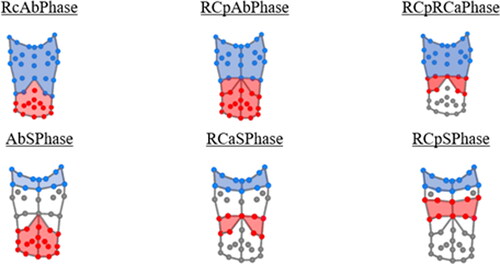
Figure 2. Illustration of the compartments related to each phase angle parameter. The blue and red areas show which sections of the thorax are involved in calculating each phase angle. The phase angle represents the synchrony between the two areas shown as described above.
Breathing pattern was analyzed, via OEP, at rest in all participants. Breathing pattern was also analyzed in EIB participants as soon as a fall in FEV1 at two consecutive time points post EVH was evidenced (see below) and again 10 min after a participant had inhaled 200 µg salbutamol. All OEP breathing pattern assessments took place after the spirometry measures at the various time points.
Eucapnic voluntary hyperpnoea (EVH) test
A spirometer (ML3500 Micro Medical Spirometer, Cardinal Health, UK) was used to measure baseline and post-EVH lung function. Forced expiratory volume in one second (FEV1), forced vital capacity (FVC), FEV1:FVC ratio (FEV1/FVC), peak expiratory flow (PEF), and forced expiratory flow between 25 and 75% of FVC (FEF25-75) were recorded in accordance with the European Respiratory Society criteria (Citation17).
The EVH challenge involved participants inhaling a dry gas mixture consisting of 74% nitrogen, 21% oxygen, and 5% carbon dioxide for six minutes. Participants aimed to achieve a target minute ventilation (VE) of 85% of their predicted maximal voluntary ventilation rate (baseline FEV1 multiplied by 30) (Citation18). Two maximal flow-volume loops were recorded 3, 5, and 10 min post-EVH challenge with the flow-volume loop with the highest FEV1 being recorded. A positive EVH result was defined as a fall by at least 10% in FEV1 from baseline at two consecutive time points (Citation6). If participants displayed this fall in FEV1, they were given 200 µg inhaled β2-agonist and performed flow-volume loop maneuvers 10 min post bronchodilator. Participants were instructed to stay in the laboratory until their FEV1 returned to within 10% of their baseline.
Data analysis
OEP data was processed using Qualisys Track Manager (v2019.2 Build 4610). Custom-built MATLAB (version R2019a) scripts were used to calculate OEP derived-breathing parameters for both population groups. The following time-derived parameters were calculated: respiratory rate (RR), inspiratory time (tI), expiratory time (tE), and total breath time (tTot).
Regional contribution parameters calculated consisted of various compartment percentage contributions to the total breath volume from the pulmonary ribcage (RCp%), abdominal ribcage (RCa%), and abdomen (Ab%).
The phase is a measure of the amount of asynchrony in the temporal movement of one torso compartment in relation to another during each breath and can be visually represented using Konno-Mead loops (Citation19). A value of zero is taken to indicate perfect synchronization between compartments during the exhale and inhale, deviations away from zero indicate asynchrony. This study calculated six measures of asynchrony between these chest compartments (). These were: phase between the ribcage and the abdomen (RcAb-Phase), between the pulmonary ribcage and the combined abdominal ribcage and the abdomen (RCpAb-Phase), and between the pulmonary and abdominal ribcage (RCpRCa-Phase). Finally, the phase between the shoulders and various compartments were calculated including the abdominal ribcage (RCaSPhase), the bottom of the pulmonary ribcage (RCpS-Phase), and the abdomen (AbS-Phase).
Statistical analysis
95% upper and lower confidence intervals were calculated for each OEP parameters across each condition i.e. rest, evoked, and recovery. A repeated measures ANOVA was used to analyze the differences between EVH conditions within EIB group. The healthy group at rest was compared to the EIB group for each of the EVH conditions using unequal variance independent samples t-tests. Checks for normality and sphericity were performed as appropriate and passed and the significance level was set at 0.05.
Results
From the 36 individuals who reported respiratory symptoms and/or had a previous diagnosis of asthma and met the inclusion criteria, 10 participants had a positive EVH challenge (). Baseline FEV1 ranged between 80 and 124% of predicted value across the EIB group. The participants included in the EIB group all experienced a reduction of ≥10% in FEV1 from baseline at two consecutive time points i.e. positive EVH result. All EIB participants experienced their second consecutive fall in FEV1 >10% baseline five minutes after competition of the EVH challenge. The mean and standard deviation FeNO for the EIB group was 61 ± 37 ppb.
The spirometry data () in the EIB group displayed a significant reduction in FEV1, FVC, and FEV1/FVC post-bronchoconstriction from resting baseline values (p < 0.05). Post-salbutamol inhaler, FEV1 (p = 0.02), FVC, and FEV1/FVC (p < 0.01) displayed an increase from the post-EVH spirometry measurements. FEV1/FVC was significantly greater in the healthy participants when compared to the EIB group (p < 0.01).
Table 2. Mean ± standard deviation spirometry data for the healthy group at rest and the EIB group across the EVH conditions.
display the mean, standard deviation, and upper and lower 95% confidence intervals for each breathing pattern metric during each condition of the EVH challenge for individuals with EIB. These tables also display the significant changes across the EVH challenge time points within the EIB group. VE was increased post-bronchoconstriction compared to rest (p < 0.01) (). RCpRCa-Phase, RCaS-Phase, and RCpS-Phase also displayed significant differences (p < 0.01) between rest and post-bronchoconstriction (). RCpS-Phase also displayed a significant difference (p = 0.03) between post-bronchoconstriction and recovery (). All other parameters were non-significant across rest, post-bronchoconstriction, and recovery.
Table 3. Exercise-induced bronchoconstriction (EIB) mean ± standard deviation (SD) and 95% confidence intervals (CI) for the basic respiratory and timing breathing parameters across each EVH challenge time points.
Table 4. Exercise-induced bronchoconstriction (EIB) mean ± standard deviation (SD) and 95% confidence intervals (CI) for the regional contribution breathing parameters across each condition for the phase angle breathing parameters across each condition.
Table 5. Comparison of the EIB group at rest, evoked, and during recovery to the healthy group at rest. * significant with p ≤ 0.05.
displays comparison between healthy individuals at rest and individuals with EIB at rest, post-bronchoconstriction, and recovery. RR, tE and tTot displayed significant differences between the two groups at rest. All other breathing parameters were non-significant between the two groups at rest. Vt, RCpRCa-Phase, RCaS-Phase, and AbS-Phase were significantly different between healthy individuals at rest and individuals with EIB post-bronchoconstriction. When comparing the healthy group at rest to the EIB group during recovery, RCpS-Phase was significantly larger for the EIB group. All other breathing parameters were non-significant.
Upper torso compartment movement
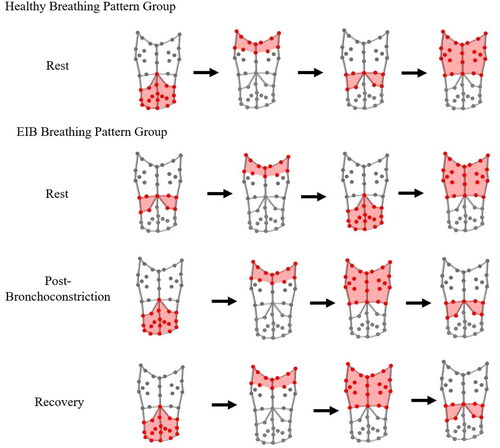
By using all of the phase angle values and signs, it is possible to determine the order in which all of the compartments move in relation to each other during a breath, providing insight into the overall coordination of the compartments. For the healthy group at rest, the abdomen seems to be the leading compartment, followed by the shoulders, the lower ribcage, and finally the upper ribcage (). For the EIB group at rest, the leading compartment was the lower ribcage, followed by the shoulders, the abdomen, and the upper ribcage. However, post-bronchoconstriction the abdomen leads the breath, followed by the shoulders, upper ribcage, and lower ribcage. During recovery, the coordination did not revert back to the resting coordination. Although the values of the breathing pattern parameters reverted back toward the resting values (see ), this compartment movement order potentially indicates that full recovery was not yet complete despite reversibility of FEV1 to within 10% of resting baseline value.
Discussion
This study, for the first time, demonstrates alterations in key OEP-derived breathing pattern parameters during bronchoconstriction induced by the EVH challenge in athletes with EIB. The key breathing pattern metrics that changed significantly pre and post EVH were the measures of asynchrony, namely RCpRCa-Phase, RCaS-Phase, and RCpS-Phase. Our findings also identify significant differences in the OEP-derived Vt, RCpRCa-Phase, RCaS-Phase, and RCpS-Phase between physically active adults without EIB at rest and with EIB during bronchoconstriction. These findings demonstrate the potential utility of novel phase angle metrics quantifying the synchrony between the shoulder compartment and other chest compartments in EIB.
The phase angles between the upper and lower ribcage (RCpRCa-Phase), between the shoulders and the lower ribcage (RCaS-Phase) and between the shoulders and the upper ribcage (RCpS-Phase) displayed a significant increase in value from rest to bronchoconstriction in the EIB group. RCpS-Phase also demonstrated a significant reduction in phase angle during recovery compared to the bronchoconstriction condition (). This indicates that during bronchoconstriction athletes with EIB display more asynchrony in relation to these phase angles compared to rest, with the upper ribcage (RCp) moving prior to the lower ribcage (RCa), and the shoulders moving before both the upper and lower ribcage.
When comparing the athletes with and without EIB at rest, there were no significant differences in any of the phase angle parameters (). Similarly, Hmeidi and colleagues (Citation20) found no significant differences between asthmatics and healthy individuals when measuring RcAb-Phase with Structured Light Plethysmography at rest. When comparing the EIB group during bronchoconstriction to the healthy group at rest, RCpRCa-Phase, RCaS-Phase, and RCpS-Phase were significantly greater in the EIB group during bronchoconstriction indicating greater asynchrony between these compartments. Individuals with different respiratory diseases exhibit distinct breathing pattern adjustments to cope with different ventilatory demands, such as exercise (Citation21). The increase in asynchrony that is associated with bronchoconstriction in individuals with EIB may be a compensatory mechanism in order to cope with the airway obstruction. OEP has previously been used to assess breathing pattern changes in asthmatics during bronchoconstriction induced by both histamine (Citation10) and methacholine (Citation11). However, phase angle parameters were not included in these studies. Post-inhaler, RCpS-Phase was significantly greater in the EIB group compared to the healthy group at rest ( and ).
As RCpS-Phase was also significantly lower post-inhaler in comparison to during bronchoconstriction, this indicates that this phase angle began to revert to its resting value post salbutamol inhaler but had not recovered completely. The other phase angles seemed to have reverted back to their resting state post-inhaler, with RCpS-Phase as the only phase angle potentially demonstrating a delayed response to the inhaler. This finding suggests that individuals with EIB who are using effective inhaler therapy and preventing bronchoconstriction may be capable of achieving an efficient breathing pattern.
This study demonstrates that although the compartment contributions were not significantly different between the groups (), the temporal movement of these compartments differs during bronchoconstriction. The order in which the compartments move during a breath may provide the basis for distinguishing between the groups. At rest, for the healthy breathing pattern group the abdomen is the first compartment to move with the upper ribcage being the final compartment (). For the EIB group at rest, the lower ribcage moves first and the upper ribcage is the last to move. Therefore, for both groups at rest, the upper ribcage is the final compartment to move. In contrast, during bronchoconstriction, the compartment order for the EIB group also changes with the abdomen now initiating the breath and the lower ribcage is the final compartment to move. This demonstrates that athletes with EIB display an altered breathing pattern in response to bronchoconstriction, with delayed movement of the lower ribcage ().
All of the regional contribution parameters in this study showed no significant changes across the conditions of the EVH challenge (). Similarly, no significant compartment contribution differences were found between those with EIB throughout the EVH challenge when compared to the individuals without EIB at rest (). This is comparable to previous research where Hmeidi and colleagues (Citation20,Citation22) found no significant differences between regional parameters when comparing asthmatic and healthy children at rest using SLP. Similarly, regional parameters, such as RcCT, have been shown to display no significant changes with the use of a bronchodilator in asthmatics when compared to a resting state (Citation20,Citation22). The findings of this study indicate that OEP-derived regional contribution parameters do not change post-bronchoprovocation during the EVH challenge in athletes with EIB, therefore, these parameters may not be essential for monitoring athletes with EIB.
The recruitment of athletes with EIB is challenging due to its under, over, and mis-diagnosis (Citation23–25). From the ten participants in this study, five individuals had a previous diagnosis of EIB/asthma and were currently taking asthmatic medication, while five individuals did not have a previous diagnosis. Additionally, six individuals with a previous diagnosis of EIB/asthma and currently taking medication were recruited but demonstrated a negative EVH test and therefore, were excluded from this study. This is in line with previous research which has demonstrated that physician-diagnosed asthma has a high rate of misdiagnosis, approximately 33% (Citation26). Two of the recruited individuals had a previous diagnosis but were unable to perform the EVH challenge as their baseline FEV1 was below 70%. The difficulty in recruiting individuals with EIB is not an uncommon issue due to the difficulty in diagnosing EIB (Citation27,Citation28). Previous studies have found that ∼15–44% of athletes recruited who reported exercise-induced respiratory symptoms demonstrated a negative provocation challenge response, while ∼8–24% of elite athletes demonstrated a positive provocation challenge response without reporting any exercise-induced respiratory symptoms (Citation8,Citation29). Although this study provides some interesting insight into breathing pattern changes in athletes with EIB, a larger sample size would be required to establish distinct cutoff points for these parameters in order to develop OEP as an alternative to spirometry and as a potential diagnostic tool for EIB in athletes. Additionally, this study only included those with mild to moderate EIB. Individuals with severe, chronic asthma may present with a similar or a unique breathing pattern profile at rest, however, in order to determine this further exploration is required using OEP.
In relation to the above point, the healthy breathing group were assumed not to have asthma or EIB based on their lack of previous history of asthma, absence of respiratory symptoms and normal lung function measurements. We acknowledge that some of this group may have presented with a positive EVH challenge had they undertaken one, as this has been previously shown (Citation23,Citation25). However, we are confident the healthy group provides a good representation of resting breathing pattern in people who do not report respiratory symptoms and do not have evidence of airway obstruction.
In conclusion, OEP breathing pattern response to the bronchoconstriction via EVH in athletes with EIB has been presented for the first time in this study, with alterations in breathing pattern coordination in line with traditional spirometry. OEP successfully identified significant differences between rest and bronchoconstriction with the following parameters: VE, RCpRCa-Phase, RCaS-Phase, and RCpS-Phase indicating greater compartment asynchronisation during bronchoconstriction leading to altered breathing mechanics. Changes in a novel asynchrony measure involving a shoulder compartment are presented for the first time. Additionally, when compared to athletes without EIB at rest, athletes with EIB demonstrated significantly larger Vt, RCpRCa-Phase, RCaS-Phase, and RCpS-Phase during bronchoconstriction. Although this preliminary data provides insight into the breathing patterns associated with EIB, a larger, more varied sample size would be required to establish these OEP-derived parameters, allowing OEP to distinguish between healthy athletes and athletes with EIB. Our data also suggest that when athletes with EIB use appropriate inhaler therapy to reverse bronchoconstriction their breathing patterns become synchronized, which suggests that athletes with EIB receiving appropriate inhaler therapy are capable of performing healthy breathing patterns.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Hull J, Ansley L, Price OJ, Dickinson JW, Bonini M. Eucapnic voluntary hyperpnea: gold standard for diagnosing exercise-induces bronchoconstriction in athletes? Sports Med. 2016;46(8):1083–1093. doi:10.1007/s40279-016-0491-3.
- Price OJ, Sewry N, Schwellnus M, Backer V, Reier-Nilsen T, Bougault V, Pedersen L, Chenuel B, Larsson K, Hull J. Prevalence of lower airway dysfunction in athletes: a systematic review and meta-analysis by a subgroup of the IOC consensus group on ‘acute respiratory illness in the athlete. Br J Sports Med. 2022;56(4):213–222. doi:10.1136/bjsports-2021-104601.
- Dickinson JW, Whyte GP, McConnell AK, Harries MG. Impact of changes in the IOC-MC asthma criteria: a British perspective. Thorax. 2005;60(8):629–632. doi:10.1136/thx.2004.037499.
- Levai IK, Hull JH, Loosemore M, Greenwell J, Whyte G, Dickinson JW. Environmental influence on the prevalence and pattern of airway dysfunction in elite athletes. Respirology. 2016;21(8):1391–1396. doi:10.1111/resp.12859.
- Hull JH, Jackson AR, Ranson C, Brown F, Wootten M, Loosemore M. The benefits of a systematic assessment of respiratory health in illness-susceptible athletes. Eur Respir J. 2021;57(6):2003722. doi:10.1183/13993003.03722-2020.
- Reier-Nilsen T, Sewry N, Chenuel B, Backer V, Larsson K, Price OJ, Pedersen L, Bougault V, Schwellnus M, Hull JH. Diagnostic approach to lower airway dysfunction in athletes: a systematic review and meta-analysis by a subgroup of the IOC consensus on ‘acute respiratory illness in the athlete’. Br J Sports Med. 2023;57(8):481–489. doi:10.1136/bjsports-2022-106059.
- Gowers W, Evans G, Carré J, Ashman M, Jackson A, Hopker J, Dickinson JW. Eucapnic voluntary hyperpnea challenge can support management of exercise-induced bronchoconstriction in elite swimmers. Transl Sports Med. 2021;4(5):657–666. doi:10.1002/tsm2.258.
- Vakali S, Vogiatzis I, Florou A, Giavi S, Zakynthinos S, Papadopoulos NG, Gratziou C. Exercise-induced bronchoconstriction among athletes: assessment of bronchial provocation tests. Respir Physiol Neurobiol. 2017;235:34–39. doi:10.1016/j.resp.2016.09.010.
- Beydon N, Davis SD, Lombardi E, Allen JL, Arets HG, Aurora P, Bisgaard H, Davis GM, Ducharme FM, Eigen H, et al. An Official American Thoracic Society/European Respiratory Society Statement: pulmonary Function Testing in Preschool Children. Am J Respir Crit Care Med. 2007;175(12):1304–1345. doi:10.1164/rccm.200605-642ST.
- Gorini M, Iandelli I, Misuri G, Bertoli F, Filippelli M, Mancini M, Duranti R, Gigliotti F, Scano G. Chest wall hyperinflation during acute bronchoconstriction in asthma. Am J Respir Crit Care Med. 1999;160(3):808–816. doi:10.1164/ajrccm.160.3.9712088.
- Filippelli M, Duranti R, Gigliotti F, Bianchi R, Grazzini M, Stendardi L, Scano G. Overall contribution of chest wall hyperinflation to breathlessness in Asthma. Chest. 2003;124(6):2164–2170. doi:10.1378/chest.124.6.2164.
- Feitosa LADS, de Britto MCA, Aliverti A, Noronh JB, de Andrade AD. Accuracy of optoelectronic plethysmography in childhood exercise-induced asthma. J Asthma. 2019;56(1):61–68. doi:10.1080/02770903.2018.1424196.
- Fregonezi G, Sarmento A, Pinto J, LoMauro A, Resqueti V, Aliverti A. Thoracoabdominal asynchrony contributes to exercise limitation in mild asthmatic subjects. Front Physiol. 2018;9:719–732. doi:10.3389/fphys.2018.00719.
- Smyth CME, Winter SL, Dickinson JW. Breathing pattern disorders distinguished from healthy breathing patterns using optoelectronic plethysmography. Transl Sports Med. 2022;2022:2816781. doi:10.1155/2022/2816781.
- American Thoracic Society Workshop. ATS workshop proceedings: exhaled nitric oxide and nitric oxide oxidative metabolism in exhaled breath condensate: executive summary. Am J Respir Crit Care Med. 2006;173(7):811–813. doi:10.1164/rccm.2601014.
- Massaroni C, Cassetta E, Silvestri S. A Novel Method to Compute Breathing Volumes via Motion Capture Systems: design and Experimental Trials. J Appl Biomech. 2017;33(5):361–365. doi:10.1123/jab.2016-0271.
- Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, Hallstrand TS, Kaminsky DA, McCarthy K, McCormack MC, et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200(8):e70–e88. doi:10.1164/rccm.201908-1590ST.
- Anderson SD, Argyros GJ, Magnussen H, Holzer K. Provocation by eucapnic voluntary hyperpnoea to identify exercise induced bronchoconstriction. Br J Sports Med. 2001;35(5):344–347. doi:10.1136/bjsm.35.5.344.
- Smyth CME, Winter SL, Dickinson JW. Novel real-time oep phase angle feedback system for dysfunctional breathing pattern training-an acute intervention study. Sensors. 2021;21(11):3714. doi:10.3390/s21113714.
- Hmeidi H, Motamedi-Fakhr S, Chadwick EK, Gilchrist FJ, Lenney W, Iles R, Wilson RC, Alexander J. Tidal breathing parameters measured using structured light plethysmography in healthy children and those with asthma before and after bronchodilator. Physiol Rep. 2017;5(5):e13168. doi:10.14814/phy2.13168.
- Wilkens H, Weingard B, Lo Mauro A, Schena E, Pedotti A, Sybrecht GW, Aliverti A. Breathing pattern and chest wall volumes during exercise in patients with cystic fibrosis pulmonary fibrosis and COPD before and after lung transplantation. Thorax. 2010;65(9):808–814. doi:10.1136/thx.2009.131409.
- Hmeidi H, Motamedi-Fakhr S, Chadwick EK, Gilchrist FJ, Lenney W, Iles R, Wilson RC, Alexander J. Tidal breathing parameters measured by structured light plethysmography in children aged 2-12 years recovering from acute asthma/wheeze compared with healthy children. Physiol Rep. 2018;6(12):e13752. doi:10.14814/phy2.13752.
- Dickinson JW, McConnell A, Whyte G. Diagnosis of exercise-induced bronchoconstriction: eucapnic voluntary hyperpnoea challenges identify previously undiagnosed elite athletes with exercise-induced bronchoconstriction. Br J Sports Med. 2010;45(14):1126–1131. doi:10.1136/bjsm.2010.072520.
- Ansley L, Kippelen P, Dickinson JW, Hull JHK. Misdiagnosis of exercise-induced bronchoconstriction in professional soccer players. Allergy. 2012;67(3):390–395. doi:10.1111/j.1398-9995.2011.02762.x.
- Molphy J, Dickinson JW, Hu J, Chester N, Whyte G. Prevalence of bronchoconstriction induced by eucapnic hyperpnoea in recreationally active individuals. J Asthma. 2014;51(1):44–50. doi:10.3109/02770903.2013.838256.
- Aaron SD, Vandemheen KL, FitzGerald JM, Ainslie M, Gupta S, Lemière C, Field SK, McIvor RA, Hernandez P, Mayers I, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. Jama. 2017;317(3):269–279. doi:10.1001/jama.2016.19627.
- Rundell KW, Wilber RL, Szmedra L, Jenkinson DM, Mayers LB, Im J. Self-reported symptoms and exercise-induced asthma screening of elite athletes: field versus laboratory exercise challenge. Med Sci Sports Exerc. 2001;32(2):309–316. doi:10.1097/00005768-200002000-00010.
- Weiler JM, Hallstrand TS, Parsons JP, Randolph C, Silvers WS, Storms WW, Bronstone A. Improving screening and diagnosis of exercise-induced bronchoconstriction: a call to action. J Allergy Clin Immunol Pract. 2014;2(3):275–280.e7. doi:10.1016/j.jaip.2013.11.001.
- Bougault V, Turmel J, Boulet LP. Bronchial challenges and respiratory symptoms in elite swimmers and winter sport athletes: airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest. 2010;138(2 Suppl):31S–37S. doi:10.1378/chest.09-1689.