Abstract
Concentrations of trace elements were measured in water (Cu, Zn, Fe, Pb, Mn, and Al), sediment (Cr, Cu, Mn, Pb, Ni, Al, Zn, Fe, and Co), and muscle tissue of nine selected fish species (Cu, Zn, Fe, and Mn) collected from eastern Kolkata (India) estuarine-sewage fed fish ponds. In water, trace elements existed in particulate phase (60–80%) with Fe as the predominant element followed by Al > Mn > Zn > Pb > Cu. The partitioning coefficients (Kd ) of the trace elements are low and fairly stable. The Pearson product moment correlation among the elements in the particulate and dissolved phase of the water column was calculated and most of the elements are correlated well (p ≤ 0.005). The trace element concentrations in sediments were in the following order: Al ≥ Fe > Mn > Zn > Cu > Ni > Cr > Pb > Co. Contamination factors (CFs) of trace elements in sediments were in the order of Pb ≥ Cu > Zn > Fe > Mn ≥ Ni ≥ Co > Al > Cr and Pollution Load Index ranges were 0.33–0.56. The CFs for Pb, Cu, and Zn are 0.92, 0.88, and 0.73, respectively, shows natural as well as anthropogenic inputs of these elements into the Kolkata sewage fed fish ponds. Accumulation of trace elements in muscle tissue of nine selected fish species were, Fe > Zn ≫ Cu > Mn Silver carp contained high Cu and Fe, American rohu contained high Zn concentration while Nylontica species contained high Mn concentration. Bio-concentration factor and bio-accumulation factor of elements, (Fe, Zn, Cu, and Mn) in fish, showed positive and negative accumulation factors when calculated against elements in the dissolved and suspended particulate matter phase of water, respectively. Iron (Fe), Mn, and Zn concentration in fish tissue were greater than WHO/FAO certified values; therefore, regular monitoring of trace elements is warranted for fish tissue collected from these estuarine-sewage fed fish ponds.
Introduction
The wetlands in the east of Kolkata (formerly Calcutta) are well known over the World for their multiple uses. These wetlands are the largest sewage fed wetlands in the World as they were included in the Ramsar List of Best Practice Wetlands (RLBPW) since November 2002. Particularly, in this region sewage-fed brackish water aquaculture has flourished since 1960 in estuarine areas 30 km east of Kolkata (Ghosh Citation1988; WBSLUB Citation1984). To the east of Kolkata, the natural wetland has been modified into a cluster of ponds that grow fish on sewage influent. Pond effluent is then used for irrigation (Ghosh and Sen Citation1987). One of the earlier reports published jointly by the World Bank and the International Development Research Centre, Canada, reused sewage in fish culture, algal and aquatic plant production, as well as energy production and have been identified as new and promising technologies that radically change the context of urban sanitation (Rybczynski, Polprasert, and McGarry Citation1978). In the course of the last decade, both natural and artificial wetlands are increasingly being identified as efficient ecosystems for improving wastewater quality, and recovering its nutrients (Chindah, Braide, and Sibeudu Citation2004; Maltby Citation1986; Mitsch and Gosselink Citation1986).
Though preventive measures have been taken to reduce the input of trace elements into oceans, rivers, estuaries, and wetlands, accumulation in different aquatic systems have been reported even today (Apeti, Robinson, and Johnson Citation2005; Paller and Littrell Citation2007; Sankar et al. Citation2006). Surface water chemistry is commonly considered for an assessment of trace element pollution in aquatic environments. However, concentrations of elements in sediments, and water provide an indication of the health quality of the associated water and wetland systems. Excessive levels of elements in the aquatic environment can affect aquatic wildlife (MacFarlane and Burchettt Citation2000; Remyla et al. Citation2008) and pose a risk to fish consumers, such as humans and other wildlife. Particularly, 12 trace elements (aluminum [Al], antimony [Sb], arsenic [As], beryllium [Be], cadmium [Cd], chromium IV [Cr], cobalt [Co], lead [Pb], manganese [Mn], mercury [Hg], nickel [Ni], and selenium [Se]) have been recognized as ‘hazardous pollutants’ by the USEPA (Glanze Citation1996). The major anthropogenic source of these trace elements are industrial sectors these include present and former mining activities, foundries and smelters, and diffuse sources such as piping, constituents of products, combustion by-products, and vehicle exhaust. Elements like Fe and Mn are required for metabolic activity in organisms, but some other elements like As, Cd, Cr, Cu, Hg, Ni, Pb, and Zn exhibit toxicity, thus necessitating regular monitoring of sensitive aquatic environments for these elements (Peerzada et al. Citation1990). Considerable attention has been paid concerning the ubiquitous occurrence of trace elements in various ecosystems and in different environmental media (Amaraneni Citation2006; Karadede, Oymak, and Unlu Citation2004; Kannan et al. Citation1993; Sajwan et al. Citation2008; Senthil Kumar et al. Citation2008; Storelli et al. Citation2005).
Since 1929, Kolkata's eastern wetlands have supported sewage fed fisheries recycling wastewater for production and culturing of fish, and the development of green belt (Jana Citation1998). The presence of different elements in wastewater has been reported in relatively high concentrations as it originates from domestic and industrial sources (Chattopadhyay, Chatterjee, and Mukhopadhyay Citation2002). The suspended particles in sewage are partly settled by traveling through its course and sedimentation. The supernatant with low suspended solids is used for fish farming. For some trace elements, toxic levels can be just above the background concentrations naturally found in the environment. One of the recent studies (Talapatraa and Banerjee Citation2007) reported that abnormality in fish collected from Kolkatta wetlands was due to high occurrences of toxic elements such as Cr, Zn, Cu, Pb, Mn, and Fe delivered through wastewater treatment. Therefore, it is important through regular monitoring for us to identify trace elements, and to take protective measures against excessive exposure during consumption by humans. Consequently, in this study, we emphasized comprehensive measurement and distribution of trace elements, also inter-trace element correlations in three major compartments (water, sediments, and fish muscle) of sewage fed fish ponds in eastern Kolkata wetlands. Further, we compared certified intake levels with available certified safety guidelines proposed by Interim Sediment Quality Guidelines (ISQG), World shale value, Tasmania Public Health Regulation, Ministry of Agriculture, Fisheries and Food (MAFF) and acceptable daily intake proposed by World Health Organization/Food and Agriculture Organization (WHO/FAO).
Materials and methods
Description of sampling area
The eastern Kolkata wetland previously was a brackish water lagoon swamp, but as fresh drainage water was supplied from Kolkata, it became suitable for raising fish. The fish farms consist of units of various sizes (70 ≥ 2800 hectares). The Kolkata population consumes approximately 13,000 tons of fish produced annually in these ponds. The wetlands lie between latitude 22°25′–22°40′ North and longitude 88°20′–88°35′ East. The present study area was located between latitude 22°34′–22°26′ North and longitude 88°23′–88°31′ East.
Sampling
To study the distribution of trace elements in the different compartments of Kolkata wetlands, sampling was conducted from December 2004 to January 2005. Water and sediment samples were collected from 12 different ponds, at approximately the mid-point of each wetlands. The mid-point is the well mixed zone of sufficient depth (4–5 ft) to move a wooden boat on the pond. The mid-points were selected after visual observation of banks of the ponds, because banks were disturbed with local human activities, shallow in depth (1–2 ft) and covered with water Hyacinths. Surface water samples were collected at a depth of 1 m using clean stainless steel buckets, which were labeled and stirred in pre acid washed polyethylene bottles, then stored. Surface sediment samples (n = 6) were collected using a Van Veen grab sediment sampler. Samples were labeled and packed in clean polyethylene bags, then stored. Nine selected fish species (n = 39) were collected from local fishermen, originating from the fish ponds where water and sediment were collected. Fish samples were labeled and stored at −4°C until pre-treatment and analysis.
Treatment of samples
Trace element concentration was estimated from both dissolved and particulate matter. The water samples were filtered using Millipore (0.45 µm pore size) filter paper. Filter papers with particulate matter were retained for particulate phase trace element estimation. The filtrate containing dissolved phase trace elements were poured into clean polyethylene containers then acidified to a pH of <2. The filter papers with particulate phase were dried at 40°C for 12 h, weighed and carefully transferred into digestion vessels and gently digested/heated with 5 mL of concentrated HNO3 in a water bath and evaporated to 5 mL. The digested sample was cooled and diluted to known volume in a volumetric flask with de-ionized distilled water and stored in pre acid washed glass bottles. The duplicate blanks were prepared from distilled water carried through all the above procedures. The particulate-bound Cu, Zn, Fe, Pb, Mn, and Al and the dissolved phase Cr, Ni, and Co elements were analyzed based on APHA, AWWA, and WEF (Citation1998); Creed and Martin (Citation1997) methods. Briefly, filter papers containing particulate phase were digested with aqua regia acid mixture. The filter papers were transferred to acid washed centrifuge tubes after adding 5 mL acid mixture, samples were refluxed in a water bath for 1–2 h (at 900°C) till complete dissolution of membrane filters and no yellow fumes were visible. The cooled digested samples were diluted to known volume and centrifuged to get clear supernatant. The supernatants were stored for further instrumental analysis. The filter paper blanks were prepared as above to check any cross contaminations.
Sediment samples were air dried, ground to a fine powder, and then passed through a plastic sieve (100 mesh size). Sieved sediment samples were processed as per USEPA method 3050 (Edgell Citation1998). Mainly Cr, Cu, Mn, Pb, Ni, Al, Zn, Fe, and Co were analyzed from the sediment samples. Five grams of sieved, air-dried sediment sample were placed in a 50 mL screw-capped centrifuge tube, and 40 mL of digestion acid and H2O2 was added and shaken for 30 min. The samples were centrifuged, and the supernatant was filtered through Whatman 42 filter paper and analyzed.
Fish samples were thoroughly washed with Milli-Q water and after removing the scales, the muscle portion was extracted for further processing. Muscle tissue was macerated into 1–2 cm clumps, dried at 70–80°C, powdered and stored until chemical analysis. Trace elements (Cu, Zn, Fe, and Mn) were analyzed by digesting the homogenized samples in a mixture of nitric, perchloric, and sulfuric acids (Honda, Tatsukawa, and Fujiyama Citation1982) then centrifuged. After being centrifuged the supernatant was filtered through leur-lock syringe filter paper (45 µm pore size), and analyzed.
Instrumental analysis
Determinations of trace elements were carried out using Flame Atomic Absorption Spectrometry (FAAS, Thermo Finnigan, UK). Performance of the instrument was checked by analyzing the reference standard material solutions (Merck NJ, USA); concurrently to check the precision of the instrument. To compensate for matrix effects between samples and standards, blank samples were analyzed in each batch. All the samples were analyzed in triplicate. The detection limits for Cu, Zn, Fe, Pb, Mn, Cr, Ni, Co, and Al were, 0.05, 0.01, 0.06, 0.10, 0.03, 0.05, 0.06, 0.05, and 0.30 mg L−1, respectively. The accuracy of the method for all the elements was checked by analysis of reference material (SW 8022), and the obtained results were comparable to the acceptable limits (). In our study, Cr, Pb, Al, and Co were less than the certified values (0% to −5%) while, Cu, Mn, Ni, Zn, and Fe were above than the certified values (+3% to +12%).
Table 1. Comparison of measured trace elements with reference value of certified standard reference material (SW-8022).
Calculations and statistical analysis
Inter-trace element correlations in the three major compartments of pond water, sediment, and fish muscle were investigated. The calculated correlations are based on Pearson product moment coefficients and presented in correlation matrices (Pentecost Citation1999). The Pollution Load Index (PLI) was computed (Tomlinson et al. Citation1980) after calculating the contamination factor (CF) based on the equation given below:
Pollution Load Index was calculated using MS excel software for each location (e.g., 0.55 at location 1) using the formula PLI = (B2 * C2 * D2 * E2 * F2 * G2 * H2 * I2)⁁(1/8). After calculating for individual locations, area-wise PLI were calculated using the following equation = (g2 * g3 * g4) ⁁ (1/3).
The inter-relationship between concentrations of dissolved phase, their total suspended matter and fish have been discussed with corresponding partitioning coefficients and bio-concentration factor (BCF) and bio-accumulation factor (BAF). BCF is defined as the trace element accumulation in an organism from its surrounding water. Therefore, BCFs can be calculated using elements in fish tissue/elements in dissolved phase of water while BAF represents the trace element accumulation in an organism from its food source. Hence, BAF of trace elements were calculated using elements in fish tissue/elements in particulate matter. Species differences of trace element content have been calculated by STATOGRAPHICS Plus 5.1 version (1994–2001) By Statistical Graphics Corporation, USA.
Partitioning coefficient
The partitioning coefficient is defined as the ratio of the particulate trace element concentration (µg kg−1) over the dissolved trace element concentration (µg L−1). The simple equation for calculating Kd = Particulate trace element concentration (µg kg−1)/Dissolved trace element concentration (µg L−1).
Results and discussions
Elements in water column
Concentration of trace elements in dissolved, particulate phase, and total phase from the water column has been shown in . Most of the elements (Cu: 76%, Pb: 69%, Zn: 60%, and Fe: 90%) existed in particulate phase relative to the total trace element load. On the other hand, Al: 52% and Mn: 68% were slightly greater in the dissolved phase than the particulate phase. Aluminum has an equal affinity with the dissolved and particulate phase binding capacity. These results also suggested that Mn is more soluble than other elements. Mn concentration in brackish water totally depends on the salinity of the water and not on the system Eh value (Jayaprakash et al. Citation2005). Mn is enriched by a factor of three at low salinity (17.8 parts per thousand) than in higher saline (35.8) conditions. This indicates that dissolved Mn adsorption could be on suspended particulate matter (SPM) and, in higher saline regions, could be derived from desorption from SPM (Knox et al. Citation1981; Manjunatha and Shankar Citation2000).
Table 2. Trace element concentrations in dissolved (µg L−1) and particulate matter (µg kg−1) in water from a sewage fed fish pond in eastern Kolkata wetlands.
Pearson product moment correlation coefficients were calculated and presented in and . Significant correlation (p < 0.05) occurred between total suspended solids (TSS) and trace elements Cu, Zn, Fe, and Pb. The strongest correlation was observed between Cu and Zn (r = 0.79), followed by Zn and Pb (r = 0.73). A considerable correlation was also observed among Pb, Cu, Zn, and Fe and among Fe, Mn, and Al (r = 0.43–0.60). Similar findings were reported by Nguyen et al. (Citation2005) and Jain and Sharma (Citation2001). While Mn and Al showed no significant correlation with TSS, both of the elements correlation was less significant with Fe (r = 0.43 and r = 0.60; ). All these results suggesting the association of trace elements with particulates could reflect contamination in the effluent that enter the ponds. Further, re-suspension of bottom sediments will cause more trace elements in the water column due to agitation by fishermen, movement of bottom dwelling fish species, wildlife, and shift in current due to winds. It was observed that there is no correlation between the trace elements of dissolved phase (data not shown). Nguyen et al. (Citation2005) reported similar results of no correlation among the dissolved elements in Balaton Lake of Hungary. Additionally, the literature reports only a few examples of correlations among dissolved elements (Munksgaard and Parry Citation2001).
Table 3. Pearson's correlation coefficients between TSS and particulate elements in the water from a sewage fed fish pond.
Table 4. Pearson's correlation coefficients between TSS and total elements in the water from a sewage fed fish pond.
Significant correlation (p < 0.05) has been noticed for TSS and total Cu, Zn, Fe, and Pb. While total Mn and Al showed negative, or no significant correlation with TSS (). Again, the Cu–Zn correlation is recognized as the strongest association (r = 0.85) suggesting that the recycling of these elements is to some extent linked. No other strong correlation was observed between the total elements with TSS. Lokeshwari and Chandrappa (Citation2006) reported similar results for a sewage fed lake in Belladur, Karnataka state in India. The strong dependence of total trace element concentrations on the amount of suspended solids reflects the importance of the particulate phase, especially for elements like Cu, Zn, Fe, and Pb, which have high kd values. Overall, the results show a strong correlation between suspended matter and total elements except Mn and Al that are observed in water column of the Eastern Kolkata wetland ().
Total trace element concentrations in water samples were found in the following order; Cu < Pb < Zn < Mn < Al ≤ Fe. Overall, the total trace element concentration of Cu, Pb, Zn, Mn, Al, and Fe were 9–48, 25–75, 27–190, 120–300, 730–4100, and 300–6600 µg L−1, respectively. It was observed that these average values for total trace element concentrations in sewage fed fish ponds were higher than recommended for aquatic life by the Canadian Council of Ministers of the Environment (CCME Citation2006) and natural elemental levels in fresh water (Ward Citation1995).
Partitioning of trace elements in water
The calculated values (mean and range) of (kd ) in Kolkata wetland water are presented in . The ranking of (kd ) mean values were observed in the following order; Fe > Pb > Cu > Zn > Mn > Al. The ranking of kd is due to specific characteristics of each trace element. The association of Fe, Pb, Zn, and Cu with high particles attached with particulate matter, while low particle association of Al and Mn allow them to remain in dissolved phase. The partitioning coefficient (kd ) of most of the elements between particulate and dissolved phases were generally low, and typical of natural waters, which were fairly stable over the Kolkata wetland waters. Similar kd values for Cu, Pb, and Zn are also observed in the north Australian coast (Munksgaard and Parry Citation2001), Scheldt estuary, UK (Baeyens et al. Citation1998) and lake Balaton, Hungary (Nguyen et al. Citation2005). Similar observations were reported in Texas and Atlantic ocean water (Benoit et al. Citation1994; Helmers Citation1996). Comparison of trace elements in water from our study with those of other studies were higher when compared to Ennore Creek, Chennai, India (Jayaprakash et al. Citation2005), Kolleru lake wetland, India (Amaraneni Citation2006), Apalachicola Bay, Florida, USA (Apeti et al. Citation2005), and Esmoriz-Paramos lagoon, Portugal (Fernandes et al. Citation2007).
Table 5. Comparison of partitioning coefficients (log K d ) of trace elements from different countries.
Elements in sediments
Concentrations of trace elements in sediment samples are illustrated in . The order of abundance of elements were observed in the decreasing order of Fe ≥ Al ≫ Mn > Zn > Cu > Ni ≥ Cr > Pb > Co, which follows a natural progressive concentration of trace elements in sediments (Turkian and Wedepohl Citation1961). This same ranking was also reported by other workers (Krupadam, Sarin, and Anjaneyulu Citation2003; Pradhan, Gauda, and Panigrahy Citation1999; Sajwan et al. Citation2008; Senthil Kumar et al. Citation2007) in different aquatic ecosystems. The concentration of iron (Fe) in this study was observed as 14,000–35,000 µg kg−1. Bottom sediments accumulate elements, which will affect the near-bottom water layer due to re-suspension or dissolution processes (Khan et al. Citation1998). Trace element contaminated sediment may act as a secondary pollution source for aquatic ecosystem and elements concentrations in sediment is also useful for the estimation of pollution trends (Forster and Wittman Citation1979; Salomons Citation1985).
Table 6. Trace element concentrations (µg kg−1 dry wt.) in sediment from sewage fed fish pond.
The Pearson's correlation coefficients are illustrated in . The correlations between elements Mn, Pb, Ni, and Al are significant at p ≤ 0.05. Among these results, the correlations between Cr and Mn (r = 0.84), Cu and Pb and Ni (r = 0.82–0.84) are the most significant. Other higher correlations are recognized for the groups, Cr–Al (r = 0.74), Pb–Ni and Fe (r = 0.69–0.73), and Ni–Fe (r = 0.70). No other significant correlations were observed between the elements in sediments of Kolkata wetland. The differences in correlations between the elements in sediments depends on physical, chemical, and biological processes in the aquatic environment as well as discharging of pollutants through sewage and other anthropogenic activities, and their effects on the partitioning of elements in the aquatic system (Baeyens et al. Citation1998). The mean concentration of all the elements in sediments does not exceed the recommended values (CCME Citation2002; GESAMP Citation1982; Turkian and Wedepohl Citation1961).
Table 7. Pearson's correlation coefficients between trace elements in sewage fed fish pond sediment.
The CF and PLI of elements in sediments
A convention for pollution assessment is the PLI obtained by measuring the trace element contents, and deriving CFs by referencing the baseline trace element levels. Another useful protocol is the CF which is defined as the ratio of observed concentration of trace element over background sediment trace element contents (Turkian and Wedepohl Citation1961). CFs for nine selected trace elements was calculated and a PLI was computed after Tomlinson et al. (Citation1980). The mean and range along with their standard deviations (SD) of CF and PLI are presented in . Although discharge of elements into this pond occurred; mean value of PLI (0.44) for the studied elements suggests that a minimal environmental or human health hazard involving these elements was occurring. Additionally, when a maximum PLI (0.56) occurs; this level is considered to produce minimum hazard to sediment dwelling organisms (Tomlinson et al. Citation1980). Therefore, it may be mentioned that sediment enriched with elements (high PLI) should be monitored during conditioning of fishponds for fish culture, and adjusted to the levels proposed by sediment quality guidelines (SQG)(CCME Citation2002).
Table 8. The CFs and PLI of trace elements in sediments from fishpond.
Comparison of trace elements in sediments from our study with those of other studies revealed similar or less concentrations than in highly urbanized Coimbatore wetlands in Tamil Nadu, India (Mathew et al. Citation2003), Kolleru lake wetland, India (Amaraneni Citation2006), Esmoriz-Paramos lagoon, Portugal (Fernandes et al. Citation2007), and Lower Hutt, New Zealand (Deely et al. Citation1992). However, the present study levels were higher when compared to Avon-Heathcote Estuary, New Zealand (Rodrigo Citation1989), Guanabara Bay, Brazil (Crapez, Neto, and Bispo Citation2003), Niger Delta, Nigeria (Chindah, Braide, and Sibeudu Citation2004), St. Louis Bay, Mississippi, USA (Elston et al. Citation2005), and Apalachicola Bay, Florida, USA (Apeti Robinson, and Johnson Citation2005).
Elements in muscles tissue of selected fish species
Nine common fish species that occupy the wetland were selected and analyzed for trace elements (Cu, Zn, Fe, and Mn) contamination. These fish species were selected based on their production and high consumption by the local population. The mean and SD values of trace elements in muscle tissue of selected fish species are presented in . The order of the trace element concentrations were: Fe ≥ Zn > Cu > Mn in the Catla, Tilapia, Silver carp, and Puti. While Rohu, Folie, and American rohu were Zn > Fe > Cu > Mn, Nylotica was Fe ≥ Zn > Mn > Cu, and Sol was Fe > Zn > Mn > Cu. The trace element hierarchy has also been reported in Taiwan coastal waters (Chen and Chen Citation1999). The range of trace elements, Cu, Zn, Fe, and Mn in fish muscles were 2.6–24, 14–66, 27–60, and 1.4–13 µg g−1, dry wt., respectively. While Mn are essential elements and are regulated by fish to maintain certain homeostatic levels (Chen and Chen Citation1999); marginally high levels of Cu, Fe, and Zn, however, are a major health concern. The tolerable intake of trace elements can be found in the United States Food and Drug Administration (USFDA Citation1993).
Table 9. Trace element concentrations (µg g−1, dry wt) in muscle tissue of fish from sewage fed fish ponds.
The trace element concentrations were markedly high in silver carp Hypophthalmichtrys molitrix (Cu: 24, Zn: 45, Fe: 59, Mn: 4.9 µg g−1, dry wt.). Trace element accumulations in muscle tissues are generally found to be species specific. The observed differences between the trace element concentrations in fish may be related to their feeding habits and the bio-concentration and bio-accumulation capacity (Farkas, Salanki, and Varanka Citation2000). Species differences of trace element concentrations were found among the nine species (p ≤ 0.05). The Cu, Zn, Fe, and Mn concentrations in muscle tissue differed greatly between species (p ≤ 0.05), showing a higher level in H. molitrix, whereas Folie showed the lowest trace element concentrations. The highest Cu, Zn, Fe, and Mn levels were in H. molitrix, American Rohu, Tilapia nylotica, and Oreochromis mossambica, respectively. Fe and Zn were higher than the other elements analyzed in fish tissue of nine species.
The water, sediment, and fish tissue element concentrations in this study were compared to those of recommended trace element standards (). Water column concentrations of Cu, Zn, Fe, Pb, and Al were several times greater than (Munksgaard and Parry Citation2001) “Canadian Council of the Ministers of Environment” and Ward (Ward Citation1995) (). Concentrations of Cu, Zn, Fe, Pb, Mn, Al, Cr, Ni, and Co in sediment in our study were comparable (GESAMP Citation1982; Salomons and Forstner Citation1984; Turkian and Wedepohl Citation1961) and lower than values stated in the ISQG and Probable Effective Level (CCME Citation2002). Concentrations of Cu, Zn, Fe, and Mn in fish muscle in this study were lower for Cu, but higher for Zn, Fe, and Mn when compared to Eustace (Citation1974); Sally, Michael, and Richard (Citation1996); WHO/FAO (Citation1984). These comparisons revealed that consuming fish from the fish ponds of eastern Kolkata wetland can have harmful effects due to slightly higher Fe, Zn, and Mn than WHO/FAO certified levels (). Concentration of Cu in fish tissue are far below the standards of trace elements concentrations set by various organizations such as Tasmania Public Health Regulation, MAFF, WHO, and FAO (). Therefore, under regular human consumption habits the intake of fish from these ponds should be monitored regularly in order to achieve the basic nutritional requirement for normal physical functions without causing adverse effects to human health.
Table 10. Comparison of resuts with recommended trace element standards in water, sediment, and fish from a sewage fed fish pond.
Concentrations of trace elements in fish species collected in this study were lower than muscle tissue of dolphins and their gut contents collected from the Ganges River (Kannan et al. Citation1993). However, observed elements were higher in the 22 fish species collected from Calicut in Kerala state, India (Sankar et al. Citation2006), Esmoriz-Paramos Lagoon, Portugal (Fernandes et al. Citation2007), toadfish from Sydney, Australia (Alquezar, Markich, and Booth Citation2006), and finfish from the Irish Coast (Bloxham et al. Citation1998).
Bio-concentration and bio-accumulation factors of elements in fish
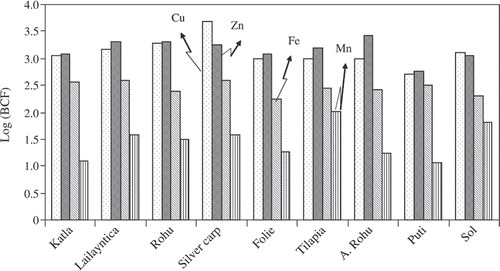
The BCF and BAF of trace element in biological samples are generally found to be organ specific. The bio-concentration and bio-magnification of the trace elements in fish may be related to their feeding habits, and the bio-accumulation capacity of each species (Farkas, Salanki, and Varanka Citation2000). The BCFs of elements in selected fish species from Kolkata fish ponds were shown in . The order of (BCF) range was observed as, Cu ≥ Zn > Fe > Mn. BCF values of Cu, Zn, Fe, and Mn in fish are 3.7, 3.4, 2.6, and 2.0, respectively. The geographical distribution of the (BCF) values for each trace element is almost stable over the ponds. However, fish from sewage fed fish ponds of Kolkata wetland accumulated more Cu; almost similar to the amount of Zn but less than Fe and Mn. Similar observations for Cu were reported elsewhere (Nguyen et al. Citation2005).
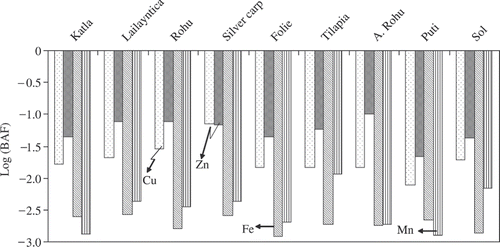
The distributions of the (BAF) values of Cu, Zn, Fe, and Mn in the fish ponds of Kolkata were illustrated in . The range and mean of the absolute (BAF) values for Cu, Zn, Fe, and Mn were observed as, −2.1 to −1.1 (−1.7), 1.7 to −1.0 (−1.3), 2.9 to −2.6 (−2.7), and −2.9 to −1.9 (−2.5), respectively. The mean value of absolute (BAF) indicates that the biodiminization of Fe and Mn from solid particulate matter was higher than Cu and Zn. Negative values of transferred BAFs revealed that trace element in the fish was not mainly from SPM. The fish analyzed in this study consumed other small aquatic wildlife (planktons, moss, crustaceans, and small fish species), as well as larger dead organic particles. Therefore, calculating the BMF against predator and prey interactions may be more appropriate. Further study is warranted for analysis of trace elements in the wildlife food webs of sewage fed fish ponds and to understand their predator–prey relationships.
Conclusions
The study reveals that municipal sewage and industrial effluent were considered to be the major sources of trace element inputs to the fish ponds of eastern Kolkata estuarine wetland. The results showed higher element concentration in the water column than recommended for the protection of freshwater aquatic life, but in sediments and fish muscle tissue, concentration of some elements was lower than recommended values. Higher concentration of total elements in water column may be due to insufficient settling conditions, and/or continuously transferred to other ponds due to high flow conditions. The partitioning coefficients (K d ) of studied trace elements between particulate and dissolved phases were typically low and stable. PLI of trace elements in sediments were in the range of 0.33–0.56 which was less than the average level that produces deleterious effects to aquatic organisms. These results revealed that consuming fish from the fish ponds of eastern Kolkata wetland can have harmful effects due to slightly higher Fe, Zn, and Mn than WHO/FAO certified levels. BCF of elements in fish showed a positive accumulation factor. Further intensive study is warranted for sewage fed fish ponds from eastern Kolkata because of possible adverse impacts, which can be expected in individuals who intake large quantity of the fish from this region particularly by Fe, Zn, and Mn. Further contamination of PCBs, DDTs, chlordanes, cyclodienes, HCHs, and several organochlorine pesticides in fish tissues should be analyzed due to greater usage of these chemicals in developing India.
Acknowledgments
Authors are very much thankful to the Chairman and Member Secretary Central Pollution Control Board for his constant encouragement and guidance to carry out this study.
References
- Alquezar , R , Markich , SJ and Booth. , DJ . 2006 . Metal accumulation in the smooth toadfish, Tetractenos glaber, in estuaries around Sydney, Australia . Environmental Pollution , 142 : 123 – 31 .
- Amaraneni , SR . 2006 . Distribution of pesticides, PAHs and heavy metals in prawn ponds near Kolleru lake wetland, India . Environmental International , 32 : 294 – 302 .
- Apeti , DA , Robinson , L and Johnson. , E . 2005 . Relationship between heavy metal concentrations in the American oyster (Crassostrea virginicia) and metal levels in the water column and sediment in Apalachicola Bay, Florida . American Journal of Environmental Sciences , 1 : 179 – 86 .
- APHA, AWWA, and WEF . 1998 . “ Standard methods for the examination of water and wastewater ” . In 9223 enzyme substrate Coliform Test, , 20th www.dac.gov.ae/NR/rdonlyres/E54F9F1E-D156-416D-887C-93E94FBC5CD4/0/Inspectoratecertificate.pdf
- Baeyens , W , Elskens , M , Gillain , G and Goeyens. , L . 1998 . Biochemical behavior of Cd, Cu, Pb and Zn in the Scheldt estuary during the period 1981–1983 . Hydrobiologia , 366 : 15 – 44 .
- Benoit , G , Oktay-Marshall , SD , Cantu , A , Hood , EM , Coleman , CH , Corapcioglu , MO and Santschi. , PH . 1994 . Partitioning of Cu, Pb, Ag, Zn, Fe, Al and Mn between filter retained particles, colloidal, and solution in 6 Texas Estuaries . Marine Chemistry , 45 : 307 – 36 .
- Bloxham , M , Rowe , A , McGovern , E , Smyth , M and Nixon. , E . 1998. Trace metal and chlorinated hydrocarbon concentrations in shellfish and finfish from Irish waters- 1996. Marine Environmental Series 2/98, Fishery Leaflet 179, Marine Institute I-18
- CCME . 2002 . “ Canadian sediment quality guidelines for the protection of aquatic life: Summary tables. Updated 2002 ” . In Canadian environmental quality guidelines, 1992 , Toronto : Canadian Council of Ministry and Environment .
- CCME . 2006 . “ Canadian sediment quality guidelines for the protection of aquatic life: Summary tables. Updated 2006 ” . In Canadian environmental quality guidelines , Toronto : Canadian Council of Ministry and Environment .
- Creed , JT and Martin. , TD . 1997 . Determination of trace elements in marine waters by stabilized temperature graphite furnace atomic absorption , Cincinnati, OH : US EPA, EPA 200.12 National exposure research laboratory office of research and development .
- Crapez , M , Neto , JAB and Bispo. , MGS . 2003 . Bacterial enzymatic activity and bioavailability of heavy metals in sediments from Boa Viagem Beach (Guanabara Bay). . Anuario do Instituto de Geociencias-UFRJ , 26 : 60 – 8 .
- Chattopadhyay , B , Chatterjee , A and Mukhopadhyay. , SK . 2002 . Bioaccumulation of metals in the East Calcutta wetland ecosystem . Aquatic Ecosystem and Health Management , 5 : 191 – 203 .
- Chen , MH and Chen , CY . 1999 . Bioaccumulation of sediment bound heavy metals in gray mullet, Liza macrolepis . Marine Pollution Bulletin , 39 : 239 – 44 .
- Chindah , AC , Braide , AS and Sibeudu. , OC . 2004 . Distribution of hydrocarbons and heavy metals in sediment and crustacean (Shrimps-Penaeus notialis) from the Bonny/New Calabar River Estuary, Niger Delta . African Journal of Environmental Management-RAGEE , 9 : 1 – 17 .
- Deely , JM , Tunnicliff , JC , Orange , CJ and Edgeriey. , WHL . 1992 . Heavy metals in surface sediments of Waiwhetu Stream, Lower Hurt, New Zealand . New Zealand Journal of Marine and Freshwater Research , 26 : 417 – 27 .
- Edgell , K . 1998. USEPA method study 37, SW-846 method 3050: Acid digestion of sediment, sludge and soils. EPA Contract No. 68-03-3254, November 1998
- Elston , R , Cake , EW Jr , Humphrey , K , Isphording , WC and Rensel. , JE . 2005 . Dioxin and heavy-metal contamination of shellfish and sediments in St . Louis Bay, Mississippi and adjacent marine waters. Journal of Shellfish Research , 24 : 227 – 41 .
- Eustace , IJ . 1974 . Zinc, cadmium, copper and manganese in species of fish and shellfish caught in the Denwent Estuary, Tasmania . Australian Journal of Marine and Freshwater Research , 25 : 209 – 20 .
- Farkas , A , Salanki , J and Varanka. , I . 2000 . Heavy metal concentrations in fish of lake Balaton lakes reservoirs . Research and Management , 5 : 271 – 9 .
- Fernandes , C , Fontainhas-Fernandes , A , Cabral , D and Salgado. , MA . 2007 . Heavy metals in water, sediment and tissues of Liza saliens from Esmoriz-Paramos lagoon, Portugal . Environmental Monitoring and Assessment , 118 : 65 – 74 .
- Forster , U and Wittman , GTW . 1979 . Metal pollution in aquatic environment , 82 – 86 . Germany : Springer Berlin . 197–210
- GESAMP (IMO/FAO/IJNESCO/WMO/IAEA/UN/IJNEP) 1982. The health of the oceans. Rep. Stand. GESAMP. 15: 108; UNEP Res. Season Rep. Study 16, 108. United Nations Environment Programme in Geneva, Switzerland
- Ghosh , D . 1988 . Proceedings of the International Seminar on Wastewater Reclamation and Reuse for Aquaculture . December 6–9 1988 , Calcutta, India.
- Ghosh , D and Sen. , S . 1987 . Ecological history of Calcutta's wetland conversion . Environmental Conservation , 14 : 219 – 26 .
- Glanze , WD . 1996 . Mosby medical encyclopedia rev. , St. Louis, MO : C.V. Mosby .
- Honda , K , Tatsukawa , R and Fujiyama. , T . 1982 . Distribution characteristics of heavy metals in the organs and tissues of striped dolphin, Stenella coeruleoalba . Agricultural Biology and Chemistry , 46 : 3011 – 21 .
- Helmers , E . 1996 . Trace metals in suspended particulars matter of Atlantic ocean surface water . Marine Chemistry , 53 : 51 – 67 .
- Jain , CK and Sharma. , MK . 2001 . Distribution of trace metals in the Hindon river system, India . Journal of Hydrology , 253 : 81 – 90 .
- Jana , BB . 1998 . Sewage-fed aquaculture: The Calcutta model . Ecological Engineering , 11 : 73 – 85 .
- Jayaprakash , M , Srinivasalu , S , Jonathan , MP and Mohan. , VRam . 2005 . A baseline study of physico-chemical parameters and trace metals in waters of Ennore Creek, Chennai, India . Marine Pollution Bulletin , 50 : 583 – 608 .
- Kannan , K , Sinha , RK , Tanabe , S , Ichihashi , H and Tatsukawa. , R . 1993 . Heavy metals and organochlorine residus in Ganges river dolphins from India . Marine Pollution Bulletin , 26 : 159 – 62 .
- Karadede , H , Oymak , SA and Unlu. , E . 2004 . Heavy metals in mullet, Liza abu, and catfish, Silurus triostegus, from the Ataturk Dam Lake (Euphrates), Turkey . Environment International , 30 : 183 – 8 .
- Khan , YSA , Hossain , MS , Hossain , SMGA and Halimuzzaman. , AHM . 1998 . An environmental assessment of trace metals in the Ganges-Brahamputra-Meghna estuary . Journal of Remote Sensing and Environment , 2 : 103 – 17 .
- Knox , S , Turner , DR , Dickson , AG , Liddicoat , MI , Whitefield , M and Butter. , EI . 1981 . Statistical analysis of estuarine profiles: Application to manganese and ammonia in the Tamar estuary . Estuarine Coast Shelf Science , 13 : 357 – 71 .
- Krupadam , RJ , Sarin , R and Anjaneyulu. , Y . 2003 . Distribution if trace metals and organic matter in the sediments of Godavary estuary of Kakinada Bay, East Coast of India . Water, Air, and Soil Pollution , 150 : 299 – 305 .
- Lokeshwari , H and Chandrappa. , GT . 2006 . Impact of heavy metal contamination of Belladur Lake on soil and cultivated vegetation . Current Science , 91 : 622 – 7 .
- MacFarlane , GB and Burchettt. , MD . 2000 . Cellular distribution of Cu, Pb, and Zn in the grey mangrove Avicemnia marina (Forsk.) . Vierh. Aquatic Botany , 68 : 45 – 59 .
- Manjunatha , BR and Shankar. , R . 2000 . Behaviour and distribution patterns of particulate metals in estuarine and coastal surface waters near Mangalore, southwest coast of India . Journal of Geological Society of India , 55 : 157 – 66 .
- Mathew , M , Mohanraj , R , Azeez , PA and Pattabhi. , S . 2003 . Speciation of heavy metals in bed sediments of wetlands in Urban Coimbatore, India . Bulletin of Environmental Contamination and Toxicology , 70 : 800 – 8 .
- Maltby , E . 1986 . Waterlogged wealth . An Earthscan Paperback . 1986 . London, , England : International Institute of Environment and Development .
- Mitsch , WJ and Gosselink , JG . 1986 . Wetlands , New York, , USA : Van Nostrand Reinhold Company .
- Munksgaard , NC and Parry. , DL . 2001 . Trace metals, arsenic and lead isotopes in dissolved and particulate phases of north Australian coastal and estuarine seawater . Marine Chemistry , 75 : 165 – 84 .
- Nguyen , HL , Leermakers , M , Elskens , M , Ridder , FD , Doan , TH and Baeyens. , W . 2005 . Correlations, partitioning and bioaccumulation of heavy metals between different compartments of Lake Balaton . Science of the Total Environment , 341 : 211 – 26 .
- Paller , MH and Littrell. , JW . 2007 . Long term changes in mercury concentrations in fish from the middle Savannah River . Science of the Total Environment , 382 : 375 – 82 .
- Peerzada , N , McMorrow , L , Skiliros , S , Guinea , M and Ryan , P . 1990 . Distribution of heavy metals in Gove Harbour, Northern Territory, Australia . Science of the Total Environmental Pollution , 10 : 19 – 38 .
- Pentecost , A . 1999 . “ Analysing environmental data ” . In Testing if a relationship occurs between two variables using correlation , 102 – 106 . England : Pearson education limited .
- Pradhan , B , Gauda , R and Panigrahy. , RC . 1999 . Distribution of some heavy metals in the sediments of the Rushikulya estuary east coast of India . Journal of Environmental Pollution , 6 : 301 – 5 .
- Remyla , SR , Ramesh , M , Sajwan , KS and Kumar. , KSenthil . 2008 . Influence of Zinc on Cadmium induced haematological and biochemical responses in a freshwater fish Catla catla . Fish Physiology and Biochemistry , 34 : 169 – 74 .
- Rodrigo , AG . 1989 . Surfacial sediment-heavy metal associations in the Avon-Heathcote Estuary, New Zealand . New Zealand Journal of Marine and Freshwater Research , 23 : 255 – 62 .
- Rybczynski , W , Polprasert , C and McGarry. , M . 1978 . “ Low-cost technology options for sanitation: A state-of-the art review and annotated bibliography ” . Ottawa, , Canada : IDRC-102e, International Development Research Centre .
- Sajwan , KS , Kumar , KSenthil , Paramasivarn , S , Compton , SS and Richardson. , JP . 2008 . Elemental status in sediment and American oyster collected from Savannah marsh/estuarine ecosystem: A preliminary assessment . Archives of Environmental Contamination and Toxicology , 54 : 245 – 58 .
- Sally , EC , Michael , SJ and Richard. , TL . 1996 . Metal contamination of angler-caught fish from the Mersey Estuary . Marine Environmental Research , 41 : 281 – 97 .
- Salomons , W . 1985 . Sediments and water quality . Environmental and Technological Letters , 6 : 315 – 26 .
- Salomons , W and Forstner. , V . 1984 . Metals in the hydrocycle , 2 Berlin-Heidelberg-New York-Tokio
- Sankar , TV , Zynudheen , AA , Anandan , R and Nair. , PGViswanathan . 2006 . Distribution of organochlorine pesticides and heavy metal residues in fish and shellfish from Calicut region, Kerela, India . Chemosphere , 65 : 583 – 90 .
- Senthil Kumar , K , Sajwan , KS , Richardson , JP and Kannan , K . 2008 . Contamination profiles of heavy metals, organochlorine pesticides, polycyclic aromatic hydrocarbons and alkylphenols in sediment and oyster collected from marsh/estuarine Savannah GA, USA . Marine Pollution Bulletin , 56 : 136 – 49 .
- Storelli , MM , Storelli , A , D'Addabbo , R , Marano , C , Bruno , R and Marcotrigiano , GO . 2005 . Trace elements in loggerhead turtles (Caretta caretta) from the eastern Mediterranean Sea: Overview and evaluation . Environmental Pollution , 135 : 163 – 70 .
- Talapatraa , SN and Banerjee. , SK . 2007 . Detection of rnicronucleus and abnormal nucleus in erythrocytes from the gill and kidney of Labeo bata cultivated in sewage-fed fish farms . Food and Chemical Toxicology , 45 : 210 – 15 .
- Tomlinson , DL , Wilson , JG , Harris , CR and Jafferey. , DW . 1980 . Problems in the assessment of heavy metal levels in estuaries and the formation of a pollution index . Helgolander Meeresunteres , 33 : 566 – 75 .
- Turkian , KK and Wedepohl , KH . 1961 . Distribution of elements in some major units of the earth's crust . Geological Society of America Bulletin , 72 : 175 – 92 .
- USFDA 1993. Guidance document for arsenic, chromium, nickel in shellfish. Center for Food Safety and Applied Nutrition. United States Food and Drug Administration. Washington, DC
- Ward , NI . 1995 . “ Environmental analytical chemistry ” . In Trace elements , Edited by: Fifield , FW and Hains , PJ . 320 – 328 . UK : Blake Academic and Professional .
- WBSLUB. 1984. Report of the fact finding committee set up by the West Bengal State Land Use Board on the problems of paddy-cum-brackishwater fish/prawn cultivation and forcible conversion of paddy lands into fisheries in parts of 24-Parganas District. Director of Land Records and Surveys, West Bengal, India
- WHO/FAO 1984. Contaminants: Joint FAO/WHO standards program. Codex Alimentarius, Vol. XVII. 1st ed. pp. 1–33. Rome, Italy