Abstract
Hepatic ethoxyresorufin-O-deethylase (EROD) activity of Oreochromis mossambicus was examined in response to naphthalene, a polycyclic aromatic hydrocarbon (PAH) as a bioindicator of exposure. This study also examined the effects of varying water parameters such as salinity, temperature, and pH on this fish model. Temperature, salinity, and pH produced cyclic changes in EROD activity which increases and/or decreases. After exposure to lower naphthalene concentrations up to 6 ppm, no marked change in activity was noted, whereas at higher concentrations EROD activity was increased. Data suggest that EROD measurement may be useful as a potential biomarker for the detection of hydrocarbon pollution.
Introduction
Currently aquatic systems are being used as dumping grounds for a variety of pollutants. Such xenobiotics accumulate within tissues of aquatic organisms due to either, direct exposure from water and sediments or, indirectly through the food chain, and result in different levels of impairments (Subramanian and Amutha 2006a). Fish, as a bioindicator species, play an increasingly important role in the biomonitoring of water pollution because they respond to more sensitive changes of the aquatic environment. Sudden fish mortality is indicative of the acute level of toxicity due to pollutants. Sublethal levels of pollutants following chronic exposure produce adverse physiological effects and tissue damage in vital organs including gill, liver, intestine, and kidneys, which can be measured by biochemical, physiological, or histological measures in fish (Mondon, Duda, and Nowak Citation2001; Subramanian and Amutha, Citation2006). Cytochrome P450 (CYP450) are the diverse multigene family of heme-containing proteins that oxidize, hydrolyze, or reduce compounds by inserting an oxygen atom into the substrate during the reaction cycle (Nebert, Puga, and Vasiliou Citation1993; Nelson et al. Citation1996). CYP 1A1 is the reliable biomarker for the polycyclic aromatic hydrocarbons (PAH) (Amutha et al. Citation2009) and ethoxyresorufin is a substrate for this enzyme. PAH are derived from petroleum, spilled fuel, street run-off, and coal tar from coal gasification, creosote treatment of wood, or coke ovens at steel plants (Ringuette et al. Citation1993). The metabolism of xenobiotics mainly occurs in liver (Daly et al., Citation1993; Guengerich et al. Citation1995).
The monooxygenase enzyme system as well as the conjugating enzymes serves to detoxify and eliminate many pollutants from the body. The monooxygenase enzymes decrease the lipid solubility of organic contaminants and thereby help to facilitate excretion of toxicants. Most of these enzymes are synthesized or activated in the liver on exposure to certain organic contaminants and thus may be used as bioindicators of specific contaminant exposure. There are two phases in the detoxification process. A polar reactive group is introduced into the organic contaminant during Phase I and thereby increasing its solubility in water to excrete or make it a more suitable substrate for Phase II conjugation reaction enzyme (Adams Citation1990; Arun and Subramanian Citation2007). Over 90% of Phase I reactions are mediated by CYP450 (Lewis, Loannides, and Parke Citation1998) The CYP450 gene responsible enzymes activate the oxidative metabolism of compounds including drugs and environmental pollutants. CYP450 formerly termed the “mixed function oxidases” are important oxidative enzymes in Phase I metabolism of the xenobiotics (Lewis Citation2001). Metabolism or biotransformation through Phase I (CYP450 dependent monooxygenase enzymes) and Phase II (conjugating enzymes) pathways is an important requisite for detoxification and excretion of lipophilic chemicals (Andersson and Förlin Citation1992; Arun et al. Citation2000). CYP4501A dependent monooxygenase ethoxyresorufin-O-deethylase (EROD) has an important function in the biotransformation of many xenobiotics and its specific induction is considered as a molecular marker in fish for exposure to PAH and planar halogenated hydrocarbons (PHH), which are usually aryl hydrocarbon receptor agonists (Stegeman and Hahn 1994; Bucheli and Fent Citation1995; Jewett et al. Citation2002).
CYP1A induction was observed with benzene, naphthalene, anthracene, acenaphthene, benzo [g,h,i,a] perylene, and fluorene, but low induction was found with fluoranthene and phenanthrene (Kennedy and Jones Citation1994; Fent Citation2003; Wang et al. 2006). CYP4501A dependent enzymes were found to be induced by polychlorinated biphenyls (PCB), PAH, dibenzofurans, and dibenzodioxins in mammals (van der Weiden et al. Citation1989; Lemaire-Gony and Lemaire Citation1992; Ueng et al. Citation1997). In the light of the above, this study was designed to investigate the induction of EROD by one of the hydrocarbons, naphthalene, under different dynamic aquatic conditions such as salinity, pH, temperature, and duration of exposure using Oreochromis mossambicus as a fish model.
Materials and methods
Animal selection
The exotic, edible euryhaline, omnivore fish, O. mossambicus, is a sturdy fish known to tolerate a wide range of aquatic environmental parameters. In general, tilapia survive in pH ranging from 5 to 10 but do best at pH range 6–9 and Mozambique tilapia grow well at salinities near or at full strength seawater (Popma and Masser Citation1999). Immature O. mossambicus fish of approximate uniform size were segregated from the stock (collected from the River Cauvery and maintained in 2 ton cement cisterns) and their length and weight (length 9–12 cm and weight 18–24 g) were measured. Fish were acclimated to lab conditions for 2 days, divided into groups of 10 individuals each and placed in tubs of 25 L of experimental medium.
CYP1A1 dependent EROD assay
Cytochrome P450 1A1 enzyme activity was measured using the EROD activity assay. EROD activity was determined from the rate of formation of resorufin from ethoxyresorufin according to the method of Lester et al. (Citation1993) and Lewis (Citation2001). Frozen liver tissue was ground into fine powder and 100 mg of sample centrifuged for 5 min at 450 ×g. The sample was decanted, supernatant discarded, and pellet collected. Lysis buffer equal to five times the cell pellet volume was added, incubated for 15 min in ice and was subsequently transferred for homogenization. The homogenate was centrifuged at 700 ×g for 10 min at 4°C, supernatant collected, and centrifuged at 10,000 ×g in a microcentrifuge for 30 min at 4°C. The supernatant is the cytosolic fraction used for analysis.
EROD assay against environmental factors
The oscillating water parameters, such as salinity, temperature, and pH are the important factors that modulate both the biological and physiological activities of in situ organisms. These parameters may affect enzymatic reactions. Thus different groups of fish were subjected to varying predetermined ranges of salinity (1–10 ppt with an interval of 1 ppt), temperature (25–40°C with an interval of 5°C) pH (6–10 with an interval of 1 pH) and differing concentrations of naphthalene from 2 to 14 ppm over a time period of 12 and 24 h exposure. The experiments were run in duplicate with a positive and negative control for reference.
Results
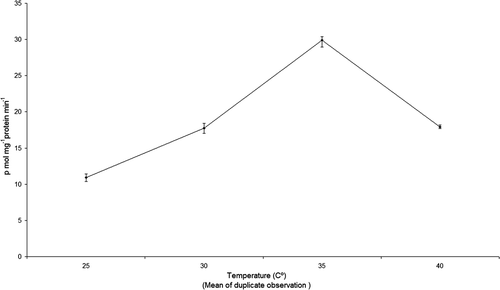
Temperature
Fish liver EROD activity at 25°C (10.9 ± 0.51 pmol mg−1 protein min−1) peaked at 35°C (29.87 ± 0.94 pmol mg−1 protein min−1) then declined at 40°C (17.94 ±0.26 pmol mg−1 protein min−1) (). Although water temperature increased hepatic EROD activity up to 35°C there was a decrease at 40°C.
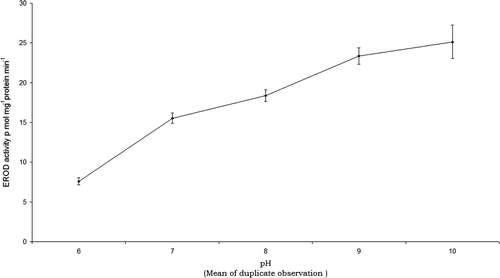
pH
In the case of pH, lower EROD activity was noted at pH 6 (7.6 ±0.42 pmol mg−1 protein min−1) and the activity gradually rose to attain a maximum at pH 10 (25.14 ± 2.11 pmol mg−1 protein min−1) ().
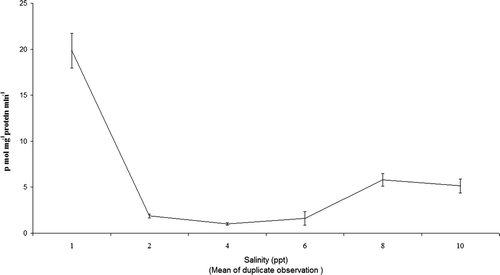
Salinity
At 1 ppt salinity the EROD activity was very high (19.84 ±1.89 pmol mg−1 protein min−1), fell at 2–6 ppt (1.5 pmol mg−1 protein min−1) and then increased at 8 and 10 ppt (5.14 pmol mg−1 protein min−1) ().
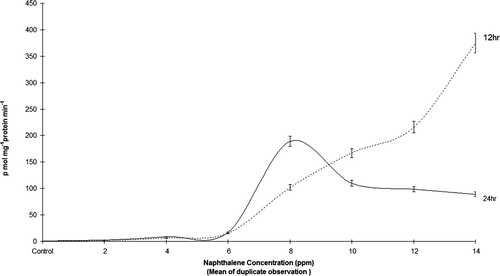
PAH (naphthalene) exposure
At 12 or 24 h naphthalene exposures, there was an increase EROD activity with lower concentrations (up to 6 ppm). Subsequently, at 12 h exposed fish liver showed a significant but gradual rise in EROD activity at higher (8–14 ppm) concentrations. In contrast the 24 h exposed fish exhibited a decrease at 8 ppm with subsequent mortality (). Further, it was noted that in 12 h naphthalene exposure EROD activity rose with increase in concentration. Whereas in 24 h naphthalene exposure, the increase in EROD activity (up to 6 ppm) declined at 8 ppm, then fell further at 14 ppm and died.
Discussion
Impact of water parameters on the enzyme activity
The use of CYP450 enzymes induction as an assessment tool for various stressors has increased in recent years. As with most biological phenomena, EROD in the tissues of an organism is influenced by a variety of internal (physiological), external (physicochemical), and temporal (duration) factors besides species specific variations (Addison et al. Citation1991; Bucheli and Fent Citation1995; Segner, Scholz, and Bohm Citation1995; Amutha 2008). Siroka et al. (Citation2005) showed that CYP450 1A1 EROD is one of the most suitable biochemical biomarkers of aquatic hydrocarbon pollution. Based on the dose related inducible efficacy of organic xenobiotics, the CYP450 1A1 gene dependent enzyme, 7-EROD was activated to reduce lethality, and is thus often used as a biomarker for monitoring organic (hydrocarbon) pollution (Andersson and Förlin Citation1985; Payne et al. Citation1985; Haux and Förlin Citation1988; McMaster et al. Citation1991). Thus EROD activity in fish liver was measured in response to some environmental conditions.
EROD activity in response to temperature
EROD activity exhibited an increasing trend from 25 to 35°C exposure, then, declined in higher temperature which may be due to enhanced metabolic activity at higher temperatures. Similar strong positive EROD activity enhancement against temperature was reported in the dab fish Limanda limanda (Goksøyr et al. Citation1994). Hanson and Larsson (Citation2007) also demonstrated increased EROD activity in response to a rise in water temperature in Perca fluviatilis. At higher temperature (30°C and 35°C), the heat stressed fish enhanced EROD for defense, but induction fails at higher temperature (40°C). This may be attributed to protein (enzyme) denaturation at 40–60°C (Lange et al. Citation1998; Van Laack and Lane Citation2000). Minchin, McIntosh, and Davies (Citation2002) suggested that the optimum temperature for most fish is typically between 20 and 25°C and pH at 7.8.
EROD activity in response to pH
EROD activity increases with rise in pH which indicates that O. mossambicus species is able to tolerate higher alkaline conditions (Popma and Masser Citation1999) and is in agreement with Van Laack and Lane (Citation2000). Since the environmental variables such as temperature and pH influence induction of EROD these need to be routinely measured throughout a study (Andersson and Koivusaari Citation1985; Willis, Edwards, and Addison Citation1991; Sleiderink et al. Citation1995; Arun et al. Citation2003).
EROD activity in response to salinity
Elevated EROD activity was observed at both low (1 ppt) and high (8–10 ppt) salinity which indicates that 2–6 ppt salinity was ideal for this species beyond which (both lower and higher side) is stressful. Subramanian, Sambasivam, and Krishnamurthy (Citation1980) reported similar effects.
EROD activity in response to PAH (naphthalene) exposure
Studies on the leaping mullet (Liza saliens) and common sole (Solea vulgaris) found a relationship between hepatic EROD activities and the concentrations of PAH in the water (Arin and Sen Citation1999; Gravato and Santos Citation2002). A similar relationship was noted in this study between EROD and naphthalene concentrations and is in agreement with findings of Orrego et al. (Citation2005). Wherein at low concentrations there is EROD induction but beyond the optimal level PAH are stressful and produce death. Thus, the concentration and duration of the toxin exposure are factors for EROD induction (Pohl and Fouts Citation1980; Shimada et al. Citation2003; Kirby et al. Citation2007).
Acknowledgments
The authors wish to record their thanks to Ministry of Earth Sciences, Government of India for the financial support, and DST-FIST and UGC-SAP for instrumentation facilities.
References
- Adams , SM . 1990 . “ Status and use of biological indicators for valuating the effects of stress on fish ” . In Biological indicators of stress in fish , American Fisheries Symposium 8 Edited by: Adams , SM . 1 – 8 . Maryland : Bethesda .
- Addison , RF , Hansen , PD , Pluta , HJ and Willis , DE . 1991 . Absence of hepatic mono-oxygenase induction in estuarine fish by Ugilec-141®: A PCB substitute based on tetrachlorobenzyl-toluenes . Marine Environmental Research , 31 : 137 – 44 .
- Amutha, C. 2008. Xenobiotics: Their detoxification in green mussel Perna viridis (Linnaeus 1758) and its nanolevel impact in Oreochromis mossambicus (Peters 1852) reproduction. PhD thesis, Bharathidasan University, India.
- Amutha , C , Bupesh , G , Ramesh , R , Kavitha , P and Subramanian , P . 2009 . Cytochrome P450-dependent mixed function oxidases (MFO) system dynamics during the poly aromatic hydrocarbon (PAH) metabolism in green mussel Perna viridis (Linnaeus 1758) . Environmental Bioindicators , 4 : 97 – 116 .
- Andersson , T and Förlin , L . 1985 . Spectral properties of substrate-cytochrome P450 interaction and catalytic activity of xenobiotic metabolizing enzymes in isolated rainbow trout liver cells . Biochemical Pharmacology , 34 : 1411 – 13 .
- Andersson , T and Förlin , L . 1992 . Regulation of the cytochrome P450 enzyme system in fish . Aquatic Toxicology , 24 : 1 – 20 .
- Andersson , T and Koivusaari , U . 1985 . Influence of environmental temperature on the induction of xenobiotic metabolism by betanaphthoflavone in rainbow trout, Salmo gairdneri . Toxicology and Applied Pharmacology , 80 : 45 – 50 .
- Arin , CE and Sen , A . 1999 . GENG cytochrome P450 and 7-ethoxyresorufin O-deethylase induction in mullet and common sole as an indicator of toxic organic pollution Bay in Izmir, Turkey . Marine Environmental Research , 48 : 147 – 160 .
- Arun , S , Thirumurugan , R , Vishakan , R , Balamurugan , S , Arivazhagan , R , Subramanian , P , Prince Vijeya Singh , J and Malarvizhi , T . 2000 . Toxicity induced biochemical modulations and Phase II xenobiotic conjugating enzyme (GST) in Oreochromis mossambicus. Asian Journal of Microbiology, Biotechnology and . Environmental Sciences , 2 : 225 – 30 .
- Arun , S , Thirumurugan , R , Visakan , R , Balamurugan , S , Arunachalam , V and Subramanian , P . 2003 . Optimal analytical conditions for catalase in freshwater prawn Macrobrachium malcolmsonii . Journal of Biotechnology and Histochemistry , 78 : 1 – 4 .
- Arun , S and Subramanian , P . 2007 . Cytochrome P450-dependent monooxygenase system mediated hydrocarbon metabolism and antioxidant enzyme responses in prawn Macrobrachium malcolmsonii . Comparative Biochemistry and Physiology C , 145 : 610 – 16 .
- Bucheli , TD and Fent , K . 1995 . Induction of cytochrome P450 in fish as a biomarker for environmental contamination in aquatic ecosystems . Critical Reviews in Environmental Science and Technology , 25 : 200 – 68 .
- Daly , AK , Cholerton , S , Gregory , W and Idle , JR . 1993 . Metabolic polymorphisms . Pharmacology and Therapeutics , 57 : 129 – 60 .
- Fent , K . 2003 . Ecotoxicological problems associated with contaminated sites . Toxicology Letters , 140–141 : 353 – 65 .
- Goksøyr , A , Beyer , J , Husøy , AM , Larsen , HE , Westrheim , K , Wilhelmsen , S and Klungsøyr , J . 1994 . Accumulation and effects of aromatic and chlorinated hydrocarbons in juvenile Atlantic cod (Gadus morhua) caged in a polluted fjord (Sørfjorden, Norway) . Aquatic Toxicology , 29 : 21 – 35 .
- Gravato , C and Santos , MA . 2002 . Juvenile Sea Bass Liver P450, EROD induction, and erythrocytic genotoxic responses to PAH and PAH-like compounds . Ecotoxicology and Environmental Safety , 51 : 115 – 27 .
- Guengerich , FP , Okazaki , O , Seto , Y and Macdonald , TL . 1995 . Radical cation intermediates in N-dealkylation reactions . Xenobiotica , 25 : 689 – 709 .
- Hanson , N and Larsson , A . 2007 . Influence of feeding procedure on biomarkers in caged rainbow trout (Oncorhynchus mykiss) used in environmental monitoring . Journal of Environmental Monitoring , 9 : 168 – 73 .
- Haux , C and Förlin , L . 1988 . Biochemical methods for detecting effects of contaminants on fish . Ambio , 17 : 376 – 79 .
- Jewett , SC , Dean , TA , Woodin , BR , Hoberg , MK and Stegeman , JJ . 2002 . Exposure to hydrocarbons 10 years after the Exxon Valdez oil spill: Evidence from cytochrome P4501A expression and biliary FACs in nearshore demersal fishes . Marine Environmental Research , 54 : 21 – 48 .
- Kennedy , SW and Jones , SP . 1994 . Simultaneous measurement of cytochrome P4501A catalytic activity and total protein concentration with a fluorescence plate reader . Analytical Biochemistry , 222 : 217 – 23 .
- Kirby , MF , Smith , AJ , Rooke , J , Neall , P , Scott , AP and Katsiadaki , I . 2007 . Ethoxyresorufin-O-deethylase (EROD) and vitellogenin (VTG) in flounder (Platichthys flesus): System interaction, crosstalk and implications for monitoring . Aquatic Toxicology , 81 : 233 – 44 .
- Lange , U , Saborowski , R , Siebers , D , Buchholz , F and Karbe , L . 1998 . Temperature as a key factor determining the regional variability of the xenobiotic-inducible. ethoxyresorufin-O-deethylase activity in the liver of dab (Limanda limanda) . Canadian Journal of Fisheries and Aquatic Sciences , 55 : 328 – 38 .
- Lemaire-Gony , S and Lemaire , P . 1992 . Interactive effects of cadmium and benzo(a)pyrene on cellular structure and biotransformation enzymes of the liver of the European eel Anguilla anguilla . Aquatic Toxicology , 22 : 145 – 60 .
- Lester , SM , Braunbeck , TA , Teh , SI , Stegeman , JJ , Miller , MR and Hinton , DE . 1993 . Hepatic cellular distribution of cytochrome P-450 IA1 in rainbow trout (Oncorhynchus mykiss): An immunohisto- and cytochemical study . Cancer Research , 53 : 3700 – 3706 .
- Lewis , DFV . 2001 . Guide to cytochromes P450 structure and function , London : Taylor & Francis .
- Lewis , DFV , Loannides , C and Parke , DV . 1998 . Cytochromes P450 and species differences in xenobiotic metabolism and activation of carcinogen . Environmental Health Perspectives , 106 : 633 – 41 .
- McMaster , ME , Van Der Kraak , GJ , Portt , CB , Munkittrick , KR , Sibley , PK , Smith , IR and Dixon , DG . 1991 . Changes in hepatic mixed-function oxygenase (MFO) activity, plasma steroid levels and age at maturity of a white sucker (Catostomus commersoni) population exposed to bleached kraft pulp mill effluent . Aquatic Toxicology , 21 : 199 – 218 .
- Minchin , A , McIntosh , AD and Davies. , IM . 2002 . BEQUALM workshop on P4501A1 activity in fish. Report on the training workshop , FRS Marine Laboratory . Aberdeen Report No. 03/02
- Mondon , JA , Duda , S and Nowak , BF . 2001 . Histological, growth and 7-ethoxyresorufin O-deethylase (EROD) activity responses of greenback flounder Rhombosolea tapirina to contaminated marine sediment and diet . Aquatic Toxicology , 54 : 231 – 47 .
- Nebert , DW , Puga , A and Vasiliou , V . 1993 . Role of the Ah receptor and the dioxin-inducible [Ah] gene battery in toxicity, cancer, and signal transduction . Annals of the New York Academy of Sciences , 685 : 624 – 40 .
- Nelson , DR , Koymans , L , Kamataki , T , Stegeman , JJ , Feyereisen , R Waxman , DJ . 1996 . P450 superfamily: Update on new sequences, gene mapping, accession numbers and nomenclature . Pharmacogenetics , 6 : 1 – 42 .
- Orrego , R , Begon , JLR , Bordajandi , JFG , Inzunza , B , Esteban , A , Gonzalez , MJ , Rivera , J and Barra , R . 2005 . EROD induction and PCDD/F levels in fish liver from the Biobio River in Chile . Chemosphere , 60 : 829 – 35 .
- Payne , JF , Fancey , L , Kiceniuk , J and Williams , U . 1985 . Mixed function oxygenases as biological monitors around petroleum hydrocarbon development sites: Potential for induction by diesel and other drilling mud base oils containing reduced levels of polycylic aromatic hydrocarbons . Marine Environmental Research , 17 : 328 – 32 .
- Pohl , RJ and Fouts , JR . 1980 . A rapid method for assaying the metabolism of 7-ethoxyresorufin by microsomal subcellular fractions . Analytical Biochemistry , 107 : 150 – 5 .
- Popma, T. and M. Masser. 1999. Tilapia life history and biology. SRAC Publication No. 283: 1–4.
- Ringuette , S , Germain , A , Gonthier , C and Perron. , F . 1993 . Presence of PAHs in the Canadian environment: An overview , Quebec Region, Montreal, PQ : Environment Canada .
- Segner , H , Scholz , S and Bohm. , R . 1995 . Carp (Cyprinus carpio) hepatocytes in primary culture: Morphology and metabolism. In La biologie des protozoaires, invertebres et poissons: Modeles experimentaux in vitro et applications [Biology of protozoans, invertebrates, and fish: In vitro experimental models and applications] , Edited by: Dorange , D , Guguen , GC and Samain , J . 77 – 82 . Brest, , France : Ifremer . Plouzane (France)
- Shimada , T , Sugie , A , Yamada , T , Kawazoe , H , Hashimoto , M , Azuma , E , Nakajima , T , Inoue , K and Oda , Y . 2003 . Dose-response studies on the induction of liver cytochromes P4501A1 and 1B1 by polycyclic aromatic hydrocarbons in arylhydrocarbon-responsive C57BL/6J mice . Xenobiotica , 33 : 957 – 71 .
- Siroka , Z , Krijt , J , Randak , T , Svobodova , Z , Peskova , G and Weissova , D . 2005 . Erythropoietic dynamic equilibrium of rats maintained after microwave irradiation . Toxicology Letters , 158 : 5 – 7 .
- Sleiderink , HM , Oostingh , I , Goksoyr , A and Boon , JP . 1995 . Sensitivity of cytochrome P450 1A induction in dab (Limanda limanda) of different age and sex as a biomarker for environmental contaminants in the southern North Sea . Archives of Environmental Contamination and Toxicology , 28 : 423 – 30 .
- Stegemian, J.J. and M.E. Hahn. 1994. Biochemistry and molecular biology of monooxygenases: current perspectives on forms, functions, and regulation of cytochrome P450 in aquatic species. In Aquatic toxicology, biochemical and cellular perspectives, ed. D.C. Malins and G.K. Ostrander, 87–206. London: Lewis Publishers
- Subramanian , P and Amutha , C . 2006 . Sewage induced alteration in the sex of Oreochromis mossambicus: A plausible cue of endocrine disruption . Toxicological and Environmental Chemistry , 88 : 515 – 26 .
- Subramanian , P and Amutha , C . 2006a . Endocrine disruption in aquatic environment: A hidden danger . Biochemical and Cellular Archives , 6 : 269 – 72 .
- Subramanian , P , Sambasivam , S and Krishnamurthy , K . 1980 . Experimental study on the salinity tolerance of Macrobrachium idea larvae . Marine Ecology Progress Series , 3 : 71 – 3 .
- van der Weiden , MEJ , Craane , LHJ , Evers , EHG , Kooke , RMM , Olie , K , Seinen , W and van den Berg , M . 1989 . Bioavailability of PCDDs and PCDFs from bottom sediments and some associated biological effects in the carp (Cyprinus carpio) . Chemosphere , 19 : 1009 – 16 .
- Van Laack , RLJM and Lane , JL . 2000 . Denaturation of myofibrillar proteins from chicken as affected by pH, temperature, and adenosine triphosphate concentration . Poultry Science , 79 : 105 – 9 .
- Ueng , YF , Kuwabara , T , Chun , YJ and Guengerich , FP . 1997 . Cooperativity in oxidations catalyzed by cytochrome P450 3A4 . Biochemistry , 36 : 370 – 81 .
- Willis , DE , Edwards , AJ and Addison , RF . 1991 . Effects of environmental pH on the hepatic mixed function oxidases in Atlantic salmon (Salmo salar) . Canadian Journal of Fisheries and Aquatic Sciences , 48 : 445 – 7 .
- Wang , X , Mu , J , Wang , IS , Lin , J and Hong , H . 2006 . Induction of ethoxyresorufin-O-deethylase (EROD) activity in the liver of black porgy (Acanthopagrus schlegeli) exposed to benzo(a)pyrene . Aquatic Ecosystem Health and Management , 9 : 9 – 53 .