Abstract
Human exposure to polychlorinated dibenzodioxins (PCDD) and dibenzofurans (PCDF), especially 2,3,7,8 tetrachlorodibenzo-p-dioxin (TCDD), was investigated in Vietnam since initial severe adverse health effects were reported in the late 1970s. The purpose of this study was to determine the effects of dioxin exposure on steroid hormones of primiparae in an Agent Orange/dioxin hot-spot and a non-exposed area in Vietnam. Sixteen primiparae (8 at each site), all of whom were aged between 20 and 30 years with infants aged between 4 and 16 weeks, agreed to participate in this study. The mean dioxin levels in breast milk of primiparae from the hot-spot area, in terms of PCDD, PCDF, and PCDD + PCDF toxic equivalents (TEQ), were significantly higher than those for the non-exposed area. PCDD TEQ, PCDF TEQ, and PCDD + PCDF TEQ levels showed a significant correlation with dehydroepiandrosterone (DHEA), androstenedione (A-dione), and estradiol (E2) in the saliva of primiparae in a combination of hot-spot and non-exposed areas in Vietnam. The dose–response curve between salivary E2 or A-dione levels and dioxin levels was U-shaped in humans. This study provides an overview of studies regarding dioxin hot-spots and effects on human health and steroid hormone levels in particular, with a focus on the toxicity attributed to dioxins and furans. Furthermore, causal evidence regarding the effects of dioxins on endocrine disruption in humans is provided.
Introduction
During the Second Indo-China War, the US military sprayed several toxic chemicals on large areas of southern Vietnam (Operation Ranch Hand), including “Agents” Orange, Blue, and White. Approximately 61% of the chemical herbicide used between 1965 and 1970 was Agent Orange, a 50/50 mixture of 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T). The 2,4,5-T used in Vietnam was contaminated with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (Stellman et al. Citation2003), the most toxic man-made substance known (Schecter et al. Citation2006), which is a potent toxicant with the ability to disrupt endocrine and reproductive systems. A single dose of TCDD induces abortion and is accompanied by a decrease in serum estradiol (E2) in the macaque (Moran et al. Citation2003). Furthermore, dioxin was found to accumulate in the adrenal glands when absorbed into the body (Li and Wang 2005). Several animal studies showed an association between maternal TCDD exposure and immune deficiency (Weisglas-Kuperus et al. Citation2000), a decrease in E2 and progesterone (P4) levels (Barsotti, Abrahamson, and Allen Citation1979), and alterations in serum testosterone (T) levels (Egeland et al. Citation1994). Similarly, Peterson, Theobald, and Kimmel (Citation1993) demonstrated that TCDD effects in rodents and other species lead to changes in steroid hormone levels. Nhu et al. (Citation2010) found that individuals exposed to high or low levels of dioxins showed contrasting indications, with salivary cortisol and cortisone levels in primiparae from the hot-spot studied being approximately 3.0–3.5-fold higher than in those from the non-exposed area.
Evaluation of the chronic effects of herbicide exposure is a complex task. Furthermore, it is often difficult to compare the results obtained as the conditions under which scientists work differ from one country to another. However, most of the conclusions drawn by Vietnamese scientists have corroborated the results of experiments conducted by scientists elsewhere in the world. Thus, several scientists reported that this herbicide produced significant health problems, and investigations by Vietnamese scientists showed that TCDD affected chromosomes and induced spontaneous abortions, low birth weight, and congenital abnormalities (Allen et al. Citation1979; Roman et al. Citation1995; Selevan, Sweeney, and Sweeney Citation2003), reduced fertility and fecundity (Allen et al. Citation1979; Barsotti, Abrahamson, and Allen Citation1979; Umbreit, Hesse, and Gallo Citation1987; Gray and Ostby Citation1995), and choriocarcinomas. Furthermore, TCDD is also a known risk factor for cancer (Fingerhut et al. Citation1991; Steenland et al. Citation1999) and increases the risk of developing diabetes mellitus and cardiovascular diseases (Steenland et al. Citation1999; Michalek, Ketchum, and Tripathi Citation2003). Five other diseases, namely soft tissue sarcoma, non-Hodgkin's lymphoma, Hodgkin's disease, chloracne, and porphyria cutanea tarda (a skin disorder produced by liver dysfunction), are known to be produced by dioxin exposure, and a further three cancers, namely respiratory cancer, prostate cancer, and multiple myeloma, may also be associated with herbicide exposure (IOM Citation1994).
The high levels of dioxins in and around former US military bases, such as Bien Hoa, Da Nang, and Phu Cat, infers that they have been identified as significant Agent Orange/dioxin hot-spots. As herbicides are known to have been spilled at those sites where soil and sediment dioxin levels are found to be high, individuals residing close to these sites are highly likely to have been exposed to such levels (Dwernychuk Citation2005; Dwernychuk et al. Citation2006). Indeed, previous studies detected elevated TCDD levels in food, wildlife, and subjects from a contaminated area (Schecter et al. Citation2001, Citation2002, Citation2003; Dwernychuk et al. Citation2002). Furthermore, studies recently demonstrated that the mean dioxin levels in soil and breast milk in a sprayed area are significantly higher than those in a non-sprayed area (Nhu et al. Citation2009; Saito et al. Citation2010).
Although there have been numerous studies on Vietnam veterans and local subjects residing in herbicide-sprayed and dioxin hot-spot areas, to date there have been few scientific studies concerning the effects of dioxin on steroid hormones, such as corticoids, and sex hormones. The purpose of this study was therefore to determine the effects of dioxin exposure on sex steroid hormones in saliva, and dioxin levels in breast milk of primiparae in an Agent Orange/dioxin hot-spot and a non-exposed area in Vietnam on the basis of epidemiological research. This study is one of the first to compare the effects of dioxin on the humans, especially sex steroid hormone levels, in a hot-spot and a non-exposed area.
Materials and methods
Study area
This study was performed in Phu Cat district (Binh Dinh province) and Kim Bang district (Ha Nam province). Phu Cat airbase is one of three dioxin hot-spots in southern Vietnam, and the local population has been living in and around the airbase since before the war. Kim Bang is located in northern Vietnam and was selected as the control site as it did not experience herbicide operations during the war.
Subjects and methods
Breast milk and saliva samples
Primiparae from these two districts aged between 20 and 30 years provided breast milk and saliva samples at their local health clinics or health centers in the morning (between 8:00 and 10:00 AM) in September 2008, immediately after waking, and were subsequently interviewed later the same morning. The local health authorities and medical staff explained the purpose of the study to the 16 primiparae selected (8 from each district). All were breast-feeding infants aged between 4 and 16 weeks and consented to donate 10–20 mL of breast milk. Samples were collected by the mothers themselves or medical staff at each local clinic and frozen immediately after collection. Saliva was collected directly in a Bakelite test tube (15 mL) after rinsing each participant's mouth with water. All samples were stored at −70°C until subsequent analysis. Mothers were asked to provide information regarding age, family income, and residence period. The body measurements for mothers (body height, body weight) were compared between the two areas. The medical ethical committee of Kanazawa University approved this study (Permission no. Health-89), and informed consent was obtained from each participant.
Analytical method
Salivary hormone analysis
Dehydroepiandrosterone (DHEA)-2H3, (100 pg), testosterone-2H3 (100 pg), estradiol-13C4 (100 pg), and progesterone-13C3 (100 pg) were added to the saliva (1–2 mL) as internal standards and the hormones extracted with ethyl acetate. The extract was applied to a Bond ElutC18 cartridge column to separate the polar (cortisol and cortisol) and less-polar (estrogen, P4, and androgen) steroid fractions. The less-polar fraction was then applied to an ion-exchange cartridge column to separate E2 and androgen completely. The E2 obtained was assayed by LC–MS/MS after derivatization, as described previously (Arai et al. Citation2010). The androgen fraction was derivatized to the picolinoyl ester and assayed by LC–MS/MS (Yamashita et al. Citation2009). The lowest analytical limits for DHEA, androstenedione, T, E2, and P4 were 10, 10, 2, 0.1, and 10 pg/assay, respectively.
Analysis of dioxin in breast milk
Breast milk samples were analyzed following previously reported procedures (Tawara et al. Citation2003; Nishijo et al. Citation2008). The fat content in breast milk was determined using the method described by Patterson et al. (Citation1987). The dioxin concentrations obtained were converted into 2,3,7,8-TCDD toxic equivalents (TEQ) using the international World Health Organization (WHO) Toxicity Equivalent Factors (TEF) from 1997 (Van den Berg et al. Citation1998) and 2005 (Van den Berg et al. Citation2006). Non-detectable (ND) and NDR (chromatographic peak detected but did not meet quantification criteria) data were not rejected.
Statistical analysis
All data are shown as the median (inter-quartile range). Spearman's rank correlation coefficients were calculated and statistical comparisons performed using the Wilcoxon signed rank tests. The significance level was set at p < 0.05. All statistical analyses were performed using the JMP®8 software package (SAS Institute, Japan).
Results
presents the characteristics of the subjects from each region showing that age, height, weight, body mass index (BMI), family income, and residence period did not differ significantly. The level of steroid hormones including DHEA, A-dione, E2, P4, and T also did not differ significantly (). The mean dioxin levels in the breast milk of primiparae from the hot-spot area, in terms of PCDD TEQ [9.1; 6.2–13.04] were significantly higher than those for subjects from the non-exposed area [3.29; 2.55–3.81]. Furthermore, the mean PCDF and PCDD + PCDF TEQ in breast milk from the hot-spot area [6.55 (4.78–7.72) and 15.65 (10.99–22.13), respectively] were significantly higher than those for the non-exposed area [2.73 (2.40–3.39) and 6.03 (4.95–6.94), respectively].
Table 1. Comparison of characteristics of primiparae from hot-spot and non-exposed regions with herbicide.
Table 2. Mean dioxin and salivary hormone level of primiparae in both areas.
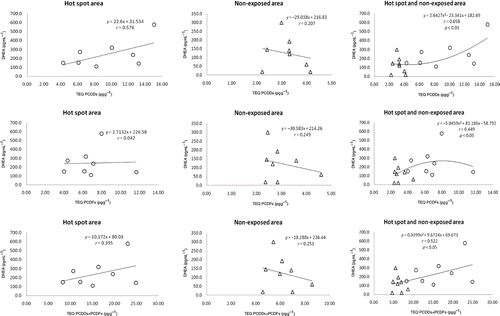
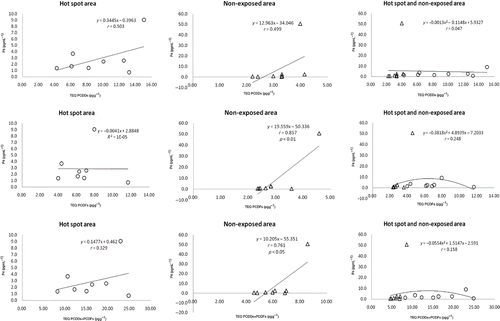
The correlation between hormone levels in saliva and dioxin levels in breast milk was subsequently determined. A plot of DHEA in saliva and dioxin in breast milk () showed a significant correlation between DHEA and PCDD, PCDF, and PCDD + PCDF TEQ for both hot-spot and non-exposed areas. The correlation between the levels of salivary A-dione and dioxin in breast milk is shown in . The dose–response curve between A-dione level and dioxin exposure in the combination of hot-spot and non-exposed areas was found to be U-shaped, thus indicating that the A-dione level decreased with an increase in PCDD + PCDF up to 15 pg g−1 PCDD + PCDF, whereas above this value the A-dione level rose with increasing concentrations of PCDD + PCDF.
Figure 2. Correlation between the levels of A-dione in saliva and dioxin in breast milk of primiparae.
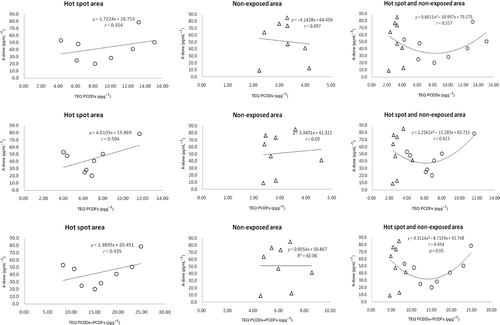
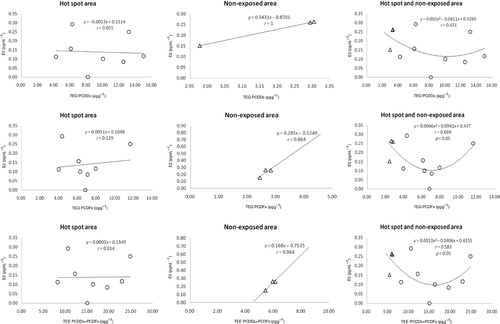
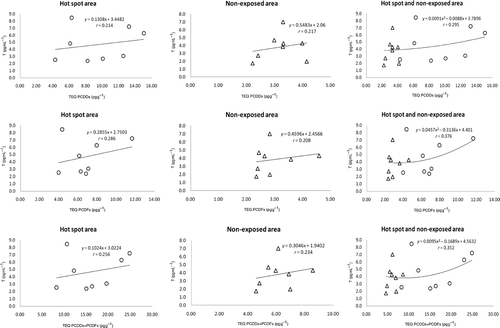
The correlation between the levels of salivary E2 and dioxin in breast milk is shown in . As above, the dose–response curve between E2 levels and dioxin exposure was also U-shaped. These results showed significant correlations between the salivary E2 level and PCDF or PCDD + PCDF TEQ in the combination of hot-spot and non-exposed areas. However, the plots of P4 and T levels in saliva and dioxin in breast milk ( and ) showed no significant correlations between P4 or T levels and PCDD, PCDF, and PCDD + PCDF TEQs in either area.
Discussion
Many herbicide-related experimental studies have been conducted in numerous countries over the last 40 years. Dwernychuk et al (Citation2002) demonstrated the apparent food-chain transfer of TCDD from contaminated soil to cultured fish pond sediments and to fish and duck tissues and finally humans, where TCDD was detected in whole blood and breast milk. Several studies reported that the half-life of dioxin in humans is 7–11 years for adults (Pirkle et al. Citation1989; Kreuzer et al. Citation1997). Despite the fact that a significant amount of time has passed since the end of the war, herbicide residues still exert adverse effects on those subjects living in the sprayed/hot-spot areas and on the country's ecosystem as a whole (Schecter et al. Citation2001; Mai et al. Citation2007). Dioxin hot-spots contain high TCDD levels due to the inevitable spillages that occurred during loading of the herbicide into crop-spraying planes during the Vietnam War (Dwernychuk Citation2005), and our study showed that mean dioxin levels in breast milk of primiparas were higher in the hot-spot than in the non-sprayed area.
Salivary steroid measurements might prove to be a convenient and non-invasive means of determining free steroid concentrations in serum and have widespread research applications in the fields of endocrinology, neuroendocrinology, and reproductive endocrinology. The merits of LC–MS/MS analysis compared to the current radioimmunoassay (RIA) method include higher sensitivity (10–100-fold higher than RIA) and accuracy. The most significant advantage of LC–MS/MS analysis, however, is the ability to determine several steroids simultaneously, thus allowing saliva to be used as the matrix for steroid hormone analysis in the field.
As given in , mean salivary A-dione and E2 levels did not differ significantly between hot-spot and non-hot spot regions. These results show that the decrease of E2 and A-dione concentrations with dioxin in the range 7–15 pg g−1 was accompanied by an increase in these levels at more than 15 pg g−1 of dioxin. In addition, the dose–response curve between A-dione or E2 levels and dioxin exposure in the combination of hot-spot and non-exposed areas was U-shaped and dioxin decreased the salivary A-dione level by approximately 50–60% in the range 7–15 pg g−1, with this fall corresponding closely to E2 levels. These reductions in A-dione and E2 levels are likely to be due to the inhibitory effects of dioxin on CYP17 and 17,20-lyase activity in the ovary rather than on aromatase activity. Indeed, Moran et al. (Citation2003) reported that the molecular target for endocrine disruption of human luteinizing granulosa cells (LGC) by dioxin was CYP17, which specifically decreases the supply of androgens for E2 synthesis, rather than aromatase. Furthermore, Li and Wang (2005) demonstrated that dioxin-like PCB126 diminished androstenedione production as well as CYP17 mRNA and 17α-hydroxylase and 17,20-lyase activity (CYP17), particularly the latter, in human adrenocortical H295H cells.
The dose–response curve between DHEA levels and dioxin exposure in the combination of hot-spot and non-exposed areas shows a correlation between the two, although the curve is neither U- nor inverted U-shaped. Nhu et al. (Citation2010) recently reported that the dose–response curve between salivary cortisol or cortisone and dioxin levels is bell-shaped in humans. It was suggested that hormones and endocrine-disrupting chemicals have a U- or inverted U-shaped response because lower concentrations of a hormone stimulate a tissue, whereas higher concentrations exert opposite effects (Vom Saal et al. Citation1997). Several studies showed that endocrine disruption often follows similar dose–response curves (U- or inverted U-shaped dose responses) (Crews and McLachlan Citation2006; Fenton Citation2006; Newbold, Padilla-Banks, and Jefferson Citation2006; Welshons, Nagel, and vom Saal Citation2006). The combined effect of these multiple actions of endocrine disrupters can, however, be difficult to interpret and is often misinterpreted due to variability in the assay system. Indeed, all hormones exert nonlinear actions on their targets, thus, inferring that the combined effects of these multiple nonlinear dose responses are not predictable (Gore, Heindel, and Zoeller Citation2006).
TCCD may affect many organs in the endocrine system in a species-specific manner, either directly or indirectly (IOM Citation2000). Indeed, Moore, Jefcoate, and Peterson (Citation1991) demonstrated that TCDD inhibited steroidogenesis by interfering with specific steps such as mobilization of cholesterol to the inner mitochondrial membrane.
E2, which is produced from androgen in the ovary, adrenal cortex, and brain, has a critical impact on reproductive and sexual function and also affects other organs and the bones. Stimulation of E2 metabolism may therefore not be the only antiestrogenic effect of TCDD. Indeed, a decrease in specific binding of E2 to the uterine estrogen receptors of TCDD-treated rodents was reported (Romkes, Piskorska-Pliszczynska, and Safe Citation1987; Astroff and Safe Citation1988). A similar study showed that TCDD reduced serum E2 concentrations but did not affect P4 secretion by human LGC (Heimler et al. Citation1998). Moreover, Moran et al. (Citation1997) also demonstrated that TCDD reduced E2 production by human LGC but exerted no marked effect on P4 production. Data showed that salivary E2 hormone levels of primiparae tended to be lower in the hot-spot area than in the non-exposed area, whereas salivary P4 hormone levels tended to be higher in the hot-spot area than in the non-exposed area.
The saliva samples used in our study, which were taken in the morning (between 8:00 and 10:00 AM) in both areas, displayed similar T levels. A recent study involving both Operation Ranch Hand and control veterans showed that serum dioxin was associated with lower T levels (Gupta et al. Citation2006). Other investigators also found an inverse association between dioxin and serum T levels (Egeland et al. Citation1994; Jonhson et al. Citation2001). Furthermore, Bookstaff et al. (Citation1990a, Citation1990b) demonstrated that TCDD inhibited the compensatory rise in the concentration of luteinizing hormone in plasma in response to low T levels in rats.
Finally, it is worthwhile noting that, even if all of these studies were to yield unequivocally positive results, only the increased rate of defects resulting from exposure would have been detected rather than their specific association with dioxin. This association must therefore remain hypothetical until a causal relationship is confirmed by separate investigations. However, such proof may be forthcoming if the newest chemical analysis methods can demonstrate the presence of variable levels of residual dioxin in human tissue. In light of this, further studies on chronic effects of herbicides on humans and the environment in Vietnam need to be continued.
Acknowledgments
This study was supported by grants from the Japan Society for the Promotion of Science (Grant-in-Aid for Scientific Research, (B) no. 17406016 and Grant-in-Aid for Scientific Research (A) no. 19209021). We thank the medical staff at Phu Cat and Kim Bang Medical Centers for their help and assistance, as well as all the women who participated in this study. The authors thank the officers of the Division for Mitigation of the Consequences of the Chemicals Used During the War on Human Health (10-80 Division), Hanoi Medical University, Vietnam, for making this study possible.
References
- Allen , JR , Barsotti , DA , Lambrecht , LK and Van Miller , JP . 1979 . Reproductive effects of halogenated aromatic hydrocarbons on non human primates . Annals of the New York Academy of Sciences , 320 : 419 – 25 .
- Arai , S , Miyashiro , Y , Shibata , Y , Kashiwagi , B , Tomaru , Y , Kobayashi , M , Watanabe , Y , Honma , S and Suzuki , K . 2010 . New quantification method for estradiol in the prostatic tissues of benign prostatic hyperplasia using liquid chromatography–tandem mass spectrometry . Steroids , 75 : 13 – 19 .
- Astroff , B and Safe , S . 1988 . Comparative antiestrogenic activities of 2,3,7,8-tetrachlorodibenzo-p-dioxin and 6-methyl-1,3,8-trichlorodibenzofuran in the female rat . Toxicology and Applied Pharmacology , 95 : 435 – 43 .
- Barsotti , DA , Abrahamson , LJ and Allen , JR . 1979 . Hormonal alterations in female rhesus monkeys fed a diet containing 2,3,7,8-tetrachlorodibenzo-p-dioxin . Bulletin of Environment Contamination and Toxicology , 21 : 463 – 9 .
- Bookstaff , RC , Kamel , F , Moore , RW , Bjerke , DL and Peterson , RE . 1990a . Altered regulation of pituitary gonadotropin-releasing hormone (GnRH) receptor number and pituitary responsiveness to GnRH in 2,3,7,8-tetrachlorodibenzo-p-dioxin-treated male rats . Toxicology and Applied Pharmacology , 105 : 78 – 92 .
- Bookstaff , RC , Moore , RW and Peterson , RE . 1990b . 2,3,7,8-Tetrachlorodibenzo-p-dioxin increases the potency of androgens and estrogens as feedback inhibitors of luteinizing hormone secretion in male rats . Toxicology and Applied Pharmacology , 104 : 212 – 24 .
- Crews , D and McLachlan , JA . 2006 . Epigenetics, evolution, endocrine disruption, health, and disease . Endocrinology , 147 ( Suppl. ) : S4 – 10 .
- Dwernychuk , LW . 2005 . Dioxin hot spots in Vietnam . Chemosphere , 60 : 998 – 9 .
- Dwernychuk , LW , Cau , HD , Hatfield , CT , Boivin , TG , Hung , TM , Dung , PT and Thai , ND . 2002 . Dioxin reservoirs in southern Vietnam – a legacy of Agent Orange . Chemosphere , 47 : 117 – 37 .
- Dwernychuk , LW , Hung , TM , Boivin , TC , Bruce , GS , Dung , PT , Son , LK Hatfield , CT . 2006 . The Agent Orange dioxin issue in Vietnam: A manageable problem . Organohalogen Compound , 68 : 312 – 15 .
- Egeland , GM , Sweeney , MH , Fingerhut , MA , Wille , KK , Schnorr , TM and Halperin , WE . 1994 . Total serum testosterone and gonadotropins in workers exposed to dioxin . American Journal of Epidemiology , 139 : 272 – 81 .
- Fenton , SE . 2006 . Endocrine-disrupting compounds and mammary gland development: Early exposure and later life consequences . Endocrinology , 147 ( Suppl. ) : S18 – 24 .
- Fingerhut , MA , Halperin , WE , Marlow , DA , Piacitelli , LA , Honchar , PA , Sweeney , MH , Greife , AL , Dill , PA , Steenland , K and Suruda , AJ . 1991 . Cancer mortality in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin . The New England Journal of Medicine , 324 : 212 – 18 .
- Gore, A.C., J.J. Heindel, and R.T. Zoeller. 2006. Endocrine disruption for endocrinologists (and others). Endocrinology 147, Suppl. no. 6: S1–3
- Gray Jr , LE and Ostby , JS . 1995 . In utero 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) alters reproductive morphology and function in female rat offspring . Toxicology and Applied Pharmacology , 133 : 285 – 94 .
- Gupta , A , Ketchum , N , Roehrborn , CG , Schecter , A , Aragaki , CC and Michalek , JE . 2006 . Serum dioxin, testosterone, and subsequent risk of benign prostatic hyperplasia: A prospective cohort study of Air Force veterans . Environmental Health Perspectives , 114 : 1649 – 54 .
- Heimler , I , Rawlins , RG , Owen , H and Hutz , RJ . 1998 . Dioxin perturbs, in a dose- and time-dependent fashion, steroid secretion, and induces apoptosis of human luteinized granulosa cells . Endocrinology , 139 : 4373 – 9 .
- IOM (Institute of Medicine) . 1994 . Veterans and Agent Orange: Health effects of herbicides used in Vietnam , Washington, DC : National Academy Press .
- IOM (Institute of Medicine) . 2000 . Veterans and Agent Orange: Toxicology , Washington, DC : National Academy Press .
- Johnson , E , Shorter , C , Bestervelt , L , Patterson , D , Needham , L and Piper , W . 2001 . Serum hormone levels in humans with low serum concentrations of 2,3,7,8-TCDD . Toxicology and Industrial Health , 17 : 105 – 12 .
- Kreuzer , PE , Csanady , GA , Baur , C , Kessler , W , Päpke , O , Greim , H and Filser , JG . 1997 . 2,3,7,8-Tetrachlorodibenzop-dioxin (TCDD) and congeners in infants. A toxicokinetic model of human lifetime body burden by TCDD with special emphasis on its uptake by nutrition . Archives of Toxicology , 71 : 383 – 400 .
- Li , LA and Wang , PW . 2005 . PCB126 induces differential changes in androgen, cortisol, and aldosterone biosynthesis in human adrenocortical H295R cells . Toxicological Sciences , 85 : 530 – 40 .
- Mai , TA , Doan , TV , Tarradellas , J , de Alencastro , LF and Grandjean , D . 2007 . Dioxin contamination in soils of Southern Vietnam . Chemosphere , 67 : 1802 – 7 .
- Michalek , J , Ketchum , N and Tripathi , RC . 2003 . Diabetes mellitus and 2,3,7,8-tetrachlorodibenzo-p-dioxin elimination in veterans of operation Ranch hand . Journal of Toxicology and Environmental Health A , 66 : 211 – 21 .
- Moore , RW , Jefcoate , CR and Peterson , RE . 1991 . 2,3,7,8-Tetrachlorodibenzo-p-dioxin inhibits steroidogenesis in the rat testis by inhibiting the mobilization of cholesterol to cytochrome P450scc . Toxicology and Applied Pharmacology , 109 : 85 – 97 .
- Moran, F.M., E. Enan, C.A. Vandervoort, D.R. Stewart, A.J. Conley, J.W. Overstreet, and B.L. Lasley. 1997. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) effects on steroidogenesis of human luteinized granulosa cells in vitro. Society for the Study of Reproduction 65, Suppl. no. 1: 56
- Moran , FM , Vandevoort , CA , Overstreet , JW , Lasley , BL and Conley , AJ . 2003 . Molecular target of endocrine disruption in human luteinizing granulosa cells by 2,3,7,8-tetrachlorodibenzo-p-dioxin: Inhibition of estradiol secretion due to decreased 17-hydroxylase/17,20-lyase cytochrome P450 expression . Endocrinology , 144 : 467 – 73 .
- Newbold , RR , Padilla-Banks , E and Jefferson , WN . 2006 . Adverse effects of the model environmental estrogen diethylstilbestrol are transmitted to subsequent generations . Endocrinology , 147 ( Suppl. ) : S11 – 17 .
- Nhu , DD , Kido , T , Naganuma , R , Sawano , N , Tawara , K , Nishijo , M , Nakagawa , H , Hung , NN and Thom , LTH . 2009 . GIS study of dioxin contamination in a Vietnamese region sprayed with herbicide . Environmental Health and Preventive Medicine , 14 : 353 – 60 .
- Nhu , DD , Naganuma , R , Suzuki , H , Kuroda , N , Kido , T , Honma , S Tai , PT . 2010 . Salivary cortisol and cortisone levels, and breast milk dioxin concentrations in Vietnamese primiparas . Toxicological and Environmental Chemistry , 92 : 1939 – 52 .
- Nishijo , M , Tawara , K , Nakagawa , H , Honda , R , Kido , T , Nishijo , H and Saito , S . 2008 . 2,3,7,8-tetrachlorodibenzo-p-dioxin in maternal breast milk and newborn head circumference . Journal of Exposure Science and Environmental Epidemiology , 18 : 246 – 51 .
- Patterson , DG Jr , Holler , JS , Belser , E , Booser , EL , Lapeza , CR and Needham , LL . 1987 . Determination of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in human adipose tissue on whole-weight and lipid bases . Chemosphere , 16 : 935 – 6 .
- Peterson , RE , Theobald , HM and Kimmel , GL . 1993 . Developmental and reproductive toxicity of dioxins and related compounds: Cross-species comparisons . Critical Reviews in Toxicology , 23 : 283 – 335 .
- Pirkle , JL , Wolfe , WH , Patterson , DG , Needham , LL , Michalek , JE , Miner , JC , Peterson , MR and Phillips , DL . 1989 . Estimates of the half-life of 2,3,7,8-tetrachlorodibenzo-p-dioxin in Vietnam veterans of Operation Ranch Hand . Journal of Toxicology and Environmental Health , 27 : 165 – 171 .
- Roman , BL , Sommer , RJ , Shinomiya , K and Peterson , RE . 1995 . In utero and lactational exposure of the male rat to 2,3,7,8-tetrachlorodibenzo-p-dioxin: Impaired prostate growth and development without inhibited androgen production . Toxicology and Applied Pharmacology , 134 : 241 – 50 .
- Romkes , M , Piskorska-Pliszczynska , J and Safe , S . 1987 . Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on hepatic and uterine estrogen receptor levels in rats . Toxicology and Applied Pharmacology , 87 : 306 – 14 .
- Saito , K , Nhu , DD , Suzuki , H , Kido , T , Naganuma , R , Sakakibara , C Tawara , K . 2010 . Association between dioxin concentrations in breast milk and food group intake in Vietnam . Environmental Health and Preventive Medicine , 15 : 48 – 56 .
- Schecter , A , Birnbaum , L , Ryan , JJ and Constable , JD . 2006 . Dioxins: An overview . Environmental Research , 101 : 419 – 428 .
- Schecter , A , Dai , LC , Päpke , O , Prange , J , Constable , JD , Matsuda , M , Thao , VD and Piskac , AL . 2001 . Recent dioxin contamination from Agent Orange in residents of a Southern Vietnam city . Journal of Occupational and Environmental Medicine , 43 : 435 – 43 .
- Schecter , A , Pavuk , M , Constable , JD , Dai , LC and Päpke , O . 2002 . A follow-up: High level of dioxin contamination in Vietnamese from Agent Orange, three decades after the end of spraying . Journal of Occupational and Environmental Medicine , 44 : 218 – 20 .
- Schecter , A , Quynh , HT , Pavuk , M , Päpke , O , Malisch , R and Constable , JD . 2003 . Food as a source of dioxin exposure in the residents of Bien Hoa City, Vietnam . Journal of Occupational and Environmental Medicine , 45 : 781 – 8 .
- Selevan , SG , Sweeney , A and Sweeney , MH . 2003 . “ Reproductive and developmental epidemiology of Dioxins ” . In Dioxins and health , Edited by: Schecter , A and Gasiewicz , TA . 765 – 825 . Hoboken, NJ : Wiley .
- Steenland , K , Piacitelli , L , Deddens , J , Fingerhut , M and Chang , LI . 1999 . Cancer, heart disease, and diabetes in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin . Journal of the National Cancer Institute , 91 : 779 – 786 .
- Stellman , JM , Stellman , SD , Christian , R , Weber , T and Tomasallo , C . 2003 . The extent and patterns of usage of Agent Orange and other herbicides in Vietnam . Nature , 422 : 681 – 7 .
- Tawara , K , Honda , R , Nishijo , M and Nakagawa , H . 2003 . Pretreatment procedure of dioxin analysis for a small volume of human breast milk . Journal of Kanazawa Medical University , 28 : 17 – 25 . (in Japanese)
- Umbreit , T , Hesse , E and Gallo , M . 1987 . Reproductive toxicity in female mice of dioxin contaminated soils from a 2,4,5-trichlorophenoxyacetic acid manufacturing site . Archives of Environment Contamination and Toxicology , 16 : 461 – 6 .
- Van den Berg , M , Birnbaum , L , Bosveld , AT , Brunstrom , B , Cook , P , Feeley , M Giesy , JP . 1998 . Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife . Environmental Health Perspectives , 106 : 775 – 92 .
- Van den Berg , M , Birnbaum , L , Denison , M , De Vito , M , Farland , W , Feeley , M Fiedler , H . 2006 . The 2005 World Health Organization re-evaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds . Toxicological Sciences , 93 : 223 – 41 .
- Vom Saal , FS , Timms , BG , Montano , MM , Palanza , P , Thayer , KA , Nagel , SC , Dhar , MD , Ganjam , VK , Parmigiani , S and Welshons , WV . 1997 . Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses . Proceedings of the National Academy of Sciences of the United States of America , 94 : 2056 – 61 .
- Weisglas-Kuperus , N , Patandin , S , Berbers , GA , Sas , TC , Mulder , PG , Sauer , PJ and Hooijkaas , H . 2000 . Immunologic effects of background exposure to polychlorinated biphenyls and dioxins in Dutch preschool children . Environmental Health Perspectives , 108 : 1203 – 7 .
- Welshons , WV , Nagel , SC and vom Saal , FS . 2006 . Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure . Endocrinology , 147 ( Suppl. ) : S56 – 69 .
- Yamashita , K , Miyashiro , Y , Maekubo , H , Okuyama , M , Honma , S , Takahashi , M and Numazawa , M . 2009 . Development of highly sensitive quantification method for testosterone and dihydrotestosterone in human serum and prostate tissue by liquid chromatography-electrospray ionization tandem mass spectrometry . Steroids , 74 : 920 – 6 .