Abstract
Large volumes of wastewater with dissolved wood components are treated in wastewater treatment plants at thermomechanical pulp mills. It has been shown previously that hemicelluloses in these wastewater streams can be recovered by membrane filtration. A serious obstacle when treating lignocellulose process streams is fouling of the membranes. Fouling not only increases operating costs but also reduces the operating time of the membrane plant. When optimizing the membrane cleaning method, it is important to know which compounds cause the fouling. In this work fouling of an ultrafiltration membrane was studied. The fouling propensity of untreated process water and microfiltrated process water was compared. Fouled membranes were analyzed using scanning electron microscopy and attenuated total reflection Fourier transform infrared spectrometry. Acid hydrolysis of membranes exposed to untreated process water and microfiltration permeate revealed that 508 mg/m2 and 37 mg/m2 of polysaccharides, respectively, remained on the membranes even after alkaline cleaning.
INTRODUCTION
Valuable high molecular mass components, such as hemicelluloses, can be recovered from biomass extracts[Citation1,Citation2] and pulp mill process streams[Citation3,Citation4] using ultrafiltration (UF). For successful operation of membrane-based applications, it is important to minimize membrane fouling.[Citation5] Unfortunately, membrane fouling is a common problem when filtering solutions containing hemicelluloses, such as pulp mill process water[Citation6–8] and wood hydrolysates.[Citation9,Citation10]
A common strategy for reducing fouling is to use hydrophilic membranes, for which the fouling substances have a lower affinity.[Citation6,Citation9,Citation11] Other methods include pretreatment of the hemicellulose solution to remove as much of the fouling substances as possible prior to UF. Pretreatment methods include adsorption,[Citation10,Citation11] coagulation,[Citation3] oxidation[Citation12], and microfiltration (MF).[Citation4]
Foulants accumulate on the membrane over time, reducing the flux, and making membrane cleaning necessary. Fouling can be classified as removable, irremovable, and irreversible fouling.[Citation13] Removable fouling caused by loosely attached foulants can be eliminated by physical cleaning, whereas irremovable fouling can be eliminated by chemical cleaning. In case of irreversible fouling, foulants cannot be removed either by physical or chemical cleaning. The cleaning procedure must be adapted to the type of substance(s) responsible for fouling in each application, reducing the amount of irreversible fouling. However, identifying the foulants can be difficult, as the amount of material deposited on the membrane surface is usually small.[Citation14]
Colloidal material in process water from a thermomechanical pulp mill was recently reported by Thuvander and Jönsson[Citation15] to have a severe detrimental effect on the flux during hemicellulose recovery by UF. Similarly, Puro et al.[Citation7] found that the fouling layer on UF membranes leading to flux decline contained extractives originating from colloids in the water after the filtration of process water from a chemical-thermomechanical pulp mill. Moreover, Puro et al.[Citation6] have shown that the fouling layer formed after filtering stone groundwood water contained extractives such as resin acids and fatty acids, which were present in dissolved form in the process water. However, the membranes studied by Puro et al.[Citation6,Citation7] were only rinsed with water prior to extraction of the foulants. Whether foulants could be removed by chemical cleaning, or if the fouling was irreversible, was thus not studied. In the study by Weis et al.,[Citation16] UF membranes were fouled by spent sulfite liquor and cleaned after several operating cycles. Analysis of the foulants causing the irreversible fouling using attenuated total reflection-Fourier transform infrared (ATR–FTIR) spectroscopy, zeta potential, and gas chromatography after Soxhlet extraction of the fouled and cleaned membranes, revealed the presence of resin acids, fatty acids, and lignin.
The objective of this investigation was to characterize the irreversible fouling, the substances remaining on the membrane after chemical cleaning of UF membranes fouled by process water from a thermomechanical pulp mill. The effects of fouling and cleaning were studied after UF of 2 feed solutions: the process water itself, and the permeate obtained after MF of the process water. The substances remaining on the UF membranes and/or within the pores, and changes in membrane characteristics after cleaning were analyzed using ATR–FTIR and scanning electron microscopy (SEM) in combination with energy dispersive X-ray analysis (EDS). In addition, acid hydrolysis of the fouled membranes was used to determine the presence and composition of fouling species of polysaccharides deposited on, and within, the membrane.
MATERIALS AND METHODS
Feed Solutions
Process water from a thermomechanical pulp mill where spruce was the primary raw material, and permeate after MF of this process water were used as feed solutions during fouling studies of a UF membrane. Suspended solids and colloidal extractives in the process water were removed by MF using a ceramic membrane with a nominal pore size of 0.2 µm (Tami Industries, Nyons, France). During MF, the transmembrane pressure (TMP) was 0.8 bar, the temperature was 80°C, and the cross-flow velocity (CFV) was 4 m/s (corresponding to a Reynolds number of 65000). The volume reduction (VR), defined as the ratio between the permeate volume and the feed volume, was 85%.
Membrane
The UF membrane used for the fouling and cleaning experiments was UFX5 pHt from Alfa Laval A/S, Nakskov, Denmark, as this membrane has been shown to be suitable for this application in a previous study.[Citation8] UFX5 pHt is a permanently hydrophilic polysulfone (PSU) membrane on a polypropylene support, and has a nominal cut-off of 5000 g/mole. New membrane samples from the same batch were used in all experiments. The membranes were mounted in a cross-flow module with an effective membrane area of 1.96 ⋅10−3 m2.
Fouling and Cleaning Procedure
Fouling and cleaning efficiency were determined by measuring the pure water flux:
| (1) | after initial preconditioning of the pristine membranes (Jnew), | ||||
| (2) | after rinsing the membranes with deionized water after UF of the feed solution (Jrinse), and | ||||
| (3) | after chemical cleaning of the fouled membranes (Jclean). | ||||
The permeate flow was measured using an electronic balance. The pure water flux was always measured at 30°C. The flux of the deionized water was measured at 0.5, 1.0, 1.5, and 2 bar TMP, and the pure water flux, L/(m2 h bar), was obtained by linear regression of the correlation between flux and pressure.
Two cleaning agents were used, the alkaline cleaning agent Ultrasil 10 and the acidic cleaning agent Ultrasil 73 (both from Ecolab AB, Älvsjö, Sweden). The pristine membranes were preconditioned by cleaning with 0.25 wt% Ultrasil 10 for 1 h at 1 bar at 50°C with a CFV of 0.2 m/s. After cleaning, the membranes were rinsed with 5 L deionized water at 50°C, and with an additional 10 L deionized water at ambient temperature, before the pure water flux, Jnew, was measured.
The membranes were then used to filter the feed solutions: process water or MF permeate. The feed volume was 4 L. The experiments were conducted at elevated temperature (75°C) and a low CFV of 0.05 m/s to accelerate fouling. The influence of pressure on the flux was studied while both the retentate and permeate were recirculated to the feed tank. The permeate was then continuously removed, while the retentate was recirculated to the feed tank. The TMP was 1 bar during UF of the process water, which is the pressure at which the flux increase leveled off when the pressure was increased, and 500 g permeate was withdrawn. As previous studies have shown that the removal of colloidal material from the process water by MF increases the UF flux,[Citation15] a different exposure time and a final VR were used during UF of the MF permeate. The TMP during UF of the MF permeate was 0.4 bar so that the initial flux was about the same as during UF of the process water, and the total volume of permeate collected was 1000 g, in order to increase the exposure time of the membrane and the amount of accumulated foulants.
The fouled membranes were rinsed with 5 L deionized water at 75°C and then rinsed, and gradually cooled down, using approximately 10 L deionized water at ambient temperature. The pure water flux of the rinsed membranes (Jrinse) was then measured to give an indication of the amount of irremovable fouling.
The membranes were then cleaned with 0.25% Ultrasil 10, at the same operating conditions as during preconditioning of the pristine membranes. As the membranes used during UF of process water was severely fouled, additional cleaning steps were performed to remove remaining foulants, so only the most recalcitrant, irreversible fouling would remain. The membranes were cleaned with 0.5% Ultrasil 73, and finally with 0.5% Ultrasil 10 at the same operating conditions as when preconditioning the pristine membranes. The pure water flux was measured after cleaning (Jclean) with each agent.
The fouling experiments were conducted in duplicate. After the membranes had been fouled and cleaned, they were removed and dried in a desiccator for later analysis. One membrane from each duplicate was used for analysis with SEM–EDS and ATR–FTIR, while the other was subjected to acid hydrolysis.
New membranes are often preserved using a humectant. This preservative is removed from the membrane during its initial use, preferably during the precondition of the membrane. However, extensive preconditioning might be required before the humectant is completely removed.[Citation17] By using a reference membrane, which is not exposed to the fouling substances but otherwise treated as the other membranes, possible influences of additional removal of preservatives during the experiments can be taken into account when studying the membrane fouling. As reference membrane, a set of pristine membranes were preconditioned as the other membranes and used to filter deionized water for 15 h at 75°C, at a CFV of 0.05 m/s and at 1 bar TMP. Both permeate and retentate were recirculated during UF of the deionized water. The membranes were then cleaned as the other membranes. The reference membranes were both used as reference for the pure water flux measurements and as a baseline for the analysis of the foulants.
Analytical Methods
The process water and the MF permeate were characterized with respect to total solids (TS), ash content, turbidity, and concentration of lignin using methods described previously.[Citation15] The polysaccharides in the feed solution were hydrolyzed into monomeric sugars by acid hydrolysis[Citation18] and measured using high-performance anion-exchange chromatography, as described previously.[Citation15]
When determining the polysaccharide content of the fouled membranes after cleaning, the edges of the dried membranes were first cut off and discarded, leaving only the 1.96⋅10−3 m2 membrane area that had been exposed to the feed solution. The membranes were then cut into pieces with an approximate size of 1 × 1 mm, and subsequently suspended in 5 mL deionized water. The content of polysaccharides on the membranes was determined using the same method that was used to analyze the feed solutions, i.e., acid was added to the mixture of deionized water and membrane pieces, hydrolyzing the polysaccharides into monomeric sugars. The content of monomeric sugars was then measured using high-performance anion-exchange chromatography.
The membrane surface structure and the content of inorganic material were examined using SEM in combination with EDS. Membrane samples were attached to double-sided adhesive carbon tape on an aluminum holder, and subsequently coated with gold, as described previously.[Citation19] The morphology of the coated membrane surface was studied using SEM (Quanta FEG 200 ESEM™, FEI, USA) at an accelerating voltage of 10 kV, and a working distance of 10 mm. The existence of inorganic substances was determined with EDS (Aztec EDS with X-Max detector, Oxford Instruments, UK). The qualitative and quantitative analyses of EDS spectra were based on internal standards using the Aztec software.
ATR–FTIR was used to identify the functional group characteristics of the membrane and the deposited foulants. An ATR accessory equipped with a diamond crystal was used with a spectrophotometer (PerkinElmer, USA). All spectra were recorded within the range 4000–500 cm−1 with 4 cm−1 resolution, and four scans were performed at room temperature (20 ± 0.5°C). The background air spectrum was subtracted and the spectra were offset corrected, normalized, and presented in transmittance units (%).
RESULTS AND DISCUSSION
The characteristics of the process water and the MF permeate, used as feed solutions, are given in . The main type of hemicellulose was galactoglucomannan. Other sugars present in the solutions were arabinan and traces of xylan (<0.05 g/L). The concentration of hemicelluloses was slightly lower in the MF permeate, while the turbidity was markedly lower. It has been shown that the turbidity of pulp mill process water after the removal of suspended material is correlated with the concentration of wood resins in the water.[Citation20,Citation21] The reduced turbidity of the MF permeate thus indicates that both suspended material and extractives are largely removed by MF.
TABLE 1 Characteristics of the process water from the thermomechanical pulp mill and the permeate after MF of the process water
Flux During Ultrafiltration
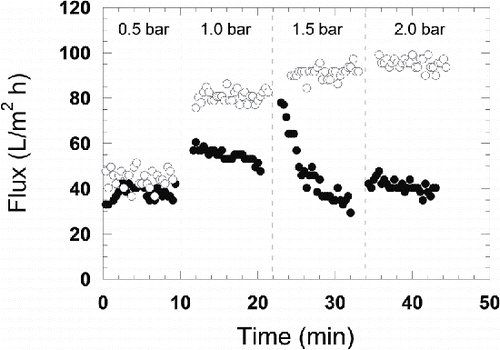
The UF experiments started by increasing the TMP gradually from 0.5 to 2 bar in steps of 0.5 bar, maintaining the same pressure for 10 min at each step. Both retentate and permeate were recirculated to the feed tank. The flux of the MF permeate increased each time the pressure was increased, although the flux started to level off at 1.5 bar, as can be seen in . In contrast, the critical flux (as defined by Bacchin et al.[Citation22]) for the process water was reached already at about 1 bar. At 1.5 bar, a severe decline in the flux of the process water was observed, as can be seen in .
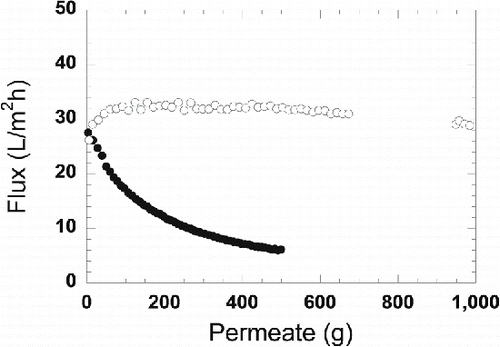
After studying the influence of TMP on flux, the pressure was reduced to 1 bar for the process water and 0.4 bar for the MF permeate, and permeate was withdrawn while the retentate was still recirculated to the feed tank. The initial flux of both the process water and the MF permeate were slightly below 30 L/m2 h. The volume of permeate withdrawn during UF of process water was 500 g, while about 1000 g was withdrawn during UF of the MF permeate, corresponding to VRs of 12.5% and 25%, respectively. Although the final VR was higher during UF of the MF permeate, the flux was almost constant during the entire experiment, whereas the flux of the process water declined over time, as shown in . The final flux of the MF permeate at a VR of 25% was 29 L/m2 h, and the flux of the process water at a VR of 12.5% was 6 L/m2 h.
Flux Recovery
Recovery of the pure water flux was used to evaluate the influence of fouling and cleaning on UF membrane performance. The flux recovery is defined as the normalized pure water flux after rinsing the membrane with deionized water, Jrinse/Jnew, and after cleaning, Jclean/Jnew. The intention of the cleaning protocols used in this work was not to find an optimal cleaning protocol, but to investigate the influence of alkaline and acidic cleaning on the removal of fouling.
The flux recovery after rinsing the membrane exposed to process water was relatively low, <50%, as can be seen in , showing that there remains a considerable amount of irremovable fouling after UF of the untreated process water. It was not possible to restore the pure water flux, despite additional acidic and alkaline cleaning, and the final flux recovery was only 80%.
FIGURE 3 Average pure water flux recovery of the duplicate experiments, where the bars show the maximum and minimum values, after rinsing the fouled membranes with deionized water, cleaning with the alkaline cleaning agent Ultrasil 10 (U10), and cleaning with the acidic cleaning agent Ultrasil 73 (U73).
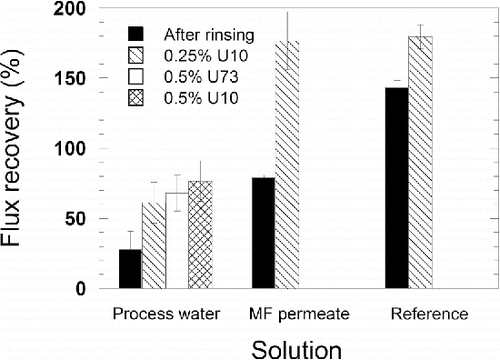
The high flux recovery of, especially, the reference membrane, but also the membrane exposed to MF permeate, could be a result of additional removal of membrane preservatives (glycerine) that still remained after the membrane preconditioning resulting in higher membrane permeability and flux recoveries above 100%. The flux recovery after rinsing the membrane exposed to MF permeate was 80%, and 140% after rinsing the reference membrane. This difference in flux recovery shows that foulants had accumulated on the membrane exposed to MF permeate. However according to the pure water measurements, the foulants on the membrane exposed to MF permeate could be removed by alkaline cleaning. The flux recovery after cleaning with 0.25% Ultrasil 10 was about 180% for both membranes.
Identification of Foulants
In order to elucidate the cause of the flux decline during UF of process water and the inadequate flux recovery after cleaning, membranes were analyzed using SEM–EDS and ATR–FTIR.
SEM
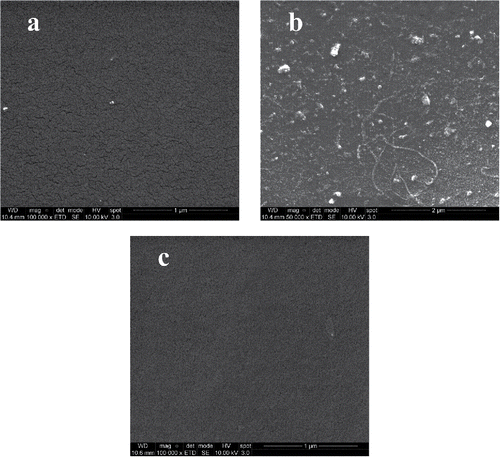
The surfaces of pristine and fouled membranes after cleaning are shown in . The membrane fouled with MF permeate () appears similar to the pristine membrane (). This is expected, both because the flux recovery after cleaning the membrane fouled with MF permeate was high (see ) and as the colloidal material in the feed was removed during the MF. However, deposits of both colloidal material and fibers can be seen on the surface of the membrane fouled by process water (). A cake of colloidal and suspended material present in the process water was thus formed on the membrane, and the deposited material was not completely removed, even after several cleaning stages, leading to the low flux recovery.
EDS
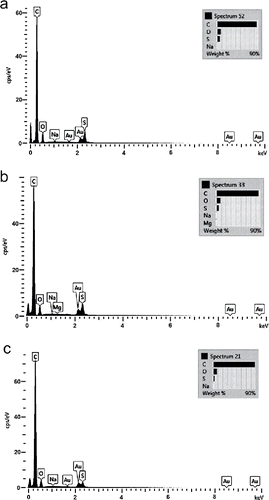
Since the process water contains inorganic material, fouling could have been due to scaling. However, the elemental composition of both the pristine and the fouled membranes was similar, as shown in . Furthermore, the amounts of ions found by SEM–EDS do not support scaling layer formation. This suggests that fouling is caused mainly by organic constituents.
ATR–FTIR
The composition of the deposits remaining after cleaning were analyzed using ATR–FTIR. Spectra obtained from the pristine membrane and the membranes that had been exposed to process water and MF permeate are shown in . The spectrum from the pristine membrane shows peaks characteristic of PSU membranes. The band assignments are summarized in . In the FTIR spectrum from the membrane exposed to process water, the characteristic bands at 1586, 1503, 1295, 1242, 1169, 1151, 1106, 873, and 834 cm−1, seen in the case of the pristine membrane, were slightly attenuated, while the bands at 1080 cm−1 and 2967 cm−1 were intensified. In addition, the membrane exposed to process water exhibited a new peak at 3366 cm−1, which was attributed to O–H stretching. The broadness of this peak (3000–3400 cm−1) suggests the presence of polysaccharides, since they contain significant numbers of –CH and –OH groups also manifest as peak intensification at 1080 cm−1 and 2967 cm−1.[Citation23–25] No significant decrease in the intensity of the peaks characteristic of PSU membranes was observed in the spectrum from the membrane exposed to MF permeate, however, the spectrum exhibited a band at 3391 cm−1, indicating the presence of polysaccharides on the membrane surface.
FIGURE 6 ATR-FTIR spectra of a) pristine reference membrane, b) membrane exposed to process water and c) membrane exposed to MF permeate.
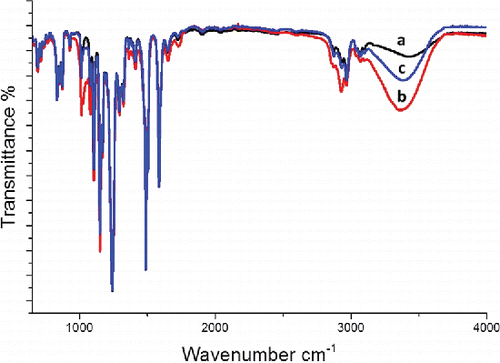
TABLE 2 ATR–FTIR peak assignments
Acid Hydrolysis of Fouled Membranes
ATR–FTIR analysis indicated that there were polysaccharides on the fouled membranes. This was also confirmed by acid hydrolysis of the membranes. The membrane exposed to process water contained the highest amount of polysaccharides: 508 mg/m2. Although the flux recovery of the membrane exposed to MF permeate was high, a small amount of polysaccharides was also found in this membrane, 37 mg/m2, as can be seen from . Interestingly, the glucan:mannan ratio of the foulants on the membranes is considerably higher than in the process water. The glucan:mannan ratios in the process water, the membrane exposed to process water, and the membrane exposed to MF permeate, were 1:3, 1:0.3, and 1:0.7, respectively. The main polysaccharide remaining on the membrane after cleaning thus seems not to be galactoglucomannan, but instead a polysaccharide containing high amounts of glucan. The main polysaccharides containing glucan in spruce are cellulose, starch, and laricinan ((1→3)-β-glucan),[Citation28] and one of these may thus be responsible for a significant proportion of the observed, irreversible fouling.
TABLE 3 Sugars detected after acid hydrolysis of membranes exposed to process water and MF permeate
CONCLUSIONS
Pretreatment with MF increases UF flux and reduces membrane fouling when isolating hemicelluloses from thermomechanical pulp mill process water with UF. After UF of untreated process water it was not possible to remove the fouling layer even after repeated cleaning with alkaline and acidic cleaning agents. The MF pretreatment removed colloidal material that attached to the UF membrane and reduced the amount of polysaccharides in the irreversible fouling layer from 502 mg/m2 to 32 mg/m2. Characteristic of the polysaccharides found in the irreversible fouling were an elevated glucan:mannan ratio compared to the dominating hemicellulose, galactoglucomannan, found in process water. This shows that a large part of the irreversible fouling is caused by another polysaccharide, containing a high amount of glucan but only found in small amounts in the process water. The findings in this work will aid the efforts in developing more efficient membrane cleaning methods in applications in pulp mills.
ACKNOWLEDGMENTS
Support for this research was provided by the Swedish Energy Agency. The authors wish to thank Stora Enso for supplying the process water, and Alfa Laval for donating the membranes. The authors would also like to thank Josefin Bucht and Sandra Farran-Lee for experimental assistance, and Anna Kaluzka for technical assistance with FTIR.
REFERENCES
- Gabrielii, I.; Gatenholm, P.; Glasser, W. G.; Jain, R. K.; Kenne, L. Separation, characterization and hydrogel-formation of hemicellulose from aspen wood. Carbohydr. Polym. 2000, 43(4), 367–374. DOI: 10.1016/S0144-8617(00)00181-8.
- Song, T.; Pranovich, A.; Holmbom, B. Separation of polymeric galactoglucomannans from hot-water extract of spruce wood. Bioresour. Technol. 2013, 130, 198–203. DOI: 10.1016/j.biortech.2012.11.149.
- Willför, S.; Rehn, P.; Sundberg, A.; Sundberg, K.; Holmbom, B. Recovery of water-soluble acetylgalactoglucomannans from mechanical pulp of spruce. Tappi J. 2003, 2(11), 27–32.
- Persson, T.; Nordin, A.-K.; Zacchi, G.; Jönsson, A.-S. Economic evaluation of isolation of hemicelluloses from process streams from thermomechanical pulping of spruce. Appl. Biochem. Biotechnol. 2007, 137, 741–752.
- Hubbe, M. A.; Metts, J. R.; Hermosilla, D.; Blanco, M. A.; Yerushalmi, L.; Haghighat, F.; Lindholm-Lehto, P.; Khodaparast, Z.; Kamali, M.; Elliott, A. Wastewater treatment and reclamation: A review of pulp and paper industry practices and opportunities. Bioresources 2016, 11(3), 7953–8091. DOI: 10.15376/biores.11.3.Hubbe.
- Puro, L.; Tanninen, J.; Nyström, M. Analyses of organic foulants in membranes fouled by pulp and paper mill effluent using solid-liquid extraction. Desalination 2002, 143(1), 1–9. DOI: 10.1016/S0011-9164(02)00215-1.
- Puro, L.; Kallioinen, M.; Mänttäri, M.; Nyström, M. Evaluation of behavior and fouling potential of wood extractives in ultrafiltration of pulp and paper mill process water. J. Membr. Sci. 2011, 368, 150–158. DOI: 10.1016/j.memsci.2010.11.032.
- Persson, T.; Jönsson, A.-S. Isolation of hemicelluloses by ultrafiltration of thermomechanical pulp mill process water-influence of operating conditions. Chem. Eng. Res. Des. 2010, 88(12), 1548–1554. DOI: 10.1016/j.cherd.2010.04.002.
- Koivula, E.; Kallioinen, M.; Preis, S.; Testova, L.; Sixta, H.; Mänttäri, M. Evaluation of various pretreatment methods to manage fouling in ultrafiltration of wood hydrolysates. Sep. Purif. Technol. 2011, 83, 50–56. DOI: 10.1016/j.seppur.2011.09.006.
- Koivula, E.; Kallioinen, M.; Sainio, T.; Antón, E.; Luque, S.; Mänttäri, M. Enhanced membrane filtration of wood hydrolysates for hemicelluloses recovery by pretreatment with polymeric adsorbents. Bioresour. Technol. 2013, 143, 275–281. DOI: 10.1016/j.biortech.2013.05.129.
- Persson, T.; Jönsson, A.-S. Fouling of ultrafiltration membranes during isolation of hemicelluloses in the forest industry. Sch. Res. Exch. 2009, Article ID 624012.
- Mänttäri, M.; Manasrah, M. A.; Strand, E.; Laasonen, H.; Preis, S.; Puro, L.; Xu, C.; Kisonen, V.; Korpinen, R.; Kallioinen, M. Improvement of ultrafiltration performance by oxidation treatment in the recovery of galactoglucomannan from wood autohydrolyzate. Sep. Purif. Technol. 2015, 149, 428–436. DOI: 10.1016/j.seppur.2015.06.001.
- Meng, P.; Chae, S.-R.; Drews, A.; Kraume, M.; Shin, H.-S.; Yang, F. Recent advances in membrane bioreactors (MBRs): Membrane fouling and membrane material. Water Res. 2009, 43, 1489–1512. DOI: 10.1016/j.watres.2008.12.044.
- Trägårdh, G. Membrane cleaning. Desalination 1989, 71(3), 325–335. DOI: 10.1016/0011-9164(89)85033-7.
- Thuvander, J.; Jönsson, A.-S. Extraction of galactoglucomannan from thermomechanical pulp mill process water by microfiltration and ultrafiltration – influence of microfiltration membrane pore size on ultrafiltration performance. Chem. Eng. Res. Des. 2016, 105, 171–176. DOI: 10.1016/j.cherd.2015.12.003.
- Weis, A.; Bird, M. R.; Nyström, M. The chemical cleaning of polymeric UF membranes fouled with spent sulphite liquor over multiple operational cycles. J. Membr. Sci. 2003, 216(1–2), 67–79. DOI: 10.1016/S0376-7388(03)00047-4.
- Wright, S.; Pellegrino, J; Amy, G. Humectant release from membrane materials. J. Membr. Sci. 2005, 246(2), 227–234 DOI: 10.1016/j.memsci.2004.07.030.
- Ruiz, R.; Ehrman, T. Laboratory Analytical Procedure LAP-014: Dilute Acid Hydrolysis Procedure for Determination of Total Sugars in the Liquid Fraction of Process Samples; NREL, Midwest Research Institute for the Department of Energy: USA, 1996.
- Zarebska, A.; Amor, Á. C.; Ciurkot, K.; Karring, H.; Thygesen, O.; Andersen, T. P.; Hägg, M.-B.; Christensen, K. V.; Norddahl, B. Fouling mitigation in membrane distillation processes during ammonia stripping from pig manure. J. Membr. Sci. 2015, 484, 119–132. DOI: 10.1016/j.memsci.2015.03.010.
- Käyhkö, J. The Influence of Process Conditions on the Deresination Efficiency in Mechanical Pulp Washing. Ph.D. Thesis, Lappeenranta University of Technology, Department of Chemical Technology, Lappeenranta, Finland, 2002.
- Krawczyk, H.; Oinonen, P.; Jönsson, A.-S. Combined membrane filtration and enzymatic treatment for recovery of high molecular mass hemicelluloses from chemithermomechanical pulp process water. Chem. Eng. J. 2013, 225, 292–299. DOI: 10.1016/j.cej.2013.03.089.
- Bacchin, P.; Aimar, P.; Field, R. W. Critical and sustainable fluxes: Theory, experiments and applications. J. Membr. Sci. 2006, 281(1–2), 42–69. DOI: 10.1016/j.memsci.2006.04.014.
- Max, J.-J.; Chapados, C. Glucose and fructose hydrates in aqueous solution by IR spectroscopy. J. Phys. Chem. 2007, 111, 2679–2689. DOI: 10.1021/jp066882r.
- Silva, F. F.; Alves, A. M. B.; de Lurdes Serrano, M.; de Sousa, A. P. M. Isolation and purification of concentrated and non-concentrated hemicellulose alkaline extracts. Sep. Purif. Technol. 2017, 173, 233–239. DOI: 10.1016/j.seppur.2016.09.033.
- Wei, X.; Wang, Z.; Wang, J.; Wang, S. A novel method of surface modification to polysulfone ultrafiltration membrane by preadsorption of citric acid or sodium bisulfite. Membr. Water Treat. 2012, 3(1), 35–49. DOI: 10.12989/mwt.2012.3.1.035.
- McCutcheon, J. R.; Hoek, E. M. V.; Bui, N.; Lind, M. L. Nanostructured Membranes for Engineered Osmosis Applications. Google Patents, US 20130105395 A1. http://www.google.com/patents/US20130105395 2013. (Accessed on 6 April 2016).
- Kumar, R.; Isloor, A. M.; Ismail, A. F.; Rashid, S. A.; Matsuura, T. Polysulfone-Chitosan blend ultrafiltration membranes: preparation, characterization, permeation and antifouling properties. RSC Adv. 2013, 3(21), 7855–7861. DOI: 10.1039/c3ra00070b.
- Sjöström, E. Wood Chemistry- Fundamentals and Applications 2nd ed.; Academic Press: San Diego, USA, 1993.