?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Determination of the chemical composition of biomaterial is important for their valued utilization in biorefinery. In this study, the chemical composition of five bamboo species, i.e., mitinga (Bambusa tulda), borak (Bambusa balcooa), rengoon (Thyrsostachys oliveri), orah (Dendrocalamus longispathus), and bajja (Bambusa vulgaris) were determined. The chemical characterization of these bamboo species can expedite a further study on the extraction of cellulose and lignin. α-cellulose content was in the range of 42.7–45.7% and Klason lignin content was 22.4–28.2%. The ash content was 1.8–4.3% for the studied five bamboo species. The α-cellulose and lignin content were similar to other non-timber spices. The ash content was lower than other non-timber species. Therefore, these species can be a potential source of raw material for biorefinery.
Introduction
Sustainability and environmental conservation are the most emergent and dominated affairs that are related to the use of renewable resources, which are devoted worldwide.[Citation1–3] The use of raw materials and products that are renewable, sustainable, and biocompatible has emerged a sturdy interest in the world. In this context, the demand for a substitute to resources, which mitigated the environmental problems, has been populated.[Citation4] Over the past few decades, bamboo has received ever more attention as a renewable, fast-growing resource.[Citation1,Citation5] Research has progressed rapidly due to its wide availability and material characteristics comparable to wood. Non-timber forest products (NTFP) like bamboo play a major role in mitigating the pressure on slow-growing forest resources and the growing demand for qualitative timber.[Citation1] Recently, there are also growing interested in the utilization of bamboo for pulp production,[Citation6,Citation7] nanofiber extraction,[Citation8] composite materials,[Citation5,Citation9–13] biofuel production,[Citation14–16] and wastewater treatment.[Citation17]
Bamboo, a perennial woody grass, plays a significant role as a material for consumer products. With its high growth rate, awide range of applications, and renewability, bamboo resources occupy a noteworthy position in the twenty-first century as a versatile and vital raw material.[Citation18] It attains maturity in 3 years as compared to wood, which takes almost more than 20 years.[Citation19] It is one of the fastest-growing plants that grow in the unproductive area. New shoots are generated from harvested clum as root structure remains alive after harvest.[Citation20,Citation21] Bamboo grows in plains, hilly, and high altitude mountainous regions, and in most kinds of soils, except alkaline soils, desert, and marsh.[Citation22] It is widely distributed in Asian countries and has become one of the most potential renewable non-woody cellulosic materials because of its high productivity, rapid growth, and easy propagation.[Citation8,Citation16,Citation20] The annual production of bamboo is approximately 20 million tons globally.[Citation23]
Chemical characterization of raw material is vital in determining its suitability for various applications and treatments. The accurate compositional analysis enables the evaluation of potential conversion yields and process economics.[Citation24] Knowledge of the physico-chemical properties of bamboo is essential for effective utilization of bamboo since Information regarding basic properties is very confined. In Bangladesh, there are many bamboo species, which are widely used as a household material. However, due to advanced processing technology and increased market demand, it has been extended to industrial applications, i.e., pulp and paper, furniture, and construction. Since many bamboo species remain unutilized, research is needed to determine their properties so that appropriate technologies can be developed to exploit them.
Therefore, the proposed study was undertaken for the chemical characterization of five bamboo species. Their proper end uses were discussed based on the results obtained from chemical analysis.
Material and methods
Raw materials
The mature (3–4 years) culms of five bamboo species, namely mitinga (Bambusa tulda), borak (Bambusa balcooa), rengoon (Thyrsostachys oliveri), orah (Dendrocalamus longispathus), and bajja (Bambusa vulgaris), were sourced from Keucia silvicultural research station, Bangladesh Forest Research Institute (BFRI), Satkania, Chattogram (92°24′E and 93°15′E longitude and 24°22′N and 25°8′N latitude), Bangladesh.
Reagent grade (≥95% purity) sodium hydroxide (NaOH), acetic acid (CH3COOH), sodium chlorite (NaClO2), and sulfuric acid (H2SO4) were received from Carolina Biological Supply Company, New York City, USA. Analytical grade (≥95% purity) benzene and ethanol were sourced from Merck KGA, Darmstadt, Germany.
Preparation of raw material
The bamboo culms were cut at 15 cm above ground level and then subdivided equally into the top, middle and basal portions according to their total length. The internodes of each portion were cut into small strips and chipped by a hammer mill. The chips were dried in the sun and milled by a Wiley mill to get fine particles and the powder of three portions mixed thoroughly to obtain a representative sample of a whole bamboo. The powders were then sieved to obtain in the range of <40 mesh to >60 mesh. This procedure was applied for all five species used in this study. The powders were stored in an air-tight container for further analysis. The sample preparation is presented in .
Chemical analysis
Chemical analysis of five bamboo species was conducted based on TAPPI standards. Solubility, extractive content, cellulose content, lignin content, and ash content were analyzed in this study.
Solubility
Water and alkaline solubility were measured. For water solubility, both cold and hot water solubility was studied. T-207 cm-99 standard was used to analyze cold and hot water solubility and alkaline solubility was determined by T-212 om-02 standard.
Cold water solubility
The sample (2 g) was placed in a 500 ml beaker, and 300 ml of distilled water was added to the beaker. After completely wetting the sample, extraction was carried out with constant stirring at room temperature (23 °C) for 48 h. The mixture was then transferred to a tared filtering crucible and washed with 200 ml of cold distilled water. After that, it was dried at 105 °C to a constant weight. The following equation was used to calculate the cold water solubility.
where A and B are before and after extraction weight (dry mass) of the test specimen, respectively.
Hot water solubility
The sample (2 g) was put in a 250 ml conical flask, and 100 ml of hot distilled water was poured into the flask. The mixture was placed in a boiling water bath and digested by the reflux condenser for 3 h. Then, the mixture was transferred to a tared filtering crucible and washed with 200 ml of hot distilled water. It was dried at 105 °C until reaching constant weight. The calculation of hot water solubility was done using the following equation.
where A and B are before and after extraction weight (dry mass) of the test specimen, respectively.
Alkaline solubility
The sample was (2 g) kept in a 500 ml conical flask, and 100 ml of 1% NaOH solution was added. The mixture was stirred with a glass rod, and it was then placed in a water bath at 97–100 °C for a period of 60 min. After that, it was transferred to a tared filtering crucible and washed with 100 ml of hot water. In the next, it was soaked in 25 ml of 10% CH3COOH for 1 min followed by soaking in 15 ml of 10% CH3COOH for 1 min. Finally, it was washed with hot water until the complete removal of the remaining acid. Alkaline solubility was calculated using the following equation.
where A and B are before and after extraction weight (dry mass) of the test specimen, respectively.
Extractive
Extractive content was determined following the T-204 cm-97 standard a 5 g sample was used for this analysis. The solvent was a mixture of benzene and ethanol in the ratio of 2:1 and kept in a flask. Soxhlet apparatus was used to extract extractive for a period of a period of 4–5 h. The solvent was evaporated and the flask was dried at 105 ± 3 °C for 1 h followed by cooling in a desiccator and weighing. The extractive content was determined using the following equation
where We, Wp, and Wb are the weight (dry mass) of the flask with the extractive, bamboo powder, and flask, respectively.
Cellulose
Holocellulose and α-cellulose were determined to identify the cellulose content of five bamboo species for this study. Holocellulose and α-cellulose were analyzed by following the standard of T 249-75 and T 203 cm-99, respectively.
Holo-cellulose
A two-gram extractive-free sample, 160 ml of distilled water, 0.2 ml of cold CH3COOH, and 1 g of NaClO2 were placed in a 250 ml flask. It was then placed into a water bath at 70–80 °C for 5 h. One gram of NaClO2 and 0.2 ml of cold CH3COOH were added to the mixture with continuous stirring in a 1 h interval. At the end of 5 h, the flasks were placed in an ice water bath and filtered into a known weight of coarse porosity fritted-glass crucible. The residue was washed with acetone followed by ethanol. The crucibles were then oven-dried at 105 °C and weighed until a constant weight was reached. The following equation was used to determine the holocellulose content.
Where W1 is alcohol-toluene extractive content (percent), W2, W3, and W4 were weight (dry mass) of samples, residue and crucible, and crucible, respectively.
α-Cellulose
A 3 g of oven-dried holocellulose sample was placed in a 250 ml Erlenmeyer flask and it was then put in a water bath maintaining a temperature of 20 °C. Then, 50 ml of 17.5% NaOH solution was poured into the flask and mixed thoroughly for one minute. After that, the specimen was allowed to react with the solution for 29 min followed by adding 50 ml of distilled water and mixing for another minute. The mixture was kept for another 5 min to complete the reaction, and it was filtered using a known weight of fritted-glass crucible with the aid of vacuum suction. The residue was washed with 50 ml of 8.3% NaOH followed by 40 ml of 10% CH3COOH, and 1000 ml of hot distilled water. The crucible was oven-dried in an oven at 103 ± 2 °C until obtaining a constant weight. α-Cellulose was determined using the following formula.
where W1 is holocellulose content (percent), W2, W3, and W4 were weight (dry mass) of holo-cellulose sample, residue and crucible, and crucible, respectively.
Lignin
Klason lignin content was analyzed based on the T-222 cm-02 standard. A 2 g of extractive free bamboo powder was placed in a beaker and 40.0 ml of 72% H2SO4 was added and stirred with a glass rod continuously until complete dispersion of the sample. It was then transferred to the flask and 1540 ml of hot water was added to the mixture. The mixture was boiled for 4 h, maintaining constant volume by the addition of hot water frequently. After that, it was kept overnight. Then, it was filtered using a known weight of fritted-glass crucible by the aid of vacuum suction and washed with hot water to remove the residual acid from the lignin. The residue containing the crucible was oven-dried at 103 ± 2 °C until obtaining a constant weight. Klason lignin content was calculated using the following equation.
where W1, W2, and W3 were the weight (dry mass) of the sample, residue and crucible, and crucible, respectively.
Ash content
The ash content was determined according to the standard of T211om-93. A 5 g of bamboo powder was put in the known oven-dry weight of the crucible. Then, it was ignited at a temperature of 575 ± 25 °C for 5 h. The crucible containing residue was placed in a desiccator to cool down and weighed. The ash content was calculated based on the following equation.
where W1, W2, and W3 were the weight (dry mass) of the sample, residue and crucible, and crucible, respectively.
Results and discussions
Cellulose
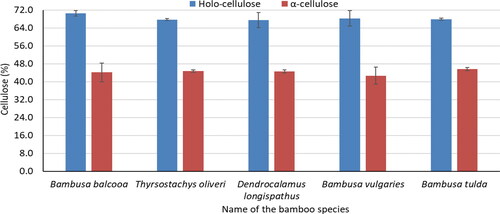
Holo-cellulose and α-cellulose content of five bamboo species are presented in . B. balcooa showed the highest amount (70.5%) of holo-cellulose and it was significantly higher than that of B. tulda, T. oliveri, D. longispathus, and B. vulgaris. α-Cellulose content was in the range of 42.7–45.7% and B. tulda had the highest content (45.7) of α-cellulose. The α-cellulose content of five bamboo species was similar to Neosinocalamus affinis (44.9%) observed in the previous study.[Citation15] It was higher than non-wood species, i.e., M. sapientum (39.4%), E. crassipes (35.2%), and a frond of C. nucifera (40.2%).[Citation25,Citation26] In comparison to wood and other bamboo species, five bamboo species showed more or less similar content of cellulose in the present study.
Lignin
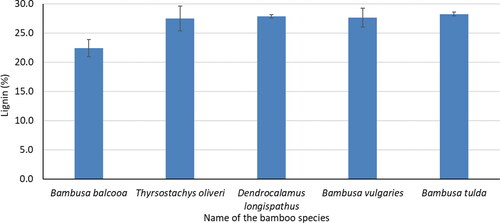
The measured Klason lignin content of five bamboo species has been shown in . The lowest Klason lignin content (22.4%) was observed for B. balcooa. It was significantly lower than the other four bamboo species. The range of Klason lignin content was 27.5–28.2 for B. tulda, T. oliveri, D. longispathus, and B. vulgaris. It was the lower and similar value of N. affinis (28.2%) obtained from the previous study. In other studies, M. sapientum (14.2%), E. crassipes (15.3%), and a frond of C. nucifera (19.9%) showed the lower value of Klason lignin content.[Citation25,Citation26] The Klason lignin content of five bamboo species was comparatively similar to other bamboo and wood species.
Solubility
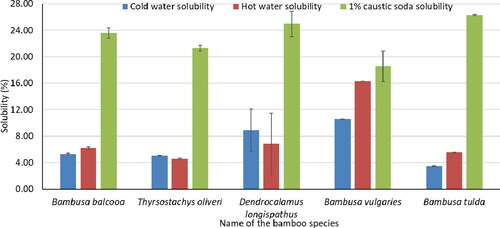
represents the solubility of five bamboo species. B. tulda showed the highest solubility (26.30%) in 1% NaOH solution. B. vulgaries was more susceptible to both cold (10.56%) and hot water (16.28%) than other species in this study. Cellulose is more resistant but xylan is soluble to NaOH,[Citation27] which may cause the higher solubility in 1% NaOH solution.
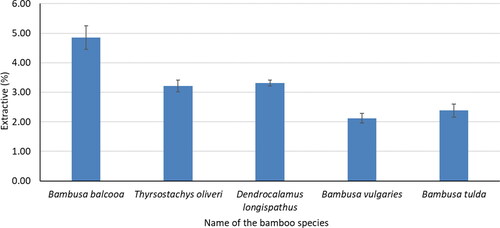
Extractive
The benzene-ethanol extractive content of five bamboo species is shown in . The highest extractive content (4.85%) was found for B. balcooa and it was the lowest (2.12%) for B. vulgaris. The hexane extractive of M. sapientum, E. crassipes, and a frond of C. nucifera was 1.1, 2.4, and 3.4%, respectively and acetone extractive of them was 2.6, 3.8, and 4.4, respectively.[Citation25] The extractive content of five bamboo species of this study was in the range of other non-wood species.
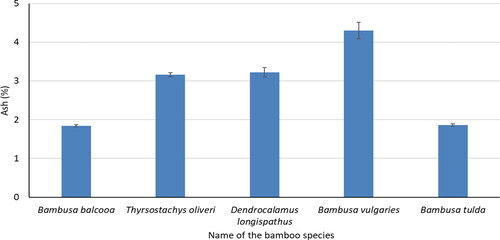
Ash
Ash content of five bamboo species has been presented in . The ash content was 1.8–4.3% for five bamboo species. The determined ash content was the highest (4.3%) for B. vulgaris and it was the lowest (1.8%) for B. balcooa. The ash content of M. sapientum, E. crassipes, and a frond of C. nucifera was 18.3, 10.2, and 5.6%, respectively.[Citation25] The bamboo species had a lower amount of ash content compared to other non-wood species obtained from previous studies.
Future prospective of five bamboo species
Cellulose and lignin are the most valuable resources for developing value-added products. The forest is the main source of cellulose and lignin leading to deforestation. Considering the environmental issues and sustainability of biorefinery, alternative resources are crucial. Non-timber forest resources can contribute to mitigating the problem of raw material shortage. Bamboo is fast-growing, and it can harvest in three years. The significant content of cellulose and lignin and less amount of ash of five bamboo species compared to other non-wood species can appeal to consider them as an alternative source of cellulose and lignin.
Conclusions
Chemical analysis has been conducted for five bamboo species in this study. Cellulose and lignin content was more or less close to the wood and other non-timber species. The ash content was lower than other non-timber species. Lower ash content and higher cellulose and lignin content of these bamboo species indicate the potential source of raw material for the biorefinery. Further studies are needed to develop a suitable cellulose and lignin extraction method for these new raw materials. It can help contribute as an alternative source of raw material in biorefinery.
Authors' contributions
Mohammad Jakir Hossain: Conceptualization, data acquisition, visualization, literature review, writing—original draft, writing—review & editing. Rupak Kumar Ghosh: Data acquisition, writing—review & editing. Atanu Kumar Das: Conceptualization, literature review, writing—original draft, writing—review & editing, visualization. Shambhu Chandra Nath: Visualization, writing—review & editing. Md. Rakibul Islam: Writing—review & editing. Shaheen Akhter: Writing—review & editing. Md. Saidur Rahman: Writing—review & editing.
Acknowledgments
The authors would like to express their gratitude to Bangladesh Forest Research Institute (BFRI) and Forest Chemistry Division for their logistic support, sincere assistance and encouragement.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Lugt, P.; Vogtländer, J.; Brezet, H. Bamboo, a Sustainable Solution for Western Europe: Design Cases LCAs and Land-Use; VSSD, Elsevier B.V., 2009.
- Tarabanko, V. E.; Kaygorodov, K. L.; Vigul, D. O.; Tarabanko, N.; Chelbina, Y. V.; Smirnova, M. A. Influence of Acid Prehydrolysis on the Process of Wood Oxidation into Vanillin and Pulp. J. Wood Chem. Technol. 2020, 40, 421–433. DOI: https://doi.org/10.1080/02773813.2020.1835984.
- Tarabanko, V. E.; Kaygorodov, K. L.; Skiba, E. A.; Tarabanko, N.; Chelbina, Y. V.; Baybakova, O. V.; Kuznetsov, B. N.; Djakovitch, L. Processing Pine Wood into Vanillin and Glucose by Sequential Catalytic Oxidation and Enzymatic Hydrolysis. J. Wood Chem. Technol. 2017, 37, 43–51. DOI: https://doi.org/10.1080/02773813.2016.1235583.
- Liu, D. G.; Song, J. W.; Anderson, D. P.; Chang, P. R.; Hua, Y. Bamboo Fiber and Its Reinforced Composites: Structure and Properties. Cellulose 2012, 19, 1449–1480. DOI: https://doi.org/10.1007/s10570-012-9741-1.
- Tong, J.; Ma, Y. H.; Chen, D. H.; Sun, J. Y.; Ren, L. Q. Effects of Vascular Fiber Content on Abrasive Wear of Bamboo. Wear 2005, 259, 78–83. DOI: https://doi.org/10.1016/j.wear.2005.03.031.
- Sridach, W. The Environmentally Benign Pulping Process of Non-Wood Fibers. Suranaree J. Sci. Technol. 2010, 17, 105–123.
- Rasheed, M.; Jawaid, M.; Karim, Z.; Abdullah, L. C. Morphological, Physiochemical and Thermal Properties of Microcrystalline Cellulose (MCC) Extracted from Bamboo Fiber. Molecules 2020, 25, 2824. DOI: https://doi.org/10.3390/molecules25122824.
- Visakh, P. M.; Thomas, S.; Oksman, K.; Mathew, A. P. Effect of Cellulose Nanofibers Isolated from Bamboo Pulp Residue on Vulcanized Natural Rubber. Bioresources 2012, 7, 2156–2168. DOI: https://doi.org/10.15376/biores.7.2.2156-2168.
- Amada, S.; Untao, S. Fracture Properties of Bamboo. Compos. B. Eng. 2001, 32, 449–457. DOI: https://doi.org/10.1016/S1359-8368(01)00022-1.
- Chiu, H. H.; Young, W. B. Characteristic Study of Bamboo Fibers in Preforming. J. Compos. Mater. 2020, 54, 3871–3882. DOI: https://doi.org/10.1177/0021998320923144.
- Jain, S.; Kumar, R.; Jindal, U. C. Mechanical-Behavior of Bamboo and Bamboo Composite. J. Mater. Sci. 1992, 27, 4598–4604. DOI: https://doi.org/10.1007/BF01165993.
- Muhammad, A.; Rahman, M. R.; Hamdan, S.; Sanaullah, K. Recent Developments in Bamboo Fiber-Based Composites: A Review. Polym. Bull. 2019, 76, 2655–2682. DOI: https://doi.org/10.1007/s00289-018-2493-9.
- Viel, Q.; Esposito, A.; Saiter, J. M.; Santulli, C.; Turner, J. A. Interfacial Characterization by Pull-out Test of Bamboo Fibers Embedded in Poly(Lactic Acid). Fibers 2018, 6, 7. DOI: https://doi.org/10.3390/fib6010007.
- Sindhu, R.; Kuttiraja, M.; Binod, P.; Sukumaran, R. K.; Pandey, A. Bioethanol Production from Dilute Acid Pretreated Indian Bamboo Variety (Dendrocalamus sp.) by Separate Hydrolysis and Fermentation. Ind. Crops Prod. 2014, 52, 169–176. DOI: https://doi.org/10.1016/j.indcrop.2013.10.021.
- Yang, H. Y.; Shi, Z. J.; Xu, G. F.; Qin, Y. J.; Deng, J.; Yang, J. Bioethanol Production from Bamboo with Alkali-Catalyzed Liquid Hot Water Pretreatment. Bioresour. Technol. 2019, 274, 261–266. DOI: https://doi.org/10.1016/j.biortech.2018.11.088.
- Sun, S. L.; Wen, J. L.; Ma, M. G.; Sun, R. C. Enhanced Enzymatic Digestibility of Bamboo by a Combined System of Multiple Steam Explosion and Alkaline Treatments. Appl. Energy 2014, 136, 519–526. DOI: https://doi.org/10.1016/j.apenergy.2014.09.068.
- Yuan, J.; Zhu, Y.; Wang, J.; Liu, Z.; Wu, J.; Zhang, T.; Li, P.; Qiu, F. Agricultural Bamboo Leaf Waste as Carbon Precursor for the Preparation of Cu-Al/Biomass Fiber Adsorption and Its Application in the Removal of Ammonia Nitrogen Pollutants from Domestic Wastewater. J. Wood Chem. Technol. 2021, 41, 137–149. DOI: https://doi.org/10.1080/02773813.2021.1914110.
- Salam, K. Bamboo for Economic Prosperity and Ecological Security; Facets of the North-East 2, 2008.
- Khalil, H.; Bhat, I. U. H.; Jawaid, M.; Zaidon, A.; Hermawan, D.; Hadi, Y. S. Bamboo Fibre Reinforced Biocomposites: A Review. Mater. Des. 2012, 42, 353–368. DOI: https://doi.org/10.1016/j.matdes.2012.06.015.
- Kleinhenz, V.; Midmore, D. J. Aspects of Bamboo Agronomy. Adv. Agron. 2001, 74, 99–153. DOI: https://doi.org/10.1016/S0065-2113(01)74032-1.
- Vogtlander, J. G.; Lugt, P. The Environmental Impact of Industrial Bamboo Products: Life-cycle Assessment and Carbon Sequestration; 2015. http://resolver.tudelft.nl/uuid:2b1f58e2-2a72-4247-bf57-49eb7d5646a1 (accessed October 20, 2021).
- Kumar, S.; Dobriyal, P. B. Treatability and Flow Path Studies in Bamboo Part I. Dendrocalamus strictus Nees. Wood Fiber Sci. 2007, 24, 113–117.
- Hu, Y.; Tang, L. R.; Lu, Q. L.; Wang, S. Q.; Chen, X. R.; Huang, B. Preparation of Cellulose Nanocrystals and Carboxylated Cellulose Nanocrystals from Borer Powder of Bamboo. Cellulose 2014, 21, 1611–1618. DOI: https://doi.org/10.1007/s10570-014-0236-0.
- Sluiter, J. B.; Ruiz, R. O.; Scarlata, C. J.; Sluiter, A. D.; Templeton, D. W. Compositional Analysis of Lignocellulosic Feedstocks. 1. Review and Description of Methods. J. Agric. Food Chem. 2010, 58, 9043–9053. DOI: https://doi.org/10.1021/jf1008023.
- Das, A. K.; Nakagawa-Izumi, A.; Ohi, H. Evaluation of Pulp Quality of Three Non-Wood Species as Alternative Raw Materials for Paper Production. Jpn. Tappi J. 2015, 69, 548–554. DOI: https://doi.org/10.2524/jtappij.1501.
- Das, A. K.; Nakagawa-Izumi, A.; Ohi, H. Quality Evaluation of Dissolving Pulp Fabricated from Banana Plant Stem and Its Potential for Biorefinery. Carbohydr. Polym. 2016, 147, 133–138. DOI: https://doi.org/10.1016/j.carbpol.2016.03.103.
- Horvath, A. L. Solubility of Structurally Complicated Materials: I. Wood. J. Phys. Chem. Ref. Data 2006, 35, 77–92. DOI: https://doi.org/10.1063/1.2035708.