Abstract
The main goal of the investigation was to measure and estimate the thermal stability of pinosylvin, pinosylvin monomethyl ether, robinetin, and dihydrorobinetin, i.e., characteristic compounds of wood of Scots pine and black locust, respectively. The pure compounds were analyzed with chromatography before and after they were steam-sterilized and oven-dried. Detailed thermogravimetric analysis was followed to check the thermal decomposition of the stilbenes and flavonoids of wood. The tested phenolic compounds were susceptible to thermal degradation. After the steam-sterilization and oven drying, the amount of investigated phenolic extractives decreased by more than a half. The thermogravimetric analysis showed that stilbenes decompose differently than flavonoids, which can be attributed to the different chemical structures. Twenty minutes of steam sterilization followed by 24 h of oven drying decreased the amount of phenolic compounds in the vials; however, after the applied thermal treatments, the stilbenes and robinetins stayed available in such quantities that they can still provide sufficient bioactivity.
Introduction
Phenolic compounds are generally presented as the main products of the secondary metabolism of plants. They are phytochemicals that can exhibit antioxidant, antimicrobial, and antifungal properties.[Citation1–5] Plant phenols as such have recently received much attention from the scientific community, because these compounds of natural origin could replace synthetic and hazardous agents in different bio-based formulations,[Citation6–8] e.g., adhesives, packaging material, foams, and preservative solutions.[Citation9] In addition to the large diversity of plant matrices, a possible source for extraction of polyphenols are different types of less-utilized biomass; among them, woody biomass has been demonstrated to have high biorefinery potential.[Citation10] Not only residues of the wood processing industry but woody biomass of the lowest quality is also considered to be one of the cheapest sources of phenolic extractives with antioxidant and antifungal properties.[Citation11] Knotwood and bark of some conifers were found to contain a large amount of lignans, phenolic acids, and flavonoids that are already accessible on the market as natural antioxidants in dietary supplements.[Citation12,Citation13] In particular, knotwood of Scots pine and heartwood of black locust have been demonstrated as potential source and raw material for extraction of phenolic compounds.[Citation14–16] Phenolic extractives obtained from sapwood, heartwood, and knotwood are generally classified as simple phenols, phenolic acids, quinones, stilbenes, flavonoids, biflavonoids, lignans, and hydrolyzable tannins and proanthocyanidins.[Citation17–19] Stilbenes and flavonoids of wood and bark in particular have been demonstrated to exhibit significant bioactive properties. These phytochemicals have proven antioxidant and antifungal activity and have already been somehow the targeted compounds of our previous investigations.[Citation4,Citation20–26] Pinosylvins (PSs) (pinosilvin and pinosilvin monomethyl ether) and robinetins (Rob) [dihydrorobinetin (DHR) and robinetin] have been shown to be abundant in pine knotwood and in black locust heartwood, respectively.[Citation14,Citation27] These extracts have also been confirmed to exhibit antioxidant and antifungal properties.[Citation28–30] The main focus of the research field is on replacing environmentally and human hazardous synthetic compounds with natural bioactive agents. The biodegradability of natural biocides at the end of the product’s life cycle has also been shown to be very important.[Citation6]
Before field testing, investigation of potential antifungal properties of phenolic compounds/extractives usually starts with a lab-scale assessment of the fungicidal properties of the relevant compounds.[Citation31–34] All these more or less standardized assays are performed in vitro under controlled and sterile conditions. Standard EN 113 is used for quantification of inherent fungal durability.[Citation35] This standard describes a methodology for determining protective effectiveness against wood destroying basidiomycetes.[Citation35] Standard EN 113 is adopted as a reference laboratory method that is frequently used for assessment of the inherent durability of wood treated with water-insoluble chemicals, organic water-insoluble or water-dispersible formulations, as well as water-soluble products.[Citation36,Citation37] The samples are exposed to wood-decaying fungi under control and sterile conditions. According to the standard, sterile conditions are provided by steam-sterilization at 121 °C for 20 min. In addition, the wood is oven-dried at (103 ± 2)°C. The standard also describes other sterilization methods, e.g., ionizing irradiation or epoxyethane-based sterilant; however, these methods are not recommended for phenolic substances (EN 113).
Since phenolic extractives are described as compounds susceptible to unwanted reactions of polymerization and oxidation and to thermal degradation,[Citation10,Citation18,Citation38–40] some doubts arise when a natural compound, a phytochemical, is tested with the relevant standard laboratory methodology. However, it has been reported that the phenolic compounds of spruce bark are relatively stable at temperatures up to 60–100 °C, with degradation ranging up to 40% after 4 h of exposure to elevated temperatures.[Citation38] As reported by Bostyn et al.,[Citation27] no degradation of Rob and DHR is observed after the extracts are submitted to 8 h of heating at 50 °C. As early as 2006, a comprehensive thermogravimetric (TG)/mass spectrometric (MS) investigation by Meszaros et al.[Citation41] revealed that the extractives decompose in the same temperature range as the natural polymers that make up the cell walls of plants. Similar findings were reported by Shebani et al.,[Citation42] who found that the thermal decomposition of hydrophilic extractives of wood occurs in a wide temperature range between 200 °C and 500 °C. Reviewing the existing literature, it quickly becomes clear that exact data on how higher temperature influences stilbenes and Robs of wood are lacking. Information on the thermal stability of the most abundant/characteristic phenolic extractives present in Scots pine knotwood and black locust heartwood is missing. This study is closely related to already published work[Citation11,Citation29,Citation43] in which we reported on the influence of the impregnation with extracts of Scots pine knotwood and black locust heartwood on the fungal decay resistance of pine and beech sapwood.
The aims of the investigation were (a) to explore the influence of two selected thermal treatments that are most frequently used for sterilization according to the EN 113 standard protocol, i.e., steam sterilization and oven drying, on the thermal stability of characteristic phenolic extractives of pine and black locust wood and (b) to study the thermal behavior of phenolic extractives of wood with thermogravimetric analysis (TGA) to reveal what temperatures affect the compounds.
Material and methods
Chemicals
Methanol [high-performance liquid chromatography (HPLC) grade] and formic acid (for LC/MS, 99%) were purchased from Merck (Sigma-Aldrich Chemie, Taufkirchen, Germany). Water and acetone, both HPLC grade, were supplied by J.T. Baker (Phillipsburg, USA), cyclohexane (99%) was provided by Carlo Erba Reagents (Chaussée du Vexin, France). PS (HPLC, ≥97%) and PSMME (HPLC, ≥97%) were from Merck (Sigma-Aldrich Chemie, Taufkirchen, Germany). Analytical HPLC standards for Rob (HPLC assay, ≥95%) and DHR (HPLC assay, ≥99%) were supplied by Extrasynthese (Genay, France).
Preparation of the extractives for chemical monitoring
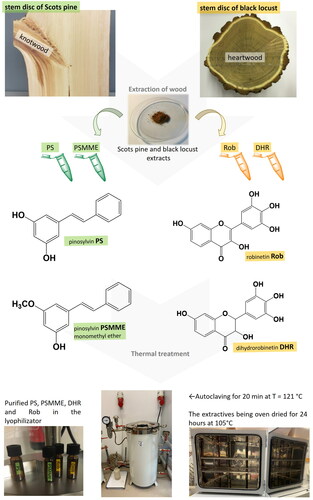
The characteristic phenolic extractives of wood of Scots and black pine, and black locust that were the focus of the present investigation have been already described in the frame of published studies.[Citation11,Citation29,Citation44] The compounds included in the analysis of thermal stability, i.e., PS and pinosilvin monomethyl ether (PSMME), Rob, and DHR, were commercially purchased (). All the phenolic extractives were prepared as 1% methanol stock solutions (wt/vol). The phenolic solutions were stored in a freezer at –25 °C before thermal treatment.
Figure 1. A: Schematic presentation of the research activities in the laboratory. PS and PSMME from knotwood of Scots pine (Pinus sylvestris), and DHR and Rob from black locust (Robinia pseudoacacia) were exposed to steam sterilization and oven drying. After each thermal treatment they were quantitatively checked on thermal decomposition by chromatographic (HPLC) and TGA.
Measuring the effect of steam-sterilization and oven drying on phenolic compounds
Each of the four phenolic compounds was exposed to steam sterilization and oven drying successively (). Between each of the thermal treatments, the phenolic solutions were qualitatively evaluated by HPLC analysis as described later. Brief description of the sample prep procedure for the analytical monitoring is as follows. First, the stock solution of an “intact” phenolic compound was prepared in methanol, i.e., the solution volume of 1 mL in an amber-colored vial of dimensions 32 mm × 11.6 mm tightly closed with a N10 polypropylene screw seal containing a silicone white/PTFE septa. The stock solutions of the intact phenolic extractives were quality checked with HPLC analysis (QC-HPLC). Then, the solvent was removed with evaporation (a vacuum chamber at 10 kPa) and the extractives were freeze dried (a lyophilizator at 4 Pa and –85 °C). Vials containing dry phenolic compounds were then steam-sterilized for 20 min at p = 220 kPa and T = 121 °C (Autoclave 300 Sutjeska, Serbia). After the steam-sterilization, the methanol solutions of extractives (V = 0.98 mL) were prepared, whereat the volume taken by the needle of QC-HPLC was deducted. After that, first monitoring of the extractives with HPLC analysis followed (1stM-HPLC). After 1stM-HPLC analysis, methanol was removed from the vials with evaporation (a vacuum chamber at 10 kPa) and the extractives were freeze dried again (a lyophilizator at 4 Pa and –85 °C). The freeze-dried extractives were then exposed to overnight oven drying at 105 °C (Kambič, SP 250-C, Semič, Slovenia). After oven drying, the methanolic solutions were prepared for HPLC analysis (V = 0.96 mL, the volume taken by the needle of QC-HPLC and 1stM-HPLC was deducted). Second HPLC monitoring followed as described earlier. Finally, the results of quantitative HPLC analyzes were studied. PS, PSMME, DHR, and Rob were monitored for HPLC chromatographic peak area/height after each step of the thermal treatment.
Chromatographic analysis
The stock solutions containing the four phenolics were first measured by HPLC. The extractive solutions were diluted and filtered through a 0.22-µm polyamide syringe filter into 1.5 mL amber HPLC vials. The extractive solutions were diluted as many times as required for the HPLC analysis to give chromatograms on which the detector response for the peaks, i.e., peak heights, were approximately 1000 milli-absorbance units (mAU). Each of the PS, PSMME, DHR, and Rob sample was measured by HPLC in six repetitions. Chromatographic analysis of PSs and Robs was performed on Thermo Scientific’s Accela HPLC system as already described.[Citation11] Briefly, the compounds of the treated solutions were separated on a Thermo Scientific Accucore ODS column with dimensions of 4.6 mm (i.d.) × 150 mm and 2.6 µm particle size. Sample trays and a column oven were thermostated at 5 °C and 30 °C, respectively. Water (A) and methanol (B), both containing 0.1% formic acid (vol/vol), were used as a mobile phase. A gradient from 5% to 95% of solvent B in 10 min was applied to elution targeted molecules. The flow rate of the mobile phase was 1000 mL/min. The separated compounds were detected with Accela photo diode array detection (PDA detector). The wavelength for monitoring the phenolic compounds was set at 280 nm, and UV spectra were recorded from 200 nm to 400 nm (). Retention times measured were tr PS = 15.3 min for PS, tr PSMME = 17.8 min for PSMME, tr DHR = 6.8 min for DHR, and tr Rob = 10.7 min for Rob. Peak assignments were achieved by comparing the retention times and UV spectra of separated compounds to those of analytical standards. A similarity of UV spectra for all the targeted compounds was larger than 0.99. Identified compounds were quantified by comparing peak areas with calibrations made with standards and analyzed under the same conditions. The linearity of the calibration curves for each standard was confirmed by the goodness of fit (R2) being larger than 0.99.
TGA analysis
The course of thermal decomposition for PS, PSMME, Rob, and DHR was determined thermogravimetrically using a Mettler Toledo TGA/DSC1 instrument from room temperature to 600 °C under dynamic airflow (50 mL min−1). The heating rate was 10 °C min−1. The masses of the samples ranged from 2 mg to 4 mg. The blank curve is subtracted. Evolved gases were transferred to a mass spectrometer (Pfeiffer Vacuum ThermoStar) via a 75-cm long heated transfer line. To lower the water content in the mass spectrometer, the sample was kept at 25 °C for 15 min at the beginning of measurement.
Statistics and graphical material
The results of the investigation were analyzed for statistical significance by Statgraphics software (StatPoint, Inc., Virginia, USA). The data were first checked for normal distribution. ANOVA was used to test differences between the means of measurements, and Fisher’s least significant difference (LSD) procedure was performed to indicate which means were significantly different from others at a 95.0% confidence level. Structural formulas of PS, PSMME, DHR, and Rob were prepared with PerkinElmer’s ChemDraw software.
Results and discussion
Influence of autoclaving and oven drying on the thermostability of wood extractives
The results of quantitative HPLC are presented in . The influence of each thermal treatment on the tested phenolic extractives was determined by comparing the chromatographic peak areas and heights.[Citation45] HPLC traces on and Citation3 represent the impact of autoclaving and oven drying on PS, PSMME, DHR, and Rob, on which the peak heights clearly represent these changes. It is clear that both steam sterilization and oven drying affected the tested phenolic compounds (ANOVA, p < 0.0001; LSD test). The most significant influence of 20 min of steam sterilization was measured on PS and DHR (, and ). After steam sterilization, the peak areas of PS and DHR decreased by 20.98% and 14.85%, respectively. The largest resistance against degradation after autoclaving was measured for PSMME, in which the peak area decreased by 3.91%. After steam sterilization, the area of the peak that was ascribed to Rob was found to be slightly higher than the peak for intact Rob, which could be explained with the relatively thermal-stable molecule of Rob at temperature of 120 °C (, ). However, Rob and DHR have already been reported as relatively resistant at low temperatures.[Citation27] Bostyn et al.[Citation27] observed no degradation of Rob and DHR after the extracts were submitted to 8 h of heating at 50 °C. A higher impact on the detector response was measured after 24 h of oven drying at 105 °C, whereby the peak area for all the characteristic phenolic extractives of Scots pine and black locust decreased by more than 50% in comparison to the initial peak area; specifically, the peak area decreased 51.51% for PS (), 53.51% for PSMME (), and 56.30% for DHR (). These results indicate that the phenolic compounds are susceptible to thermal degradation, especially with more prolonged exposure to higher temperatures. In terms of all the necessary steps of sterilization that are required by, e.g., standard EN 113, the results of present study clearly show the impact of autoclaving on the stability of the wood low-molecular polyphenols, although it has already been demonstrated in our previous studies that sterilization itself does not affect the characteristics of phenolics in such way that these compounds do not show antifungal properties or do not increase the resistance of wood against fungal decay.[Citation11,Citation46] Thermogravimetric analysis was performed in the next step, by which the actual course of thermal degradation for the four phenolic extractives of wood was checked.
Figure 2. HPLC-PDA chromatograms of PS and PSMME exposed to autoclaving and oven drying. (a and d), A PDA detector response of intact PS and PSMME (native); (b and e), detector response of PS and PSMME after autoclaving for 20 min at p = 220 kPa and T = 121 °C; (c and f), detector response of PS and PSMME after the 20 min steam sterilization followed by the overnight oven drying at T = 105 °C.
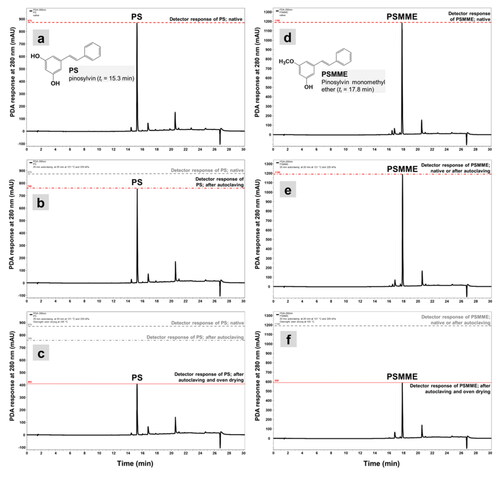
Figure 3. HPLC-PDA chromatograms of DHR and Rob exposed to autoclaving and oven drying. (a and d), a PDA detector response of intact DHR and Rob (native); (b and e), detector response of DHR and Rob after autoclaving for 20 min at p = 220 kPa and T = 121 °C; (c and f), detector response of DHR and Rob after 20 min steam sterilization followed by overnight oven drying at T = 105 °C.
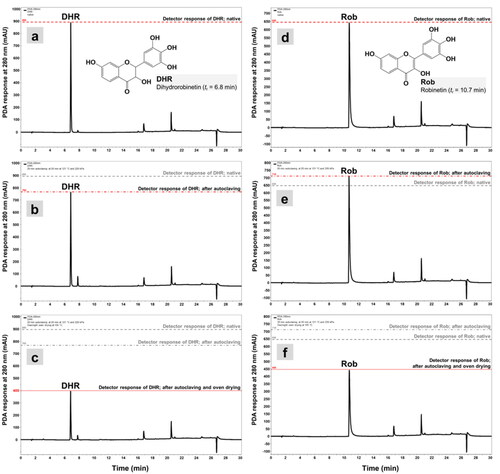
Table 1. HPLC results showing a decrease of the HPLC peak areas (Δ area%) due to steam sterilization (20 min at p = 220 kPa and T = 121 °C) and oven drying (24 h at T = 105 °C).
Results of TGA analysis
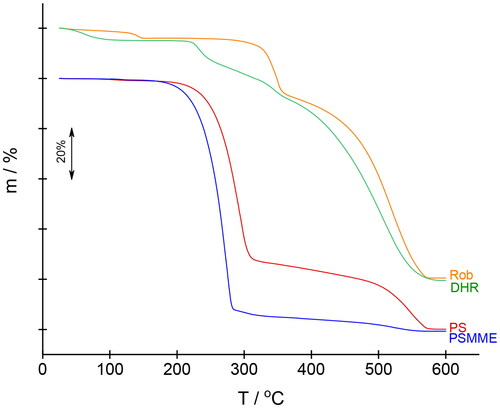
shows the TG-MS curves of individual compounds, whereas shows a comparison of all TG curves. Onset temperatures and temperatures at which decomposition of organic matter reaches 5% are given in . The thermal decomposition of PS occurs in several successive steps. From room temperature to 200 °C, there is a small mass loss (1.8%) but, surprisingly, intense signals typical of benzene (m/z = 78, 51)[Citation47] were detected in the mass spectrum in this temperature range. We ascribe these signals to the evaporation of the solvent that was used for recrystallization.[Citation48,Citation49] Decomposition of PS starts at temperatures higher than 200 °C. Mass loss is large in the first step (more than 70%), during which benzene, CO2, and H2O are evolved. Decomposition then progresses slowly up to 480 °C (mass loss between 320 °C and 480 °C is only 8%). In the last step, the evolution of CO2 predominates, and the sample loses another 19.5% from 480 °C to 580 °C. Thermal decomposition of PSMME starts at a slightly lower temperature compared to PS (see ). Evaporation of the recrystallization solvent is barely noticeable (see arrow on the mass peaks 77 and 51 at around 120 °C). The onset decomposition temperature is 230 °C, and degradation is nearly complete at 290 °C (mass loss reaches 92% up to this temperature). The same species are evolved as during the thermal decomposition of PS. The small amount of residue decomposes slowly in a temperature range from 290 °C to 470 °C. After that, the rate of mass loss increases, and the sample decomposes completely up to 560 °C; CO2 is mainly released in the last small step. We ascribe the small mass loss (2.3%) in the TG curve of Rob at around 140 °C to the evaporation of water and CO2. The latter is probably present due to an oxidized solvent residue. Thermal decomposition starts at 335 °C and occurs in two successive steps: from 280 to 365 °C, the mass loss is 22.0%, the evolution of water and CO2 takes place. The degradation continues up to 575 °C, with 100% mass loss. For DHR, dehydration of adsorbed moisture occurs below 100 °C. The decomposition of DHR starts at 225 °C. At the beginning of more than two overlapping successive steps, CO2 and water are evolved, while in the last step, which begins at 380 °C, CO2 again predominates. However, during the thermal decomposition of Rob and DHR, no fragments of the benzene ring are observed, only a signal for water and carbon dioxide is observed. This means that Rob and DHR burn completely, oxidizing to water and carbon dioxide. Rob is more thermally stable than DHR. The Rob molecule is planar because all carbon atoms are sp2 hybridized. Due to this planar structure, delocalization of electrons and thus resonance stabilization of the molecule is possible, resulting in better thermal stability of Rob. From it can clearly be seen that the thermal decomposition of stilbenes differs from the thermal decomposition of flavonoids. A comparison of the thermal decomposition of PS and PSMME shows that PS is more thermally stable than PSMME. These results correspond with the results of HPLC analysis (). When the temperature of thermal decomposition is reached, stilbenes start to decompose much faster than do flavonoids. Comparison of Rob and DHR shows that Rob is more thermally stable than DHR. Most importantly, the thermal decomposition of flavonoids occurs in several, or at least two stages, with the formation of decomposition intermediates (). Compound content decreases during 24 h exposure in the oven (105 °C) as determined by HPLC chromatography, while TGA results show no weight loss in this temperature range. Of course, the two methods differ because the samples in the oven are exposed to high temperatures for a long period of time, whereas in TGA the samples are heated at a constant rate of 10 °C min–1. Despite the difference in sample processing methods, we assume that exposure to the oven results in the formation of oligomers that are not detected by the HPLC method. In terms of all the HPLC and TGA results, the tested phenolic extractives of wood can be described as natural compounds with relatively high resistance to temperature decomposition. It is true that autoclaving, and especially oven drying, affected the quantity of the tested phenolic extractives in the glass vials; however, it can be said that even after the sterilization process, stilbenes and flavonoids remain in a carrier matrix in sufficient amounts so that they can still perform a specific function there, i.e., as natural fungistatic compounds and/or natural antioxidants.
Figure 4. Thermogravimetric and mass spectrometric (TG-MS) curves of stilbenes PS and PSMME, and flavonoids Rob and DHR. 18, water (H2O); 44, CO2; 77 and 51, C6H5.
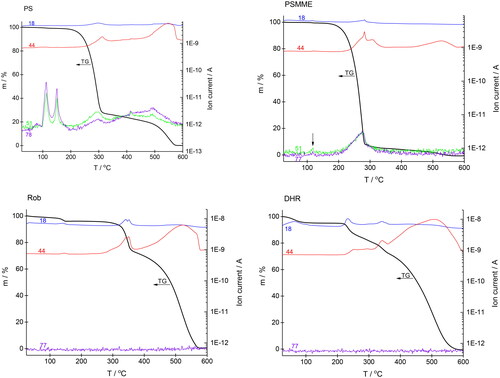
Table 2. Thermo-gravimetrical analysis of investigated phenolic compounds.
Conclusions
The results of the present investigation demonstrate the impact of the most commonly used laboratory techniques for sterilization and drying, i.e., steam sterilization and oven drying, on the thermal stability of PS, PSMME, DHR, and Rob. The targeted phenolic compounds extracted from devaluated woody biomass were found to be relatively resistant to conventional lab-scale thermal treatments. The results of chromatographic (HPLC) and thermogravimetric (TG) analysis showed that stilbenes and flavonoids start to decompose above a temperature of 200 °C. The decomposition paths for stilbenes and flavonoids were shown to differ significantly. The TG curves for robinetines were described by more shoulders on TG curves indicating more decomposition steps in the case of flavonoids. This can be explained by the differences in chemical structure between the 1,2-diphenylethene and 2-phenyl-1,4-benzopyrone molecules. The results of TGA analysis of the pure compounds also confirmed that phenolic extractives of wood decompose in a wide temperature range, i.e., from 200 °C to 570 °C, meaning that low-molecular polyphenols decompose in the same temperature range as the structural components of a wooden cell wall.[Citation41] The investigation showed that the phenolic extractives are susceptible to thermal decomposition at higher temperatures. However, at the same time, their thermal stability was shown to be relatively high, and they could therefore be exposed to the temperatures that are characteristic for the processes of wood preservation and of producing packaging material. The literature reports that the thermal resistance of plant polyphenols can even be enlarged by encapsulation to protect them from thermal degradation during the sterilization and baking process.[Citation9] Further research activities are needed to provide more accurate conclusions on this.
Declaration of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Part of the work was performed in the frame of the transnational WoodWisdom ERA-NET project PINOBIO. Many thanks also to Dr. Boštjan Lesar and Andreja Žagar for many useful tips and professional assistance, and to Mr. Martin Cregeen for language editing.
Additional information
Funding
References
- Laoung-On, J.; Jaikang, C.; Saenphet, K.; Sudwan, P. Phytochemical Screening, Antioxidant and Sperm Viability of Nelumbo Nucifera Petal Extracts. Plants (Basel) 2021, 10, 1375. DOI: 10.3390/plants10071375.
- Plumed-Ferrer, C.; Väkeväinen, K.; Komulainen, H.; Rautiainen, M.; Smeds, A.; Raitanen, J. E.; Eklund, P.; Willför, S.; Alakomi, H. L.; Saarela, M.; et al. The Antimicrobial Effects of Wood-Associated Polyphenols on Food Pathogens and Spoilage Organisms. Int. J. Food Microbiol. 2013, 166, 163–163. DOI: 10.1016/j.ijfoodmicro.2013.06.031.
- Oleson, K. R.; Schwartz, D. T. Extractives in Douglas-Fir Forestry Residue and Considerations for Biofuel Production. Phytochem. Rev. 2016, 15, 985–1008. DOI: 10.1007/s11101-015-9444-y.
- Välimaa, A.-L.; Honkalampi-Hämäläinen, U.; Pietarinen, S.; Willför, S.; Holmbom, B.; von Wright, A. Antimicrobial and Cytotoxic Knotwood Extracts and Related Pure Compounds and Their Effects on Food-Associated Microorganisms. Int. J. Food Microbiol. 2007, 115, 235–243. DOI: 10.1016/j.ijfoodmicro.2006.10.031.
- Štular, D.; Savio, E.; Simončič, B.; Šobak, M.; Jerman, I.; Poljanšek, I.; Ferri, A.; Tomšič, B. Multifunctional Antibacterial and Ultraviolet Protective Cotton Cellulose Developed by in Situ Biosynthesis of Silver Nanoparticles into a Polysiloxane Matrix Mediated by Sumac Leaf Extract. Appl. Surf. Sci. 2021, 563, 150361. DOI: 10.1016/j.apsusc.2021.150361.
- Singh, T.; Singh, A. P. A Review on Natural Products as Wood Protectant. Wood Sci. Technol. 2012, 46, 851–870. DOI: 10.1007/s00226-011-0448-5.
- Barbero-López, A.; Akkanen, J.; Lappalainen, R.; Peräniemi, S.; Haapala, A. Bio-Based Wood Preservatives: Their Efficiency, Leaching and Ecotoxicity Compared to a Commercial Wood Preservative. Sci. Total Environ. 2021, 753, 142013. DOI: 10.1016/j.scitotenv.2020.142013.
- Schultz, T. P.; Nicholas, D. D. Naturally Durable Heartwood: Evidence for a Proposed Dual Defensive Function of the Extractives. Phytochemistry 2000, 54, 47–52. DOI: 10.1016/S0031-9422(99)00622-6.
- Albuquerque, B. R.; Heleno, S. A.; Oliveira, M. B. P. P.; Barros, L.; Ferreira, I. C. F. R. Phenolic Compounds: current Industrial Applications, Limitations and Future Challenges. Food Funct. 2021, 12, 14–29. DOI: 10.1039/d0fo02324h.
- Holmbom, B. Extraction and Utilisation of Non-Structural Wood and Bark Components. In Biorefining of Forest Resources; Alén, R., Ed.; Paper Engineers’ Association/Paperi ja Puu Oy: Helsinki, 2011; pp 178–224.
- Vek, V.; Balzano, A.; Poljansek, I.; Humar, M.; Oven, P. Improving Fungal Decay Resistance of Less Durable Sapwood by Impregnation with Scots Pine Knotwood and Black Locust Heartwood Hydrophilic Extractives with Antifungal or Antioxidant Properties. Forests 2020, 11, 1024. DOI: 10.3390/f11091024.
- Tavčar Benković, E.; Grohar, T.; Žigon, D.; Švajger, U.; Janeš, D.; Kreft, S.; Štrukelj, B. Chemical Composition of the Silver Fir (Abies Alba) Bark Extract Abigenol® and Its Antioxidant Activity. Ind. Crops Prod. 2014, 52, 23–28. DOI: 10.1016/j.indcrop.2013.10.005.
- Willför, S.; Nisula, L.; Hemming, J.; Reunanen, M.; Holmbom, B. Bioactive Phenolic Substances in Industrially Important Tree Species. I. Knots and Stemwood of Different Spruce Species. Holzforschung 2004, 58, 335–344. DOI: 10.1515/HF.2004.052.
- Willför, S.; Hemming, J.; Reunanen, M.; Holmbom, B. Phenolic and Lipophilic Extractives in Scots Pine Knots and Stemwood. Holzforschung 2003, 57, 359–372. DOI: 10.1515/HF.2003.054.
- Sergent, T.; Kohnen, S.; Jourez, B.; Beauve, C.; Schneider, Y. J.; Vincke, C. Characterization of Black Locust (Robinia pseudoacacia L.) Heartwood Extractives: Identification of Resveratrol and Piceatannol. Wood Sci. Technol. 2014, 48, 1005–1017. DOI: 10.1007/s00226-014-0656-x.
- Magel, E.; Jay-Allemand, C.; Ziegler, H. Formation of Heartwood Substances in the Stemwood of Robinia pseudoacacia L. II. Distribution of Nonstructural Carbohydrates and Wood Extractives across the Trunk. Trees: Struct. Funct. 1994, 8, 165–171. DOI: 10.1007/BF00196843.
- Fengel, D.; Wegener, G. Wood: chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin-New York, 1989; p. 613.
- Kai, Y. Chemistry of Extractives. In Wood and Cellulosic Chemistry, Hon, D. N. S., Shiraishi, N., Eds.; Marcel Dekker, Inc.: New York, 1991; pp 215–255.
- Pietarinen, S. P.; Willfor, S. M.; Vikstrom, F. A.; Holmbom, B. R. Aspen Knots, a Rich Source of Flavonoids. J. Wood Chem. Technol. 2006, 26, 245–258. DOI: 10.1080/02773810601023487.
- Belt, T.; Hanninen, T.; Rautkari, L. Antioxidant Activity of Scots Pine Heartwood and Knot Extractives and Implications for Resistance to Brown Rot. Holzforschung 2017, 71, 527–534. DOI: 10.1515/hf-2016-0232.
- Smith, A. L.; Campbell, C. L.; Walker, D. B.; Hanover, J. W. Extracts from Black Locust as Wood Preservatives: Extraction of Decay Resistance from Black Locust Heartwood. Holzforschung 1989, 43, 293–296. DOI: 10.1515/hfsg.1989.43.5.293.
- Sablík, P.; Giagli, K.; Pařil, P.; Baar, J.; Rademacher, P. Impact of Extractive Chemical Compounds from Durable Wood Species on Fungal Decay after Impregnation of Nondurable Wood Species. Eur. J. Wood Prod. 2016, 74, 231–236. DOI: 10.1007/s00107-015-0984-z.
- Lu, J. R.; Venalainen, M.; Julkunen-Tiitto, R.; Harju, A. M. Stilbene Impregnation Retards Brown-Rot Decay of Scots Pine Sapwood. Holzforschung 2016, 70, 261–266. DOI: 10.1515/hf-2014-0251.
- Pietarinen, S.; Willför, S.; Ahotupa, M.; Hemming, J.; Holmbom, B. Knotwood and Bark Extracts: Strong Antioxidants from Waste Materials. J. Wood Sci. 2006, 52, 436–444. DOI: 10.1007/s10086-005-0780-1.
- Harju, A. M.; Venäläinen, M. Measuring the Decay Resistance of Scots Pine Heartwood Indirectly by the Folin-Ciocalteu Assay. Can. J. For. Res. 2006, 36, 1797–1804. DOI: 10.1139/x06-074.
- Tavčar Benković, E.; Žigon, D.; Mihailović, V.; Petelinc, T.; Jamnik, P.; Kreft, S. Identification, in Vitro and in Vivo Antioxidant Activity, and Gastrointestinal Stability of Lignans from Silver Fir (Abies alba) Wood Extract. J. Wood Chem. Technol. 2017, 37, 467–477. DOI: 10.1080/02773813.2017.1340958.
- Bostyn, S.; Destandau, E.; Charpentier, J.-P.; Serrano, V.; Seigneuret, J.-M.; Breton, C. Optimization and Kinetic Modelling of Robinetin and Dihydrorobinetin Extraction from Robinia Pseudoacacia Wood. Ind. Crops Prod. 2018, 126, 22–30. DOI: 10.1016/j.indcrop.2018.09.049.
- Vainio-Kaila, T.; Zhang, X.; Hanninen, T.; Kyyhkynen, A.; Johansson, L. S.; Willfor, S.; Osterberg, M.; Siitonen, A.; Rautkari, L. Antibacterial Effects of Wood Structural Components and Extractives from Pinus Sylvestris and Picea Abies on Methicillin-Resistant Staphylococcus aureus and Escherichia coli O157: H7. BioResources 2017, 12, 7601–7614. DOI: 10.15376/biores.12.4.7601-7614.
- Vek, V.; Poljansek, I.; Humar, M.; Willfor, S.; Oven, P. In Vitro Inhibition of Extractives from Knotwood of Scots Pine (Pinus sylvestris) and Black Pine (Pinus nigra) on Growth of Schizophyllum commune, Trametes versicolor, Gloeophyllum trabeum and Fibroporia vaillantii. Wood Sci. Technol. 2020, 54, 1645–1662. DOI: 10.1007/s00226-020-01229-7.
- Hosseinihashemi, S. K.; HosseinAshrafi, S. K.; Goldeh, A. J.; Salem, M. Z. M. Antifungal and Antioxidant Activities of Heartwood, Bark, and Leaf Extracts of Robinia pseudoacacia. BioResources 2015, 11, 1634–1646. DOI: 10.15376/biores.11.1.1634-1646.
- Lindberg, L. E.; Willför, S. M.; Holmbom, B. R. Antibacterial Effects of Knotwood Extractives on Paper Mill Bacteria. J. Ind. Microbiol. Biotechnol. 2004, 31, 137–147. DOI: 10.1007/s10295-004-0132-y.
- Lee, D. G.; Lee, S. J.; Rodriguez, J. P.; Kim, I. H.; Chang, T.; Lee, S. Antifungal Activity of Pinosylvin from Pinus Densiflora on Turfgrass Fungal Diseases. J. Appl. Biol. Chem. 2017, 60, 213–218. DOI: 10.3839/jabc.2017.034.
- Fernandez-Costas, C.; Palanti, S.; Charpentier, J. P.; Sanroman, M. A.; Moldes, D. A Sustainable Treatment for Wood Preservation: Enzymatic Grafting of Wood Extractives. ACS Sustainable Chem. Eng. 2017, 5, 7557–7567. DOI: 10.1021/acssuschemeng.7b00714.
- Celimene, C. C.; Micales, J. A.; Ferge, L.; Young, R. A. Efficacy of Pinosylvins against White-Rot and Brown-Rot Fungi. Holzforschung 1999, 53, 491–497. DOI: 10.1515/HF.1999.081.
- EN 113. Wood Preservatives – Test Method for Determining the Protective Effectiveness against Wood Destroying basidiomycetes – Determination of the Toxic Values; British Standards; CEN (European Committee for Standardization), Brussels, 1997.
- Humar, M.; Lesar, B. Fungicidal Properties of Individual Components of Copper–Ethanolamine-Based Wood Preservatives. Int. Biodeter. Biodegrad. 2008, 62, 46–50. DOI: 10.1016/j.ibiod.2007.06.017.
- Kutnik, M.; Suttie, E.; Brischke, C. European Standards on Durability and Performance of Wood and Wood-Based Products – Trends and Challenges. Wood Mater. Sci. Eng. 2014, 9, 122–133. DOI: 10.1080/17480272.2014.894574.
- Volf, I.; Ignat, I.; Neamtu, M.; Popa, V. I. Thermal Stability, Antioxidant Activity, and Photo-Oxidation of Natural Polyphenols. Chem. Pap. 2014, 68, 121–129. DOI: 10.2478/s11696-013-0417-6.
- Umezawa, T. Chemistry of Extractives. In Wood and Cellulosic Chemistry; Hon, D. N. S., Shiraishi, N., Eds.; Marcel Dekker, Inc.: New York, 2000; pp 213–241.
- Sehlstedt-Persson, M.; Karlsson, O. Natural Durability and Phenolic Content in Dried Scots Pine Heartwood. BioResources 2010, 5, 17.
- Meszaros, E.; Jakab, E.; Varhegyi, G. TG/MS, Py-GOMS and THM-GIC/MS Study of the Composition and Thermal Behavior of Extractive Components of Robinia Pseudoacacia. J. Anal. Appl. Pyrolysis 2007, 79, 61–70. DOI: 10.1016/j.jaap.2006.12.007.
- Shebani, A. N.; van Reenen, A. J.; Meincken, M. The Effect of Wood Extractives on the Thermal Stability of Different Wood Species. Thermochim. Acta 2008, 471, 43–50. DOI: 10.1016/j.tca.2008.02.020.
- Vek, V.; Poljanšek, I.; Balzano, A.; Humar, M.; Oven, P. Extraction of Fungitoxic Compounds from Low-Quality Wood Biomass. Presented at 4th International Conference on Technologies & Business Models for Circular Economy (TBMCE 2021); University of Maribor, Faculty of Chemistry and Chemical Engineering: Portorož, Slovenia, 2021; p 1
- Poljansek, I.; Oven, P.; Vek, V.; Raitanen, J. E.; Hemming, J.; Willfor, S. Isolation of Pure Pinosylvins from Industrial Knotwood Residue with Non-Chlorinated Solvents. Holzforschung 2019, 73, 475–484. DOI: 10.1515/hf-2018-0152.
- Vek, V.; Poljanšek, I.; Oven, P. Variability in Content of Hydrophilic Extractives and Individual Phenolic Compounds in Black Locust Stem. Eur. J. Wood Prod. 2020, 78, 501–511. DOI: 10.1007/s00107-020-01523-y.
- Zimmer, K.; Melcher, E. A Screening Study on Extractive Content and Composition of Scots Pine Heartwood of Three Stands with Close Proximity and Their Resistance against Basidiomycetes. Int. Wood Prod. J. 2017, 8, 45–49. DOI: 10.1080/20426445.2016.1271091.
- NIST Mass Spectrometry Data Center, William E. Wallace, director, “Mass Spectra” in NIST Chemistry WebBook, NIST Standard Reference Database Number 69, Eds. P.J. Linstrom and W.G. Mallard, National Institute of Standards and Technology, Gaithersburg MD, 20899, https://doi.org/10.18434/T4D303, (retrieved September 12, 2022)
- Laavola, M.; Nieminen, R.; Leppänen, T.; Eckerman, C.; Holmbom, B.; Moilanen, E. Pinosylvin and Monomethylpinosylvin, Constituents of an Extract from the Knot of Pinus Sylvestris, Reduce Inflammatory Gene Expression and Inflammatory Responses in Vivo. J. Agric. Food Chem. 2015, 63, 3445–3453. DOI: 10.1021/jf504606m.
- Albrecht, H.; Sheers, E. H. The Isolation of Trans-3,5-Dimethoxystilbene from Tall Oil. J. Am. Chem. Soc. 1954, 76, 603–604. DOI: 10.1021/ja01631a082.