Abstract
The collapse of the World Trade Center (WTC) buildings #2 (South Tower), #1 (North Tower), and #7 created an enormous collapse pile which emitted intense plumes of acrid smoke and dust until roughly mid-December, when the last spontaneous surface fire occurred. We collected particles by size (8 modes, ≈12 to 0.09 micrometers diameter) and time (typical resolution of 1 to 3 h) from October 2 until late December at the EML 201 Varick Street site roughly 1.8 km NNE of the collapse site and 50 m above ground level. Here we show some of the 70,000 mass and elemental data from the time period October 2 through October 30. Identification of a WTC collapse pile source for aerosols seen at the receptor site were based upon the simultaneous presence of finely powdered concrete, gypsum, and glass with intense very fine combustion mode mass episodes concurrent with winds from the southwest quadrant. The results, derived from seven independent beam-based analytical techniques, showed that while PM10 and PM2.5 24 h values rarely, if ever, violated federal air quality standards, WTC-derived plumes swept over lower Manhattan Island, resulting in intense aerosol impacts of duration a few hours at any one site. The WTC plume resembled in many ways those seen from municipal waste incinerators and high temperatures processes in coal-fired power plants. The size fractions above 1 micrometer contained finely powdered concrete, gypsum, and glass, with sootlike coatings and anthropogenic metals, but little asbestos. Composition in the very fine size range (0.26 > Dp > 0.09 μm) was dominated by sulfuric acid and organic matter, including polycyclic aromatic hydrocarbons (PAHs) and their derivatives, and glasslike silicon-containing aerosols. Many metals were seen in this mode, most, but not all, at low concentrations. The concentrations of very fine silicon, sulfur, and many metals, as well as coarse anthropogenic metals, decreased markedly during October, probably in association with the cooling of the collapse piles. Values of very fine elements seen in May, 2002 at the WTC site were only a few percent of October values.
INTRODUCTION
The collapse of the World Trade Center (WTC) buildings #2 (South Tower), #1 (North Tower), and #7 on September 11, 2001 is an unprecedented event in numerous ways. Yet the prompt and massive emissions of smoke and dust in the first days after the collapse were in accord with common understanding of such phenomena. However, the continuing emission of these plumes, especially after the heavy rains of September 14 and the increasingly effective efforts of fire suppression in mid- and late September, are not fully understood. Factors which are essential for an in-depth analysis are the chemical composition of the materials that could be aerosolized and the energy sources available in the collapse piles. In this regard, the kinetic energy of the two aircraft is negligible (<1%) compared to the chemical energy in the roughly 25,000 liters of fuel in each plane (some of which was burned outside the buildings). The gravitational potential energy of the collapse was capable of raising the entire mass of debris only a few degrees K. The largest energy sources available are the combustible materials present in the buildings and furnishings and a significant body of fuel, especially under WTC #7, in the form of diesel fuel for emergency electrical generators and large quantities of oil in various forms in the Consolidated Edison substation, also under WTC #7. Very high temperatures occurred in the burning floors of the buildings prior to collapse and during the first few days of active surface fires, as shown by the melting of metals. Later, infrared surveys showed surface temperatures in the collapse pile were as high as 30 K above ambient in October, and much higher subsurface temperatures were inferred from the lower portions of removed steel beams glowing red. The subsurface of the collapse piles remained hot for months despite use of massive amounts of water to cool them, with the last spontaneous surface fire occurring in mid-December.
Satellite and photographic observations of the aerosol plumes are extremely useful in characterizing plume transport, especially in the early days when few other measurements were performed or are available. On the evening of September 11, the area of lower Manhattan Island was blanketed with a dark gray smoke. On September 12 at 11:30 AM, the Enhanced Thematic Mapper Plus (ETM+) aboard the Landsat 7 satellite showed a dispersed plume moving WNW to SSW in a broad plume over roughly 120° angular dispersion, while later that same day IKONOS showed a whitish coherent plume no more than about 0.3 km wide lofting above the buildings as it moved south towards open water (CitationIKONOS 2001). Heavy rains occurred on September 14, which helped the Fire Department of New York (FDNY) extinguish surface fires while wetting the massive dust deposits. The plume detected by IKONOS on September 16 was much less intense and much darker than the plume of September 12, and the lofting is not as evident. All of these were consistent with the improved conditions on the collapse pile observed during rescue operations. In late September, rescue operations gradually ceased and recovery operations began and with them greatly increased fire suppression efforts, including wetting agents and use of heavy equipment to begin unpeeling the collapse pile.
EXPERIMENTAL TECHNIQUES
In response to the continuing emissions from the collapse site, a DELTA Group 8-DRUM sampler was prepared and air expressed to the Department of Energy's Environmental Measurement Laboratory (EML) on 201Varick Street, New York, a 12 story building roughly 1.8 km NNE of the collapse site. The unit was sited on the roof at an elevation of roughly 50 m above the street but lower than most of the surrounding buildings. Sampling began on October 2 at 12:20 PM and continued in operation with two changes in drums until late December, when it was shipped back to Davis. Other than a few hours of uncertainty at the beginning of the study (backlash in gears), samples were collected during over 99% of all available sampling periods in October.
SAMPLE COLLECTION
Samples were collected in a slotted rotating drum impactor (CitationLundgren 1967), a technique in which the UC Davis Air Quality Group and DELTA Group have specialized for the past 30 years (some examples are in CitationCahill et al. 1974, 1981, 1989; CitationFlocchini et al. 1976; CitationFeeney et al. 1975; CitationBarone et al. 1978; CitationFlocchini et al. 1981; CitationReid et al. 1994; and summarized in CitationCahill and Wakabayshi 1993) totaling almost 17,000 site days of sampling and analysis, most recently in international studies of aerosol transport (CitationReid et al. 1993; CitationPerry et al. 1997, 1999) including the large study, ACE-Asia 2001. The present version, an 8 stage rotating drum impactor called a DRUM (Davis Rotating-drum Unit for Monitoring) was developed (CitationCahill et al. 1985; CitationRaabe et al. 1988), modified (O. Raabe, private communication, 4 October 1997), and labeled the DELTA 8 DRUM. This sampler used the validated models of Raabe et al. (1989) for one of the two cases that can be derived analytically, a long slot (CitationMarple et al. 1986). Note that in Raabe et al. (1989) a universal relationship was developed that provided the shape and cut point of all stages on a single graph, assuming that certain aerodynamic limits, such as on the Reynold's number, were respected. The behavior of the sampler was validated by comparing the predicted pressure drop on each stage to the observed pressure drop, which was on the average within 2% for Stages 2 through 7. The sampler operated at 10.0 L/min, maintained by a critical orifice on Stage 8 ().
Parameters of the DELTA DRUM slotted drum impactor
The sampler was operated on a 42 day rotation schedule, delivering 8 lightly greased MylarTM strips 168 mm long. All stages were coated with a thin (few μg/cm2) layer of Apiezon-LTM grease applied in a 2% toluene solution (CitationWesolowski et al. 1978; CitationCahill 1979). This has proven highly effective in preventing mis-sizing of coarse, dry particles with less than one part in 5,000 of soil mass present at 10 μm diameter appearing at 0.34 μm (CitationCahill et al. 1985).
The physical slot length L was 0.605 cm and the effective slot length in the deposit was 0.65 cm for all stages, but mass is seen distributed laterally, especially on Stage 1, as would be expected due to the shape of the slots which vary significantly from the theoretical “infinite slit” description. However, we measured the effective length of the slit, and the laterally distributed mass was found to come from locations close to the end of the slot. Thus, no correction must be included for this effect when converting from areal density of deposit (μg/cm2) to concentration (μg/m3) as long as the effective slot length is used.
The effective time resolution Δt is thus set by the width of the deposit and the rotation rate of the DRUM sampler. It is best for the finest stages, which for the 6 week rotation rate (4 mm per 24 hr day for this work) delivers a time resolution of about 1 h. The time resolution on the coarser stages is not as great as the physical dimension of the slot. The observed width of deposit W (d) is much smaller on the coarse stages than the slot width W (s), resulting in improved time resolution, ΔTime, three to four times better than would have been expected from the slot width alone. This difference decreases in the finer stages until the observed width is about 85% of the slot width. The DRUM protocol delivered a time resolution approximating 1 to 1.5 h for the fine (PM2.5) stages, although better resolution could be obtained using a deconvolution protocol.
SAMPLE ANALYSIS
Samples were received at Davis shipped on the drums used to collect the particles. Samples were dismounted according to DRUM protocols and transferred to stiff plastic frames for analysis. The frames were coded to establish the time at which the samples were taken. Due to the nature of the study, we were unable to incorporate automatic time-marking protocols on the strips during sampling thus, increasing the uncertainty with which absolute times can be determined.
Seven separate nondestructive techniques routinely used by DELTA Group collaborators were applied to the WTC samples:
-
Optical: color photography (macro lens) (in air) at UCD.
-
Mass: soft beta ray transmission for mass and quality assurance (Mass β) (in air), at UCD.
-
Mass: scanning transmission ion microscopy (STIM) to measure high temporal resolution aerosol mass profiles (Mass STIM) (in vacuum) at Center for Accelerator Mass Spectrometry, Lawrence Livermore National Laboratory (LLNL).
-
Hydrogen: Proton elastic scattering analysis (PESA) (in vacuum) at LLNL.
-
Elemental: Na-U, synchrotron x-ray fluorescence (in vacuum) (S-XRF), digital Si (Li) analysis at Advanced Light Source, Lawrence Berkeley National Laboratory (LBNL).
-
Speciated organic matter: laser desorption ionization time-of-flight mass spectrometry (LDITOF/MS) (in vacuum) at UCD.
-
Size and morphology: scanning electron microscopy (SEM) with X-ray analysis (in vacuum, using small pieces of the strip) at UCD.
We will emphasize in this report mass data from scanning transmission ion microscopy (STIM), hydrogen data from proton elastic scattering analysis (PESA), and elemental data from Synchrotron-induced X-Ray Fluorescence (S-XRF), while other results will be used to help clarify and interpret these primary data.
Mass by STIM
Mass was measured in vacuum by STIM using the 3 MeV proton microprobe of the Center for Accelerator Mass Spectrometry (CAMS), Lawrence Livermore National Laboratory (LLNL). This method is described in CitationBench et al. (2002), which establishes the accuracy and precision of STIM on equivalent samples in some detail. Mass was measured by the energy loss of the proton as it passes through the Mylar substrate (480 μg/cm2), the grease coating (nominally 10 μg/cm2), and the deposit with a minimum detectable level equivalent to about 0.5 μg/m3 in clean DRUM samples. The STIM proton beam, which can be as narrow as 1 μm, was set at a width of 125 μm to match the width of deposit of the sampler which was equivalent to 45 min time resolution for the 4 mm/day DRUM rotation rate (). Thus, the analysis width is slightly less than the width of deposit, giving an equivalent time resolution of about 1 h taken every 45 min for the finest DRUM stages. The measurement was made in a vacuum, which removes unbound or lightly bound water and some fraction of volatile constituents, primarily organic matter and some nitrates.
While relative time accuracy is excellent (<3 h in 4 weeks), absolute times are uncertain because of the finite width of the beginning and ending deposits on the Mylar strip, backlash in the gearing, etc. Normal DELTA Group protocols call for a blank region in the middle of each strip for time and blank calibration and for a fixed ultrafine after filter, neither of which could be implemented in the short time available. Thus, absolute times are given with an estimated maximum error of 6 h.
Hydrogen by PESA
Hydrogen was measured by PESA (CitationCahill et al. 1989, 1990, 1996; CitationMalm et al. 1994) at CAMS, LLNL, which is also described in CitationBench et al. (2002). The time and spatial resolution was identical to that of the STIM analyses, roughly 1 h resolution taken every 45 min. The strips had already been exposed to vacuum in the STIM analyses and were additionally exposed to vacuum during the PESA analyses, which removes unbound or lightly bound water and some fraction of volatile constituents, primarily organic matter and some nitrates. Thus, the remaining hydrogen is primarily found in inorganic ions and organic matter. With knowledge of the chemical state of major ions, the remaining hydrogen has been found to be closely associated with organic matter (CitationCahill et al. 1989, 1990, 1996). This has been independently verified in over 100,000 ambient aerosols sampled in the past 14 years as part of IMPROVE (Interagency Monitoring of Protected Visual Environments) quality assurance protocol (CitationMalm et al. 1994; CitationIMPROVE 1988–2002), which directly compares Organic Matter by Carbon (OMC) by combustion to organic matter via PESA (OMH). Despite the fact that these measurements for IMPROVE were made on different substrates (fired quartz for OMC, stretched Teflon for OMH) by different laboratories, with OMC in air and OMH in vacuum, the results are very well correlated, with a slope with ±10% of unity.
Elemental by S-XRF
The concentration of elements from sodium through molybdenum and selected heavy elements was established by synchrotron X-ray fluorescence (S-XRF) on the DELTA Group Beam Line 10.3.1. X-ray microprobe at the Advanced Light Source, Lawrence Berkeley National Laboratory (ALS, LBNL). S-XRF is X-ray fluorescence in which the primary excitation source consists of polarized X-rays from a synchrotron. We have used this technique on samples from the Kuwaiti oil fires at the Stanford Linear Accelerator (CitationCahill 1992; CitationReid 1994), and it is becoming more widely appreciated (CitationKhodzher et al. 2000) as a tool for aerosol analysis. The advantages are many, including removal of more than 95% of the X-ray background due to the polarization of the beam and the ability to focus the beam into small areas due to ALS's extraordinary source brightness. Through use of a “white” beam covering 4 keV < Ex < 18 keV, possible because of the polarization, all elements between sodium and uranium can be simultaneously measured. However, in realistic ambient samples, the elements from molybdenum through the rare earths and some heavy elements usually have serious interferences and are not routinely quantified. The measurements are made in a vacuum, which allows elements as light as carbon to be seen, but self absorption corrections make quantification very difficult. The X-rays are detected by a digital Si(Li) detector and electronics, and calibration is accomplished through 65 thin National Institute of Standards and Technology (NIST) traceable elemental standards and 12 certified elements, Al-Pb, in NIST Standard Reference Materials (SRMs) 1832 and 1833. The results are reduced in the DELTA Group version of the International Atomic Energy Agency's preferred code, AXIL. Accuracy and precision have been verified by repeated analyses and blind intercomparisons with IMPROVE (0.99 ± 0.04) and California Air Resources Board (CARB) (1.02 ± 0.11) filters (CitationDQAP ver. 7.02, 2002). The minimum detectable limit in a 30 s analysis is around 0.2 ng/cm2 for most elements other than the exceptions (above), which converts to 0.004 ng/m3 for the 6 week DRUM sampler used in this study. An example of the integration of these data with mass by STIM and hydrogen by PESA is given in CitationBench et al. (2002).
RESULTS
Single Particle Analysis
The impaction of particles onto the lightly greased flat Mylar substrate made single particle analysis possible for both coarse and fine particles. Samplers were analyzed on the actual substrates without attempts at removal, which necessitates removing a small portion of the DRUM strip onto a SEM stem for analysis.
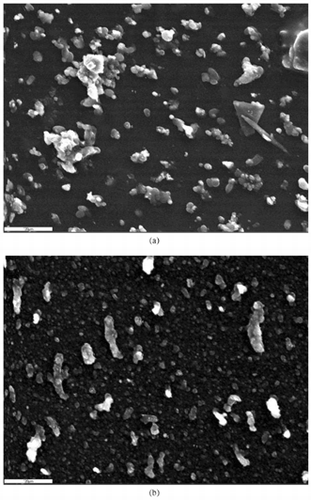
shows an example of particles from aerodynamically defined stage, Stage #2, from 5.0 to 2.5 μm diameter, while shows the same period but for particles from 0.26 to 0.09 μm diameter. The SEM data represent the period that occurred on October 4, a period which will later be assigned to WTC plume impact, and had the highest value for very fine silicon. In , in addition to the large number of particles in the aerodynamically selected 5 to 2.5 μm range, there are what appear to be large agglomerated flakes that would normally be aerodynamically sized along their thinnest aspect, as would the one fiber roughly 25 μm long. In all the pictures taken, only one other asbestos-like fiber was seen, 120 μm long, collected in the coarsest size mode ≈12 to 5.0 μm equivalent aerodynamic diameter. shows the silicon particles are indeed very fine, 0.26 > Dp > 0.09 μm diameter. The electron beam of the SEM system was used to detect numerous silicon-containing particles below the surface of roughly 3 μm-thick grease antibounce coating. The silicon particles appear to have been imbedded in the grease of the surface coating because of the high (sonic) speed of impact during collection, while the sulfate particles stayed on the hydrophobic grease surface. The presence of large amounts of sulfur was confirmed in the elongated particles, which appear to be generated by postcollection aggregation. The alignment was parallel to the air flow over the sample. This is in accord with both the PESA hydrogen data and the LDITOF/MS data, which shows that the primary sulfur species was sulfuric acid and thus contains large amounts of water at the ambient relative humidity present on October 4.
FIG. 1 Scanning electron microscopy of particle episodes. SEM coarse, very fine particles collected on October 4 by the DRUM sampler as a function of aerodynamic size, (a) coarse (5.0 μm > Dp > 2.5 μm) and (b) very fine (0.26 μm > Dp > 0.09 μm). Compositional analysis of coarse particles was consistent with finely ground concrete, glass, and gypsum. Only two fibers that were potentially asbestos were found in these analyses. Very fine particles showed large sulfate-rich aggregates aligned roughly with the direction of the air flow across the drum, consistent with postcollection agglomeration of highly hydrated sulfuric acid. Silicon was the only element seen in particles <0.3 μm, often imbedded in the grease coating.
Mass
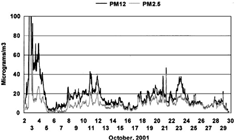
The mass data are voluminous (over 7,000 values) and are posted on the DELTA Group web site (http://delta.ucdavis.edu) in the World Trade Center file. presents results for coarse particles, roughly PM12 (12 to 0.09 μm diameter), and fine particles, roughly PM2.5, (2.5 to 0.09 μm diameter), both lacking the ultrafine component below 0.09 μm diameter.
FIG. 2 Mass data, approximately PM10 and PM2.5, versus time. DRUM data and STIM mass analysis from October 2 through October 30, 2001, at the 201 Varick Street site, sorted into approximately PM10 (closer to PM12) and PM2.5 mass fractions, although both measurements neglect any ultrafine mass below 0.09 μm diameter. The data are taken every 45 min, giving a roughly 1 h averaging period. The 1 h average of the PM10 and PM2.5 peaks on October 3 were 247 μg/m3 and 196 μg/m3, respectively.
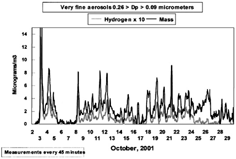
One of the most unusual aspects of the mass data was the high levels of very fine aerosols, 0.26 > Dp > 0.09 μm, measured at this site (). This results in an extraordinarily large number of particles that have the ability to penetrate deep into the lung. Plotted on the same graph are the results of the hydrogen measurement by PESA (over 7,000 values) taken shortly after and on the same system as used for the STIM analysis. Generally, there was an extremely high correlation between the mass and hydrogen data, which also provides an estimate of the precision of the two techniques (CitationBench et al. 2002). The hydrogen data have been multiplied by 10.
FIG. 3 Mass and hydrogen data, very fine particles, versus time. Very fine (0.26 > Dp > 0.09 μm) DRUM data for mass by STIM and hydrogen by PESA, times 10, October 2 through October 30. The data are taken every 45 min, giving a roughly 1 h averaging period. The 1 h average of the very fine mass was peak on October 3 was 58 μg/m3. The measurements were done independently and in a vacuum, which removes unbound water. If we use the S-XRF sulfur data and the LDITOF/MS speciated sulfate data were to correct for hydrogen bound in sulfates and nitrates, we get an approximate organic mass that is roughly hydrogen times 10.
Elemental Analysis
Elemental analysis was performed on all DRUM samples by S-XRF, yielding low detectable limits for element sodium through zirconium and some heavy metals, with limited information of the remaining trace elements. The amount of information contained in the 55,000 values (plus an equal number of uncertainties and minimum detectable limits) prevents a full accounting in this report. Thus, we will only highlight some of the more significant findings in this publication.
COARSE PARTICLES
The mass distribution was bimodal, with peaks in the coarse mode (5.0 > Dp > 2.5 μm) and very fine mode (0.26 > Dp > 0.09 μm). Such a distribution is not typical of ambient aerosols, which tend to have a mass minimum around 2.5 μm and very low values for very fine aerosols <0.26 μm. We will treat each mode separately. In we present the plots of major crustal elements and noncrustal elements in the size fraction 5.0 > Dp > 2.5 μm. Averages of crustal elements are more typical of soil except for the excesses (enrichment factor of 6) for calcium. Finely powdered concrete is a probable source for the Ca excess, based on WTC building inventories of friable materials by mass.
FIG. 4 Coarse crustal elements versus time. Three hour averages of the typically earth crustal elements aluminum, silicon, calcium, and iron from October 2 to October 30, 2001 in the coarse mode (5.0 > Dp > 2.5 μm). This size range typically represents the peak of the mass distribution in calcium-rich periods. The time integration for this and all later elemental data was 3 h.
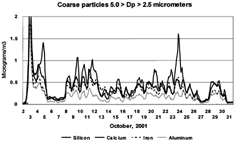
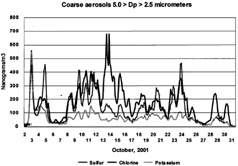
FIG. 5 Coarse noncrustal elements versus time; S, Cl, K. The coarse mode (5.0 > Dp > 2.5 μm) sulfur, chlorine, and potassium are plotted versus time. Radical changes are seen in these ratios versus time, such as on October 3 and October 5. The chlorine peaks had little associated sodium except for on October 14, which was associated with an east wind, when the elemental ratios were consistent with NaCl and a presumed oceanic salt signature.
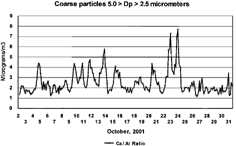
In , the supermicron (5.0 > Dp > 2.5 μm) excess calcium signatures of the mineral aerosol are presented, using the Al/Ca ratio. Similar results are obtained using the Ca/Si and Ca/Fe ratio to calculate the noncrustal calcium excess. The anomalously fine mineral aerosols shown in have a presumed major source from cement dust, the largest friable building component by mass. The use of both Ca/Si and Ca/Fe ratios corrects for the presence of another friable material, glass, but the results are essentially equivalent in identifying the calcium excess. Likewise, sulfur appears in the coarse mode, correlated with the excess calcium sulfate aerosols in the 5.0 to 2.5 μm size mode. The sulfur/calcium correlation is excellent, in two periods: r 2 ≈ 0.9, Ca/S ratio of 6.1 ± 0.2 seen on October 3, and r2 ≈ 0.8, Ca/S = 3.5 ± 0.5 for all other periods. Gypsum, Ca2SO4, used in the wallboard that was used in the core of the buildings, is a potential source for this material.
FIG. 6 Coarse calcium/aluminum ratio versus time. The coarse mode (5.0 > Dp > 2.5 μm) calcium and aluminum are plotted versus time. The graph represents approximately the earth crustal enrichment factor. Values above 3 were seen on 10 occasions and are consistent with a strong calcium excess typical of concrete, as expected from the WTC concrete inventory and settled dust data of CitationLioy et al. (2002).
The noncrustal elements are showed in . The large chlorine episode on October 14 begins with an oceanic sea salt signature, namely winds from the ocean (120°) and a Na/Cl molar ratio of close to 1.0. Otherwise, there is excess chlorine for all other chlorine peaks.
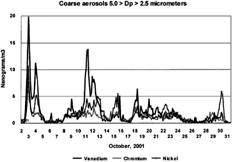
presents the noncrustal elements vanadium, chromium, and nickel. All three have extremely high enrichment factors versus Earth crustal materials.
FIG. 7 Coarse noncrustal elements versus time; V, Cr, Ni. The coarse mode (5.0 > Dp > 2.5 μm) vanadium, chromium, and nickel are plotted versus time. All of these elements have extremely high Earth crustal enrichment factors, and thus are not soil or concrete derived. Note the steady diminution of coarse vanadium versus time.
VERY FINE PARTICLES
plots the major crustal species in the very fine mode, 0.26 > Dp > 0.09 μm for comparison with . Note that there is an enormous enhancement of silicon relative to all other crustal elements showing that the form is not soil. This is illustrated in , in which we plot the Al/Si ratio versus time.
FIG. 8 Very fine crustal elements versus time. Crustal elements in the very fine mode, (0.26 > Dp > 0.09 μm). Silicon dominates all other elements, unlike the coarse mode aerosols shown above in .
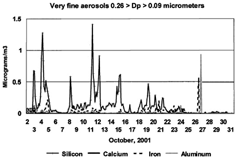
FIG. 9 Al/Si ratio for very fine particles versus time. The total dominance of silicon in very fine (0.26 > Dp > 0.09 μm) particles can be also expressed as an “enrichment factor” compared to normal Earth crustal ratios. This also confirms that the very fine silicon is not a sampling artifact of coarse particle modes.
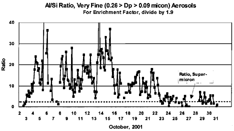
shows the same silicon, but this time plotted against sulfur, while shows vanadium, nickel, and chromium. Clearly, there is a relationship between the very fine silicon and sulfur, but the ratio varies widely. Note, the Si/S ratio decreases throughout October.
FIG. 10 Very fine sulfur and silicon versus time. DRUM data and S-XRF elemental analysis for very fine (0.26 > Dp > 0.09 μm) sulfur and silicon, October 2–30, 2001. (For mass of sulfate, times 3.0; for H2SO4, times 3.06; for mass of SiO2, times 2.14).
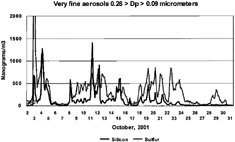
FIG. 11 Very fine vanadium, nickel, and chromium versus time. Elemental data are presented for the very fine (0.26 > Dp > 0.09 μm) mode for vanadium, nickel, and chromium, October 2–30.
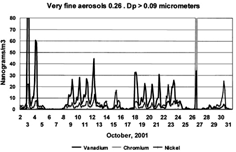
The vanadium and nickel retain a relatively constant ratio, V/Ni ≈ 5, for all peaks seen in October except the last one, where it dropped to ≈ 1.2 on an east wind trajectory.
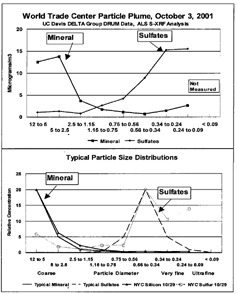
PARTICULATE SIZE
The size distribution of major elements was anomalous with almost all mass either coarse or very fine, unlike typical ambient aerosols seen in scores of studies since 1972 (CitationWhitby 1978). This is shown in , which gives the size profile for the particle episodes of October 3 though 5, in contrast to the typical ambient size distribution for these species show in . In , we also plot a non-WTC episode on October 29, which except for excess very fine sulfur, looks like a typical size distribution. The presence of so much mass in the very fine size fraction thus leads to a very high particulate surface area and number of particles. The coarse mode has a relatively large amount of mass in the vicinity of 3.5 μm, with high potential for deposition in the bronchial tract.
DISCUSSION
In this section, we treat four topics: (1) which of the aerosol episodes seen in the PM10, PM2.5, and elemental data were most likely derived in all or part from the WTC collapse piles; (2) what are the most likely sources within the collapse piles that generated the episodes; (3) how the WTC-derived episodes seen at Varick Street compare to similar sampling at other sites and times around the world; and (4) what supplemental measurements are being made that have or could reduce the uncertainties in the data interpretation.
Identification of Episode Origins at the WTC Collapse Site
The repeated presence of intense aerosol plumes of short duration at the Varick Street site could arise from a number of local and regional sources within and upwind of New York City. Clearly, full interpretation of this enormous data set (circa 7,000 mass, 7,000 hydrogen, over 50,000 elements) will require close analysis with local data that are only now being developed as current investigations progress. In this analysis, we will attempt to identify probable sources for the PM10 and PM2.5 concentrations presented earlier in .
Many of the normal source identification protocols that could, in principle, be used to identify the origins of these aerosol events are not possible in this work. Only one sampler of this type was operated, and thus no direct upwind-downwind comparison is possible. No prior measurements of this type have been made in New York, but data have been recently generated (May 2002) in New York with identical instrumentation. Similar data have been generated in Baltimore as part of the Supersite program which will aid comparisons in future papers. Similar size- and time-resolved compositional data are available at Shenandoah National Park as part of the IMPROVE program (Cahill and Wakabayashi 1996) which is mostly useful in characterizing regional upwind sources that typically dominate aerosols in the eastern US each summer. In this regard, the urban IMPROVE site in Washington, DC and Shenandoah National Park are essentially identical each summer in the period July–September for PM2.5 mass and almost every measured parameter (CitationIMPROVE 1998–2002), and we expect this to be largely true of New York City. Potentially strong local sources, thermal electric power plants, were all operating on natural gas in this period, and thus would not be a major source of sulfur, vanadium, and nickel, which they would be if operating on heavy fuel oil.
The wind rose, and direct plume transport studies lack detailed meteorology at the source and local measurements at the receptor site. Complexities include unmeasured parameters such as street canyon transport and plume loft, which are the focus of continuing studies by other groups but are unavailable at present. Standard 24 h PM2.5 data are available but are of limited use in identifying short-duration aerosol events, especially events dominated by particles below 0.26 μm diameter. Standard statistical source-receptor techniques cannot be used since the WTC aerosol source was uncharacterized. In this regard, the detailed measurements of CitationLioy et al. (2002) represent settled dust from the immediate collapse, which we expect would be very different from the continuing emissions from the hot collapse pile.
In the face of these uncertainties, we have chosen to use as a basis for identification of potential WTC-derived aerosol plumes those enhanced aerosol concentrations deriving from the southwest quadrant that have probable WTC source materials (coarse Ca, coarse S) and are divergent from standard ambient size resolved aerosol data (very fine mass) and from background values at times when persistent prevailing winds would not allow major WTC impacts.
The most extreme divergence from standard ambient aerosols is mass in the very fine mode, 0.26 > Dp > 0.09 μm. In we present the highly time-resolved very fine mass and hydrogen. Very fine mass below 0.26 μm diameter are typically associated with high temperature and industrial sources (CitationU.S. EPA 1996 and references therein). Further, the very high diffusion rates of these particles generally make their residence times in the atmosphere short, favoring relatively local high temperature sources.
The highest time resolution data available, the size-resolved mass data from STIM, has an effective time resolution of roughly 1 h. The very fine mode 0.26 > Dp > 0.09 μm was generally at very low levels outside of the events, giving a sharp event–nonevent signature. For background we used values during October 6 and 7 with winds from the northwest and west, which had very fine mass 0.26 > Dp > 0.09 μm averaging 0.26 μg/m3. For all nonevent periods, totaling 25 days, masses averaged 1.8 μg/ m3, while in the 18 events, totaling about 96 h, masses averaged 6.9 μg/m3, or about 25 times background values. In below, we have identified 18 such aerosol events based on very fine mass levels greater than 4.0 μg/m3 that persisted for at least 2 h and listed them by date, since no more than one event was present on any given day.
Comparison of the chosen aerosol events versus all nonevent hours
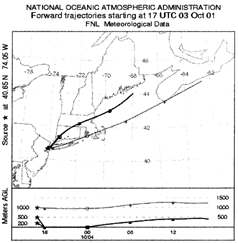
HYSPLIT regional backward isentropic trajectories were used to identify the quadrant from which the air mass originated (CitationHYSPLIT 2002). One hour peak values were used for wind direction and wind velocity. HYSLIT forward isentropic trajectories were used to show transport from the WTC downwind. (), while providing information on plume behavior in the vertical dimension. Note the strong subsidence of the trajectories from the WTC site as the air parcels follow the potential temperature. A similar pattern was seen on September 13, October 4, October 5, October 23, and October 24, dates when surface haze layers were seen at LaGuardia, measured at downwind air sampling sites along the trajectory, and widely perceived by residents of Manhattan. From HYSPLIT, we can then approximate 11 AM lapse rate: −− = very stable, rapidly descending isentropic trajectories; − = stable, descending isentropic trajectories; 0 = neutral; + = unstable, rising isentropic trajectories; and ++ = very unstable with rapidly rising isentropic trajectories. For Episodes 8, 10, 15, and 16, the actual peak hours were used for this calculation. We have also included the presence of ground level haze at LaGuardia (LGA) airport which lies at an angle of roughly 235° from the WTC.
FIG. 13 HYSPLIT isentropic forward trajectories for October 3, 2001. The NOAA ARL HYSPLIT trajectory analysis was performed hourly for the period October 2 through October 6, 2001. On the morning of October 3, the incoming air at 200, 500, and 800 m entered the New York City region from the southwest (225°) and exited to the northeast across lower Manhattan Island, Brooklyn, and Queens, crossing over LaGuardia airport where surface hazes were observed. AGL, above ground level.
Finally, in , we present the coarse calcium and coarse sulfate values. The sulfur/calcium correlation is excellent in two periods: r2 ≈ 0.9, Ca/S ratio of 6.1 ± 0.2 seen on October 3; and r2 ≈ 0.8, Ca/S = 3.5 ± 0.5 for all other periods. Three hour averaging times for species are used, but background values are a 2 day average. Mass is in μg/m3 and other concentrations are in ng/m3.
None of these parameters is by itself definitive proof of a WTC source. For example, WTC-enhanced cement dust was distributed widely over lower Manhattan Island, so that it could be present on winds from other directions. However, there had been several days of rainfall since September 11, (September 14, 20, 28, and 30, as well as October 1, 6, 16, and 17) which would tend to suppress the dust. Despite massive use of water starting later September, the WTC collapse pile itself remained hot and dusty until at least December 14, when the last spontaneous surface fire occurred.
The presence of surface haze layers of short (typically 3 h) duration at LaGuardia Airport provides supportive evidence on WTC plumes. These hazes were observed on September 13, 22, and 24, and October 3, 4, 5, 23, and 24. The haze episodes were typically about 3 h in extent, and in all cases the surface-based hazes occurred at times of stable atmospheric conditions and descending isentropic trajectories that would hold the aerosols close to the ground. This was confirmed on September 13, when a haze event was also seen at LaGuardia Airport. Two air-monitoring sites, PS 64 in lower Manhattan and PS 199 in Queens, lie on a rough line between the WTC site and LaGuardia. Each showed one hour PM2.5 concentrations above 100 μg/m3 (U.S. EPA briefing paper, September 10–15, 2001). No other site in the New York area reported one hour PM2.5 values greater than 50 μg/m3 at any time in this 5 day period. This result is in accord with numerous photographs from ground and satellites showing a characteristically narrow aerosol plume that rose rapidly to roughly 500 m and then passed over much of the area without visual evidence of ground contact.
While the probable association of the aerosol events to the WTC source is hypothesized in these tables, the confounding presence of potential sources upwind of the WTC collapse site cannot be neglected. In we compare the aerosol event of October 3 with concurrent 24 h sampling at two sites on Staten Island, Fresh Kills and PS 44, and in New York City at Battery Park, Manhattan Borough Community College, and PS 64 (CitationU.S. EPA 2001). The wind direction of 225° at 0700 hours local allows for the WTC plume to impact Varick Street but the lofting of the plume from the hot collapse pile may reduce ground level concentrations in ways we cannot calculate with the available data, including lack of measurements of temperature in the plume itself as it issued from the WTC site. Nevertheless, the 24 h values measured at Varick Street in nonplume periods, 25.3 μg/m3, almost exactly matches the average upwind PM2.5 mass values from Staten Island, 24.9 μg/m3. Thus, all mass above about 25 μg/m3 at the local EPA sites and at Varick Street cannot be assigned to regional background, but must be a local source. Thus, there is no inconsistency between the high time resolution DRUM mass data and the 24 h EPA filter measurements.
Comparison of PM2.5 mass values along wind trajectory for October 3, 2001
All the EPA values were at elevations close to ground level, but our data were taken at 50 m. However, qualitative observations of WTC pollutants such as odor and smoke were widely observed in New York on these days, leading to a NYC Health Department Press release issued on October 5 reporting that “despite the smoky conditions in areas of lower Manhattan that are close to the World Trade Center site, test results from ongoing monitoring of airborne contaminants indicates that the levels continue to be below the level of concern to public health” despite “the presence of dust and smoke odor in the downtown area” (CitationMullin and Butler 2001).
The very high levels on October 3 require an explanation. Two possible explanations lie in changes of emission rate and/or changes in transport efficiency. We have no detailed information from site activities that can be used to derive changes in the emission rate. Heavy equipment was in constant use, uncovering hot subsurface zones in the collapse pile, and perhaps enhancing emissions. In terms of transport efficiency, the HYSPLIT isentropic trajectories at 200 m and 500 m were rapidly descending, reaching the ground in only a few kilometers. This was the most extreme case seen in the entire period and is most likely a major part of an explanation for the low level transport of the plume. It is also possible that the cooling of the site after 2 days of light rain and massive fire fighting efforts would reduce plume lofting, thus increasing downwind surface concentrations. Significantly, the highest 24 h PM value seen by the EPA at sites just north of the WTC (Site A) occurred on October 3, over 400 μg/m3 (CitationU.S. EPA 2002). It is also important to appreciate that the Varick Street values were taken at an elevation of about 50 m and thus could well be impacted directly by a slightly lofted plume from the still hot WTC collapse site.
While much of lower Manhattan south and west of the Varick Street receptor site, other than intense activities at the WTC collapse site itself, was relatively inactive in October 2001, there were a very large number of idling diesel trucks lined up almost as far north as the sampling site waiting to take debris loads to the Hudson River transport site. In addition, many diesel generators were in use on the WTC site for power water pumping, etc. Diesel exhaust tends to peak in the very fine mode, roughly the same as Stage 8 of the DRUM sampler, 0.26 > Dp > 0.09 μm (CitationCahill and Zielenska 2003). Information of the relative influence of the diesel source can be gained from two sources, meteorology and sulfur/mass ratios. First, since many of the trucks were much closer to the receptor site than the WTC site, truck exhaust would reach the receptor site more frequently than WTC aerosols, giving broader periods of influence than the more remote WTC aerosols, which only occur in sharp narrow peaks. This would broaden the sulfur peaks relative to other species, which is not observed. Second, recent measurements of the sulfur/mass ratio in very fine size modes from diesel exhaust using “road” (non-California) fuel (CitationCahill and Zielenska 2003) give a ratio of roughly 0.3% as opposed to the average value seen in the 9 highly probable WTC events, 10.3%. Thus, we conclude that diesel exhaust was at most a minor component of the observed very fine aerosol episodes at the Varick Street receptor site.
We confidently propose a dominant WTC collapse pile source for 5 episodes (October 3, October 4, October 5, October 23, and October 24). Four more episodes on October 10, 11, 12, and 20 have a high probability of a WTC collapse pile source, while episodes on October 26, and 28 are likely from other sources in Manhattan and north-northwest of the city. The remaining episodes are likely to have important WTC components, but may include other sources.
Likely Sources of Aerosols from the WTC Collapse Pile Episodes
With major episodes identified as having a probable WTC sources, we can examine other components of the aerosol not used in the source identification and additional methods that clarify the nature of the WTC aerosol plumes, although in many cases the mechanisms involved in generating the aerosols are uncertain (). As before, the events are labeled by the day on which they occurred.
Additional aerosol parameters sorted by event
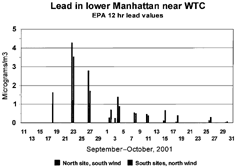
There are several points to note in . The ratio very fine sulfur to very fine mass was quite stable in the 9 highly probable WTC episodes (±17%), despite a 20-fold decrease in concentrations, while the VF H/VF mass and VF V/VF mass were both stable to ±35%. These data also strengthen the association with other events to a WTC source. Note that the mass (by STIM), hydrogen (by PESA), and sulfur and vanadium (by S-XRF) were all done at different times in two different laboratories and with different integration periods, all of which could increase variability in the highly time-variable episodes. The VF Si/VF mass was about 3 times more variable than hydrogen of vanadium. The meteorology, including subsiding isentropic trajectories, was almost identical during October 3 to 5 versus October 23 and 24. The coarse nonsoil calcium and sulfur were essentially the same on October 3 to 5 and October 23 and 24, and haze was seen at LaGuardia at about the same intensity, 14.1 km versus 12.3 km. The ratio of very fine mass to very fine sulfur was also constant, 9.8 versus 11.1, in this period. Thus, the conclusion is that these two periods both strongly sampled the WTC collapse pile plumes. However, other parameters, especially the very fine components normally associated with high temperature combustion sources, decreased radically in the same period, mass by a factor of 5.2, silicon by 12.9, sulfur by 5.3, vanadium by 7.2, and many other very fine aerosols had similar behavior. Coarse vanadium, largely absent in the immediate collapse dust (Lioy et al. 2001), decreased by a factor of 5.1, marking a sharp decrease in the V/Ca ratio, but lead only decreased by a factor of 2.2. The same behavior was seen in the even-longer record for lead aerosols measured by the EPA close to the source and shown in (CitationU.S. EPA 2001a). We interpret these decreases as a consequence of the cooling collapse pile, which in turn strengthens the association of these species with the WTC source. In we have also included data taken in May 2002 at 4 indoor sites in lower Manhattan Island, three of which were open to the outdoor air, and three of which were immediately adjacent to the WTC site. Very fine particles penetrate buildings easily, and these measurements should be similar to outdoor values. The very low levels of silicon and vanadium in May further strengthen the association of silicon and vanadium, and most of the sulfur, to the WTC collapse piles. Note that there was still heavy construction and diesel sources at the WTC site during these measurements.
FIG. 14 EPA lead values versus time. Values for lead taken by the EPA at sites close to the World Trade Center collapse site, September and October, 2001. The data are sorted by wind direction to represent downwind values.
The aerosols identified as having an origin in the WTC collapse piles are extraordinary in almost every regard, from the species mix to the concentrations in the very fine mode. Nevertheless, there are similarities to other aerosol sources that we propose could explain some of the features observed, while the local meteorology and low temperature plume behavior could explain the variable ground level concentration values. These hypotheses will need to be evaluated by more detailed information on emission factors from the WTC collapse piles and complex meteorological analysis in the Manhattan street canyons.
The major coarse particle mass is explainable in terms of the WTC building inventories of potentially friable materials (cement floors, gypsum in the dry wall, glass windows, etc.) and is similar to the data of Lioy et al. (2001) in many regards. Differences could well occur, however, since the coarsest particles described in detail in our report (5.0 to 2.5 μm) are still about 10 times finer than those that dominated the Lioy et al. (2001) compositional data. A major compositional difference is that our particles have more metals, consistent with the results of CitationNatusch and Wallace (1974) and CitationNatusch et al. (1974), which showed that condensed metal vapors from coal combustion tended to form relatively uniform layers on more refractory materials. This gives relatively higher metal to refractory ratios as particles become smaller, which is consistent with our results.
These ratios indicate a different source than merely finely ground crustal materials. SiO2 can potentially change to either vapor SiO, as in coal fired power plant plumes, or SiCl4, to be reoxidized to SiO2 to form the very fine glassy particles seen in the optical and elemental data. The boiling point of pure SiO2 is 1725°C, but that of SiCl4 is 12°C. It is also clear that by October 24 these very fine silica particles have been greatly diminished despite evidence of direct plume impact via coarse particles and meteorology.
We propose the unusual ratios of fine metals seen in the WTC plumes may reflect a phenomenon well known in waste incinerators. The volatility temperature that dominates emission of metals is generally far lower than the boiling point of the pure metals due to formation of molecular species (CitationBarton et al. 1990; CitationSeeker and Koshland 1990). Further, the presence of chlorine in the waste greatly depresses the volatility temperature of some metals, but not others. For example, CitationSeeker (1990) showed that many federally regulated metals are affected by 10% chlorine in the waste (). In addition, vanadium, normally very refractive but not a Federal Register 1986 EPA-regulated metal, converts to vapor VCl4 with a boiling point of 147°C. Some of the metals are naturally volatile at temperatures below 315°C (especially Se, Cd, As, and Hg) even without the presence of chlorine. Thus, these metals and metal compounds can leave the WTC collapse pile as gasses and vapors, providing both very fine (and presumably ultrafine) metals directly and coating larger and more refractory particles. Both effects would decrease in time as the collapse piles cooled, as both we and the EPA observed. In this regard, the relatively large chlorine inventory in the WTC buildings from plastics, including the ubiquitous PVC, and chlorine-bleached paper and our observation of persistent high levels of nonseal salt chlorine aerosols () including, some in the submicron modes, lends support to this hypothesis.
Volatility temperature of federally regulated metals in waste incinerators with and without 10% chlorine (CitationSeeker 1990; CitationU.S. EPA 1996)
Comparison of WTC-Derived Episodes with Prior Data at Other Sites
With these assignments, we can compare the 3 h average data for very fine particles with those measured at other sites and times using equivalent sampling and analysis protocols in . In all cases, each comparative value represents the highest 3 h average concentration in a field exercise of at least 3 weeks duration. Mass, hydrogen (in organic matter and sulfuric acid), Si, S, V, Ni, Cu, As, and Se occurred at unprecedented levels in the very fine size range from WTC-derived aerosol episodes. Working with the American Lung Association of New York City we performed an indoor air study at one site in midtown Manhattan and three sites directly adjacent to the WTC. The 55 Liberty Street site has excellent contact with outdoor air, but very fine particles penetrate buildings very much like a gas. The peak 3 h values are similar to non-WTC data from October, 2001, and are generally only a few percent of peak WTC episodes, except for Sulfur. Midtown sulfur values were only 1/6 to the WTC site values. There was still heavy use of diesel equipment in the WTC pit in May, which may be the source.
Summary of mass and compositional data for peak values of sulfur between October 2 and October 30, 2001, with comparisons to concentrations of very fine particles sampled in our other campaigns and in the literature. Note the values in New York in May, 2002, at the 55 Liberty Street near the WTC site. The values are far smaller that in October, 2001, for all elements except sulfur, but we note that there was heavy use of diesels in the pit in May, 2002. The sulfur numbers in midtown Manhattan were always lower than 110 ng/m3
Information from Supplemental Analyses
The analyses begun in this report are being continued in several areas. There are archived but unanalyzed DRUM strips for the period October 31 through mid-December 2001 which, when analyzed, will help clarify the effects of cooling the WTC collapse pile and excavating into deeper debris horizons. Although we present here some of the data from the finest DRUM stage from May 2002, there are additional DRUM strip sites both remote and adjacent to the WTC site awaiting further analysis. But the major continuing effort is analysis of the existing DRUM strips of October 2 through October 30 using the compositionally sensitive laser desorption ionization time of flight mass spectrometry (LDI TOF/MS). While all results must be considered preliminary at this time, we present LDI TOF/MS results for aerosols on October 4. All of these values greatly exceed background values taken on October 6 and 7. The analysis of the samples by laser desorption ionization time-of-flight mass spectrometry (LDITOF/MS) focuses primarily on the PAHs and their derivatives. The samples are analyzed with both positive and negative polarity, and the spectra are complex. In this report, we can only report on preliminary results of these analyses. show the negative ion spectra from the WTC October 3 to 5, 2001.
FIG. 15 Negative ion LDI TOF/MS spectra for WTC course and very fine particles, positive ion LDI TOF/MS spectra for coarse particles, October 4. The analyses strongly confirm the presence of acidic sulfate, consistent with sulfuric acid, as well as relatively low values for nitrates.
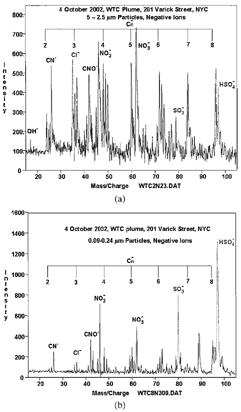
The positive ions detected correspond to Na+, K+, and very large mass ions which have the similar masses as Polycyclic Aromatic Hydrocarbons (PAH) compounds found in diesel soot. A small amount of the PAH compounds observed may contain heteroatom functional groups. The negative ions detected correspond to small carbon clusters (up to C10), chloride, hydroxide, nitrite, nitrate, CO2H−, CN−, CNO−, SO− 2, SO− 3, and HSO− 4. The presence of CN− and CNO− indicate there are some nitrogen-containing organic compounds, not necessarily that cyanide is present in the samples themselves. In many cases, the nitrite and nitrate ion signals are of comparable size, indicating the source is most likely a mixture of nitrous and nitric acids formed when NO2 created in the fire dissolved in water. In a few of the samples from October 3–5 the signal from the nitrate ion is much larger than the signal from nitrate; this indicates there are nitrated organic compounds in the sample.
The sulfur oxides appear to originate from sulfuric acid; the ratio of the intensities of HSO− 4/SO− 3 remains relatively constant. This conclusion is supported by data on hydrogen content by PESA and the sulfur content by S-XRF, both measured in a vacuum, which leads to an attribution by mass balance of most of the very fine sulfur to the form sulfuric acid. The constant ratio of mass to sulfur, 10.4 ± 1.9 for the 9 highly probable WTC episodes (), and the establishment of a dominant form of sulfuric acid by the H/S ratio and TOF/MS data, shows that 29% ± 5% of the very fine mass was sulfuric acid throughout the month of October.
This information, together with some measure of nitrates and nitrites, allows a calculation of total organic matter (CitationCahill et al. 1989; CitationCahill et al. 1990), which has been validated by comparisons with organic matter via combustion as part of the IMPROVE program (CitationMalm et al. 1994). The result shows that in the very fine mode, 0.26 > Dp > 0.09 μm, organic matter makes up about 1/3 (30% ± 6%) of the measured mass in the WTC-attributed peaks, with concentrations ranging from 1.0 to 9.3 μg/m3. In the clean period of October 6 and 7, the value was 0.04 μg/m3.
There also appears to be some organic acid present in the samples; this is indicated by the signal at 45 amu in the TOF/MS spectra which corresponds to CO2H− fragment. The presence of hydrocarbons functionalized by heteroatoms is worrisome. The toxicity of these compounds is higher than the toxicity of the PAH compounds themselves. It should be noted, without observing the parent peak ions, this method is unable to determine how these hydrocarbons are functionalized.
CONCLUSIONS
In this work, we have isolated and characterized the nature of the aerosol plumes coming from the WTC collapse site in the period between October 2 and October 30, 2001. The key finding is the plumes were generally both coherent and elevated, thus not generally impacting ground-based sites in New York City away from the WTC collapse pile. However, under certain meteorological conditions, the plumes could ventilate to the ground, leading to periods of sharply elevated coarse, fine, and very fine particulate mass over periods of a few hours 1.8 km from the WTC collapse site and beyond. The WTC plume data were in semiquantitative accord with EPA 24 h PM2.5 measurements, confirming that the 24 h PM10 and PM2.5 mass standards were not violated even during periods of plume impact. However, plume impacts delivered a very different type of the aerosol exposure, which is thus dependent on the fraction of time a plume is present at a given site.
The coarse particulate mass, finer than typical soils, appeared to be derived from hot portions of the collapse pile itself as it persisted even after periods of rain and despite increasingly effective efforts to wet and cool the pile. The particles were primarily finely powdered concrete, gypsum from dry wall, and rounded glass shards, with a mass peak in the 2.5 to 5 μm diameter range, as well as above 12 μm which we did not measure. They had components including sulfates, soot, and metals consistent with anthropogenic coatings derived from the hot collapse piles. This conclusion is supported by the rapid decrease in lead versus distance in the EPA data, indicative of lead in or on a coarse particle. The noncrustal metals associated with coarse particles declined steadily during October and were largely gone late in the month, unlike the more volatile lead which continued to show elevated levels that, however, never approached 24 h EPA standards.
The measured mass of very fine particles peaked in the very finest range measured 0.26 to 0.09 μm aerodynamic diameter. Based on the size distribution, it is likely that there were components in the ultrafine mode <0.09 μm diameter, but these particles were not collected. The levels of mass and several of the very fine components were the highest we have recorded in a variety of studies, including on the ground in the oil fields of Kuwait, June 1991 (CitationReid et al. 1994). Note that a study in May 2002 at the 55 Liberty Street near the WTC site showed very low concentrations of essentially all very fine species except sulfur, possibly reflecting heavy use of diesels in the WTC pit at that time. Midtown Manhattan values of sulfur never exceeded 110 ng/m3. The particulate mass of WTC events was dominated by organic matter (30% ± 6%) and sulfuric acid (29% ± 5%), but included metals consistent with chlorine depression of volatility temperatures, as shown by highly correlated V/Ni values. A variety of organics were observed, including many derivatives of PAHs.
Very fine silicon particles, similar to those recently seen near coal-fired power plants, were a major component, about 10%, and may be derived either from similar high temperature processes or the formation of volatile halosilanes such as SiCl4. Due to the combination of relatively high mass and smaller-than-usual size, the number and surface concentration are unusually high both absolutely and on a per μg of mass basis. Since these particles are poorly soluble in lung fluids, they will likely have long retention times in the lung and most likely be cleared through macrophage ingestion and transport through the blood stream, although the data are available only for the even smaller ultrafine particles (CitationU.S. EPA 1996). Very fine particle silicon concentrations dropped sharply during October, far faster than the sulfates. Very fine metals were routinely seen, but while most were at low concentrations, some metals (V, Ni, Cu, As, Se, Br, and Hg) occurred at unprecedented levels in the very fine size range.
This study shows the value of highly time-resolved, size-resolved, and compositionally resolved aerosol data in aerosol emission events do not match the typical ambient aerosol patterns. In such situations, it may not be appropriate to base the estimated impact on health derived from the results of epidemiological studies based on 24 hour averages. A model based on acute industrial exposures may be more appropriate if extended to susceptible populations, i.e., young, old, and sick people. A person could, in a few hours, be subject to materials in amounts and composition that they would not have had to endure in years of typical ambient conditions. While the impacts of the plumes at sites away from the WTC collapse pile were episodic, that is not true for workers at the site itself, for which our data, when scaled to on-site conditions, could be relevant to health impact investigations. Finally, while the WTC event is hopefully unique, there have been in the past 30 years many similar types of events that deviate strongly from typical ambient conditions, including industrial accidents, major fires, dust storms, and the Mt St. Helens eruption, that would have benefited from increased information on particle size and composition as a function of time.
Acknowledgments
The authors would like to gratefully acknowledge the scores of people and numerous organizations that contributed their time and effort to this work, all of which was done on a volunteer basis. The work benefited greatly from sampler and analytical developments occasioned by the ACE-Asia study NSF ATM 0080225, support from the center for Accelerator Mass Spectrometry, LLNL, and resources Center for X-ray Optics of the Advanced Light Source, LBNL. This work was performed in part under the auspices of the U.S. Department of Energy by University of California, Lawrence Livermore National Laboratory, under Contract No. W-7405-Eng-48. We would like to especially thank William Wilson, U.S. EPA, for his careful review and thoughtful discussions which led to significant improvements in the article.
Notes
a Analysis by synchrotron x-ray fluorescence, Advanced Light Source, Lawrence Berkeley National Laboratory.
b Analysis by proton induced x-ray emission, Crocker Nuclear laboratory, University of California, Davis.
c Analysis by scanning transmission ion microscopy, Center for Accelerator Mass Spectroscopy, Lawrence Livermore NL.
d Analysis by proton elastic scattering analysis, Center for Accelerator Mass Spectroscopy, Lawrence Livermore NL.
REFERENCES
- Barone , J. B. , Cahill , T. A. , Eldred , R. A. , Flocchini , R. G. , Shadoan , D. J. and Dietz , T. M. 1978 . A Multivariate Statistical Analysis of Visibility Degradation at Four California Cities . Atmos. Environ. , 12 : 2213 – 2221 .
- Barton , R. G. , Clark , W. D. and Seeker , W. R. 1990 . Fate of Metals in Waste Combustion Systems . Combust. Sci. Tech., , 74 : 327 – 342 .
- Bench , G. , Grant , P. G. , Ueda , D. , Cliff , S. S. , Perry , K. D. and Cahill , T. A. 2002 . The Use of STIM and PESA to Measure Profiles of Aerosol Mass and Hydrogen Content Respectively, Across Mylar Rotating Drums Impactors Samples, . Aerosol Science and Technology , 36 : 642 – 651 .
- Cahill , T. A. 1979 . “ Comments on Surface Coatings for Lundgren-Type Impactors ” . In Aerosol Measurement , Edited by: Lundgren , DaleA. pp. 131 – 134 . Gainesville, FL : University Presses of Florida .
- Cahill , T. A. 1995 . “ Compositional Analysis of Atmospheric Aerosols ” . In Particle-Induced X-Ray Emission Spectrometry , Edited by: Johansson , S. A. E. , Campbell , J. L. and Malmqvist , K. G. pp. 237 – 311 . John Wiley & Sons, Inc. Chemical Analysis Series .
- Cahill , T. A. , Eldred , R. A. , Motallebi , N. and Malm , W. C. 1989 . Indirect Measurement of Hydrocarbon Aerosols Across the United States by Nonsulfate Hydrogen-Remaining Gravimetric Mass Correlations . Aerosol Sci. Technol. , 10 : 421 – 429 .
- Cahill , T. A. , Flocchini , R. G. , Feeney , P. J. and Shadoan , D. J. 1974 . Comparison of Equal-Velocity Ion Beams for Elemental Analysis by Ion-Excited X-Ray Emission . Nuclear Instruments Methods , 120 : 193 – 195 .
- Cahill , T. A. , Goodart , C. , Nelson , J. W. , Eldred , R. A. , Nasstrom , J. S. and Feeney , P. J. 1985 . “ Design and Evaluation of the Drum Impactor ” . In Proceedings of International Symposium on Particulate and Multi-phase Processes , Edited by: Ariman , T. and Veziroglu , T. N. Washington, D.C. : Hemisphere Publishing Corporation .
- Cahill , T. A. , Kusko , B. H. , Ashbaugh , L. L. , Barone , J. B. , Eldred , R. A. and Walther , E. G. 1981 . Regional and Local Determinations of Particulate Matter and Visibility in the Southwestern United States during June and July, 1979. Symposium on Plumes and Visibility. Grand Canyon, AZ, 1980 . Atmos. Environ. , 15 : 2011 – 2016 .
- Cahill , T. A. , Surovik , M. and Wittmeyer , I. 1990 . Visibility and Aerosols During the 1986 Carbonaceous Species Methods Comparison Study . Aerosol Sci. Technol. , 12 : 149 – 160 .
- Cahill , T. A. , Wakabayashi , P. and James , T. 1996 . Chemical State of Sulfate at Shenandoah National Park During Summer, 1991 . Nuclear Instruments and Methods in Physics Research B: Beam Interactions with Materials and Atoms , 109/110 : 542 – 547 .
- Cahill , T. A. and Wakabayashi , P. 1993 . “ Compositional Analysis of Size-Segregated Aerosol Samples ” . In Measurement Challenges in Atmospheric Chemistry , Edited by: Newman , Leonard . pp. 211 – 228 . American Chemical Society . Chapter 7
- Cahill , T. A. , Wilkinson , K. and Schnell , R. 1992 . Composition Analyses of Size-Resolved Aerosol Samples Taken from Aircraft Downwind of Kuwait . J. Geophys. Res. , 97 ( D13 ) : 14513 – 14520 . Spring, 1991
- Feeney , P. J. , Cahill , T. A. , Flocchini , R. G. , Eldred , R. A. , Shadoan , D. J. and Dunn , T. 1975 . Effect of Roadbed Configuration on Traffic Derived Aerosols . J. Air Pollution Control Association , 25 : 1145 – 1147 .
- Flocchini , R. G. , Cahill , T. A. , Shadoan , D. J. , Lange , S. J. , Eldred , R. A. , Feeney , P. J. , Wolfe , G. W. , Simmeroth , D. C. and Suder , J. K. 1976 . Monitoring California's Aerosols by Size and Elemental Composition . Environ. Sci. Technol. , 10 : 76 – 82 .
- Flocchini , R. G. , Cahill , T. A. , Pitchford , M. L. , Eldred , R. A. , Feeney , P. J. and Ashbaugh , L. L. 1981 . Characterization of Particles in the Arid West . Atmos. Environ. , 15 : 2017 – 2030 .
- Khodhzer , T. V. , Obolkin , V. A. , Potemkin , V. L. , Bufetov , N. S. , Tomza , U. and Rahn , K. A. 2000 . Nuclear Inst. Meth. , A448 : 413 – 418 .
- Lioy , P. J. , Weisel , C. P. , Millette , J. R. , Eisenreich , S. , Vallero , D. , Offenberg , J. , Buckley , B. , Turpinm , B. , Zhong , M. , Cohen , M. D. , Prophete , C. , Yang , I. , Stiles , R. , Chee , G. , Johnson , W. , Porcja , R. , Alimokhtari , S. , Hale , R. C. , Weschler , C. and Chen , L. C. 2002 . Characterization of the Dust/Smoke Aerosol that Settled East of the World Trade Center (WTC) in Lower Manhattan after Collapse of the WTC 11 September, 2001 . Environmental Health Perspectives , 110 ( 7 ) : 703 – 714 .
- Lundgren , D. A. 1967 . J. Air Pollution Control Association , 17 : 225
- Malm , W. C. , Sisler , J. F. , Huffman , D. , Eldred , R. A. and Cahill , T. A. 1994 . Spatial and Seasonal Trends in Particle Concentration and Optical Extinction in the United States . J. Geophys. Res. , 99 ( D1 ) : 1347 – 1370 .
- Marple , V. , Rubow , K. , Anath , G. and Fissan , H. 1986 . J. Aerosol Sci. , 17 : 489 – 494 .
- Natusch , D. F. S. , Wallace , J. R. and Evans , C. A. 1974 . Science , 183 : 202
- Natusch , D. F. S. and Wallace , J. R. 1974 . Science , 186 : 695
- Perry , K. , Cahill , T. A. , Eldred , R. A. , Dutcher , D. D. and Gill , T. E. 1997 . Long-Range Transport of North African Dust to the Eastern United States . J. Geophys. Res.—Atmos. , 102 ( D10 ) : 225 – 11,238 . 11
- Perry , K. D. , Cahill , T. A. , Schnell , R. C. and Harris , J. M. 1999 . Long-Range Transport of Anthropogenic Aerosols to the NOAA Baseline Station at Mauna Loa Observatory, Hawaii . J. Geophys. Res. Atmos. , 104 ( D15 ) : 18,521 – 18,533 .
- Raabe , O. G. , Braaten , D. A. , Axelbaum , R. L. , Teague , S. V. and Cahill , T. A. 1988 . Calibration Studies of the DRUM Impactor . J. Aerosol Sci. , 19 ( 2 ) : 183 – 195 .
- Reid , J. S. , Cahill , T. A. , Gearhart , E. A. , Flocchini , R. G. , Schweitzer , J. S. and Peterson , C. A. 1993 . Elemental Analysis of Kuwaiti Petroleum and Combustion Products . J. Nuclear Geophys. , 7 ( 1 ) : 81 – 86 .
- Reid , J. S. , Cahill , T. A. and Dunlap , M. R. 1994 . Geometric/Aerodynamic Equivalent Diameter Ratios of Ash Aggregate Aerosols Collected in Burning Kuwaiti Well Fields . Atmos. Environ. , 28 ( 13 ) : 2227 – 2234 .
- 1990 . Combustion Byproduct Formation: An Overview. Special Issue on Toxic Combustion Byproducts . Combustion Sci. Technol. , 74 : 1 – 6 .
- Wesolowski , J. J. , John , W. , Devor , W. , Cahill , T. A. , Feeney , P. J. , Wolfe , G. and Flocchini , R. 1978 . “ Collection Surfaces of Cascade Impactors ” . In X-ray fluorescence analysis of environmental samples Edited by: Dzubay , T. pp. 121 – 130 . Ann Arbor Science, Ann Arbor, MI
- Whitby , K. T. 1978 . Atmos. Environ , 12 : 135 – 159 .