Abstract
Simultaneous multielement graphite furnace atomic absorption spectrometry was used to determine Al, As, Cd, Cr, Cu, Fe, Mn, Ni, Pb, Sb, Se, and Zn in ambient air sampled at 170 L·min−1 for 30 min and collected as a slurry after dynamic preconcentration. In PM20 samples collected at College Park, MD, results were > 2σ above system blank in > 95% of samples for Al, Cd, Cu, Cr, Fe, Mn, Pb, and Zn, and > 2σ above system blank in > 80% of samples for As, Ni, and Se. Analyses of slurries of NIST SRM 1648, Urban Particulate Matter, were typically within 10% of expected values for all elements except Al, Cr, and Fe, elements for which deviations were mostly due to difficulties in transferring large particles. This problem will be reduced for urban fine particulate matter samples (PM2.5). Trends in the concentrations of elemental source markers were readily correlated with wind direction and other meteorological factors to identify the influences of local industrial emissions, including motor vehicle traffic, coal- and oil-fired power plants, and municipal incinerators. Factor analysis was applied to the 88-sample data set to extract 7 factors: urban dust, meteorological factors, incinerators, coal-fired power plants, Tour Bus emission, unknown As source, and oil-fired power plants. Factor analysis was also applied to an 18-sample data set representing 2.5 h averages of the 30 min data to simulate the effect of longer sample collection times. Only 6 factors were extracted from this data set, which shows that increased temporal resolution enhances the power of factor analysis to resolve sources. These results indicate that a wealth of detailed information is revealed at this level of temporal resolution.
Introduction
Measurements of the elemental composition of atmospheric aerosol particles are needed for compliance monitoring, studies of environmental deposition, and source attribution by receptor modeling techniques (CitationGordon 1988). It is important to determine temporal variations in aerosol concentrations to assess health exposure effects and to improve correlations between source emission rates and ground-level measurements. Recent findings linking metals with inflammatory response in vivo (CitationCarter et al. 1997; CitationCosta and Dreher 1997; CitationAdamson et al. 2000; CitationPrieditis and Adamson 2002) and in vitro (CitationBecker et al. 1996; CitationMonn and Becker 1999) systems underscores the importance of the former. Elements of environmental interest are typically found on particles < 2.5 μm in diameter (i.e., those particles that are more efficiently deposited in the lungs (CitationICRP 1994) and include toxins (e.g., As, Cd, Cr, Cu, Ni, and Se) and many elements (e.g., Al, Cd, Fe, Mn, Ni, Sb, and Se) that are useful marker species for receptor modeling (CitationGordon 1988). Due to the low concentrations of these elements, typically 0.1–10 ng · m−3 in rural air samples (CitationWu et al. 1994), continuous or semicontinuous monitoring systems have been virtually nonexistent. Current methods collect particulate matter samples on low-blank substrates at flow rates of 6–100 L · min−1 and require collection times of 12–24 h to accumulate sufficient mass for analysis by multielement techniques such as X-ray fluorescence (CitationWagman et al. 1977), proton-induced X-ray emission (CitationJohansson and Campbell 1988), instrumental neutron activation (CitationOndov et al. 1990), or inductively coupled plasma mass spectrometry (ICP-MS) (CitationMontaser 1998). Such time periods are far longer than those for changes in source emission rates and meteorological conditions, which obviates the detection of plumes—and thus the evaluation of short-term exposure maxima—and reduces the power of multivariate correlation techniques for source apportionment. Reducing the sample collection time from 24 h to 12 h has been shown to improve greatly the ability of factor analysis to resolve sources (CitationLioy et al. 1989). Thus, convenient collection/analysis schemes able to provide improved temporal resolution are needed. Single-particle mass spectrometry (CitationCarson et al. 1995; CitationGard et al. 1997) provides the ultimate in temporal resolution but provides only qualitative information about the composition of the particles.
Other analytical methods investigated for more rapid determination of elemental composition include Inductively Coupled Plasma-Atomic Emmission Spectroscopy (ICP-AES) and graphite furnace atomic absorption spectrometry (GFAAS) For example, CitationGomes et al. (1996) used an air plasma ICP-AES to analyze ambient aerosol sampled at 15 L · min−1 for compliance with threshold limit values but reported that detection limits (DLs) were generally about 1000-fold greater than ambient concentrations due to the molecular background emission and cool plasma conditions. Some improvement in DLs for elements in stack emissions were reported using ICP-AES with a hotter argon plasma (CitationSeltzer and Meyer 1997). Again, molecular background emission caused DLs to be 100- to 1000-fold greater than ambient concentrations.
GFAAS has been shown to be amenable to solids analysis (CitationMiller-Ihli 1993). Furthermore, DLs for many elements by GFAAS exceed those of ICP-AES and in fact rival those of ICP-MS for a fraction of the cost. Consequently, applications of GFAAS for ambient aerosol monitoring have been more successful than ICP-AES. CitationChakrabarti et al. (1987) filtered ambient air through a porous graphite probe at 0.1 L · min−1 and then inserted the probe directly into the graphite furnace for analysis. Sampling times were 10–20 min for Pb, Cd, Zn, and Mn, 1–5 h for Cu, Cr, and Ni, and 3–4 days for V. Higher flow rates could not be used because of the high pressure drop across the graphite probe. Sneddon and coworkers (CitationSneddon 1985; CitationLee et al. 1996) have developed a system for collecting atmospheric particles directly or to the interior surface of the graphite furnace tube by inertial impaction. The small orifice size of a graphite furnace tube limits the system to a single nozzle which is incapable of collecting particles < 0.3 μm in diameter, wherein lie primary emissions from high-temperature combustion sources (CitationOndov and Wexler 1998). Detection limits (CitationSneddon 1986) were generally 10- to 20-fold greater than needed for analysis of ambient air. CitationLiang et al. (1990) also used direct inertial impaction, followed by GFAAS analysis with similar results. Direct analysis of ambient aerosol by ICP-MS has not been reported.
The work presented here describes a GFAAS technique suitable for multielemental analysis of ambient aerosol samples collected for 30 min using dynamic aerosol preconcentration (CitationKidwell and Ondov 2001). The objective of this work is to develop a quantitative, low-cost, multielement method capable of providing subhourly temporal resolution for identification of short-term trends in elemental concentrations in ambient air and improved resolution of emission sources by statistical methods such as factor analysis, especially advanced factor analysis methods (CitationPaatero and Tapper 1994) that benefit from large data sets. Herein we discuss analytical methodology, data quality, and interpretations of three 15 h series of 30 min measurements of 11 elements in atmospheric PM collected in College Park, MD.
Experimental
Sampling Apparatus
The apparatus for collecting 30 min atmospheric aerosol particle samples for elemental analysis has been described previously (CitationKidwell and Ondov 2001). Briefly, condensational growth by direct steam injection is used to grow particles with diameters as small as 0.084 μm (herein all diameters are aerodynamic) to 3–4 μm in diameter at a flow rate of 170 L · min−1. Theoretical calculations suggest particles as small as 0.01 μm in diameter are collected. The grown particles, now droplets, are concentrated into the 10 L · min−1 minor flow of a virtual impactor and then separated from the airstream using a real impactor. The droplets accumulate in the bottom of the real impactor in a liquid slurry at rate of about 0.2 mL · min−1. The overall collection efficiency for the prototype system, including particle losses to interconnection and transfer tubing walls, as measured using monodisperse fluorescent polystyrene latex particles (Polysciences, Inc., Warrington, PA), was 40% for particles initially 0.1–0.5 μm in diameter and gradually increased with size to 68% for 3–5.9 μm particles, and is likely similar for larger particles. Following a 30 min collection period, the slurry is hydraulically delivered to an X–Y fraction collector (Foxy, Isco, Lincoln, NE) and stored in glass sample vials for subsequent analysis.
Sample Collection
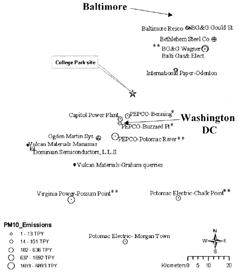
Ambient aerosol samples were collected at College Park, MD, a suburb of Washington, DC. There is very little heavy industry in the vicinity, and air quality is dominated by emissions from motor vehicles, coal-fired power plants, municipal incinerators, and regionally transported material (CitationKowalczyk et al. 1982). Major point sources () in the area include a coal-fired power plant and an incinerator in Alexandria, VA, 21 km southwest, and an incinerator, a steel mill, and other heavy industry in Baltimore, MD, 40 km northeast. The sampling site was on the University of Maryland campus, with the sampling inlet 1.5 m above ground level and adjacent to a parking lot which receives a large amount of motor vehicle traffic from 8 a.m. to 9 a.m. each work day. Samples were collected every 30 min from 05:00 to 19:00 for 3 days. Prior to sampling, each vial was precleaned with 10% nitric acid (Ultrex II ultrapure reagent, J. T. Baker, Phillipsburg, NJ), rinsed with 18.2 MΩ · cm−1 water, and dried in a laminar flow hood in a Class 100 clean room. A 20 μL aliquot of 70% nitric acid was dispensed into each vial to prevent trace metal losses to the walls of the vial. To determine the mass of slurry collected, each vial was weighed before and after sample collection. Laboratory and field blanks were collected in sample vials containing 20 μL of 70% nitric acid and 5 mL of high-purity water. The field blank vials were uncovered for 5 h, i.e., the same length of time that sample vials were uncovered, while the laboratory blank vials remained covered. System blanks were collected at the beginning and end of each daily sampling period by placing an absolute fluted filter capsule (3 μm pore Model 12116, Gelman Laboratory, Ann Arbor, MI) at the sampler inlet. Temperature, relative humidity, and barometric pressure were recorded at the sampler inlet, while wind speed and direction were recorded on a 9 m tower on the roof of the 4 story meteorology building, 200-m from the inlet. The building is on an elevated area of the campus and is well above the wakes of most trees and buildings on campus.
FIG. 1 Area map showing the sampling location in College Park, MD, and the locations of various sources of airborne particles. Major source concentrations are in South Baltimore to the Northeast, and Washington, DC, and Virginia to the Southwest. These include coal- and oil-(*)fired power plants, power plants with both coal- and oil-fired boilers (**), incinerators, a steel mill, and quarries.
No size segregation was attempted for the sampled aerosol, but the theoretical cutpoint of the sampling tube was about 20 μm. Particle size data was collected simultaneously using a forward-scattering laser spectrometer (Model CSAS-100, Particle Measuring Systems, Inc., Boulder, CO) and indicated a mass median aerodynamic diameter of 7 μm for coarse particles. However, the submicrometer size distribution is not well defined in this instrument. Particle mass concentrations inferred from these data ranged from about 10–40 μg/m3. Mass-to-liquid ratios for the system vary with particle number concentration and size distribution. A value of 20 μg/mL was typically observed.
Elemental Analyses
Samples were analyzed in triplicate by GFAAS on a SIMAA 6000 (Perkin Elmer Corp., Norwalk, CT), which uses 4 lamps to determine up to 6 elements simultaneously. Spectral interferences and differing sample concentrations, however, limit the possible combinations of elements that may be determined simultaneously. To determine elements of interest in atmospheric particulate matter, three groups of elements were selected (). Elements in the first two groups were chosen for their similar atomization temperatures, while those in Group 3 were chosen for their relatively high concentrations. A mixed matrix modifier of 5 μg Pd and 3 μg magnesium nitrate was added to each sample prior to firing the furnace. The limited dynamic range of GFAAS necessitated a 10-fold dilution of each sample prior to analysis for Al, Fe, and Zn.
TABLE 1 Graphite furnace atomization parameters
Calibration was performed, approximately after every 15 samples, using 5-point calibration curves generated by automatic dilutions of two standard stock solutions. For Group 1 and 2 elements, these stock solutions were prepared by manual dilution, by mass, of GFAAS Mixed Standard (No. N9300244, Perkin Elmer Corp., Norwalk, CT), a multielement standard containing Ag, Al, As, Ba, Be, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, Sb, Se, and Tl. Group 3 stock solutions were prepared by manual dilution, by mass, of single-element standard solutions (High Purity Standards, Charleston, SC) of Al, Fe, and Zn. To determine the integrity of the analytical technique, two National Institute of Standards and Technology (NIST) standard reference materials (SRMs) were analyzed following each calibration: 1643d, Trace Elements in Water, and 1648, Urban Particulate Matter. Standard Reference Material 1643d was diluted 5-fold with high-purity water prior to analysis. Standard Reference Material 1648 was prepared as a slurry of approximately 1.5 mg in 100 mL of 0.5% nitric acid to match the sample matrix and expected aerosol mass loadings for ambient samples. Results of analyses of SRM 1648 were used to determine the analytical efficiency for each element, which is defined as the ratio of measured values to certified values for the SRM.
Each aerosol sample was analyzed in three replicates, and the average and standard deviation of the three peak areas were recorded for each element. The area of the reagent blank signal was subtracted, and the blank-corrected area was then transformed using the calibration function to determine the concentration in ng · mL−1. The mass of each sample vial was measured three times before and after sample collection to determine the sample mass. For each sample, the elemental concentration was multiplied by the sample mass, assuming a density of 1 g · mL−1, to determine the elemental mass.
The air flow rate, measured by a mass flow meter, was recorded as 1 min averages of readings taken every 2 s. The volume of air sampled at ambient conditions was calculated from ambient temperature and pressure and the mass flow rate data. The uncertainty in the volume is calculated from the standard deviation of the 1 min flow rate averages. The elemental mass for each sample was divided by this air volume to determine ambient air concentration in ng · m−3. This concentration was then corrected for collection and analytical efficiencies (described above). Analytical efficiencies derived for SRM1648 are likely reasonably applied to atmospheric PM collected herein, as the mean particle sizes were similar for both fine and course particles in these materials.
Collection efficiencies were taken to be 40% for elements associated with predominately fine particles (i.e., Se); 68% for elements predominately associated with course particles (i.e., Al, Cr, and Fe); and 54 ± 14% for elements (As, Cu, Cd, Mn, Ni, Pb, and Zn) having both fine and course components, based largely on size distributions for these elements developed by CitationDivita Jr. (1993) for College Park.
Factor Analysis
Factor analysis was performed with the computer program StatView (SAS Institute, Inc., Cary, NC) using the principal components method. This method assumes that all variation is due to discreet factors, with no residuals. Herein, the parameters to be factored were expressed as
Results and Discussion
Analytical Performance
The SIMAA 6000 GFAAS performance results are summarized in , wherein we list DL, characteristic mass, and limit of linearity for each element. System DLs were calculated based on 3 standard deviations of the mean from the 9 laboratory and 9 field blanks. There were no differences in concentrations between the laboratory and field blanks. As indicated in , DLs determined here were typically 3- to 8-fold greater than Perkin-Elmer (PE) product literature single-element values. This is due to the necessary compromise of furnace conditions for multielement analysis. The DL for Al is particularly high, presumably due to the low intensity of the multielement hollow cathode lamp used. The DL for Sb is also high because the atomization temperature of 1900°C given by the SIMAA 6000 product literature was too low. Incomplete atomization was also observed for Se at 1900°C. This apparently had little effect on the calibration, but this could explain the low measured values in SRM 1648 discussed below. Further tests using an atomization temperature of 2450°C suggest a DL of 30 pg for Sb, which agrees well with the literature value of 25 pg. Unfortunately, this was not discovered until after the samples had been analyzed, therefore no usable Sb data are available for the samples.
TABLE 2 Instrument performance of SIMAA 6000 GFAAS
For all elements except Zn, second-order functions were fit to 5 calibration points. Due to extreme nonlinearity for Zn, a second-order function could be used only on the lowest 4 calibration points. In GFAAS, characteristic mass (CM) is used to evaluate instrument performance and sensitivity and is defined as the mass necessary to give an integrated absorbance of 0.0044 A · s. The CM for each element was calculated using the calibration curve and is compared with PE literature values in . For all elements except Al, the CM compares very well with the literature values. Again, the very high CM for Al is attributed to the low intensity of the multielement hollow cathode lamp. To determine the limit of linearity (LOL), the second-order calibration curves through 5 points (4 for Zn) were compared to linear fits through the lowest 4 points (3 for Zn). The LOLs were calculated as the point where the two curves diverge by 10% and are listed in . For most elements, the linear dynamic range extends about 2 orders of magnitude above their respective DLs.
Analyses of SRM 1643d (Trace Elements in Water, see ) indicates good agreement for all elements, except Al, for which our measured values exceeded the certified value. However, Al was accurately measured in the GFAAS Mixed Standard. Considering this, it appears that our SRM 1643d was contaminated with Al. Our analyses of SRM 1648 (urban particulate matter; see ) agree with NIST-certified values for As, Cd, Ni, Pb, and Zn but are consistently low for other elements, particularly Al, Cr, and Fe, which we attribute to poor atomization and transfer efficiencies. The results of these analyses show that the analytical method is valid for dissolved solutions, but poor atomization and transfer efficiency of the large (10 μm) particles contained in SRM 1648 and the similarly sized atmospheric particles collected herein. This effect is reduced by sonication prior to analysis and should be reduced for fine atmospheric particulate matter (i.e., particles < 2.5 μm in diameter), as smaller particles are more efficiently transferred. Furthermore, we believe that statistical sampling of the relatively few large particles, especially in aerosol slurry samples, leads to the large standard deviations for Al, Cr, and Fe that are predominately associated with large particles.
TABLE 3 Results for analyses of SRM 1643d, trace elements in water
TABLE 4 Results for analyses of SRM 1648, urban particulate matter
Meteorological Conditions
Meteorological conditions of temperature, relative humidity, wind speed, and wind direction for each of the three dates on which the samplers were collected are shown in as the average and standard deviation over the 30 min sample collection period. Temperature and relative humidity followed typical diurnal cycles with relative humidity (RH) falling during the day as temperatures increased. On the evening of November 21, a warm front passed through the area, along with a small amount of rain. Although there was no rain during sampling on November 22, fog developed at approximately 4:00 a.m. and persisted until 11:00 a.m. Consequently, the ground was quite damp, which greatly reduced resuspension of soil and urban dust.
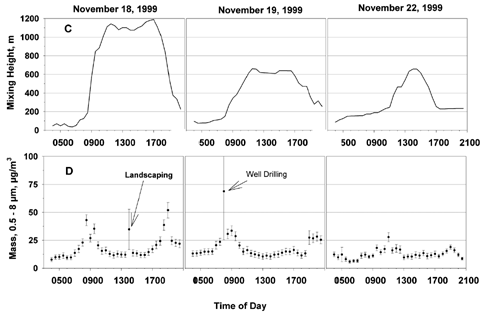
FIG. 2 (A) Thirty minute averages and standard deviations of temperature (closed circles) and relative humidity (open circles), (B) wind speed (open circles) and direction (filled circles), (C) mixing height, and (D) PM mass are shown for the three sampling periods. Urban dust constituents are clearly elevated during morning and evening traffic periods. Dust from nearby landscaping and well-drilling activities are also evident.
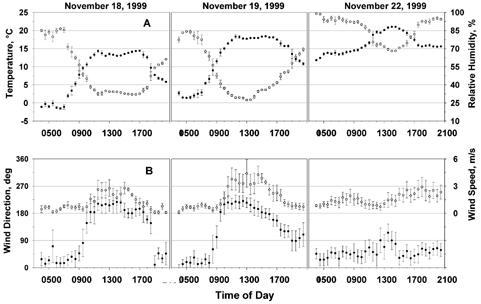
Winds on November 18 and 19 were calm during the early morning hours, changing to light from the south and southwest beginning at 9:00 a.m. each day. On November 18, winds were from the south from 10:30 a.m. until 11:30 a.m., southwest from 11:30 a.m. to 3:00 p.m., then turned southerly from 3 p.m. until 5:30 p.m., and later became calm at 7 p.m. On November 19, winds were from the southwest from 10 a.m. until 1 p.m. and gradually shifted to easterly by 7 p.m. Wind speeds from 10 a.m. to 6 p.m. were generally greater than those on November 18 and ranged from 1.5–4 m s−1. On November 22, winds were light (1–2 m s−1) from the northeast until 12:30 p.m., when the wind direction shifted to the east. At 3 p.m., the wind returned to northeasterly until the end of the sampling period at speeds ranging from 2–3 m s−1.
Mixing heights were estimated from soundings taken at 7 a.m. each day at Dulles International Airport, 45 km west of the site. For each 30 min sampling interval, the average air temperature measured at the sampling site was used to expand a theoretical parcel of air adiabatically until it crossed the sounding temperature. The corresponding pressure was then converted to altitude above ground level using the hydrostatic equation. Due to the large inherent uncertainty in this method, the data are only used as a guide for trends in mixing height. As shown in , the nocturnal inversion is evident at the beginning of each of the 3 days. On November 18, the mixing height increased rapidly to an estimated 1100 m from 8 a.m. to 11 a.m., where it remained until about 5 p.m. On November 19, there was a more gradual increase in mixing height to 600 m from 8 a.m. to 11 a.m. On November 22, the morning inversion lasted until 11 a.m.
Ambient Aerosol Results
Total mass on particles 0.5–8 μm in diameter was estimated from the laser spectrometer data and is plotted in as 30 min averages and standard deviations of the 1 min raw data. Local traffic tends to resuspend soil and urban dust, which are large particles and, therefore, have a large influence on the aerosol mass loadings. Dust suspended by the morning rush hour is clearly evident on November 18 and 19 from 7 a.m. to 10 a.m., but the evening rush hour was less pronounced. On both of these days, however, there was a basketball game on campus, resulting in heavy local traffic from 6 p.m. to 7 p.m. The spike in mass at 2 p.m.
on November 18 is attributed to local landscaping work. The large spike at 8 a.m. on November 19 is attributed to well-drilling operations approximately 20 m from the sampling site. The damp conditions on November 22 limited the resuspension of particles, which reduced the mass loadings and obscured the rush hour influences.
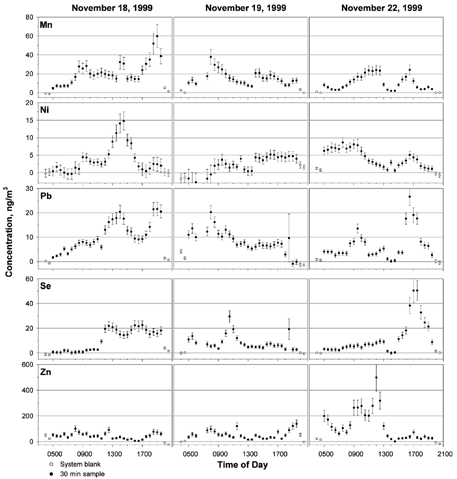
Ambient elemental concentrations for the three sampling periods are shown in . The first and last two points (shown as empty circles) in each plot are system blank values, and the points in between them represent system blank-corrected 30 min average elemental concentrations. The solid line represents 5 h averages of the data to simulate results for longer collection periods. On November 19, data is not available for the 6:30 a.m. and 7:00 a.m. periods due to a problem with the fraction collector, which resulted in the loss of those samples. Uncertainties were propagated as one standard deviation from the following sources: analytical replicates, reagent and system blank subtraction, calibration function, collection efficiency, analytical efficiency, and measurement of collected sample mass and air volume. Relative standard deviation (RSD) is defined as the ratio of the standard deviation to the measured value. For analytical measurements based on three replicate analyses, RSD varied by element, but was generally < 10% for samples. Elements present in concentrations much greater than the DL (i.e., Al, Cu, Fe, Mn, and Zn) had average RSDs of < 5%, while elements with lower concentrations (i.e., As, Cd, and Ni) had average RSDs > 15%. For reagent and system blanks, RSDs were typically 20 to 40%. However, most samples were well above blank, so this had little effect on the overall uncertainty. The calibration data were excellently correlated, typically r2 > 0.99, so uncertainties from calibrations were < 1% RSD for all elements. The largest contributions to overall uncertainty were the RSDs for collection (20%) and analytical (5–10%) efficiencies. The RSDs for sample mass and volume were < 0.002% and < 0.1%, respectively. Uncertainties in each of these measurements were propagated throughout the calculations to give overall average RSDs of 20–43%.
FIG. 3 Ambient aerosol concentrations measured in College Park, MD, on November 18, 19, and 22, 1999. Each point represents one 30 min collection period. Solid lines are 5 h averages of the 30 min samples. The solid lines in each of the plots are 5 h averages. High concentrations of Fe at 7:00 p.m. coincided with a running tour bus, parked 20 m from the sampling inlet.
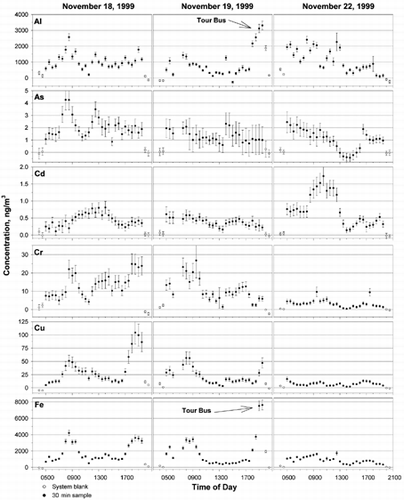
As mentioned above, system blanks at the beginning and end of each daily sample collection period were averaged and subtracted from that period's samples. Blank ratios presented in show that system blanks were generally 2- to 5-fold greater than laboratory blanks. System blanks for Cu, Fe, and Mn were substantially larger at the end of sampling than at the beginning (i.e., at 04:00 and 20:00), which we attribute to memory effects caused by particle adhesion to the impactor collection surfaces. Therefore, only the second of the two consecutively collected system blank samples were averaged for blank subtraction. The average concentrations of elements over the entire sampling period were typically 2- to 16-fold greater than laboratory blanks and 1.5-to 5-fold greater than system blanks. For all samples, results were > 2σ bove system blank in > 95% of samples for Al, Cd, Cu, Cr, Fe, Mn, Pb, and Zn, and were > 2σ bove system blank in > 80% of samples for As, Ni, and Se. Analysis of the steam used for condensation growth showed it to be cleaner than the reagent blank. Therefore, we expect that the system blank can be substantially lowered, and the DLs improved, by adding a wash cycle between samples.
TABLE 5 Ratios of elemental concentrations determined in samples and laboratory and system blanks
Large concentrations of Al, Cr, Cu, Fe, and Mn, and concomitant resuspended dust during the morning (08:00–10:00 h) and evening (17:00–20:00 h) rush hours of November 18 and 19 indicate the influence of motor vehicle traffic. The evening rush hours were somewhat extended due to heavy local traffic for basketball games. Of particular interest are the very high Fe concentrations of 7500 ng · m−3 at 7:00 p.m. on November 19. Fe is enriched in motor vehicle emissions relative to other elements (Ondov et al. 1982) and correlates well with the local traffic patterns. During this time, a charter bus was parked with its motor idling approximately 20 m from the sampling inlet. There is no corresponding spike in total mass for these samples, which indicates the Fe was present on small particles emitted from the charter bus.
Emissions from coal-fired power plants (CFPPs) are typically identified by enriched levels of Se and, to a lesser extent, As and Pb (Gordon 1998). For these samples, however, As and Se do not correlate well, which indicates that As may be emitted from another source, possibly steel production. The largest excursion in Se concentrations occurred on November 22, when winds carried air from the direction (NE) of Baltimore. This likely represents the influence of the large Brandon Shores (924 ton PM10/yr) and smaller BG&E Wagner (294 ton PM10/yr) power plants on the Patapsco River in South Baltimore (open circle below Baltimore in , 41 km and 59° (measured from North) from College Park). The former is the closest power plant in this direction.
On both November 18 and 19, southwesterly winds were accompanied by substantial excursions in the Se concentration as the mixing height rose and winds rotated clockwise through the quadrant Southeast of College Park. As shown in , four major coal-fired power plants lie at wind angles ranging from 155° to 212°, (i.e., in order of increasing angle, Chalk Point, 155°, 52 km; Morgantown, 183°, 65 km; Potomac River, 205°, 21 km; and Possum Point, 212°, 57 km. Benning Road, 175° and 9.9 km from College Park, was not operated on November 18 or 19). Plumes from one or more of these plants undoubtably influenced the air at College Park on both days. Comparable maxima in the Se concentrations were observed on both days; however, the Se peak on November 19 was a sharp peak lasting only 2 h, while the Se concentrations November 18 were elevated for 8 h. The sharper peak on November 19 compared to November 18 may be due to the increased wind speeds on November 19, which caused less lateral dispersion of the plume. Additionally, the mixing height was apparently lower on November 19, which may have hindered plume penetration into the surface boundary layer. There is an apparent correlation of Se and Pb on these two days, though peaks in Pb tend to have a broader distribution and are displaced in time compared to the Se peaks, indicating two sources. As discussed below, Factor Analysis reveals that Pb is loaded onto the power plant component.
Oil-fired power plants (OFPPs) are identified by enriched levels of Ni and V (CitationMroz 1976), though V was not measured for these samples due to the very high atomization temperatures required. On November 18, Ni concentrations begin to rise at 12:30 PM to 6-fold above background when the winds were from the southwest and south. This likely indicates the influence of the PEPCO Benning Road OFPP, located 10 km south southwest of the sampling site. On November 19, however, no peak in Ni is observed when the winds were from the south. Discussions with PEPCO confirmed that the Benning Road plant operated on November 18 but not on November 19.
Interestingly, the rise in Ni concentration on November 18 coincided with a 25% dip in the Se concentration. Examination of generator load logs for the various coal-burning plants indicated that the load on Potomac River Unit 5 was operated at 88% capacity until 08:00, after which it was reduced by 17% until it was brought to a maximum of 92% capacity between 14:00 and 15:00, resulting in early morning and afternoon emission peaks. Chalk Point Unit 4 load was also reduced at 08:00, but this facility probably lies too far to the East to have affected Se concentrations at College Park. For transport of the early morning emission peak from Potomac River to College Park, the wind angle would have first been favorable at about 09:30, i.e., about 2 h before the first Se peak appeared at 11:30. At a distance of 21 km, this would correspond to an average transport time of about 3 m/s. The second Se peak occurred at about 16:00, i.e., 1 or 2 h after the afternoon emission peak, corresponding to a comparable or perhaps a 50% greater wind speed. Such transport speeds are reasonably consistent with the measured wind speeds during these periods. The influence of the Potomac River plume at College Park has been definitively demonstrated with an intentional tracer (CitationOndov et al. 1992). The juxtaposition of the Ni peak and Se minimum is, perhaps, the result of the oil-fired unit being brought online to make up for power lost by the coal-fired units. However, influences from one or more of the other plants might have contributed to the observed pattern of Se concentrations.
On November 22, light winds from the northeast transported aerosol from Baltimore to the sampling site. During this period, Cd and Zn concentrations were elevated (4- and 15-fold for Cd and Zn, respectively) which is consistent with influence from the Baltimore RESCO (CitationGreenberg et al. 1978), 40 km northeast of the sampling site. Medical incinerators in the same area may have also contributed. Arsenic, Mn, and Ni were also elevated during the morning and late afternoon but decreased around 13:00, along with Cd and Zn. Because the wind speed and direction showed little variation during this time period, the concentration decrease is attributed to vertical dispersion of the Baltimore plume due to the increasing mixing height. When the mixing height decreased starting at 15:00, some elemental concentrations began to increase again. Finally, the influence of CFPPs is indicated by concentrations of Se and Pb rising 10-fold above background from 15:00–19:00 on November 22. Three CFPPs operated by Baltimore Gas & Electric are located northeast of the sampling site: H. A. Wagner and Brandon Shores, 40-km distant, and C. P. Crane, 41-km distant.
Factor Analysis
Factor analyses were performed for the 30 min data and for 2.5 h averages of the 30 min data. The results for the former are shown in . The 2.5 h averages were included to simulate results that would be obtained with other sampling methods. Averages over longer periods of time were not possible due to the small number of samples available. Fifteen species were included: Al, As, Cd, Cr, Cu, Fe, Mn, Ni, Pb, Se, Zn, total mass (for particles 0.5–8 μm in diameter), mixing height, wind direction, and wind speed.
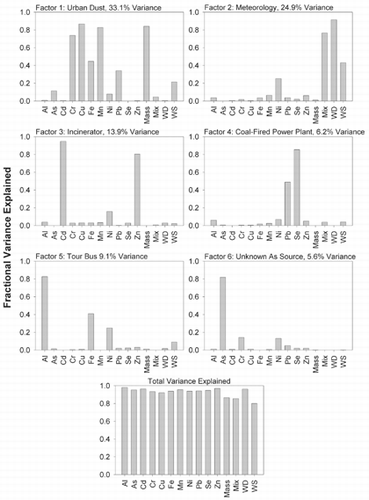
FIG. 4 Principle components analysis results for 30 min data set. Factors identified correspond to readily observed peaks in the concentration time series of source marker elements.
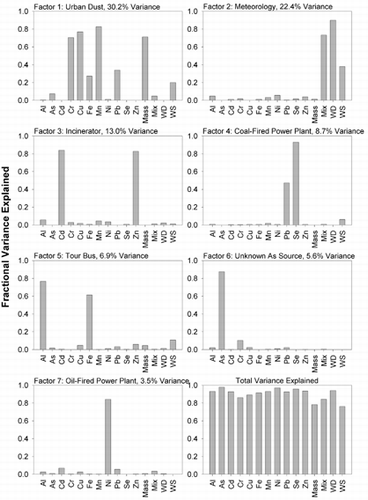
For the 30 min samples, > 80% of the variance is explained by seven factors: urban dust, meteorology (i.e., wind speed and direction, and mixing height), incinerator, an Fe and Al source, an unknown As source, and an OFPP. In assigning sources we are guided by composition and, in part, the knowledge that PCA factors are strongly influenced by maxima in the time series. As might be expected, urban dust composed of Cr, Cu, Fe, Mn, and Pb has the largest influence on variance and is associated with large particles which carry most of the PMTSP on Factor 1. There is also a correlation with wind speed, suggesting that wind contributes to resuspension of the urban dust. The meteorological components—wind speed and mixing height—are primarily on Factor 2 and not other factors, which indicates there is little influence of meteorology on the sources represented by these other factors. Municipal incineration is easily identified by the Cd and Zn signatures on Factor 3. Likewise, CFPP emissions are identified by Se and Pb on Factor 4. We believe that Factor 5, with Al and Fe as the primary components, represents the influence of the diesel bus which parked 20 m from the inlet of our sampler on the evening of November 19. During this period the Fe concentration was nearly 8000 ng/m3, i.e., twice that of any other period and 16000-fold greater than its background concentration. As with the urban dust factor, there is also a small contribution from wind speed. Factor 6 shows the influence of an As source, though its identity has not been determined. As previously mentioned, As is typically attributed to steel production and CFPPs, but these data indicate that it is emitted from a different source. These observations are consistent with those of CitationCaffrey (1997), who identified an As/Sb source on the basis of size-spectra of elements derived from Micro-Orifice Impactor sampling in the Chicago area. Suarez and Ondov, 2002, also detected an As/Sb source in the Baltimore area. Finally, emissions from OFPPs are evident on Factor 7 with Ni as the primary component.
The results for the 2.5 h averages () show a greater fraction of variance explained, and only six factors were identified: urban dust, meteorology, incinerator, CFPP, tour bus, and the unknown As source. Variance explained by Ni was spread across all factors instead of being concentrated on an OFPP factor. Previously, CitationLioy et al. (1989) argued that greater temporal resolution (in their case, 12 h versus 24 h) enhanced the power of factor analysis to resolve sources. Our study suggests that increasing temporal resolution to 30 min increases the resolving power over 2.5 h averages.
Conclusions
These data suggest that 30 min resolution permits far greater resolution of emission sources than customary integral 24 h measurements. In the prototype system, detection limits were governed largely by system blanks, rather than analytical sensitivity, for most elements. While the overall average relative standard deviations ranged from 20–43%, we expect substantial improvements in the future through redesign of various components and by rinsing the system between sample collections. Lastly, as no size-segregating inlet was used, much of the Al and Fe was surely on large particles, i.e., 5–20 μm in diameter, as evidenced by laser spectrometer data. The use of a 2.5 μm inlet will likely improve the analytical efficiency for the system by removing these coarse particles
Acknowledgments
This research has been supported by a grant from the US Environmental Protection Agency's Science to Achieve Results (STAR) program, Contract Number R-825269-01-0. Although the research described in the article has been funded wholly or in part by the US Environmental Protection Agency's STAR program through grant (number), it has not been subjected to any EPA review and therefore does not necessarily reflect the views of the Agency, and no official endorsement should be inferred.
Notes
a Perkin-Elmer product literature for analysis of blank solution containing 0.2% nitric acid.
b Measured values for analysis of blank solution containing 0.5% nitric acid.
c Results reported for atomization temperature of 1900ˆC, followed by results for 2450ˆC atomization in parentheses. Limit of linearity could not be determined with 1900ˆC atomization due to poor calibration curve fits.
REFERENCES
- Adamson , I. Y. , Prieditis , H. , Hedgecock , C. and Vincent , R. 2000 . Zinc is the Toxic Factor in the Response to an Atmospheric Particulate Sample . Toxicol. Appl. Pharmacol. , 166 ( 2 ) : 111 – 119 .
- Becker , S. , Soukup , J. M. , Gilmour , M. I. and Devlin , R. B. 1996 . Simulation of Human and Rat Alveolar Macrophages by Urban Air Particulates: Effects on Oxidant Radical Generation and Cytokine Production . Toxicol. Appl. Pharmacol , 141 : 637 – 648 .
- Caffrey , P. F. 1997 . Size Distributions, Sources, and Dry Deposition of Atmospheric Particles in Southern Lake Michigan , College Park : University of Maryland . Ph.D. Thesis
- Carson , P. G. , Neubauer , K. R. , Johnston , M. V. and Wexler , A. S. 1995 . On-Line Chemical Analysis of Aerosols by Rapid Single-Particle Mass Spectrometry . J. Aerosol Sci , 26 : 535 – 545 .
- Carter , J. D. , Ghio , A. J. , Samet , J. M. and Devlin , R. B. 1997 . Cytokine Production by Human Airway Epithelial Cells After Exposure to an Air Pollution Particle is Metal-Dependent . Toxicol. Appl. Pharmacol. , 146 : 180 – 188 .
- Chakrabarti , C. L. , Xiuren , H. , Shaole , W. and Schroeder , W. H. 1987 . Direct Determination of Metals Associated with Airborne Particulates Using a Graphite Probe Collection Technique and Graphite Probe Atomic Absorption Spectrometric Analysis . Spectrochim. Acta , 42B : 227 – 1233 .
- Costa , D. L. and Dreher , K. L. 1997 . Bioavailable Transition Metals in Particulate Matter Mediate Cardiopulmonary Injury in Healthy and Compromised Animal Models . Environ. Health Persp , 105 : 1053 – 1060 .
- Divita , F. Jr. 1993 . Size Distributions and Sources of Submicrometer Atmospheric Particles in Washington, DC, and Philadelphia, PA , College Park, MD : University of Maryland . Ph.D. Thesis
- Gard , E. , Mayer , J. E. , Morrical , B. D. , Dienes , T. , Fergenson , D. P. and Prather , K. A. 1997 . Real-Time Analysis of Individual Atmospheric Aerosol Particles: Design and Performance of a Portable ATOFMS . Anal. Chem , 69 : 4083 – 4091 .
- Gomes , A.-M. , Sarrette , J.-P. , Madon , L. and Almi , A. 1996 . Continuous Emission Monitoring of Metal Aerosol Concentrations in Atmospheric Air . Spectrchim. Acta , 51B : 1695 – 1705 .
- Gordon , G. E. 1988 . Receptor Models . Environ. Sci. Technol. , 22 : 1132 – 1142 .
- Greenberg , R. R. , Zoller , W. H. and Bordon , G. E. 1978 . Composition and Size Distributions of Particles Released in Refuse Incineration . Environ. Sci. Technol. , 12 : 566 – 573 .
- International Commission on Radiological Protection (ICRP) . 1994 . Human Respiratory Tract Model for Radiological Protection; A Report of Committee 2 of the ICRP , Oxford : Pergamon Press .
- Johansson , S. A. E. and Campbell , J. L. 1988 . PIXE: A Novel Technique for Elemental Analysis , New York : John Wiley & Sons .
- Kidwell , C. B. and Ondov , J. M. 2001 . Development and Evaluation of a Prototype System for Collecting Sub-Hourly Ambient Aerosol for Chemical Analysis . Aerosol Sci. Technol. , 35 : 596 – 601 .
- Kowalczyk , G. S. , Gordon , G. E. and Rheingrover , S. W. 1982 . Identification of Atmospheric Particulate Sources in Washington, D.C., Using Chemical Element Balances . Environ. Sci. Technol , 16 : 79 – 90 .
- Lee , Y.-I. , Smith , M. V. , Indurthy , S. , Deval , A. and Sneddon , J. 1996 . An Improved Impaction-Graphite Furnace System for the Direct and Near Real-Time Determination of Cadmium, Chromium, Lead, and Manganese in Aerosols and Cigarette Smoke by Simultaneous, Multielement Atomic Absorption Spectrometry . Spectrochim. Acta , 51B : 109 – 116 .
- Liang , Z. , Wei , G.-T. , Irwin , R. L. , Walton , A. P. , Michel , R. G. and Sneddon , J. 1990 . Determination of Subnanogram per Cubic Meter Concentrations of Metals in the Air of a Trace Metal Clean Room by Impaction Graphite Furnace Atomic Absorption and Laser Excited Atomic Fluorescence Spectrometry . Anal. Chem , 62 : 1452 – 1457 .
- Lioy , P. J. , Zelenka , M. P. , Cheng , M.-D. and Reiss , N. M. 1989 . The Effect of Sampling Duration on the Ability to Resolve Source Types Using Factor Analysis . Atmos. Environ , 23 : 239 – 254 .
- Miller-Ihli , N. J. 1993 . Advances in Ultrasonic Slurry Graphite Furnace Atomic Absorption Spectrometry . Fresenius J. Anal. Chem , 345 : 482 – 489 .
- Monn , C. and Becker , S. 1999 . Cytotoxicity and Induction of Proinflammatory Cytokines from Human Monocytes Exposed to Fine (PM2.5) and Course Particles (PM10–2.5) in Outdoor and Indoor Air . Toxicol. Appl. Pharmoacol. , 155 : 245 – 252 .
- Montaser , A. 1998 . Inductively Coupled Plasma Mass Spectrometry , New York : Wiley-VCH .
- Mroz , E. J. 1976 . The Study of Elemental Composition of Particulate Emissions from an Oil-Fired Power Plant , College Park : University of Maryland . Ph.D. Thesis
- Ondov , J. M. , Dodd , J. A. and Tuncel , G. 1990 . Nuclear Analysis of Trace Elements in Size-Classified Submicrometer Particles from a Rural Airshed . Aerosol Sci. Technol , 13 : 249 – 263 .
- Ondov , J. M. , Kelly , W. R. , Holland , J. Z. , Lin , Z. C. and Wight , S. A. 1992 . Tracing Fly Ash Emitted from a Coal Fired Power Plant with Enriched Rare-Earth Isotopes: An Urban Scale Test . Atmos. Environ. , 26B : 453 – 462 .
- Ondov , J. M. and Wexler , A. S. 1998 . Where Do Particulate Toxins Reside? An Improved Paradigm for the Structure and Dynamics of the Urban Mid-Atlantic Aerosol . Environ. Sci. Technol. , 32 : 2547 – 2555 .
- Paatero , P. and Tapper , U. 1994 . Positive Matrix Factorization: A Non-Negative Factor Model with Optimal Utilizatoin of Error Estimates of Data Values . Environmetrics , 5 : 111 – 126 .
- Prieditis , H. and Adamson , I. Y. 2002 . Comparative Pulmonary Toxicity of Various Soluble Metals Found in Urban Particulate Dusts . Exp Lung Res , 28 ( 7 ) : 563 – 576 .
- Seltzer , M. D. and Meyer , G. A. 1997 . Inductively Coupled Argon Plasma Continuous Emissions Monitor for Hazardous Air Pollutant Metals . Environ. Sci. Technol. , 31 : 2665 – 2672 .
- Sneddon , J. 1985 . Use of an Impaction-Electrothermal Atomization Atomic Absorption Spectrometric System for the Direct Determination of Cadmium, Copper, and Manganese in the Laboratory . Anal. Lett , 18 : 1261 – 1280 .
- Sneddon , J. 1986 . Direct and Near Real Time Determination of Metallic Compounds in the Atmosphere by AA . Am. Lab. , 18 ( 3 ) : 43 – 50 .
- Suarez , A. E. and Ondov , J. M. 2002 . Ambient Aerosol Concentrations of Elements Resolved by Size and by Source: Contributions of Some Cytokine-Active Metals from Coal-Fired Power Plants . Energy & Fuels , 16 : 562 – 568 .
- Wagman , J. , Bennett , R. L. and Knapp , K. T. 1977 . X-ray Fluorescence Analysis of Environmental Samples , Edited by: Dzubay , T. G. Ann Arbor, MI : Ann Arbor Science .
- Wu , Z. Y. , Han , M. , Lin , Z. C. and Ondov , J. M. 1994 . Chesapeake Bay Atmospheric Deposition Study, Year 1: Sources and Dry Deposition of Selected Elements in Aerosol Particles . Atmos. Environ , 28 : 1471 – 1486 .