Abstract
For a period of six years (1995–2000) the scavenging ratio, which is the ratio of a pollutant's concentration in water to its concentration in air, collected at an urban site in the Spanish Basque Country was studied. The aerosol is characterized by SO4 2− and NO3− with 1.79 and 1.61 μg m−3, respectively. Greater fractions of SO4 2−, NO3−, and NH4+ ions were present in the fine particle range, while greater fractions of other ions appeared in the coarse range. The most important species found in the precipitation is SO4 2− with 3.0 mg l−1. NO3−, Ca2+, and Cl− are the second most important ions. The volume-weighted mean concentration of H+ is 4.6 μg l−1 (pH = 5.3). The concentration of all analyzed ions (except H+) decreases throughout the rain event, showing the washout phenomenon of the rainwater. The scavenging ratio for the anthropogenic ions NO3−, SO4 2−, NH4+, and K+ is lower than the scavenging ratio for the marine-terrigenous ions, Cl−, Na+, and Ca2+.
INTRODUCTION
Atmospheric aerosol is the main source of most of the nonreactive mineral species found in precipitation. The main process for the incorporation of aerosols into precipitation include the incorporation in cloud water (nucleation) and the collection via raindrops in the in-cloud and below-cloud regions (inertial impaction, interception, and diffusion; CitationTsai et al. 1990).
The concept of scavenging ratios is based on the simplified assumption that the concentration of a component in precipitation is related to the concentration of that compound in the air. Thus, scavenging ratios can be calculated on a mass basis (CitationKasper-Giebl et al. 1999),
The scavenging ratio is fundamentally affected by the following factors: (1) particle size and hygroscopicity for aerosols, which is itself dependent upon many factors including chemical speciation and atmospheric life history; and (2) cloud type and precipitation intensity. Moreover, while much of the pollutant incorporation into precipitation occurs at cloud level, concentrations of airborne species are normally measured at the ground. The atmosphere is frequently not well mixed over this vertical distance, especially for components with a strong ground-level source and short lifetime (e.g., ammonia). For these reasons, scavenging ratios vary greatly in time and space (CitationHarrison and Allen 1991).
One important condition for the reliability of the scavenging ratio is to verify simultaneous rainwater and aerosol sampling at the same location and over a long period in order to avoid important deviations in the results.
We are not aware of any other studies on this subject carried out in the north of Spain (the part of the country with the highest levels of annual precipitation). The present study aims at providing data related to this parameter, which is so important for the field.
In this article, the scavenging ratio calculated from rainwater and aerosol ionic content in an urban area over a period of six years (1995–2000) is presented for different chemical species.
EXPERIMENTAL
The sampling point is located at Vitoria, an urban area in the Spanish Basque Country (). Vitoria is a town of 200,000 inhabitants, located in the south of the Basque Country (Northern Spain) at an altitude of 580 m above sea level. It is moderately industrialized. However, the northern area of the Basque Country is a highly industrialized region (40–70 km from Vitoria). About half of the Basque Country is forested ().
A six-stage cascade impactor was used to measure the particle size distribution. Each measurement was carried out once a week over 24 h (from Wednesday at 10:00 a.m. to Thursday at 10:00 a.m.) in order to integrate the dynamic cycle of the atmospheric mixing layer. shows the diameter ranges for the particles in each stage of the cascade impactor. The airflow used was 1 m3 h−1.
TABLE 1 Diameter particle range collected by the cascade impactor
The design of the cascade impactor has been described in detail previously (CitationEncinas et al. 1994; CitationEncinas and Casado 1996, 1999). In order to avoid a situation where the particles bounce off the impactor, the impactor was made of electrostatic nylon. The collection surfaces were 0.4 μm Nuclepore filters. The soluble matter was extracted with 5 ml of deionixed H2O using the ultrasonic method.
The rainwater samples were collected using an sequential “wet-only” collector (giving 0.13 mm fractions of precipitation). The collector features a plate equipped with a resistance that maintains the plate's temperature at 120°C. When there is precipitation, the first raindrops that touch the plate will evaporate, and a servomechanism opens the cupola. The date and time of opening are registered, and the first 10 ml of rain (0.13 mm of precipitation) are sent through a system of valves to the conductivity cell, where the temperature is measured. When these two parameters have been registered, the 10 ml of rainfall are injected into the first test tube of the tray. When this is done, the tray automatically turns until the second tube is placed below the injector. The process is repeated until the end of the rain event, when the water on the plate evaporates and the cupola closes.
Once the samples were collected, they were analyzed within 24 h or, if that was not possible, kept in a refrigerator at a temperature below 4°C.
The Cl−, NO3−, SO4 2−, Na+, NH4+, K+, Ca2+, and Mg2+ ions were the species determined in both the soluble extracts from the filter and the rainwater samples. The analytical technique was the ionic chromatography with conductimetric detection (CitationCasado et al. 1989, 1995, 1996; CitationEncinas and Casado 1999; CitationEzcurra et al. 1998). Moreover, the pH was measured in the rainwater samples.
The ionic concentrations from the filters have been corrected for blanks. The mean concentrations of the different ions analyzed at each event, weighed by volume, have been calculated using the fraction samples.
RESULTS
The study of the scavenging ratio comprises the period between January 1995 and December 2000. During this period, a total of 127 samples (762 filters) were collected in the cascade impactor.
For the rainwater, 4380 mm of precipitation were registered at Vitoria. The precipitation collected in the wet-only collector was 4220 mm, distributed among 637 events, which correspond to 96% of the total registered precipitation.
Size Distribution of Atmospheric Particulate Fraction
Fine particle range, integrated both in the Aitken nucleus mode and in the accumulation mode (D< 2.5 μm), is of a distinct anthropogenic nature—that is, the particles are either the outcome of nucleation and coagulation processes, or are emitted directly by anthropogenic sources (such as combustion processes, industrial activity, etc.). Coarse or sedimentable particle range (D>2.5 μm) are produced mainly by natural processes. It includes sea-salt aerosols, which are produced by direct dispersal of ocean water, and crustal aerosols, which originate from the solid surface of the earth (CitationMorawska et al. 1999; CitationParmar et al. 2001).
shows the average ionic concentrations measured in each stage of the cascade impactor.
FIG. 2 Average ionic concentrations of atmospheric particulate fraction collected at Vitoria. Period: January 1995 to December 2000. Units: μg m−3.
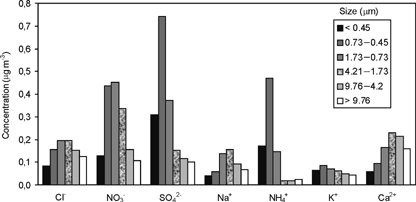
As can be seen from , the Mg2+ ion was not included in the study because its concentration is below the detection limit in the majority of the samples collected.
The maximum concentrations of anthropogenic ions, NO3−, SO4 2−, and NH4+, are logically in the fine particles mode (D< 1.73 μm). Of the total mass of SO4 2− and NH4+ ions, 41% and 55% respectively, are in the 0.45–0.73 μm range. For NO3−, 55% of the total mass are in the 0.45–1.73 μm range. This result is probably due to the absorption of the HNO3 gas by the coarse particles carrying the Ca2+ ion, which have a basic character. Similar results have been reported in other sites in the Spanish Basque Country (CitationCasado et al. 1996; CitationEncinas et al. 1994; CitationEncinas and Casado 1996, 1999) and by other authors (CitationPratt and Krupa 1985; CitationOhta and Okita 1990).
Both marine ions (Cl− and Na+) and a crustal ion (Ca2+) are present in higher concentration levels in the coarse particles range, as can be expected from their mechanical origin. Of the total mass of Cl− and Na+ ions, 60% and 70%, respectively, are in the 0.73–4.21 μm range. For the Ca2+ ion, the percentage of the total mass in the 1.73–9.76 μm range is 48%. There is not a distinct size distribution for the K+ ion.
Total Atmospheric Particulate Fraction
The total atmospheric particulate fraction was calculated as the addition of the average ionic concentrations in each stage of the cascade impactor. shows the total atmospheric particulate fraction collected at Vitoria during the study period.
FIG. 3 Total atmospheric particulate fraction calculated as the addition of the average ionic concentrations in each stage of the CRA-88 impactor. Period: January 1995 to December 2000. Units: μg m−3.
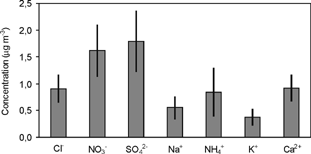
The anthropogenic ions, NO3− and SO4 2−, are the most important species found in the atmospheric particulate fraction, with 1.61 and 1.79 μg m−3, respectively. This was expected due to the urban character of the sampling point. Ca2+, Cl−, and NH4+ are the second-most important species, with 0.92, 0.91, and 0.85 μg m−3, respectively. These five ions represent on the whole 87% of the water-soluble aerosol mass collected at Vitoria during the study period.
In order to determine the marine influence on the composition of aerosols, sea-salt ratios were calculated using Na+ as reference, assuming all Na+ to be of marine origin (CitationParmar et al. 2001). These sea-salt ratios are given in .
TABLE 2 Sea salt ratio values of atmospheric particulate fraction components and the corresponding values for sea water
It is evident from that the sea-salt ratios for SO4 2−, K+, and Ca2+ are higher than the seawater ratios, which indicates incorporation of nonmarine constituents in aerosols. The Cl−/Na+ ratio is similar to the marine aerosol ratio, denoting the marine source as the only one for the Cl− ion in the atmospheric particulate fraction. CitationEncinas and Casado (1999) stated that the Cl−/Na+ ratio at Salvatierra () was 2.0 due to the presence of a waste incinerator that affects Cl− concentration in the atmospheric particulate fraction collected at this site.
Precipitation
The volume-weighted mean concentrations of the different ions analyzed in the precipitation samples collected at Vitoria are represented in .
FIG. 4 Volume-weighted mean concentrations of precipitation collected at Vitoria. Period: January 1995 to December 2000. Units: mg l−1. H+ concentration is expressed in μg l−1.
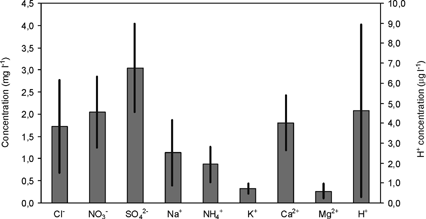
The precipitation at Vitoria is characterized by SO4 2− with 3.0 mg l−1. The second-most important species are NO3−, Ca2+, and Cl− with 2.0, 1.8, and 1.7 mg l−1, respectively. On the whole these four ions represent 77% of the ionic content of rainwater.
The volume-weighted mean concentration of H+ is 4.6 μg l−1, which corresponds to a mean pH value of 5.3. Consequently, the precipitation at Vitoria has neutral characteristics. The pH value of rain events varies between 4.0 and 7.9, with 17% of the samples showing a pH value less to 5.3. Rain events with strong acidity (pH ≤ 4.5) represent only 3% of the total number of samples, and they proceeded mainly from the European continent (CitationEncinas and Casado 1995).
shows the concentration percentage of the analyzed ions throughout the ten first fractions of the rain events. The rest of the collected fractions were omitted in order to reduce the complexity of the figure.
FIG. 5 Average concentration percentage of ionic species found in the ten first fraction of precipitation.
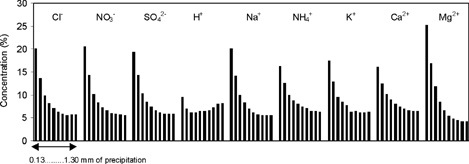
The concentration of all analyzed ions (except H+) decreases throughout the rain event, as can be seen from , which implies that there is an important washout process in the first millimeter of precipitation. Percentage values between 25% (for Mg2+) and 16% (for NH4+) are collected in the first 0.13 mm of precipitation. The concentration percentage drops gradually to an approximate value of 6%.
Finally, the concentration percentage of H+ initially decreases in the three first samples and then increases progressively, probably due to a neutralization effect by alkaline substances (CitationCasado et al. 1992).
Scavenging Ratio
The scavenging ratio for each analyzed species from the precipitation and aerosol data set was calculated as in EquationEquation (1) and represented in . In order to avoid errors that may derive from the variability of conditions throughout the collecting period, only the impactor measurements that preceded a rain event have been considered.
FIG. 6 Scavenging ratios (W) calculated as the relationship between the ionic concentrations in the precipitation and aerosol collected at Vitoria.
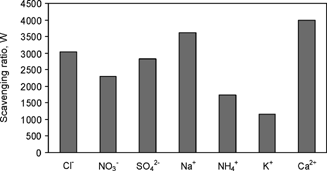
As stated by CitationJaffrezo and Colin (1988), aerosol particle size seems to be one of the main factors that determine scavenging efficiency; depending on the diameter of the particle, the collection efficiency between a raindrop and a particle is predicted to vary over several orders of magnitude.
The anthropogenic NO3−, SO4 2−, NH4+, and K+ ions present the lower scavenging ratios, as can be expected because of their size distributions. The higher W(SO4 2−) compared with W(NO3−) and W(NH4+) was probably due to the particulate SO4 2− from the marine source.
Moreover, the NO3−, SO4 2−, and NH4+ scavenging ratios are influenced by the presence of gaseous compounds such as HNO3, SO2, and NH3. Many studies have been made regarding the contribution of gases to rainwater concentrations (CitationChaumerliac et al. 2000; CitationCrutzen and Lawrence 2000; CitationGraedel 1984; CitationVoisin et al. 2000). Thus, CitationKasper-Giebl et al. (1999) stated that precipitation sulfate originates predominately from particulate sulfate (89–96%). Reactive gas-phase scavenging of SO2 is only of minor importance. Precipitation NO3−, on the other hand, is predominantly formed by gas-phase scavenging of HNO3 (88–96%), while particulate NO3− contributes to a much lesser extent (4–12%). Precipitation NH4+ is formed by comparable amount of particulate NH4+ (49–79%) and NH3 (51–21%). Therefore, W(NO3−) and W(NH4+) based on particulate matter only have to be regarded as an upper limit of the actual value.
On the other hand, the marine (Cl− and Na+) and crustal (Ca2+) ions are in the coarse particle range; therefore, they present a high value of the scavenging ratio.
compares our results with those of the reference. In this chart we also include the scavenging ratios obtained a Olaeta and Salvatierra, two municipalities located near Vitoria (see ). The results obtained at Vitoria are similar to the ones obtained for other areas. However, the scavenging coefficient of the Cl− ion at Olaeta and Salvatierra is higher due to the gaseous HCl from a waste incinerator located near the stations (CitationCasado et al. 1996; CitationEncinas and Casado 1999). This waste incinerator does not affect the scavenging ratios of the Vitoria station.
TABLE 3 Scavenging ratios in other areas
CONCLUSIONS
The ratio of the pollutant's concentration in water to its concentration in air (named scavenging ratio) was studied at Vitoria, an urban site in the Spanish Basque Country, over a period of six years (1995–2000).
The aerosol is characterized by SO4 2− and NO3− with 1.79 and 1.61 μg m−3, respectively. The aerosol size distribution presents SO4 2− and NH4+ in the fine particle range (D< 0.73 μm) and the rest of the ions in the coarse range (D > 0.73 μm), as can be expected from their respective origins. The NO3− ion presents a high concentration in the 0.45–1.73 μm range, probably due to the absorption of the HNO3 gas by the coarse particles that carry the Ca2+ ion, which have a basic character.
The most important species found in the precipitation is SO4 2− with 3.0 mg l−1. NO3−, Ca2+, and Cl− are the second-most important species. The volume-weighted mean concentration of H+ is 4.6 μg l−1 (pH= 5.3). The concentration of all analyzed ions (except H+) decreases throughout the precipitation event, showing the washout phenomenon of the rainwater.
The scavenging ratio for the anthropogenic ions, NO3−, SO4 2−, NH4+, and K+, is lower than the one for the marine-terrigenous ions, Cl−, Na+, and Ca2+. Therefore, the higher the particle diameter, the higher the scavenging ratio.
Acknowledgments
This work is a part of the “Cuantificación y estudio del depósito contaminante atmosférico en la CAPV y sus posibles efectos sobre las poblaciones de Pinus radiataD. Don” programme. The Basque Government sponsors this program.
REFERENCES
- Casado , H. , Encinas , D. and Lacaux , J. P. 1992 . The Moderating Effect of Ca2+ Ion on the Acidity in Precipitation . Atmos. Environ. , 26A ( 6 ) : 1175
- Casado , H. , Encinas , D. and Lacaux , J. P. 1995 . Evaluation of Origin and Distribution of Wet Deposition Recorded in the Spanish Basque Country . J. Environ. Sci. Health , A 30 ( 4 ) : 695 – 712 .
- Casado , H. , Encinas , D. and Lacaux , J. P. 1996 . Relationship Between the Atmospheric Particulate Fraction and the Ionic Content of Precipitation in an Area Under Influence of a Waste Incinerator Located in the Basque Country (Spain) . Atmos. Environ. , 30 ( 10/11 ) : 1537 – 1542 .
- Casado , H. , Ezcurra , A. , Durana , N. , Albala , J. L. , García , C. , Ureta , I. , Lacaux , J. P. and Pham , V. D. 1989 . Chemical Composition of Acid Rain in the North of Spain: The EPOCA Program . Atmos. Res. , 22 : 22 – 297 .
- Chaumerliac , N. , Leriche , M. and Audiffren , N. 2000 . Modeling of Scavenging Processes in Clouds: Some Remaining Questions About the Partitioning of Gases Among Gas and Liquid Phases . Atmos. Res. , 53 ( 1–3 ) : 29 – 43 .
- Crutzen , P. J. and Lawrence , M. G. 2000 . The Impact of Precipitation Scavenging on the Transport of Trace Gases: A 3-dimensional Model Sensitivity Study . J. Atmos. Chem. , 37 ( 1 ) : 81 – 112 .
- Encinas , D. and Casado , H. 1995 . “ Eight Years Studying Bulk and Wet Deposition in the Spanish Basque Country ” . In Studies in Environmental Science 64. Acid Rain Research: Do we have enough answers? , Edited by: Heij , G. J. and Erisman , J. W. pp. 407 – 410 . Elsevier Science . edited by
- Encinas , D. and Casado , H. 1996 . “ Chemical Composition of the Aerosol According to the Particles Size Observed on Three Sites in the Basque Country (Spain) ” . In Proc. of the Fourteenth International Conference on Nucleation and Atmospheric Aerosols , Edited by: Kulmala , M. and Wagner , P. E. pp. 691 – 694 . Elsevier Science . edited by
- Encinas , D. and Casado , H. 1999 . Rain-Aerosol Coupling in an Rural Area in the Basque Country(Spain): Scavenging Ratios . Aerosol Sci. Technol. , 30 ( 5 ) : 411 – 419 .
- Encinas , D. , Casado , H. , Lacaux , J. P. and Pham , V. D. 1994 . Concentration and Size Distribution of Atmospheric Particulate Matter on a Forested Area in the Basque Country (Spain) . J. Environ. Sci. Health , A 29 ( 1 ) : 99 – 114 .
- Ezcurra , A. , Casado , H. , Lacaux , J. P. and García , C. 1998 . Relatonships Between Meteorological Situations and Acid Rain in Spanish Basque Country . Atmos. Environ. , 22 ( 12 ) : 2779 – 2786 .
- Graedel , T. E. 1984 . Effects of Below-Cloud Gas Scavenging on Raindrop Chemistry Over Remote Ocean Regions . Atmos. Environ. , 18 ( 9 ) : 1835 – 1842 .
- Harrison , R. M. and Allen , A. G. 1991 . Scavenging Ratios and Deposition of Sulphur, Nitrogen and Chlorine Species in Eastern England . Atmos. Environ. , 25A ( 8 ) : 1719 – 1723 .
- Harrison , R. M. and Pio , C. A. 1983 . A Comparative Study of the Ionic Composition of Rainwater and Atmospheric Aerosols: Implications for the Mechanism of Acidification of Rainwater . Atmos. Environ. , 17 ( 12 ) : 2539 – 2543 .
- Jaffrezo , J. L. and Colin , J. L. 1988 . Rain-Aerosol Coupling in Urban Area: Scavenging Ratio Measurement and Identification of Some Transfer Processes . Atmos. Environ. , 22 ( 5 ) : 929 – 935 .
- Kane , M. M. , Rendell , A. R. and Jickells , T. D. 1994 . Atmospheric Scavenging Processes Over the North Sea . Atmos. Environ. , 28 ( 15 ) : 2523 – 2530 .
- Kasper-Giebl , A. , Puxbaum , H. and Kalina , M. F. 1999 . Scavenging Ratios for Sulfate, Ammonium and Nitrate Determined at Mt. Sonnblick (3106 m a.l.s.) . Atmos. Environ. , 33 ( 6 ) : 895 – 906 .
- Morawska , L. , Thomas , S. , Jamriska , M. and Johnson , G. 1999 . The Modality of Particle Size Distributions of Environmental Aerosols . Atmos. Environ. , 33 ( 27 ) : 4401 – 4411 .
- Ohta , S. and Okita , T. 1990 . A Chemical Characterization of Atmospheric Aerosol in Sapporo . Atmos. Environ. , 24A ( 4 ) : 815 – 822 .
- Parmar , R. S. , Satsangi , G. S. , Kumari , K. M. , Lakhani , A. , Srivastava , S. S. and Prakash , S. 2001 . Study of Size Distribution of Atmospheric Aerosol at Agra . Atmos. Environ. , 35 : 693 – 702 .
- Pratt , G. C. and Krupa , S. V. 1985 . Aerosol Chemistry in Minnesota and Wisconsin and Its Relation to Rainfall Chemistry . Atmos. Environ. , 19 ( 6 ) : 961 – 971 .
- Tsai , W. T. , Altwicker , E. R. and Asman , W. A. H. 1990 . Numerical Simulations of Wet Scavenging of Air Pollutants—II. Modeling of Rain Composition at the Ground . Atmos. Environ. , 24 ( 9 ) : 2485 – 2498 .
- Voisin , D. , Legrand , M. and Chaumerliac , N. 2000 . Scavenging of Acidic Gases (HCOOH, CH3COOH, HNO3, HCl, and SO2) and Ammonia in Mixed Liquid-Solid Water Clouds at the Puy de Dôme Mountain (France) . J. Geophys. Res. Atmos. , 105 ( D5 ) : 6817 – 6835 .