Abstract
The particle formation and emission in the combustion of pulverized olive residue (orujillo) is studied in this work. The fuel has been burned in controlled combustion conditions in an entrained flow reactor. A bimodal distribution with mode peaks at 155 nm and 110 μm is found for fly ashes. Coarse particles have been characterized by laser diffractometry and SEM, while fines have been analyzed by low-pressure impaction, DMA, SEM, and X-ray diffraction. Particles with Dp < 1 μm are composed of only K2SO4 and KCl in the same mass proportion, and possibly K3PO4 (less than 7% in mass). The use of a new particle sampling probe and a TEM allowed a detailed study of the formation of these particles when flue gases cool down. K2SO4 is experimentally found to start nucleation over 900°C, while KCl is not observed at this temperature. Condensation of KCl on these nuclei is observed in a sample taken at 560°C. These “formation steps” are in good agreement with both theoretical calculations by other authors and a simple equilibrium schema shown here.
INTRODUCTION
Biomass combustion is acquiring, by itself or in cocombustion processes, an increasing importance within the electricity and heat generation system, and a number of by-products previously not used or burned in low-efficiency boilers are being incorporated into the power market. As a relevant example of this fact, a number of combustion and gasification processes are being developed for orujillo, a residue of the olive oil production process, in order to use the huge amounts generated every year in the Mediterranean countries. In Spain, two medium-size (∼16 MWe each) power plants fired with pulverized orujillo have been recently built. Even though many of the technologies and knowledge developed for coal plants can be adapted to the combustion of solid biomass, these alternative fuels present some distinct features that require specific developments. Among them are the processes of aerosol formation, deposition, and emission.
The long- and even short-term human toxicity and environmental impact of combustion-generated aerosols has been clearly established (e.g., CitationNeas 2000; CitationMaynard and Maynard 2002), and regulations on the final power plant particulate matter emissions tend to be increasingly strict, especially for the finest fraction (PM2.5). Moreover, aerosols are strongly related to biofuel boiler and heat exchanger corrosion/fouling phenomena (e.g., CitationNielsen et al. 2000a; CitationEskola et al. 1998; CitationPeltola et al. 1999). As a result, there exists a growing interest (both public and industrial) in investigating the specific aerosol-related phenomena in biomass combustion.
Most biomass fuels are characterized by high-alkali contents (a comprehensive review is available in CitationJenkins et al. 1998; CitationSkrifvars et al. 1998)—as will be shown later, orujillo is an outstanding example—leading to high concentrations of fine aerosols in the flue gases. A number of authors in the last decade have reported on the characteristics of aerosols produced in the combustion of a variety of biomass fuels and in a wide range of boiler scales, from domestic heat producers to large electricity generation plants (CitationChristensen and Livbjerg 1996; CitationPagels et al. 2003; CitationWieser and Gaegauf 2000; CitationJohansson et al. 2003; CitationValmari et al. 1998; CitationKurkela et al. 1998). In most cases, a bimodal particle size distribution (PSD) is found, with a fine particle mode ∼0.1–0.5 μm and a supermicron mode typically in the range 1–100 μm. The relative importance of both modes is strongly affected by the type of combustion system; the cited works were accomplished at various types of fluidized bed combustors (FBC) or grate boilers, in which coarse-particle removal as bottom ash increases the submicron/supermicron fraction ratio with respect to the pulverized fuel configuration. Moreover, the known fact that flue gas-cleaning devices present a minimal efficiency for particles 0.1–1 μm (CitationMcElroy et al. 1982; CitationNielsen et al. 2002) makes the fine fraction even more significant within the final emissions. K, S, Cl, and Na (in the case of sodium-enriched fuels) account for most of the fine particle mass, mainly in the form of sulphates or chlorides.
The description and understanding of aerosol formation in these systems is, however, still far from complete. It is generally accepted that fine particles are generated in a manner similar to that described for coal combustion, i.e., by volatilization and subsequent nucleation/condensation of a part of the fuel mineral matter (see, e.g., CitationFlagan and Friedlander 1978; CitationQuann and Sarofim 1982; CitationNeville and Sarofim 1982). CitationSkrifvars et al. (1998) assumed that, for the case of woody chips and forest residue combustion, all (and only) inorganic matter soluble in water or a weak acid leach (as part of the chemical fractionation analysis; CitationBaxter et al. 1993) volatilized. CitationSkrifvars et al. (1998) used thermodynamic equilibrium considerations to predict the composition of the fine particles so generated. CitationChristensen and Livbjerg (1996, 2000) and CitationChristensen et al. (1998) developed a particle-formation model in straw combustion based on equilibrium calculations, gas-phase reaction kinetics, and nucleation/condensation equations; using their own field emission data, they concluded that K2SO4 nucleated at ∼800°C, and KCl condensed later on these nuclei. Little experimental evidence is available in the literature, however, for this process. CitationLind et al. (1998) sampled forest residue/willow flue gases at ∼820°C and ∼150°C by dilution in N2 and impaction, finding K2SO4 and KCl at both temperatures; the KCl detected at 820°C was assumed to be a result of a sampling artifact (condensation during dilution). CitationValmari et al. (1998) compared direct impaction of willow combustion flue gases at 650°C and 160°C, and they found that no chlorine was present at the higher temperature, while small quantities appeared after flue-gas cooling.
The aim of the present study is to characterize the final particulate emissions under controlled pulverized orujillo combustion conditions and to experimentally study and describe the particle-formation process. Orujillo being a good exponent of the special features of biomass fuels, the results and conclusions of this work should be useful for understanding of the aerosol generation in a general biomass combustion system.
EXPERIMENTAL
Entrained Flow Reactor
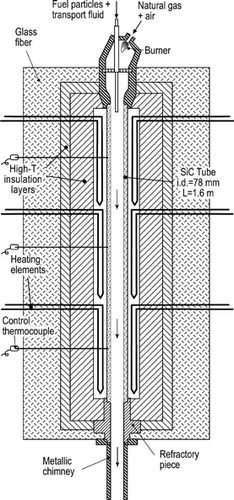
The experiments were conducted in a down-fired entrained flow reactor (EFR) whose principal characteristics are shown in . The 1.6 m long, 78 mm i.d. SiC tube is externally heated by 3 levels of 4 electrical resistances, assuring a uniform temperature along the reactor. The fuel particles are pneumatically transported and enter the tube through an insulated and cooled injector. The feeding rate is controlled by a low-rate solid-fuel–feeding device designed ad hoc for this facility; it consists of a continuously weighed silo from which a variable speed rotary valve extracts small amounts of fuel. Before injection, the air + particles stream is passed through an agitation chamber for disgregation and filtering of pulses in the feeding. The injector tip can finally be located at any point along the upper half of the EFR axis. Special attention has been paid to the stability of the fuel feeding rate, monitored by both laser extinction at the injector tip and oxygen level at the EFR exit. The coflowing gas stream is combustion products from a natural gas/air burner, installed over the heated tube, so that the fuel particles burn in an atmosphere representative of practical combustion systems, in terms of gas composition and temperature (both independently controllable). By adding pure O2, N2, or both to the transport air or the coflow, a broad range of combustion conditions can be reproduced.
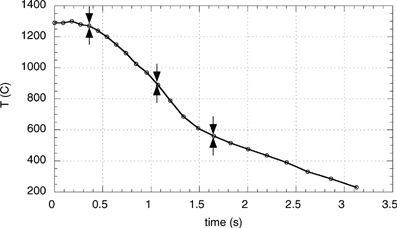
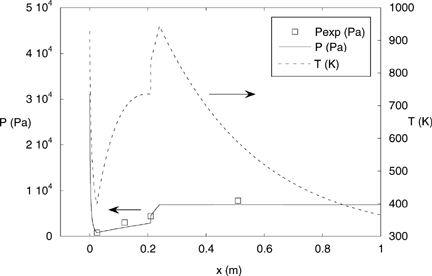
The heated tube is followed by an 18 cm long refractory, slightly insulated tube and a 30 cm long air-cooled chimney which provide a gas cooling rate that is similar to those found in real systems. The measured gas temperature evolution with time is presented in .
Aerodynamic-Quenching Particle Sampling (AQPS) Probe
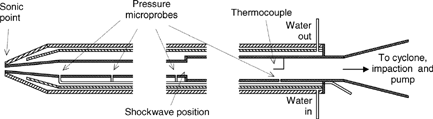
Submicron particles were sampled by means of a new probe, which is intended to minimize errors due to sampling artifacts in the presence of condensable species, in order to obtain a sample that is representative of the particle population in the undisturbed flow. presents its main features. It consists of a tiny (1.5 mm) nozzle entrance followed by a low-angle divergent cone, a 20 cm straight tube (6 mm i.d.) ending in a sudden expansion, and finally a 1 m long tube (10 mm i.d.). All sections are externally cooled by a water jacket and covered by an outer insulating layer. Four pressure taps connected to an absolute pressure sensor and an internal thermocouple (see ) serve to characterize the flow inside the probe.
Choked conditions are achieved at the inlet orifice when the gas + particle sample is aspirated by means of a vacuum pump. The flow is accelerated (Mach number increases) along the subsequent diverging section, causing a sudden decrease in static temperature (up to 108 K/s) and pressure (down to 2000 Pa). The resulting freezing of gas reactions is known as aerodynamic quenching and allows the original gas composition to remain unchanged. However, the main effect sought in this application is the fast reduction of pressure in order to reduce dramatically the rates of the physical phenomena that can modify the particle population characteristics (condensation, agglomeration).
The one-dimensional model proposed by CitationColkett III et al. (1982) has been used to design the probe and evaluate the evolution of velocity, temperature, and pressure. The following equations (CitationShapiro 1953) show the terms accounting for the effect of section changes, heat exchange and friction with walls on the flow Mach number (M), pressure (P), and temperature (T), respectively:
shows the predicted evolution for the pressure along the probe, together with measurements obtained when sampling gases at 1300 K, 105 Pa. The good agreement between both (and especially the fact that the measured pressure upstream of the predicted shockwave position is lower than the measured pressure downstream) assures the presence of supersonic flow and thus the aerodynamic quenching. As shown in , the final total pressure in this calculation is ∼8000 Pa; the vacuum pump is regulated during sampling to achieve this value. The calculated temperature profile for this case is also included in . These profiles are in good qualitative agreement with those shown by CitationColkett III et al. (1982).
FIG. 4 Measured and predicted pressure and static temperature evolution with length along the AQPS probe.
A cyclone separator (d50∼1 μm) located at the end of the probe and a preimpactor collect the particles over 0.5 μm. A tube constriction focuses the remaining particles into a narrow section in which a Transmission Electron Microscope (TEM) grid, coated with a thin (∼30 nm) commercial polymer film is immersed for a short time (typically 0.5–2 s) by means of a pneumatic actuator. Calculations (based on CitationFernandez de la Mora 1996; CitationHinds 1982) predict significant collection of particles by inertial impaction (Stk > 0.15) down to 20 nm; in practice, as it will be shown later, particles of ∼15 nm appear in great number on the film.
As an important feature of this probe, the calculated particle residence time within it (i.e., lapse between sampling and collection on the TEM grid) is ∼0.01 s, which is much shorter than those found in conventional isokinetic or dilution-based probes.
Analysis of the Samples
Particle Emission.
A low-angle, 20 cm long converging section allowed for direct connection of the chimney end to a cyclone (d50∼2 μm) followed by an 11-stage Berner-type low pressure cascade impactor (BLPI) (Hauke LPI 25/0,018/2). Manufacturer's aerodynamic cut diameters calibration (0.018, 0.036, 0.07, 0.14, 0.27, 0.54, 1.1, 2.1, 4.1, 8.1, and 16 μm) was assumed to be correct. Filtered air was added to the postcombustion gases through a “T” located between the cyclone and the cascade impactor, to reach the operational BLPI volume flow rate (determined by a critical nozzle), which is slightly greater than the throughput of the EFR (dilution ratio ∼1:1.2). The cyclone–BLPI system was maintained at ∼100°C by an external electrical heater to avoid water condensation. Calculations of CitationValmari et al. (1998) show that cut diameter variations due to impactor temperature changes from 20°C to 160°C are of minor importance for Dae < 0.2 μm; no further correction has been applied to the indicated values. According to the chemical composition of the sample obtained, a particle density of 2.3 kg/m3 has been assumed in the derivation of the Stokes particle diameter (particle diameter, Dp, thereafter) from the aerodynamic diameters. No further PSD data deconvolution was performed, as the described impactor stage efficiencies are steep functions of particle diameter (e.g., CitationFernandez de la Mora 1996).
The PSD of the particles collected in the cyclone was measured with a laser difractometer (Malvern Mastersizer S); ultrasound was previously applied to the samples to avoid particle aggregation. Fraunhoffer diffraction theory was followed for the deconvolution of diffraction patterns, instead of Mie theory, due to the uncertainty in the refractive index and the minimum particle diameter expected, given by the cyclone cut diameter. The impactor aluminium substrates were weighed before and after the experiment, thus determining the fine particle distribution for particles in the range (Dae) 18 nm–16 μm. The stages corresponding to particles over 5 microns were strongly affected by the presence of the cyclone, and the diffractometer data are considered more reliable for this range. Impactor deposits of stages 1–6 and 8–9 were also chemically analyzed by means of a scanning electron microscope (SEM) equipped with an X-ray energy-dispersive spectrometer (XEDS; JEOL JSM 6400), suitable for quantitative elemental composition determination above Z = 12 (Mg). Previous coating of the samples with a thin gold layer was necessary for analysis. Selected samples were also analyzed in an X-ray difractometer (D-Max Rigaku system) for qualitative identification of their major crystalline compounds. The thermogravimetric method developed by CitationMayoral et al. (2001) was followed to determine the ash content of the cyclone-retained sample and, by comparison with the fuel ash content, the combustible fraction remaining in the particles.
Finally, samples collected through a classical N2-dilution probe (dilution ratio 1:6) at the chimney exit were brought to a differential mobility analyzer (DMA; TSI 3071A), connected to a charge neutralizer and a condensation nuclei counter (CNC; TSI 3022), for the determination of the PSD in the range 20–700 nm. The DMA was preceded by a precyclone and a preimpactor that retained particles coarser than the upper limit of the measurement range, and by a laminar flow, silica gel diffusion drier. The sampling line was maintained over 120°C to avoid condensations.
Particle Formation.
Samples collected with the abovedescribed AQPS probe were analyzed for both morphology and qualitative chemical composition in a TEM-XEDS (JEM2000FXII). The polymer film is essentially transparent to the electron beam.
METHOD AND TESTS
Pretreatment and Properties of the Fuel
Orujillo was received as a relatively dry, ground material, with sizes ranging from a few microns to several millimeters. The fuel was repetitively sieved (until no significant variation of the PSD with iteration was detected) to select only the particles in the 300–400 μm range, so that all the particles burn with a similar rate along the EFR. This facilitates the interpretation of the results, avoiding uncontrolled effects due to the presence of particles in a wide range of burnout states.
Proximate and ultimate ASTM analysis as well as inorganic matter composition were performed for the sieved orujillo. Results are summarized in .
TABLE 1 Proximate and ultimate ASTM analysis and inorganic matter composition of the sieved orujillo
Tests
Natural gas, primary combustion air, and transport air mass flow rates were adjusted so that the fuel particles burn in combustion conditions similar to those found in industrial pulverized fuel boilers. O2 molar fraction at the particle injection point was 0.08 (db). Fuel consumption causes oxygen depletion, controlled and regulated by a sufficiently low fuel mass rate (40 g/h in the reported work). Oxygen mass fraction (db) at the exit (or “excess oxygen”) was maintained in 0.05 for these experiments. Total gas flow rate was 1.1 Nm3/h. Reactor wall and combustion gas temperature were set to 1300°C. The maximum particle residence time inside the heated tube, calculated through the particle momentum equation, is around 2 s.
The total experiment duration (1 h) for emission characterization was selected to collect a mass of sample at the impactor stages sufficient for weighing and analysis. Sampling for DMA measurements was carried out at the chimney exit (T∼200°C); 5 independent measurements were averaged to give a single result. The longest particle residence time was used, in order to allow for complete burning of the fuel particles.
The AQPS probe was used for sampling at 3 points within the heated tube and the subsequent refractory tube and chimney, corresponding to gas temperatures of 1300°C, 900°C, and 560°C (marked in ).
RESULTS AND DISCUSSION
Particulate Matter Emission
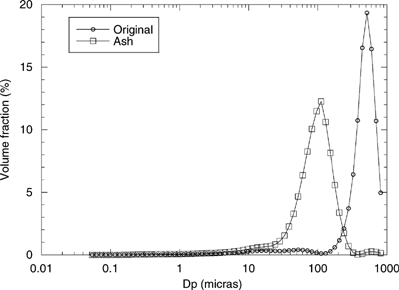
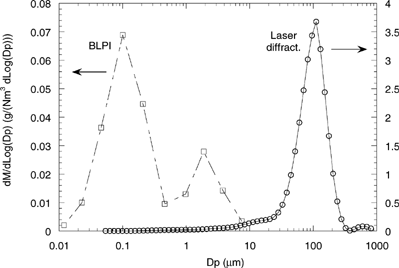
shows the global PSD of particles exiting the EFR as measured by the BLPI and the laser difractometer. Each independent measurement has been normalized by the relative amount of mass collected in the cyclone and the total mass collected on the stages of the low-pressure impactor. From thermogravimetric analysis, the combustible fraction of the major mode is known to be less than 5%, and no further significant combustion-related particle evolution is expected; then, this would be a measure of the final emission for this fuel at the previously described combustion conditions. A clear bimodal distribution is found, with mode peaks in Dp∼155 nm and 110 μm (according to the fit of the experimental data of to lognormal distributions); approximately 3% of the total particulate matter is contained in the finest mode. Other authors (e.g., CitationChristensen and Livbjerg 1996; CitationPagels et al. 2003) have reported similar-to-considerably-higher submicron content fractions in biomass combustion emissions. The higher fine/coarse ratios might be ascribed to the fact that most of these studies were accomplished on FBC facilities, in which removal of coarse particles as “bottom ash” radically increases the relative importance of the finest particles within the final particulate emissions. Normalization of the submicron mass by the total mineral matter entering the boiler is necessary for comparison between the “submicron production tendency” of different fuels or combustion conditions. In this case, as essentially all the mineral matter entering the EFR is collected in the cyclone or the BLPI, approximately 3% of the inorganic matter in the fuel is emitted in the form of particles smaller than 1 μm. This value is comparable with published submicron fraction measurements in FBC plants at locations prior to the process cyclone (CitationValmari et al. 1999). The supermicron mode is also shifted to larger diameters with respect to previous results for biomass-fired boilers, the type of combustion being again responsible for this fact.
FIG. 5 Particle size distribution of the final emissions. Note the difference between the scales for both modes. (dLog10Dp equals 0.0664 for the laser diffractometer and ∼0.31 for the BLPI).
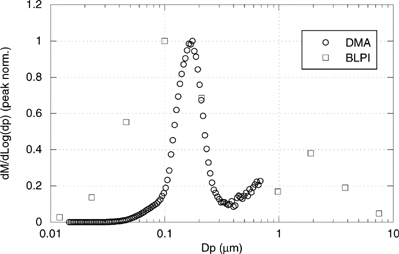
shows the DMA-derived PSD (mass basis) measurement of the submicron mode together with the BLPI result. Both curves display a reasonable agreement; the slight shift of the DMA-derived curve towards greater values (its mode peak being 174 nm, while the BLPI lognormal fit peak is ∼155 nm) might be explained by coagulation occurring along the sampling line (probe, cyclone, desiccator). It is worth noting that these results refer to the emission prior to any flue-gas–cleaning device (such as bag filters or ESP), which would greatly affect the final emitted PSD.
FIG. 6 DMA-derived PSD distribution (mass basis) compared with the BLPI result. Both series have been normalized by their peak height.
Deposits on the seven lower stages of the BLPI (i.e., corresponding to particles with Dp < 1 μm) are white, while the rest (and the cyclone deposit) are light brown. Chemical composition of the samples collected at the BLPI stages is presented in ; the analysis of the original fuel mineral matter is also shown to facilitate comparison. It is evident from this figure that qualitative differences exist between the two modes of the distribution: while coarse particles essentially retain the original characteristics of the fuel (Ca, Si, K, Al, Fe, P), fines are only composed of K, S, Cl, and small amounts of P (apart from possible elements with Z ≤ 11, not detected by SEM-XEDS). The approximate average mass and molar ratios of these elements in the submicron fraction are:
FIG. 7 Chemical composition (in a mass basis) of deposits on BLPI stages 1 through 6, and 8 and 9, compared with the mineral matter composition of the original fuel.
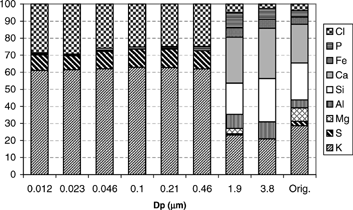
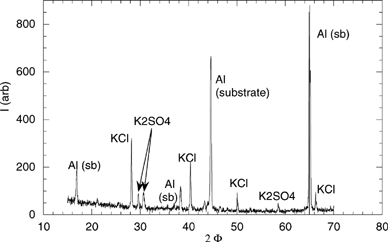
FIG. 8 X-ray diffractogram of a sample from stage 4 of the BLPI (dp = 100 nm). The aluminum substrate peaks are identified.
The observed differences between both modes clearly reveal their correspondence to different particle-formation mechanisms. Similar results were found in other works. In particular, those reported by CitationChristensen and Livbjerg (1996) for straw combustion in FBC almost coincide with the data presented here for pulverized orujillo. CitationValmari et al. (1998) found a much lower relative content of Cl (or KCl) in fine particles produced in a wood chip/willow FBC (probably due to the lower chlorine content of willow wood).
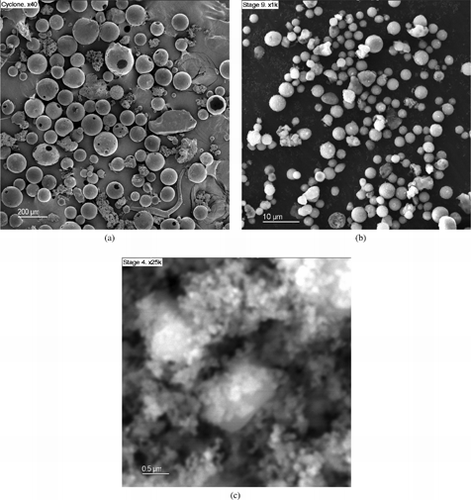
Morphological differences are also observed between the supermicron and submicron modes, as shown in the SEM micrographs of and the TEM pictures shown later. Samples collected in the cyclone and on the higher impactor stages (Dp > 1 μm) are composed of isolated spherical particles (, respectively), while those of the lower stages (Dp < 1 μm) are constituted of “amorphous” aggregates of particles (). It is not possible on the SEM images to elucidate whether aggregation occurred before impaction or during the growth of the deposit.
Physicochemical Evolution of the Particles
Supermicron Mode.
Due to the typical mode of biomass fuels combustion (FBC), supermicron particles emitted in those processes have not traditionally received much attention. In the case of pulverized orujillo, however, up to 97% of the emitted mass has been found to be in the coarse mode ().
shows the PSD of the unburned fuel and that of the ash collected at the cyclone (), measured by means of the laser difractometer. The PSD change observed in is similar to those typically found in coal combustion (apart from the size scale change), usually well modelled by particle fragmentation and later mineral matter coalescence during combustion (see, e.g., CitationSarofim et al. 1977). Indeed, the sphericity of the particles in indicates melting and supports coalescence.
Submicron Particles.
shows three TEM images corresponding to samples collected using the AQPS probe at 1300°C, 900°C, and 560°C. The sampling time (2 s) was sufficient for allowing average TEM-XEDS analysis. Individual analyses of single particles have also been accomplished in some cases.
At 1300°C, a huge number of very small, almost electron-transparent particles (mean size ∼14 nm, as determined by digital image processing with the UTHSCSA 1ImageTool program), account for most of the mass present in the image. Potassium is the only detected element over background in the analysis of a group (∼400) of these particles (again, elements with Z ≤ 11 are beyond the capabilities of the detector). The sparse, bigger, and dense/opaque particles also present here are composed of Ca, K, Si, Fe, and Al as major elements, and correspond to the lower tail of the supermicron mode, already present at this temperature.
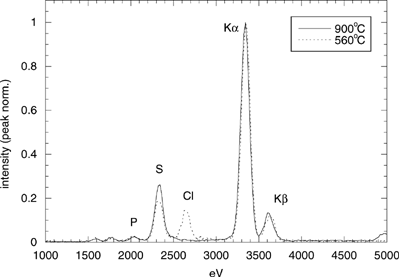
At 900°C (), the smallest particle mode is still present, in less concentration and with similar characteristics to those described for 1300°C (Dp∼12 nm, K as the only detectable element observed). However, a new mode, composed of crystalline particles, appears in this sample. The mean corpuscle size, measured by semiautomatic digital processing of this image, is ∼130 nm. Individual analysis of isolated particles (i.e., not included in an aggregate), although not giving a high signal, indicate that K and S are their major elements. shows the TEM-XEDS spectrum corresponding to the analysis of a big (∼200) group of particles. Clearly K and S are the most significant peaks, P being much less relevant. TEM micrographs of samples collected in a shorter “immersion” time (0.5 s) show that most of the particles are isolated; therefore, the observed aggregation in b is attributed to particle accumulation on the TEM grid film.
FIG. 11 TEM pictures of samples collected in the EFR using the AQPS probe at (a) 1300°C, (b) 900°C, and (c) 560°C. (Continued)

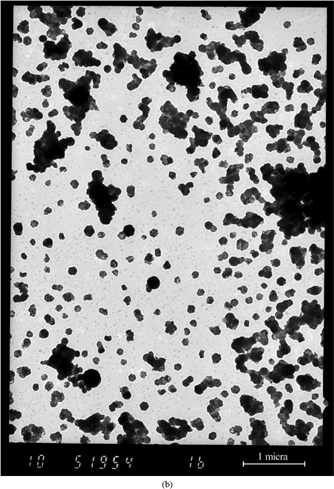

Finally, at 560°C, the ultrafine particles previously observed have totally disappeared (the grey background being due to the own absorption of the polymer film), and the “new mode” described appears to be composed by rounder particles, slightly bigger in corpuscle diameter (∼145 nm), compared to those observed at 900°C. also presents a general spectrum of this sample. Comparison with the previous result remarkably shows the appearance of Cl as temperature decreases from 900°C to 560°C. Again, shorter grid immersion in the sampled particle flux shows that particles are mostly isolated, with only a fraction of them forming small (2–3 corpuscles) aggregates.
FIG. 12 TEM-XEDS spectra of samples collected at 900°C and 560°C. Both curves have been normalized by their K (Kα) peak height. The small peaks to the left of P are spurious due to the measurement technique.
Rapid sample damage under a concentrated TEM electron beam prevented the study of single-particle composition dependence on radial position, a technique that has been shown to be a powerful tool (CitationMaynard 2000) for the understanding of the inner particle structure.
Submicron Particle Formation Model
CitationSkrifvars (1998) and CitationChristensen and Livbjerg (1996, 2000) used thermodynamic equilibrium considerations to explain/ predict the observed final emissions (including for the latter works HCl (g) and SO2 (g)) in different biomass combustion systems. In the particle formation model developed by CitationChristensen et al. (2000), gas reaction kinetics are also taken into account. Not much empirical data are available for the reactions considered here, apart from those inferred by this group to fit their own field measurements.
A pure, simpler thermodynamic equilibrium scenario is shown instead in . The NASA CEA program (CitationMcBride and Gordon 1996), based on Gibbs free-energy minimization, has been used for these calculations (compound and element physical data being mostly based on JANAF tables). Input data for calculations include results extracted from the final particulate emissions (submicron fraction) for K, S, and Cl, as well as experimental gas conditions at the exit of the heated tube. Up to 300 gaseous and condensed species were considered in the equilibrium calculations; apart from the typical combustion products, those in were the only ones with molar fraction over 10−8. summarizes the input data and some of the species considered.
TABLE 2 Input data and extract of the species considered in the thermodynamic equilibrium calculations
As shown in , KOH(g), SO2(g), and HCl(g) are the only stable species at high temperatures. When temperature decreases, KOH(g) reacts with SO2(g) and through nucleation forms K2SO4(s) (approximately at 1000°C), while KCl (g) is formed from KOH(g) and HCl(g). At ∼800°C, KCl(g) condenses to KCl(s). This simple schema serves to explain most of the features of the experimental results on submicron particle formation:
| • | The ultrafine particles observed in a are most probably constituted of KOH(s) nucleated along the probe. Neither K2SO4 nor KCl, with melting points of 1067°C (www.chemsoc.org) and 790°C (CitationPerry and Green 1997), respectively, are present in this sample. | ||||
| • | K2SO4 starts nucleating at T ∼1000°C. The temperature at the second sampling point is ∼900°C, which is above the KCl melting point, and thus KCl condensation is not yet active (see ). The ultrafine particles remaining may indicate that some KOH is still present at this temperature, supporting kinetical limitation for the formation rate of K2SO4 from KOH and SO2–SO3 (CitationChristensen and Livbjerg 1996; CitationValmari et al. 1998). | ||||
| • | Between the second and the third sampling points, condensation of KCl on the existing K2SO4 particles occurs; significantly, as shown in , the amount of chlorine detected at 560°C clearly increases with respect to the previous sampling point. Also, the previous crystalline aspect of the particles has been substituted by a rounded appearance, and mean size is found to be slightly larger; both effects might be ascribed to condensation of significant amounts of KCl on the particles already present at 900°C. The diminution of the sulphur peak is due to the decrease of its relative importance. | ||||
FIG. 13 Equilibrium distribution of gaseous (dashed curves) and condensed (continuous curves) species in the EFR as a function of temperature.
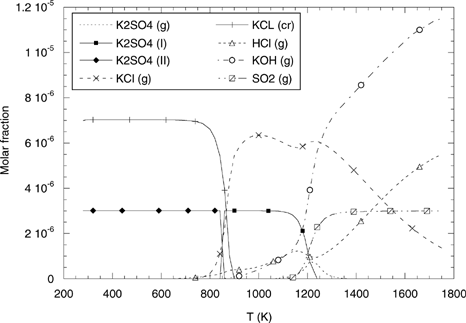
Some uncertainty exists about the KCl melting temperature: while the references cited above state its value in the range 776–790°C, transition of KCl(g) to condensed phase in the equilibrium calculations shown in seems to occur around 600°C. In the data reported by CitationValmari et al. (1998), impaction at 650°C did not reflect condensed KCl, while it was detected (in small amounts) at 160°C. It is not clear whether this is an indication that equilibrium predictions are more accurate or if, being the actual melting point in the range 776–790°C, the effective phase change is delayed due to limited condensation rate of KCl. The calculations due to CitationChristensen and Livbjerg (1996, 2000) and CitationChristensen et al. (1998) support the second possibility, by suggesting that condensation may be “delayed” and extended through a wide temperature range, down to 500°C or less, depending on the flue gas cooling curve.
Condensation of inorganic vapors on the supermicron mode particles during the flue gas cooling is, if it exists, of minor importance. shows that no appreciable amounts of sulfur and little (∼1%) of chlorine are included in the smallest (2–4 μm) particles in this mode; if condensation is assumed to be the reason for the presence of Cl in the coarser particles, progressively lower concentrations should be expected in these particles as diameter increases, since greater particles offer a lower surface/mass ratio. This is in good agreement with the calculations of CitationChristensen and Livbjerg (1996), which indicate that 100% of the heterogeneously condensed KCl (and 90% of the K2SO4) end on the fine particles.
CONCLUSIONS
Particulate emissions from pulverized orujillo combustion in realistic conditions were characterized in an entrained flow reactor. A bimodal distribution was found, with mode peaks in 155 nm and 110 μm, the former accounting for approximately 3% of the emitted mass. Coarse particles result from a process of fragmentation of the original fuel particles and melting and coalescence of its inorganic matter; they essentially retain the characteristics of the original mineral matter regarding elemental composition.
On the contrary, fine particles are composed of only K2SO4, KCl (in the same mass proportion), and possibly K3PO4 (accounting for less than 7% of the total mass in this range). Morphological differences were also observed between both modes. These results agree with previous field measurements for other biomass fuels and boiler configuration, thus showing the generality of the processes leading to the formation of these particles.
The AQPS probe has allowed for detailed characterization of the fine-particle formation mechanism by sampling flue gases at successively lower temperatures along the cooling process. K2SO4 is found to nucleate between 1300°C and 900°C, with KCl not appearing at these temperatures. Instead, KCl is clearly detected at 560°C as a condensed layer on the previously existing K2SO4 particles.
The same experimental series shows that little sampling artifact is introduced by the use of this probe in such an unstable system (which includes condensable species). However, further studies are needed to establish clearly the extent of these artifacts and the applicability of this kind of probe to similar situations and processes. Also, the use of the EFR allowed the study of a particular process in well-controlled combustion conditions. The method followed here should be useful for other fuels and situations.
Finally, a simple equilibrium schema was shown to explain the main features previously observed in the experimental results, including the formation steps described.
Acknowledgments
The authors would like to acknowledge the collaboration of Dr. J. J. Rodriguez-Maroto, J. L. Dorronsoro, and L. Ojeda during the experiments. The fuel samples were supplied by Foster-Wheeler Energia S.A. This work was funded by the Spanish Ministry of Science and Technology through grant BFM2001-1314-C03-03.
Notes
*Normalized (i.e., the sum of the elements in the list is 100).
REFERENCES
- Baxter , L. L. , Miles , T. R. , Miles , T. R. Jr. , Jenkins , B. M. , Richards , G. H. and Oden , L. L. 1993 . “ Transformations and Deposition of Inorganic Matter in Biomass Boilers ” . In Second International Conference on Technologies for a Clean Environment , Edited by: Carvalho , M. G. pp. 9 – 15 . Lisbon, , Portugal : Commission of European Communities . edited by
- Christensen , K. A. and Livbjerg , H. 1996 . A Field Study of Submicron Particles from the Combustion of Straw . Aerosol Sci. Technol. , 25 : 185 – 199 .
- Christensen , K. A. and Livbjerg , H. 2000 . A Plug Flow Model for Chemical Reactions and Aerosol Nucleation and Growth in an Alkali-Containing Flue Gas . Aerosol Sci. Technol. , 33 : 33 – 470 .
- Christensen , K. A. , Stenholm , M. and Livbjerg , H. 1998 . The Formation of Submicron Aerosol Particles, HCl and SO2 in Straw-Fired Boilers . J. Aerosol Sci. , 29 ( 4 ) : 421 – 444 .
- Colkett , M. B. III , Chiapetta , L. , Guile , R. N. , Zabielski , M. F. and Seery , D. J. 1982 . Measurements; Diagnostics. Internal Aerodynamics of Gas Sampling Probes . Combustion Flame , 44 : 3 – 14 .
- Eskola , A. J. , Jokiniemi , J. K. , Lehtinen , K. E. J. and Vakkilainen , E. 1998 . “ Modelling Alkali Salt Deposition on Kraft Recovery Boiler Heat Exchangers in the Superheater Section ” . In International Chemical Recovery Conference pp. 469 – 486 . Tampa, FL 1–4 June
- de Fernandez , la and Mora , J. 1996 . Drastic Improvement of the Resolution of Aerosol Size Spectrometers via Aerodynamic Focusing: The Case of Variable-Pressure Impactors . Chem. Eng. Comm. , 151 : 101 – 124 .
- Flagan , R. C. and Friedlander , S. K. 1978 . “ Particle Formation in Pulverized Coal Combustion—A Review ” . In Recent Developments in Aerosol Science , Edited by: Shaw , D. T. pp. 25 – 59 . New York : John Wiley . edited by
- Hinds , W. C. 1982 . Aerosol Technology. Properties, Behavior and Measurement of Airborne Particles , New York : John Wiley & Sons .
- Jenkins , B. M. , Baxter , L. L. , Miles , T. R. Jr. and Miles , T. R. 1998 . Combustion Properties of Biomass . Fuel Proc. Technol. , 54 : 54 – 17 .
- Johansson , L. S. , Tullin , C. , Leckner , B. and Sjövall , P. 2003 . Particle Emission from Biomass Combustion in Small Combustors . Biomass Bioenergy , 25 : 435 – 446 .
- Kurkela , J. , Latva-Somppi , J. , Tapper , U. , Kauppinen , E. I. and Jokiniemi , J. 1998 . Fly Ash Particle Formation in Fluidized Bed Combustion of Wood-Based Fuels . J. Aerosol Sci. , 29 (Suppl.1) : S571 – S572 .
- Lind , T. , Valmari , T. , Kauppinen , E. , Maenhaut , W. and Huggins , F. 1998 . “ Ash Formation and Heavy Metal Transformations During Fluidised Bed Combustion of Biomass ” . In Series Thermal Biomass Utilization , Graz, , Austria : BIOS . edited by
- Maynard , A. D. 2000 . Overview of Methods for Analyzing Single Ultrafine Particles . Philos. Trans. Roy. Soci. London , 358 : 2593 – 2610 .
- Maynard , A. D. and Maynard , R. L. 2002 . A Derived Association Between Ambient Aerosol Surface Area and Excess Mortality Using Historic Time Series Data . Atmos. Environ. , 36 : 36 – 5561 .
- Mayoral , M. C. , Izquierdo , M. T. , Andrés , J. M. and Rubio , B. 2001 . Different Approaches to Proximate Analysis by Thermogravimetry Analysis . Thermochimica Acta , 370 : 91 – 97 .
- McBride , B. J. and Gordon , S. 1996 . Computer Program for Calculation of Complex Chemical Equilibrium Compositions and Applications , NASA . RP-1311
- McElroy , M. W. , Carr , R. C. , Ensor , D. S. and Markowski , G. R. 1982 . Size Distributions of Fine Particles from Coal Combustion . Science , 215 : 215 – 13 .
- Neas , L. M. 2000 . Fine Particular Matter and Cardiovascular Disease . Fuel Proc. Technol. , : 55 – 67 .
- Neville , M. and Sarofim , A. F. 1982 . The Stratified Composition of Inorganic Submicron Particles Produced During Coal Combustion . Proc. Comb. Inst. , 19 : 1441 – 1449 .
- Nielsen , H. P. , Baxter , L. L. , Sclippab , G. , Morey , C. , Frandsen , F. J. and Dam-Johansen , K. 2000b . Deposition of Potassium Salts on Heat Transfer Surfaces in Straw-Fired Boilers: A Pilot-Scale Study . Fuel , 79 : 131 – 139 .
- Nielsen , H. P. , Frandsen , F. J. , Dam-Johansen , K. and Baxter , L. L. 2000a . The Implications of Chlorine-Associated Corrosion on the Operation of Biomass-Fired Boilers . Prog. Energy Combustion Sci. , 26 : 283 – 298 .
- Nielsen , M. T. , Livbjerg , H. , Fogh , C. L. , Jensen , J. N. , Simonsen , P. , Lund , C. , Poulsen , K. and Sander , B. 2002 . Formation and Emission of Fine Particles from Two Coal-Fired Power Plants . Combustion Sci. Technol. , 174 : 174 – 79 .
- Pagels , J. , Strand , M. , Rissler , J. , Szpila , A. , Gudmunson , A. , Bohgard , M. , Lillieblad , L. , Sanati , M. and Swietlicki , E. 2003 . Characteristics of Aerosol Particles Formed During Grate Combustion of Moist Forest Residue . J. Aerosol Sci. , 34 : 34 – 1043 .
- Peltola , K. , Hiltunen , M. , Blomquist , J. P. , Skrifvars , B. J. , Kurkela , J. , Latva-Somppi , J. and Kauppinen , E. I. 1999 . “ Fouling of the Cooling Surfaces in Biofuel-Fired Fluidized Bed Boilers ” . In Proc. of the 15th International Conference on Fluidized Bed Combustion 9 – 12 May . Savannah, Georgia, , USA
- Perry , R. H. and Green , D. W. 1997 . Perry's Chemical Engineers' Handbook , 7th ed. , New York : McGraw-Hill .
- Quann , R. J. and Sarofim , A. F. 1982 . Vaporization of Refractory Oxides During Coal Combustion . Proc. Comb. Inst. , 19 : 1429 – 1440 .
- Sarofim , A. F. , Howard , J. B. and Padia , A. 1977 . The Physical Transformation of the Mineral Matter in Pulverized Coal Under Simulated Combustion Conditions . Combust. Sci. Technol. , 16 : 16 – 187 .
- Shapiro , A. H. 1953 . The Dynamics and Thermodynamics of Compressible Fluid Flow , New York : Ronald Press .
- Skrifvars , B.-J. , Blomquist , J.-P. , Hupa , M. and Backman , R. 1998 . “ Predicting the Ash Behaviour During Biomass Combustion in FBC Conditions by Combining Advanced Fuel Analyses with Thermodynamic Multicomponent Equilibrium Calculations ” . In 15th Annual International Pittsburgh Coal Conference Pittsburgh, PA, , USA 14–18 September, paper 218
- Valmari , T. , Kauppinen , E. I. , Kurkela , J. , Jokiniemi , J. K. , Sfiris , G. and Revitzer , H. 1998 . Fly Ash Formation and Deposition During Fluidized Bed Combustion of Willow . J. Aerosol Sci , 29 ( 4 ) : 445 – 459 .
- Valmari , T. , Lind , T. M. , Kauppinen , E. I. , Sfiris , G. , Nilsson , K. and Maenhaut , W. 1999 . Field Study on Ash Behaviour During Circulating Fluidized-Bed Combustion of Biomass. 1. Ash Formation . Energy Fuels , 13 : 379 – 389 .
- Wieser , U. and Gaegauf , C. K. 2000 . “ Nanoparticle Emissions of Wood Combustion Processes ” . In 1st World Conference and Exhibition on Biomass for Energy and Industry Sevilla, , Spain 5–9 June