Results of recent research show that particulate matter (PM) composition and size vary widely with both space and time. Despite the variability in PM characteristics, which are believed to influence human health risks, the observed relative health risk estimates per unit PM mass falls within a narrow range of values. Furthermore, no single chemical species appears to dominate health effects; rather the effects appear to be due to a combination of species. Non-PM factors such as socioeconomic status and lifestyle are also believed to affect the health risk, although accounting for these confounding factors is challenging. Airborne PM is also responsible for a number of effects aside from human health, such as alterations in visibility and climate. Because the PM problem is associated with a range of societal issues such as energy production and economic development, making progress on reducing the effects of PM will require integrated strategies that bring together scientists and decision makers from different disciplines to consider tradeoffs holistically.
Introduction and Background
In response to epidemiology studies published over the past 20 years, at least three research communities have been intensively studying airborne particulate matter (PM).Footnote 1 These efforts have been coordinated by approaching the Source–Airborne Concentration–Receptor–Exposure–Dose–Health Effects paradigm (adopted from the CitationNational Research Council 2001, p. 24) from different perspectives or along different parts of the paradigm. The interests of the atmospheric sciences community include the emissions of particles and precursors from sources, their transport and transformation in air to receptor locations, and finally removal from the atmosphere. The interests of the exposure community are to examine the pathways by which pollutants, particulate matter in this case, approach and enter the body, for example by relating exposures to PM concentrations at central locations and to other factors. Both the atmospheric sciences and exposure communities approach the paradigm from left to right, although beginning from different points along the paradigm. In contrast, the health effects community has studied health outcomes, including hospital admissions, school absences, disease rates and deaths in human populations, and potential mechanisms of biological actions in laboratory settings. In general, health effects scientists approach the paradigm from right to left, attempting to correlate an observed adverse health effect with dose or exposure measures. For the most part, research results are reported in scientific publications and conferences for each community separately. Over the years, there has been little effort to integrate information from these diverse groups in a substantive way (CitationNational Research Council 1998, Citation1999, Citation2001, Citation2004; CitationPhalen 2002). While a major attempt took place in 1998 at a workshop in Chapel Hill (CitationAlbritton and Greenbaum 1998), little has occurred after that meeting until recently.
The first major integrative conference covering these topics was the specialty conference of the American Association for Aerosol Research held in Pittsburgh, Pennsylvania in April 2003. Entitled Particulate Matter: Atmospheric Sciences, Exposure and the Fourth Colloquium on PM and Human Health, the conference was co-chaired by the authors of this article. The overall goal of the conference was to bring together health and exposure scientists with atmospheric scientists, air quality managers, and policy makers to allow for enhanced communications and exchange of information among these groups. As a result of this meeting, we now have an opportunity to summarize the state of scientific understanding regarding several important questions related to PM and human health.
The objective of this paper is to provide such a summary in concise form, focusing on the eight key questions that comprised the framework of the specialty conference. We cite many relevant papers in this field, most of which were either presented at the conference or were cited during the presentations. We also provide examples of important data presented at the conference that have improved our understanding of particulate matter air pollution.
For detailed information about specific topics mentioned in this review paper, the reader is referred to special issues of five journals devoted to papers presented at the conference: Aerosol Science and Technology; Atmospheric Environment; Journal of the Air & Waste Management Association; Journal of Geophysical Research—Atmospheres; and Inhalation Toxicology. Additional details regarding papers presented at the conference and organization of the conference can be found at http://www.aaar.org/PM2003/PM03confinfo.htm.
A. What Are the Policy Perspectives Linking PM Emissions, the Atmosphere, and Effects?
Atmospheric PM is a highly variable and complex mixture of particles and gases. Primary particles are emitted directly from sources, while secondary particles are formed in the atmosphere from gaseous emissions. Both primary particles and precursor gases can be emitted from natural and anthropogenic sources. The U.S. EPA National Ambient Air Quality Standards (NAAQS) for PM2.5 are 65 μ g/m3 for a 24-hour average, and 15 μ g/m3 for an annual average (CitationU.S. EPA 2004a). On December 17, 2004, the U.S. EPA designated 224 counties plus the District of Columbia as nonattainment for the annual average PM2.5 NAAQS (http://www.epa.gov/pmdesignations/). Approximately 95 million people live in these nonattainment areas. Only a few counties, mostly in California, exceed the 24-hour average PM2.5 NAAQS, but these counties also exceed the annual average PM2.5 NAAQS.
Several factors make it difficult to establish policies for reducing levels of atmospheric PM. First, PM2.5 and precursor gases can be transported over long distances, allowing mixing of the two over space and time as well as mixing of urban and rural pollution. This makes it difficult to identify which sources are producing the primary particulate matter material and precursor gases. For example, recent data on the ratio of 13C to 14C in PM2.5 near Houston suggest that up to 75% of the organic carbon component of PM2.5 is associated with modern carbon or biomass related combustion, as opposed to carbon from fossil fuels (CitationLemire et al. 2002). This result is unexpected, considering the great amounts of oil, gas, and other fossil fuels used in Houston, Texas, and suggests that additional work is needed to understand biogenic sources. It is also known that greater than 75% of the PM2.5 sulfate and organic carbon in several Eastern cities is derived from regional sources in upwind areas, not due to local sources (CitationCabada et al. 2004; CitationDutkiewicz et al. 2004; CitationModey et al. 2004; CitationTang et al. 2004; CitationTanner et al. 2004). This suggests it may be difficult to meet the NAAQS by relying only on local controls in many urban areas.
Second, even when the most important sources have been identified, it is difficult to estimate the emissions under all possible conditions. This is especially true of NH3, where emissions from livestock manure, fertilizers, and soil can vary by orders of magnitude as atmospheric and surface conditions change (CitationAnderson et al. 2003). NH3 is often the limiting constituent in the production of particles by the conversion of acid gases to their ammonium salts (e.g., NH4HSO4, (NH4)2SO4, NH4NO3) (CitationTakahama et al. 2004).
Third, atmospheric chemistry can be nonlinear. As an example, the chemical interactions between nitrogen oxides, sulfur oxides, and ammonia can lead to counterintuitive results. Reductions of SO2 from sources will generally reduce SO4 2 − levels, but might increase NO3 − due to the availability of the NH3 that was associated with the sulfate before SO2 reductions occurred. Reductions of NOx can change the atmospheric system in complex ways and can either increase or decrease SO4 2 −, NO3 −, and O3 (CitationPandis 2004, pp. 3–14 and 3–15). The effects of various control strategies on concentrations of organic compounds are also unknown. Therefore, the ultimate impacts of abatement efforts on human health and welfare effects are difficult to predict.
Fourth, some events can lead to excessive PM2.5 concentrations that cannot be readily reduced by human intervention. The dust storms in Africa in 2001 and forest fires in eastern Canada in 2002 were huge sources of particles and precursor gases that exacerbated the PM problem in the eastern United States. Dry conditions in the Southwest can cause soil in the surrounding regions to contribute to PM2.5 mass in urban areas.
Fifth, economic and other tradeoffs associated with control actions may be substantial. As PM standards become more stringent, compliance costs escalate, and a decrease in economic productivity becomes more likely. However, such a decrease may be balanced or overcome by reduced health care and other costs associated with the adverse effects of PM (CitationU.S. EPA 1999).
The EPA is establishing a National Core Network (NCORE) to improve monitoring for PM2.5. A diagram illustrating the concept of the NCORE system is shown in . The sites established at levels 1, 2, and 3 provide a range of monitoring and research capabilities to further our ability to understand and regulate PM. NCORE involves multi-pollutant monitoring to address multiple objectives, such as identifying nonattainment areas and quantifying the specific chemical constituents in PM. The latter is important so that PM mass at a receptor can be apportioned back to its sources. Furthermore, NCORE provides support to epidemiology and toxicology studies so that physical and chemical characteristics most harmful to human health can be identified. At present, there is a lack of agreement between results of ambient epidemiological studies and toxicology studies in the lab, which will be discussed below. Information on the NCORE program can be obtained from the CitationU.S. EPA (2004b).
FIG. 1 Proposed NCORE Program. Figure courtesy of Richard Scheffe (CitationU.S. EPA 2004b).
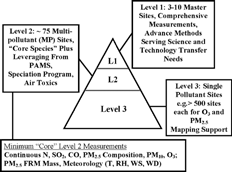
It is of interest that the European Union has adopted the “precautionary principle” regarding regulation of PM. Simply stated, existing PM levels are assumed to be responsible for at least a fraction of the illness observed in polluted areas; it is also assumed that the incidence of illness can be decreased by reducing PM2.5 concentrations (CitationBuringh 2003). Furthermore, since PM2.5 is correlated with PM10 in Europe, the EU is considering whether a single standard for PM is sufficient, unlike the United States which has separate standards for PM2.5 and PM10 (CitationU.S. EPA 2004a). The United States also is in the process of replacing the current PM10 standard, which includes PM2.5, with a standard for coarse particles (PMc). This will result in two separate standards in the United States, one for PM2.5 and one for PMc (CitationU.S. EPA 2004c).
B. What Are the Physical and Chemical Characteristics of PM? What Health Effects Are Associated with Specific Characteristics?
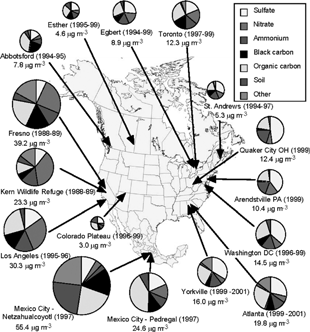
shows the composition of PM2.5 at various sites in the United States, Canada, and Mexico (CitationBlanchard 2004). Based on annual average results, the most abundant chemical species in PM in the East are SO4 2 − and organic material, while the most abundant species in the West are NO3 − and organic material. The absolute magnitude, however, has a seasonal dependence based on the volatility of some of the species and the influence of photochemical production of secondary species. Sites on both sides of the United States have smaller but significant amounts of NH4 + which neutralizes much of the SO4 2 − as well as NO3 − in the particles. Elemental carbon (EC) and crustal material (typically considered the sum of oxides of the trace elements most abundant in soil, e.g., Si, Al, Fe, Ca, etc.) comprise varying fractions depending on location, but usually each contributes less than 10–15% of the PM2.5, with higher EC levels in urban areas and higher soil dust in the western United States. The “other” category includes particles such as trace metals from fossil fuel combustion, natural bioaerosols such as from microbial, plant, and animal sources, and water associated with the particles.
FIG. 2 PM2.5 concentration and chemical composition at various sites in North America (CitationBlanchard 2004). Chemical species for each site are presented clockwise in the pie charts in the same order as in the legend. Reprinted from Chapter 6, –16 of the Final NARSTO Report with permission from Cambridge University Press. Copyright 2004 Envair.
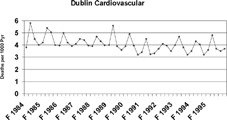
Several studies have attempted to isolate the health effects of specific chemical components in PM2.5. Most of these efforts have not been able to implicate individual chemical species, but rather have found that a variety of chemical as well as physical properties of aerosols, such as particle number, surface area, and mass, are associated with health effects (CitationHarrison and Yin 2000). For example, greater numbers of people in cities become ill when airborne concentrations of PM2.5 mass and PM2.5 SO4 2− increase (e.g., CitationThurston et al. 1994; CitationSchwartz et al. 1996; CitationPope 2000). Examples of associated illnesses include respiratory problems, changes in heart rhythms, heart attacks, and severe respiratory and heart malfunctions leading to death. There also are more absences at work and at school when airborne concentrations increase. In the Harvard six-cities study, CitationDockery et al. (1993) show that increases in PM2.5 mass and PM2.5 SO4 2 − are associated with increases in death rates (). This includes death rates from all causes and death specifically from respiratory and heart problems, as well as from lung cancer. In Holland, CitationHoek et al. (2002) report that death rates increase when there are increasing airborne concentrations of black smoke (black or dark particles emitted from incomplete combustion and often referred to as soot, such as emitted from diesel sources) and pollutants emitted from motor vehicles. Using data obtained prior to and following a 1990 law prohibiting sale of coal in the city of Dublin, CitationClancy et al. (2002) quantify decreases in concentrations of black smoke () along with decreases in death rates from lung cancer and from lung and heart ailments (). However, the Dublin results must be confirmed in order to eliminate the effects of other factors that have recently improved public health.
FIG. 3 Increased mortality rate ratios due to fine particles (FP), which are particles less than 2.5 microns in aerodynamic diameter, and sulfate particles from the Harvard Six-Cities Study (CitationDockery et al. 1993). Concentrations on the x-axis are given in μ g/m3. The y-axis shows the ratio of the death rate in each city (deaths/year normalized by population) to the lowest death rate measured, namely that in Portage, Wisconsin. Reprinted with permission from New Engl. J. Med. 329:1753–1759, 1993. Copyright 1993, Massachusetts Medical Society. All rights reserved.
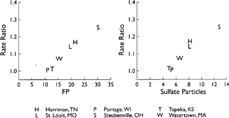
FIG. 4a Reduction in concentrations of black smoke in Dublin, Ireland, following a city-wide ban on sales of coal (CitationClancy et al. 2002). Reprinted with permission from Elsevier (Lancet. 360:1210–1214, 2002).
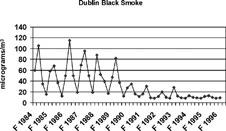
FIG. 4b Reduction in cardiovascular mortality in Dublin, Ireland, following a city-wide ban on sales of coal (CitationClancy et al. 2002). The y-axis shows deaths per 1000 person years. Reprinted with permission from Elsevier (Lancet. 360:1210–1214, 2002).
CitationPope (2000) highlights four categories of chemical constituents likely to be responsible for the observed health associations: emissions from combustion of fossil and biomass fuels, particles generated by high temperature industrial processes such as smelting, products of chemical reactions in the atmosphere such as SO4 2 − and NO3 −, and fine particles from soil and other sources. Pope's conclusions have some support from factor analysis studies (e.g., CitationGrahame and Hidy 2004). In contrast, dose-response correlations are absent and the harmful agents are still uncertain. Research is needed to determine whether there are human health effects from specific chemical species like polynuclear aromatic hydrocarbons and certain trace metals that are toxic at high concentrations, but are found in only trace concentrations in PM. Furthermore, little is known about the health effects of ultrafine particles (geometric diameter less than 0.1 micrometers), which can be emitted directly from sources or formed in the atmosphere by nucleation from precursor gases (CitationWoo et al. 2001; CitationStanier et al. 2004). The roles of gases such as NO2, CO, and volatile organic compounds in producing effects or modifying PM effects are also poorly understood. Finally, the roles of coarse particles and the ever-present biological aerosols require additional research.
C. What Are the Sources of Precursor Gases and PM That Are Potentially Causing Health Effects?
Current estimates of the global emissions of PM2.5 are shown in (CitationScheffe 2003). Both natural and anthropogenic sources are responsible for particles with potential to cause health effects. For example, natural emissions include gaseous sulfur from volcanoes as well as from decaying vegetation, which can form secondary sulfate particles in the atmosphere. Anthropogenic sources such as coal and oil acids, elemental carbon, heavy metals, and organic species emitted from coal and oil combustion.
TABLE 1 Global emissions of PM2.5 (CitationScheffe 2003)
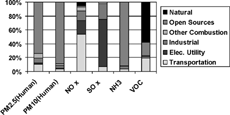
The 1999 national emission inventory for the United States, including PM2.5, PM10, and several gaseous pollutants, is presented in (CitationHidy and Pace 2004). The inventory shows the fraction of emissions in each of six major categories. Over 70% of the PM2.5 emissions are in the “open sources” category, referring to emissions from road dust, wind blown dust, and other fugitive sources.
FIG. 5 1999 National emissions in the U.S. This figure was prepared by R. Scheffe from data in Chapter 4, Table 4.3 of the Final NARSTO Report (CitationHidy and Pace 2004) and natural emission data from CitationU.S. EPA (1998). No data from natural sources are included for PM2.5 or PM10. Data from the NARSTO Report are used with permission from Cambridge University Press, Copyright 2004 Envair.
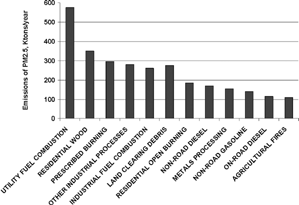
A more detailed inventory for 2001 focusing on primary anthropogenic PM2.5 is shown in (CitationU.S. EPA, 2004d). The largest source category averaged over the United States is utility fuel combustion, which is mainly coal burning for electricity production accounting for 570 Ktons/yr. The other two high temperature process categories, namely industrial fuel combustion and metals processing, bring the total emissions for high temperature processes to 970 Ktons/yr. Three mobile source categories include non-road diesel, non-road gasoline, and on-road diesel for a total of 400 Ktons/yr. The remaining categories in are all diffuse area sources with a total of 1180 Ktons/yr. Thus the total PM2.5 emissions represented in the figure are 2550 Ktons/yr.
FIG. 6 Anthropogenic sources of PM2.5 according to the national emission inventory for 2001. Figure courtesy of Andy Miller (Redawn from CitationMiller 2003). All values are given in Ktons (thousand English tons) per year. Data available from http://www.epa.gov/ttn/chief/trends/.
Understanding the health effect impacts of sources of PM2.5 requires approaching the problem from different directions. For example, some studies have attempted to correlate epidemiological data with emissions using source apportionment methods. However, such attempts are limited due to lack of knowledge as to what is actually inhaled by affected individuals as well as the limited ability of source apportionment methods to accurately identify and quantify specific sources of concern. Other studies have used the collection of PM2.5 emitted directly from sources in toxicology studies to check the toxicity of emissions from those specific sources. However, particle attributes may change during atmospheric transport and during storage and recovery from filters, and furthermore mixtures of particles from different sources can impact toxicity. Thus, it also is necessary, and likely more appropriate, to conduct toxicology studies using ambient particles along with co-pollutant gases to fully understand the links between source emissions and effects (CitationNational Research Council 1998, p. 74; CitationMiller 2003).
When sufficient data are available, the use of receptor models can identify possible sources of the particles. Several types of models are available; some are useful when the emissions of species unique to the source are known, while others can identify source categories based entirely on ambient data. The former approach is known as the Chemical Mass Balance, where specific trace metals, organic compounds, and molecular markers can be used in source apportionment resulting in identification of specific source types. The latter approach, known as Factor Analysis, includes statistical methods that can explore the structure of a dataset to identify possible sources (CitationHopke 2003). An example of Factor Analysis is Positive Matrix Factorization, an approach that has been applied to ambient and indoor PM data; recent work has shown that this method can avoid many of the uncertainties associated with Principal Component Analysis (CitationZhou et al. 2004a). Positive Matrix Factorization can be used with noncompositional data such as particle size distributions (CitationZhou et al. 2004a) and can accomodate the combination of data from instruments operating at different sampling time intervals (CitationZhou et al. 2004b). However, the ability for receptor models to identify sources is based on the chemical species and physical characteristics (e.g., size distribution) available from the monitoring studies. Currently, national routine monitoring networks provide data only for a limited number of species and source categories, including nonspecific categories for secondary aerosols (e.g., sulfate and nitrate). Nevertheless, the size of the networks, having multiple sites in a given area or region, also provides strength to the receptor model analyses.
Although a wealth of data exists on emissions from stationary and mobile sources, there are still large uncertainties in our ability to estimate accurately emissions from other sources, since size distributions and chemical composition changes as meteorology, co-pollutants, and operating conditions vary. Furthermore, the measurements can be affected by sampling conditions and techniques (CitationLipsky et al. 2002). Emissions from natural sources and anthropogenic non-combustion sources are generally less well understood than those from anthropogenic combustion.
D. When and Where Are People Exposed to PM?
People are exposed to PM on a continual basis, while in their residences and workplaces, while commuting, and during recreation and other leisure activities. Recent research has attempted to quantify these exposures using personal monitors, where people carry sampling instruments as they go about their daily activities. Data from personal monitors have been compared with 24-hour average exposures estimated from stationary PM samplers at central ambient monitoring sites; the latter appear to represent personal exposure reasonably well for SO4 2 − and in some cases for total PM2.5 mass (CitationU.S. EPA 2003; CitationSamet et al. 2000; CitationSuh 2003). However, estimating personal exposure to many other chemical species in PM, as well as estimating short-term peak exposure, require consideration of concentrations in each microenvironment. There also is a need to explore personal exposures that are unusual (e.g., indoor vacuum cleaning) with respect to proximity to sources, or that occur during activities that generate high levels of pollutants. The problem is complicated because the susceptible populations only have been generally identified, and their locations and activities during exposure are poorly documented.
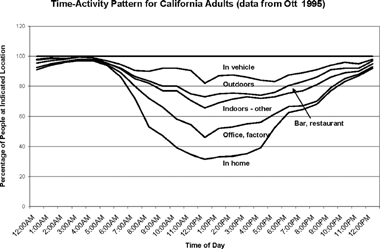
CitationKlepeis et al. (2001) estimate that people in the United States spend an average of 87% of their time indoors. The number of possible indoor environments is apparent from , reporting where people in California spend their time on a typical workday (CitationOtt 1995). From these data, it is clear that modeling exposure is a complex task, as it is affected by people's activity patterns, characteristics of their residence (indoor sources, proximity to outdoor sources, and type of housing), characteristics of their workplace, socioeconomic status, and topography/ meteorology in their living and working locations. Nevertheless, modeling exposure both indoors and outdoors is important: CitationLong et al. (2000) suggest that some types of PM generated indoors can be hazardous. For example, data from CitationNaumova et al. (2002) and CitationWeisel et al. (2005) show lower levels of soil dust, SO4 2 −, and NO3 − indoors than outdoors, but much higher organic PM concentrations. However, the question still remains as to why adverse health effects consistently correlate with ambient data collected at a central monitoring location.
FIG. 7 Percentage of the population at different locations as a function of time on a typical workday, presented in the form of a stacked vertical chart. Data are taken from CitationOtt (1995) and pertain to California. Printed with permission from J. Exposure Analysis and Environ. Epidemiol. 5:449–472, 1995. Copyright 1995, Macmillan Publishers Ltd.
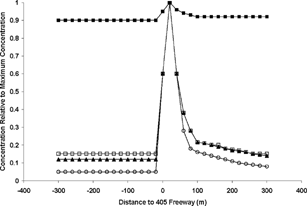
People also may be exposed to excessive levels of PM while in traffic. CitationZhu et al. (2002a) have shown that the airborne concentrations of CO, EC, and particle number are much greater at a site on a Los Angeles freeway than a short distance away (). Consistent with this result, CitationSioutas (2003) shows that EC concentrations in vehicles in LA traffic are 10–30 times greater than the urban background. He also reports ultrafine PM number concentrations as high as a half million counts per cm3 air in traffic, suggesting that a 24-hour exposure of ultrafine PM may be dominated by the fraction of time commuters spend driving. Exposures to ultrafine particles may be especially high in winter, when mixing heights are lower and dispersion is poorer, both of which can result in higher concentrations of ultrafine particles. Rapid particle growth and turbulent mixing in summer result in much lower number concentrations, with the possible exception of exposures near significant sources.
FIG. 8 Airborne concentrations relative to the maximum concentration for several species as a function of distance from a major freeway (CitationZhu et al. 2002a). Upwind is to the left of 0, while downwind is to the right. The symbols refer to the following species: black squares (PM), white squares (particle number), black triangles (black carbon), and white circles (carbon monoxide). Reprinted with permission from the Air and Waste Management Association.
Understanding the key factors influencing human exposure is necessary if we are to develop successful control strategies to reduce health impacts of PM. This will require source-receptor modeling to identify specific source impacts at receptors, in conjunction with state-of-the-art personal and ambient monitoring. For example, new developments in continuous monitors have improved the ability to identify sources though receptor-modeling tools. Identifying the roles of outdoor and indoor aerosols in total personal exposure also is important, as well as quantifying emissions from indoor sources and understanding atmospheric processes indoors.
E. Who Are the Susceptible Subgroups Affected by PM? What Host Characteristics Underlie Their Susceptibilities?
Based on associations developed through epidemiology studies, people exposed to a high ambient PM concentration appear to have a range of responses: most people show no clinical effects, others may become mildly or even seriously ill, and a few may die. The factors responsible for these striking differences in susceptibility are not well understood. However, people weakened by illnesses such as cardiovascular disease, asthma, or diabetes may be more susceptible, as well as people with nutritional deficiencies (e.g., CitationCosta 2003). Furthermore, PM10 epidemiologic data summarized by CitationSchwartz (2003) suggest that susceptibilities vary with race (whites are more susceptible than blacks), gender (females are more susceptible than males), and age (the elderly and the very young are more susceptible than young adults). Different susceptible groups have been identified in different studies, and even within each group there is wide variation in susceptibility.
One way to approach susceptibility is in terms of dosimetry considerations, that is, identifying factors causing the dose of PM to be higher in certain individuals (CitationBennett 2003). Some people with chronic obstructive pulmonary disease are known to have higher total deposited doses of particles in their lungs. They also have more uneven deposition, which produces local regions of much higher dose than that seen in normal people. Individuals whose lungs have impaired clearance functions, that is, reduction in the activity of the mucous membrane that clears particles deposited on the airway walls, may be more susceptible to the effects of deposited particles. CitationCassee (2003) notes that people who breathe mainly through their mouths rather than their noses can receive higher particle doses to their bronchial and alveolar airways.
Genetic factors also can be responsible for significant differences in susceptibility. CitationKleeberger (2003) summarizes experiments underway using animal models to identify genetic factors. Some diseases that increase susceptibility can be transmitted genetically, and laboratory models can be used in certain cases to identify the genes associated with these diseases. The recent availability of a large variety of genetically engineered rodent models permits valuable studies of both mechanisms of PM action and factors that modify susceptibility.
Toxicological studies can provide information on factors related to susceptibility, but as of yet such research has focused on the effects of PM on animals that are old or have chemically damaged lungs (CitationCassee 2003). Nevertheless, such studies can be crucial in understanding the specific damage done by inhaled particles. For example, CitationFrampton (2003) summarizes several pathways by which PM can cause damage to cells in the lung as well as to other tissues in the heart, liver, bone marrow, and brain.
Much remains to be studied on susceptibility to PM. For the present, only broad subgroups of the population can be characterized as most sensitive. The underlying reasons why some individuals suffer adverse effects and others do not are, in most cases, still unknown.
F. What Relations Exist between PM and Human Welfare?
Two important aspects of human welfare in the context of PM are climate change and visibility (i.e., the ability to see an object in the distance). The effects of PM on climate change are complex, with both direct and indirect effects. Direct effects involve scattering and absorption of the sun's energy by atmospheric PM. In some cases, the presence of particles may increase the reflectance of solar energy back to space above the reflectance expected based on the earth's normal albedo. This increased reflectance results in a net cooling of the earth. In other cases, the particles may increase the absorption of radiation, resulting in a net warming effect. Factors such as the angle of the sun and the particle size, shape, and composition determine whether the direct effect causes cooling or warming. For example, ammonium sulfate particles have high reflectance and hence cause cooling, while EC has high absorption and is therefore believed to result in warming. In fact, EC may be the second most important anthropogenic atmospheric constituent contributing to global warming, after CO2 (CitationJacobson 2002; CitationHansen and Sato 2001).
The indirect effects relate to changes in cloud cover caused by particles serving as cloud condensation nuclei. A greater fraction of cloud cover means a greater reflectivity of solar energy back to space, resulting in cooling. The characteristics of the clouds themselves also can be affected by PM. CitationAdams (2003) has shown that formation of clouds in regions of high PM concentration results in large numbers of tiny cloud droplets, yielding brighter clouds that persist for longer time periods. Formation of clouds in clean air tends to produce smaller numbers of large droplets, resulting in clouds that are less reflective than those with smaller droplets. Hygroscopic particles such as ammonium sulfate are efficient cloud condensation nuclei and can have a significant indirect effect.
Visibility decreases when airborne particles scatter light, thereby reducing the contrast in light intensity between a distant object and the background sky. The reduction can be striking: one can see objects 200 kilometers away or more in clean, dry air, but polluted air can restrict visibility to less than a kilometer. CitationMalm (2003) reports that reduced visual range can have a marked influence on the psychological well being of people, increasing stress and degrading the enjoyment of outdoor leisure activities.
Some of the same constituents of PM that affect climate change also affect visibility. Elemental carbon is an example, participating in atmospheric warming as well as visibility reduction due to light absorption. Another example is ammonium sulfate, which can scatter light back into space, so that energy is lost from the atmosphere resulting in a cooling effect; this same scattering also reduces visibility. Thus there is a close link between these two different phenomena.
Extensive visibility studies have been conducted in the western states, where terrain features and dry air make even slight degradation noticeable. CitationMalm (2003) reports that particles reducing visibility in the rural West are primarily ammonium sulfate, ammonium nitrate, organic compounds, soil, and coarse dust particles. The hygroscopicity of each type of particle, i.e., the tendency to grow by uptake of water in high humidity conditions, affects the light scattering and hence the visibility. It is necessary to account for changes in relative humidity to which the particles have been previously exposed: particles in dry air that formerly experienced high humidity may be different from particles of the same composition in dry air that have never been in a humid atmosphere (CitationPandis 2004, pp. 3–7 and 3–8).
G. What Relations Exist between the PM Problem, Other Air Quality Problems, and Issues Such as Energy Use and Economic Development?
Several studies have examined which pollutants have increasing concentrations when hospital admissions for cardiac and respiratory problems increase. In general, these studies show correlations between hospital admissions and daily variations in measures of PM mass, particle number, or certain components of PM, as well as correlations with pollutant gases such as SO2, O3, and CO. However, the results are not consistent across studies, suggesting that there may be complex interactions among various pollutants that affect health, or that non-PM factors, which vary with PM levels, are causal (CitationValberg and Watson 1998).
In addition to global climate change discussed above, local climate also is linked to PM. For example, high elemental carbon concentrations in some Asian cities affect the hydrologic cycle due to absorption of the sun's energy (CitationINDOEX 2003). Reducing airborne concentrations of this species can allow precipitation to become closer to normal in addition to potentially reducing health effects.
Production of electricity is a significant anthropogenic source of PM2.5 (). There is currently a complex regulatory schedule for reducing emissions of SOx, NOx, and Hg from power plants throughout the U.S. Once these reductions are achieved, airborne PM should be reduced significantly—expected in most cases to be below the NAAQS. However, the residual PM will have a greater fraction of carbon, as the regional strategies for reducing SO2 and NOx do not address this component. EPA recognizes the need for a robust strategy to control all of the major PM2.5 components; while they are leading the regional program with a focus on SOx and NOx emissions from stationary sources, there are now significant new national rules for the major anthropogenic carbonaceous sources, namely on-road and off-road diesel engines (CitationBachmann 2003).
Future efforts will most likely integrate air quality management into broader programs, such as urban planning, energy management, land use, and mitigation of global climate change. presents a tentative schedule showing that PM2.5 attainment must be achieved in some regions as early as 2010; the EPA review of the PM2.5 standard scheduled for that year is likely to bring new issues to bear such as whether enforcement should be based on shorter averaging times, e.g., 1-hr, employing continuous monitors (CitationBachmann 2003).
TABLE 2 U.S. EPA tentative regulatory schedule for O3 and PM2.5 (CitationBachmann 2003)
H. How Can We Evaluate Our Progress in Reducing PM Health and Welfare Effects? How Can We Incorporate This Information in Refining Strategies for Reducing Exposure?
Measures of health effects for evaluating progress generally include reduction in death rates, hospital admissions, or other health endpoints as a result of decreasing PM2.5 concentrations. There have been several “studies of opportunity” where health effects in a population are monitored before and after major changes in emissions, due to fuel use changes, a labor strike, or new regulations. As shown earlier in , there is a correlation between black smoke and cardiovascular disease: the original data show that a 70% drop in black smoke concentration results in a 10% reduction in this disease (CitationClancy et al. 2002). CitationPope (1992) reports a 4–5% decrease in mortality when airborne PM10 fell by 50 μ g/m3 due to a steel mill strike in Utah. Deaths from all causes fell 1.8–2.8% in Hong Kong after 1990 when a regulation went into effect limiting the sulfur content of fuel to 0.5% (CitationHedley et al. 2002). Such studies are confounded by concurrent changes in lifestyle, which have also improved public health.
One of the main challenges in evaluating progress is having consistent data on PM2.5 and its effects over a long period of time—generally several years. With the assumption that decreasing PM concentrations will reduce adverse health effects, we can evaluate progress by noting lower PM levels as a result of decreasing emissions. Some of the most detailed studies have been conducted in the South Coast Air Basin in California, where automotive emissions have been decreasing for the past two decades. CitationBlanchard (2003) reports data for Azusa, California, showing that total suspended particles decreased an average of 2.0 μ g/m3 per year during 1980–2000, PM10 decreased an average of 1.6 μ g/m3 per year during 1985–2000, and PM2.5 decreased an average of 1.2 μ g/m3 per year during 1988–2000. Similarly, SO4 2 − in eastern U.S. has decreased by almost 30% from 1989–2000, although NO3 − at the same sites has been steady (CitationBlanchard 2004). The lack of reductions in ambient nitrate may be due to the more limited reductions in NOx relative to SO2, a greater increase in NOx emissions from, for example, a greater number of motor vehicles, or due to nitrate substitution, that is, as sulfate concentrations decline, more ammonia is available to react with nitric acid and form aerosol nitrate.
Despite the wealth of data available, CitationMauderly (2004) observes that we still do not know how much of the total health burden in the general population is due to PM exposure. Although our knowledge is advancing, there is still considerable uncertainty about the level of health risk per unit of PM and the relative roles of PM and co-pollutants. Similarly, although many PM characteristics have been shown to be important in laboratory studies our lack of careful comparisons of composition-dose-response relationships leaves considerable uncertainty regarding the roles of size and chemical composition (CitationU.S. EPA 2004d). Making progress on these fronts will require more complete records of health measures and ambient concentrations over longer periods of time.
CONCLUSIONS
Reducing the health (and welfare) effects associated with ambient PM is not a simple undertaking. It involves understanding not only the effects of PM, but also the linkages between PM (or precursors) emitted from sources and how that PM makes its way through the air and into the human body. Each step along the way between source and health effect is complicated and makes it difficult to link the observed effect back to the specific source or even source type. For example, roughly half the total global emissions of PM2.5 and precursor species (∼ 500 Tg/year) are emitted from anthropogenic sources with the other half from natural sources. The distribution of those emissions in air and resulting PM varies greatly in size and composition, and thus, PM2.5 concentrations vary widely over space and time.
The health effects of PM are thought to be strongly associated with particle size, composition, and concentration, even though relative risk estimates indicate that the risk per unit PM mass falls within a limited range of values for these parameters. As well, a combination of species and daily variations in PM mass and composition are believed to contribute to the toxicity of the particulate matter air pollution. Furthermore, measuring the relevant parameters and quantifying the health effects are extremely challenging, as numerous external factors, including meteorology and socioeconomic aspects of the human lifestyle, strongly affect human morbidity and mortality. Identifying and correcting for the effects of confounding factors remain a difficult task.
People are exposed to PM2.5 from many sources as they go about their daily activities, spending time in their homes, at work, in recreation, and in traveling. This is further complicated by the knowledge that some individuals or segments of the population are more susceptible to PM exposures, due to factors such as respiratory habits (e.g., mouth breathing versus nose breathing), pre-existing diseases, or genetics. Given all these complications, it is interesting to note that fixed monitoring stations at a central urban site seem to provide reasonable estimates of total exposure of an individual to PM2.5 mass and some secondary species like SO4 2 −. However, determining concentrations of other species such as metals and organic compounds may require measurements in each microenvironment.
The PM pollution problem is still further complicated by human welfare and socioeconomic effects. Two important PM welfare effects include climate change and visibility degradation. High PM levels can influence the way light and heat energy are transmitted though the atmosphere, and thus impact the earth's radiation balance that controls climate. Particles also absorb and scatter light, resulting in hazy skies and poor visibility. Some species, like sulfate and elemental carbon, influence both phenomena as well as likely health effects. The PM problem also is closely linked to issues such as production of energy and economic development. Energy production stimulates the economy but also can result in higher PM2.5 levels, making consideration of tradeoffs important. Future efforts are likely to integrate air quality management into urban planning and development as well as efforts to mitigate global climate change.
Finally, evaluating progress in reducing the effects of PM requires data collection over long time periods and detailed statistical analyses. Several programs are underway to monitor atmospheric PM and to track changes in emissions, ambient concentrations, and effects as well as to communicate the data to the broader scientific community.
ACKNOWLEDGMENTS
The authors express their sincere thanks to the sponsors of the 2003 conference Particulate Matter: Atmospheric Sciences, Exposure and the Fourth Colloquium on PM and Human Health: U.S. Environmental Protection Agency (primary sponsor), American Association for Aerosol Research (host association for the conference), Air and Waste Management Association, American Chemistry Council, American Petroleum Institute, Center for Occupational and Environmental Health at UC Irvine, California Energy Commission, Department of Energy—National Energy Technology Laboratory, EPRI, Ford Motor Company, Health Effects Institute, International Society for Aerosols in Medicine, Mid-Atlantic Region Air Management Association, NOAA Aerometry Laboratory, NARSTO, National Institute of Environmental Health Sciences, National Science Foundation, New York State Energy Research and Development Authority, the South Coast Air Quality Management District, and Southern Company.
The support of the exhibitors is also appreciated: BGI, Grimm Technologies Inc., Magee Scientific, Met One Instruments, MSP Corporation, RJ Lee Group, Rupprecht and Patashnick, SKC Inc., Taylor and Francis, Thermo-Electron Corporation, TSI, and URG Corporation.
The Organizing and Science Advisory Committees greatly assisted the conference in many ways. The AAAR staff, especially Ann Marie Smith, Elizabeth McDannell, and Celeste McNair, did an excellent job in handling the logistics of the conference. Comments on the manuscript were kindly provided by John Bachmann, Charlie Blanchard, Morton Lippmann, Joe Mauderly, Andy Miller, Gary Norris, Arden Pope, Costas Sioutas, and Ron Williams.
The United States Environmental Protection Agency through its Office of Research and Development partially funded and collaborated in the research described here under assistance agreement No. X3-83091401-0 to the American Association for Aerosol Research, Mt. Laurel, New Jersey. It has been subjected to Agency review and approved for publication. Mention of trade names or commercial products does not constitute an endorsement of recommendation for use.
Notes
Particulate matter refers to small particles consisting of solid or liquid droplets suspended in air. EPA currently regulates particles in two size ranges to help protect public health. These include PM10 and PM2.5. PM10 refers to particles less than 10 μ m in aerodynamic diameter (about 1/10th the diameter of a human hair), while PM2.5 (fine particles) refers to particles less than 2.5 μ m in aerodynamic diameter. Because PM10 includes PM2.5, EPA is in the process of promulgating new standards for coarse particles (PMc). This refers to particles with aerodynamic diameters between 2.5 μ m and 10 μ m.
REFERENCES
- Adams , P. 2003 . Presentation at the Conference “Particulate Matter: Atmospheric Sciences, Exposure, and the Fourth Colloquium on PM and Human Health,” . March 31–April 4 2003 , Pittsburgh, PA.
- Albritton , D. L. and Greenbaum , D. S. 22–23 July 1998 . Atmospheric Observations: Helping to Build the Scientific Basis for Decisions Related to Airborne Particulate Matter. Report of the PM Measurements Research Workshop , 22–23 July , Chapel Hill, NC : Published by the Health Effects Institute and the Aeronomy Laboratory of the National Oceanic and Atmospheric Administration . available from the Health Effects Institute, Cambridge, MA
- Anderson , N. J. , Strader , R. and Davidson , C. I. 2003 . Airborne Reduced Nitrogen: Ammonia Emissions from Agriculture and Other Sources . Environ. Internat. , 29 : 277 – 286 .
- Bachmann , J. D. 2003 . Presentation at the Conference “Particulate Matter: Atmospheric Sciences, Exposure, and the Fourth Colloquium on PM and Human Health,” . March 31–April 4 2003 , Pittsburgh, PA.
- Bennett , W. 2003 . Presentation at the Conference “Particulate Matter: Atmospheric Sciences, Exposure, and the Fourth Colloquium on PM and Human Health,” . March 31–April 4 2003 , Pittsburgh, PA.
- Blanchard , C. L. 2003 . Presentation at the Conference “Particulate Matter: Atmospheric Sciences, Exposure, and the Fourth Colloquium on PM and Human Health,” . March 31–April 4 2003 , Pittsburgh, PA.
- Blanchard , C. L. 2004 . “ Spatial and Temporal Characterization of Particulate Matter ” . In Particulate Matter Science for Policy Makers: A NARSTO Assessment , Edited by: McMurry , P. , Shepherd , M. and Vickery , J. United Kingdom : Published by Cambridge University Press . Chapter 6, ISBN is 0521842875. (EPRI report version available at http://www.cgenv.com/Narsto link to PM Science and Assessment)
- Buringh , E. 2003 . Presentation at the Conference “Particulate Matter: Atmospheric Sciences, Exposure, and the Fourth Colloquium on PM and Human Health,” . March 31–April 4 2003 , Pittsburgh, PA.
- Cabada , J. C. , Rees , S. , Takahama , S. , Khlystov , A. , Pandis , S. N. , Davidson , C. I. and Robinson , A. L. 2004 . Mass Size Distributions and Size Resolved Chemical Composition of Fine Particulate Matter at the Pittsburgh Supersite . Atmos. Environ. , 38 ( 20 ) : 3127 – 3141 . [CROSSREF]
- Cassee , F. 2003 . Presentation at the Conference “Particulate Matter: Atmospheric Sciences, Exposure, and the Fourth Colloquium on PM and Human Health,” . March 31–April 4 2003 , Pittsburgh, PA.
- Clancy , L. , Goodman , P. , Sinclair , H. and Dockery , D. W. 2002 . Effects of Air Pollution Control on Death Rates in Dublin, Ireland: An Intervention Study . Lancet. , 360 : 1210 – 1214 . [PUBMED] [INFOTRIEVE] [CROSSREF]
- Costa , D. 2003 . Presentation at the Conference “Particulate Matter: Atmospheric Sciences, Exposure, and the Fourth Colloquium on PM and Human Health,” . March 31–April 4 2003 , Pittsburgh, PA.
- Dockery , D. W. , Pope , C. A. III , Xu , X. , Spengler , J. D. , Ware , J. H. , Fay , M. E. , Ferris , B. G. and Speizer , F. E. 1993 . Mortality Risks of Air Pollution: A Prospective Cohort Study . New Engl. J. Med. , 329 : 1753 – 1759 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Dutkiewicz , V. A. , Quershi , S. , Khan , A. , Ferraro , V. , Schwab , J. , Demerjian , K. and Husain , L. 2004 . Sources of Fine Particulate Sulfate in New York . Atmos. Environ. , 38 ( 20 ) : 3179 – 3189 . [CROSSREF]
- Frampton , M. 2003 . Presentation at the Conference “Particulate Matter: Atmospheric Sciences, Exposure, and the Fourth Colloquium on PM and Human Health,” . March 31–April 4 2003 , Pittsburgh, PA.
- Grahame , T. J. and Hidy , G. M. 2004 . Using Factor Analysis to Attribute Health Impacts to Particulate Pollution Sources . Inhal. Toxicol. , 16 ( S1 ) : 143 – 152 . [PUBMED] [INFOTRIEVE] [CROSSREF]
- Griffin , R. J. , Cocker , D. R. III , Flagan , R. C. and Seinfeld , J. H. 1999 . Organic Aerosol Formation from Oxidation of Biogenic Hydrocarbons . J. Geophys. Res. , 104 : 3555 – 3567 . [CSA] [CROSSREF]
- Hansen , J. E. and Sato , M. 2001 . Trends of Measured Climate Forcing Agents . Proc. Natl. Acad. Sci. , 98 : 14,778 – 14,783 .
- Harrison , R. M. and Yin , J. 2000 . Particulate Matter in the Atmosphere: Which Particle Properties are Important for its Effects on Health? . Sci. Total Environ. , 249 : 85 – 101 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Hedley , A. J. , Wong , C. , Thach , T. , Stefan , M. , Lam , T. and Anderson , H. R. 2002 . Cardiorespiratory and All-causes Mortality after Restrictions on Sulphur Content of Fuel in Hong Kong: An Intervention Study . Lancet. , 360 : 1646 – 1652 . [PUBMED] [INFOTRIEVE] [CROSSREF]
- Hidy , G. M. and Pace , T. 2004 . “ Emissions Characterization ” . In Particulate Matter Science for Policy Makers: A NARSTO Assessment , Edited by: McMurry , P. , Shepherd , M. and Vickery , J. United Kingdom : Cambridge University Press . Chapter 4, ISBN 0521842875. (EPRI report version available at http://www.cgenv.com/Narsto link to PM Science and Assessment)
- Hoek , G. , Brunekreef , B. , Goldbohm , S. , Fischer , P. and van den Brandt , P. A. 2002 . Association between Mortality and Indicators of Traffic-Related Air Pollution in the Netherlands: A Cohort Study . Lancet. , 360 : 1203 – 1209 . [PUBMED] [INFOTRIEVE] [CROSSREF]
- Hopke , P. K. 2003 . Recent Developments in Receptor Modeling . J. Chemometrics , 17 : 255 – 265 . [CSA] [CROSSREF]
- INDOEX . 2003 . Indian Ocean Experiment, An International Field Experiment in the Indian Ocean , INDOEX . Information available at http://www-indoex.ucsd.edu
- Jacobson , M. Z. 2000 . A Physically Based Treatment of Elemental Carbon Optics: Implications for Global Direct Forcing of Aerosols . Geophys. Res. Lett. , 27 : 217 – 220 . [CSA] [CROSSREF]
- Jacobson , M. Z. 2002 . Control of Fossil-Fuel Particulate Black Carbon and Organic Matter, Possibly the Most Effective Method of Slowing Global Warming . J. Geophys. Res. , 107 ( D19 ) : 4410 [CROSSREF]
- Kamens , D. 2003 . Personal communication . Cited by Richard Scheffe
- Kiehl , J. T. and Rodhe , H. 1995 . “ Modelling Geographical and Seasonal Forcing Due to Aerosols ” . In Aerosol Forcing of Climate, , Edited by: Charlson , R. J. and Heintzenberg , J. pp. 281 – 296 . John Wiley and Sons .
- Kleeberger , S. 2003 . Presentation at the Conference “Particulate Matter: Atmospheric Sciences, Exposure, and the Fourth Colloquium on PM and Human Health,” . March 31–April 4 2003 , Pittsburgh, PA.
- Klepeis , N. E. , Nelson , W. C. , Ott , W. R. , Robinson , J. , Tsang , A. M. , Switzer , P. , Behar , J. V. , Hern , S. and Engelmann , W. 2001 . The National Human Activity Pattern Survey (NHAPS): A Resource for Assessing Exposure to Environmental Pollutants . J. Expos. Analysis and Environ. Epidem. , 11 ( 3 ) : 231 – 252 . [CSA] [CROSSREF]
- Lemire , K. R. , Allen , D. T. , Klouda , G. A. and Lewis , C. W. 2002 . Fine Particulate Matter Source Attribution for Southeast Texas Using 14C/13C Ratios . J. Geophys. Res. , 107 ( D22 ) : 4613 [CROSSREF]
- Lipsky , E. , Stanier , C. O. , Pandis , S. N. and Robinson , A. L. 2002 . Effects of Sampling Conditions on the Size Distribution of Fine Particulate Matter Emitted from a Pilot-scale Pulverized-coal Combustor . Energy and Fuels , 16 ( 2 ) : 302 – 310 . [CROSSREF]
- Long , C. M. , Suh , H. H. , Kobzik , L. , Catalano , P. J. , Ning , Y. Y. and Koutrakis , P. 2000 . A Pilot Investigation of the Relative Toxicity of Indoor and Outdoor Fine Particles: In Vitro Effects of Endotoxin and Other Particulate Properties . Environ. Health Perspect. , 109 : 1019 – 1026 . [CSA]
- Malm , W. C. 2003 . “ Fundamentals of Visibility ” . In Handbook of Weather, Climate, and Water: Atmospheric Chemistry, Hydrology, and Societal Impacts , Edited by: Potter , T. D. , T. D. and Colman , B. R. pp. 285 – 329 . New York : Wiley Interscience, John Wiley & Sons . Chapter 16
- Mauderly , J. L. 2004 . “ Health Effects of Complex Mixtures: Where Are We and Where Do We Need to Be? ” . In Effects of Air Contaminants on the Respiratory Tract—Interpretations from Molecules to Meta Analysis, , pp. 43 – 52 . INIS Monographs, Fraunhofer IRB Verlag .
- Miller , A. 2003 . Presentation at the Conference “Particulate Matter: Atmospheric Sciences, Exposure, and the Fourth Colloquium on PM and Human Health,” . March 31–April 4 2003 , Pittsburgh, PA.
- Modey , W. K. , Eatough , D. J. , Anderson , R. R. , Martello , D. V. , Takahama , S. , Lucas , L. J. and Davidson , C. I. 2004 . Ambient Fine Particulate Concentrations and Chemical Composition at Two Sampling Sites in Metropolitan Pittsburgh: A 2001 Intensive Summer Study . Atmos. Environ. , 38 : 3165 – 3178 . [CROSSREF]
- National Research Council . 1998, 1999, 2001, 2004 . Research Priorities for Airborne Particulate Matter I, II, III, IV , Washington, D.C. : National Academy Press .
- Naumova , Y. Y. , Toten , L. A. , Eisenreich , S. J. , Turpin , B. J. , Colome , S. , Morandi , M. , Weisel , C. , Stock , T. , Winer , A. , Alimokhtari , S. , Kwon , J. , Maberti , S. , Shendell , D. and Wall , S. 2002 . Polycyclic Aromatic Hydrocarbons in the Indoor and Outdoor Air of Three Cities in the U.S.: Results from the RIOPA Study . Environ. Sci. Technol. , 36 : 2552 – 2559 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Ott , W. R. 1995 . Human Exposure Assessment: The Birth of a New Science . J. Exposure Analysis and Environ. Epidemiol. , 5 : 449 – 472 . [CSA]
- Pandis , S. N. 2004 . “ Atmospheric Aerosol Processes ” . In Particulate Matter Science for Policy Makers: A NARSTO Assessment , Edited by: McMurry , P. , Shepherd , M. and Vickery , J. Cambridge, , UK : Cambridge University Press . Chapter 3, (EPRI report version available at http://www.cgenv.com/Narsto link to PM Science and Assessment)
- Phalen , R. F. 2002 . The Particulate Air Pollution Controversy: A Case Study and Lessons Learned , pp. 81 – 94 . Boston, MA : Kluwer Academic Publishers .
- Pope , C. A. III . 2000 . What Do Epidemiologic Findings Tell Us about Health Effects of Environmental Aerosols? . J. Aerosols in Medicine , 13 : 335 – 354 . [CSA]
- Pope , C. A. III , Schwartz , J. and Ransom , M. R. 1992 . Daily Mortality and PM10 Pollution in Utah Valley . Arch. Environ. Health , 47 : 211 – 217 . [PUBMED] [INFOTRIEVE] [CSA]
- Pope , I. C. A. II . 2003 . Presentation at the Conference “Particulate Matter: Atmospheric Sciences, Exposure, and the Fourth Colloquium on PM and Human Health,” . March 31–April 4 2003 , Pittsburgh, PA.
- Samet , J. M. , Dominici , F. , Zeger , S. L. , Schwartz , J. and Dockery , D. W. May 2000 . “ The National Morbidity, Mortality, and Air Pollution Study, Part 1: Methods and Methodologic Issues ” . In Research Report 94, May , May , Boston, MA : Health Effects Institute .
- Scheffe , R. 2003 . Presentation at the Conference “Particulate Matter: Atmospheric Sciences, Exposure, and the Fourth Colloquium on PM and Human Health,” . March 31–April 4 2003 , Pittsburgh, PA.
- Schwartz , J. , Dockery , D. W. and Neas , L. M. 1996 . Is Daily Mortality Associated Specifically with Fine Particles? . J. Air Waste Manag. Assoc. , 46 : 927 – 939 . [PUBMED] [INFOTRIEVE] [CSA]
- Schwartz , J. 2003 . Presentation at the Conference “Particulate Matter: Atmospheric Sciences, Exposure, and the Fourth Colloquium on PM and Human Health,” . March 31–April 4 2003 , Pittsburgh, PA.
- Seinfeld , J. H. and Pandis , S. 1998 . Atmospheric Chemistry and Physics , New York : John Wiley and Sons .
- Sioutas , C. 2003 . Presentation at the Conference “Particulate Matter: Atmospheric Sciences, Exposure, and the Fourth Colloquium on PM and Human Health,” . March 31–April 4 2003 , Pittsburgh, PA. Information taken from Zhu et al. (2002a), Zhu et al. (2002b), and Zhu et al. 2004.
- Stanier , C. O. , Khlystov , A. Y. and Pandis , S. N. 2004 . Nucleation Events during the Pittsburgh Air Quality Study: Description and Relation to Key Meteorological, Gas Phase, and Aerosol Parameters . Aerosol Sci. Technol. , 38 ( S1 ) : 253 – 264 . [CSA] [CROSSREF]
- Suh , H. 2003 . Presentation at the Conference “Particulate Matter: Atmospheric Sciences, Exposure, and the Fourth Colloquium on PM and Human Health,” . March 31–April 4 2003 , Pittsburgh, PA.
- Takahama , S. , Wittig , A. E. , Vayenas , D. , Davidson , C. I. and Pandis , S. N. 2004 . Modeling the Diurnal Variation of Nitrate during the Pittsburgh Air Quality Study . J. Geophys. Res. , 109 : D16S06 [CROSSREF]
- Tang , W. , Raymond , T. , Wittig , A. E. , Davidson , C. I. , Pandis , S. N. , Robinson , A. L. and Crist , K. 2004 . Spatial Variations in PM2. 5 Concentrations in the Pittsburgh Air Quality Study . Aerosol Sci. Technol. , 38 ( S2 ) : 80 – 90 . [CROSSREF]
- Tanner , R. L. , Parkhurst , W. J. , Valente , M. L. and Phillips , W. D. 2004 . Regional Composition of PM2. 5 Aerosols Measured at Urban, Rural and Background Sites in the Tennessee Valley . Atmos. Environ. , 38 : 3143 – 3153 . [CROSSREF]
- Thurston , G. D. , Ito , K. , Hayes , C. G. , Bates , D. V. and Lippman , M. 1994 . Respiratory Hospital Admissions and Summertime Haze Air Pollution in Toronto, Ontario: Consideration of the Role of Acid Aerosols . Environ. Res. , 65 : 271 – 290 . [PUBMED] [INFOTRIEVE] [CROSSREF]
- Turpin , B. 2003 . Presentation at the Conference “Particulate Matter: Atmospheric Sciences, Exposure, and the Fourth Colloquium on PM and Human Health,” . March 31–April 4 2003 , Pittsburgh, PA.
- U.S. EPA . January 15 1998 . Particulate Matter Research Needs for Human Health Risk Assessment to Support Future Reviews of the National Ambient Air Quality Standards for Particulate Matter , January 15 , Research Triangle Park, NC : Office of Research and Development . EPA Report EPA/600/R-97/132F
- U.S. EPA . 1999 . The Benefits and Costs of the Clean Air Act, 1990 to 2010 , Washington, DC : Office of Air and Radiation . November EPA Report EPA/410-R-99-001
- U.S. EPA . 2003 . Exposure of High Risk Sub-Populations to Particles: Final Report (APM-21) , Edited by: Wallace , L. A. , Williams , R. W. , Rodes , C. , Liu , L. J. S. and Brown , K. Research Triangle Park, NC : Environmental Protection Agency . Report EPA/600/R-03/145
- U.S. EPA . 1997 . Part II, Environmental Protection Agency, 40 CFR Part 50, National Ambient Air Quality Standards for Particulate Matter; Final Rule . Federal Register , 62 ( 138 ) July 18 40 CFR 50. Available at http://www.epa.gov/ttnamti1/files/cfr/recent/pmnaaqs.pdf
- U.S. EPA . April 2004b . The National Ambient Air Monitoring Strategy, , April , Research Triangle Park, NC : Office of Air Quality Planning and Standards . 2004, Available at http://www. epa.gov/ttn/amtic/monstratdoc.html
- U.S. EPA . 2004c . Review of the National Ambient Air Quality Standards for Particulate Matter: Policy Assessment of Scientific and Technical Information, , Research Triangle Park, NC : Office of Air Quality Planning and Standards . OAQPS Staff Paper—Second Draft, Available at http://www.epa. gov/ttn/naaqs/standards/pm/data/pm_;staff_;paper_;2nddraft.pdf
- U.S. EPA . 2004d . Air Quality Criteria for Particulate Matter. Fourth External Review Draft , Research Triangle Park, NC : Office of Research and Development . EPA Report, EPA/600/P-99/002. aD, bD
- Valberg , P. A. and Watson , A. Y. 1998 . Alternative Hypotheses Linking Outdoor Particulate Matter with Daily Morbidity and Mortality . Inhal. Toxicol. , 10 : 641 – 662 . [CROSSREF]
- Weisel , C. P. , Zhang , J. J. , Turpin , B. J. , Morandi , M. T. , Colome , S. , Stock , T. H. , Spektor , D. M. , Korn , L. , Winer , A. , Alimokhtari , S. , Kwon , J. , Mohan , K. , Harrington , R. , Giovanetti , R. , Cui , W. , Afshar , M. , Maberti , S. and Shendell , D. 2005 . Relationship of Indoor, Outdoor, and Personal Air (RIOPA) Study: Study Design, Methods and Quality Assurance/Control Results . J. Expo. Anal. Environ. Epidemiol. , 15 : 123 – 137 . [PUBMED] [INFOTRIEVE] [CROSSREF]
- Woo , K. S. , Chen , D. R. , Pui , D. Y. H. and McMurry , P. H. 2001 . Measurement of Atlanta Aerosol Size Distributions: Observations of Ultrafine Particle Events . Aerosol Sci. Technol. , 34 : 75 – 87 . [CSA]
- Zhou , L. , Kim , E. , Hopke , P. K. , Stanier , C. O. and Pandis , S. 2004a . Advanced Factor Analysis on Pittsburgh Particle Size-Distribution Data . Aerosol Sci. Technol. , 38(S1) : 118 – 132 . [CROSSREF]
- Zhou , L. , Hopke , P. K. , Paatero , P. , Ondov , J. M. , Pancras , J. P. , Pekney , N. J. and Davidson , C. I. 2004b . Advanced Factor Analysis for Multiple Time Resolution Aerosol Composition Data . Atmos. Environ. , 38 : 4909 – 4920 . [CROSSREF]
- Zhu , Y. , Hinds , W. C. , Kim , S. and Sioutas , C. 2002a . Concentration and Size Distribution of Ultrafine Particles Near a Major Highway . J. Air Waste Manag. Assoc. , 52 : 1032 – 1042 . [PUBMED] [INFOTRIEVE] [CSA]
- Zhu , Y. , Hinds , W. C. , Kim , S. , Shen , S. and Sioutas , C. 2002b . Study on Ultrafine Particles and other Vehicular Pollutants Near a Busy Highway . Atmos. Environ. , 36 : 4375 – 4383 . [CROSSREF]
- Zhu , Y. , Hinds , W. C. , Kim , S. , Shen , S. and Sioutas , C. 2004 . Seasonal Trends of Concentration and Size Distributions of Ultrafine Particles Near Major Freeways in Los Angeles . Aerosol Sci. Technol. , 38 : 5 – 13 . [CSA] [CROSSREF]