Three different probes, respectively based on aerodynamic quenching, N2 dilution, and thermophoresis, were used for particle sampling in biomass postcombustion gases from a laboratory reactor at different temperatures (from 1300 to 560°C). The different artifacts caused by the use of each probe were identified and compared. While the classical dilution sampling resulted in the formation (inside the probe) of particles comparable in size and composition to those already existing in the sampled gases, the aerodynamic quenching particle sampling (AQPS) probe always caused the nucleation of vapors into very small (15–20 nm) particles. The difference is attributed to the much higher cooling rate (∼ 108 K/s) at the entrance of the probe. The preliminary results of the thermophoretic probe also reveal significant deviations between the collected sample and the actual particle population in the sampled gas. In general, the results shown here highlight the difficulties in obtaining representative samples in this type of systems, as well as the existence of a variety of possible sampling artifacts, whose interpretation is not straightforward. The AQPS probe is found to provide better results than the other methods considered, and its use might enable the study of both the condensed and vapor phase in hot gases containing important amounts of condensable inorganic vapours.
INTRODUCTION
The formation, emission, and deposition of fine (<1 μ m) particles from the combustion of solid fuels has received considerable attention due to their intimate relation with the initiation and growth of boiler deposits, as well as with human health and environmental hazards. Experimental data on the properties of particulate emissions are available in the literature: for coal combustion see e.g. CitationMcElroy et al. (1982), CitationQuann et al. (1990), CitationNielsen et al. (2002), and for biomass combustion see e.g. CitationChristensen and Livbjerg (1996), CitationValmari et al. (1998), CitationJohansson et al. (2003). In most cases, particulate emissions from real boilers that burn different types of coal and biomass show a distinct submicronic mode. Also, theoretical models have been proposed for the fine-particle formation process for both coal and biomass combustion. See, e.g., CitationSarofim et al. (1977), CitationFlagan and Friedlander (1978) for the fundamental processes occurring in the combustion of coal; the most comprehensive model for biomass combustion, developed by CitationChristensen (1995), is described in a subsequent section.
Few experimental studies have been devoted to the detailed description of these processes in biomass combustion because of the intrinsic difficulties encountered in sampling the high-temperature mixture of gases, particles, and inorganic vapors in which the formation of the particles takes place. Because the saturation pressures of these vapors essentially vanish at low or even moderate temperatures, nucleation and/or condensation processes are expected when the temperature of the sample is reduced, which leads to some modification of the particle's properties. Therefore, reliable methods for the characterization of particles in the presence of condensable species are needed to investigate the aerosols produced in these combustion systems.
The extraction method usually employed, i.e., dilution of the sampled gases in an inert, cold gas (typically N2), is expected to assure the quenching of chemical reactions by a rapid temperature decrease accompanied by a reduction of the partial pressure of the reactants. This technique, suitable for most applications (e.g., sampling of gases or charring particles in flames), when applied to post-combustion gases, necessarily implies gas-to-particle conversion of the inorganic vapors present. This unavoidable artifact may lead to an unacceptable distortion of the actual particle population and characteristics at the sampling point.
Several examples of this bias in N2-dilution probes have been reported: CitationLind et al. (1998) sampled forest residue/willow flue gases at ∼ 820°C and ∼ 150°C, finding K2SO4 and KCl in particles at both temperatures. The KCl detected at 820°C was assumed to be a result of a sampling artifact (i.e., condensation during dilution). In a similar combustion system, CitationValmari et al. (1999) aspired gases at ∼ 840°C and diluted them after filtering with a quartz filter to induce the condensation/nucleation of the vapors contained in the gas sample. The study of the particles so formed provided information on the vapor phase at the sampling temperature (e.g., KCl was the main compound found in such particles). Finally, CitationDavis et al. (1998) reported the formation of metal nuclei by homogeneous nucleation of vapour entering the probe.
Strand et al. (Citation2003, Citation2004) specifically addressed the use of dilution probes for sampling in the presence of condensable species, and provided evidence of dramatic modifications of the particle population in some cases. They used a new dilution-probe design with dilution ratios of DR = 1:6 to 1:100 for sampling in hot gases containing KCl vapor and seeded with SiO2 particles. Low DR sampling at 780°C resulted in a distinct KCl nucleation mode, which was thought to form inside the probe; for DR > 60, this mode seemed to disappear, which the authors hypothesized was due to vapor condensation on the inner walls of the probe. These results were reproduced in a real biomass boiler, and showed Cl depletion in fine particles when high (>1:60) dilution ratios were used.
CitationJiménez and Ballester (2004) used a new probe, based on aerodynamic quenching, to collect fine particles at different temperatures along the cooling section of a drop-tube furnace. The obtained results, briefly summarized later in this paper, are reasonably consistent with the calculations of Christensen and Livbjerg (Citation1996, Citation2000) and CitationChristensen et al. (1998) for a similar case, which suggests that this probe might be a valid tool for the description of aerosol formation processes.
This paper presents some recent experiments with the new probe, and intends to yield a deeper understanding of its effect on the mix gas + particles + vapors sampled. The sampling artifacts produced by the use of this probe and a dilution probe in the same postcombustion system are presented and discussed. Finally, some preliminary experiments performed with a thermophoretic probe are described.
EXPERIMENTAL METHODS
Entrained Flow Reactor (EFR)
The combustion facility used in these experiments has been described in more detail elsewhere (CitationJiménez and Ballester 2004) and will be briefly described here. Pulverized biomass is transported pneumatically in air and injected axially into a tubular combustion chamber by means of an insulated injector. The solids feeding system consists of a rotary valve that extracts the fuel contained in a hopper, which is continuously weighed by a load cell, followed by an agitation chamber that filters out pulsations in the feed rate. The temperature along the length of the tube is controlled by a set of electrical-resistance heaters. A natural gas burner located upstream of the injection point determines the gas composition of the combustion atmosphere. The heated tube is followed by an insulated refractory tube and an air-cooled chimney. The measured gas temperature evolution with time (approximately 600 K/s from 1300°C down to ∼ 600°C, and ∼ 250 K/s afterwards) is similar to those found in real systems.
Aerodynamic-Quenching Particle Sampling (AQPS) Probe
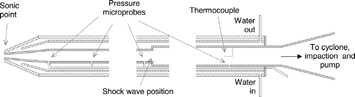
A new probe, based on aerodynamic quenching (CitationColkett et al. 1982; CitationBowman 1977), was used for submicron particle sampling (). Both temperature and pressure of the sampled gas rapidly decreases in the diverging section downstream of the probe entrance because of the supersonic flow. The inner walls are cooled so that the stagnation temperature is significantly reduced before reaching sudden expansion. The calculated “residence time” of the particles from sampling to impaction is ∼ 0.01 s, while the gas cooling rate near the probe inlet is ∼ 108 K/s. The reader is directed to the work of CitationColkett et al. (1982) and CitationJiménez and Ballester (2004) for a more detailed description of the evolution of the gas temperature and pressure along the probe.
A cyclone separator (d50 ∼1 μ m), located at the end of the probe and designed ad hoc for this system, and a preimpactor collect the particles over 0.5 μ m. The inner preimpactor pieces are coated with a felt-paper greased with resin to avoid particle re-entrainment. An orifice focuses the remaining particles into a narrow section in which a TEM grid coated with a thin film is immersed for a short time by means of a pneumatic actuator. Several TEM grid films were considered and tested. Pure, carbon coatings (continuous or in a lacy form) offer better performance in TEM observation, but they are too rigid for this application and were damaged by the impact of the particles. The flexibility of the commercial polymer film used (Formvar, EMS) reduced this problem, and was further avoided by supporting the grid on a thin metal plate, slightly greater in diameter than the grid. However, the collection of particles greater than ∼1 μm upstream of the impactor is still necessary to prevent excessive film damage. The collected particles are not uniformly distributed over the grid, but typically form a ring-shaped or circular spot on it.
Dilution Probe
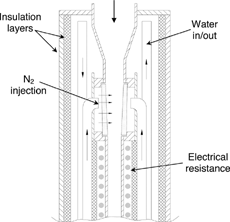
shows the main features of the dilution probe used in this study. An 18-mm diameter orifice is followed by a convergent section and a sintered bronze tube (6 mm i.d., ∼ 23 mm long) through which pure N2 is injected and mixed with the sampled gases. The sample mass-flow rate is obtained as the difference between the reading of the mass flowmeters used for the N2 and the diluted sample streams. The mixture is maintained above 120°C by means of longitudinal-resistance heaters to prevent condensation of water and to minimize particle losses by thermophoresis. The probe is protected by a water jacket and an outer mullite cover. Its total length is approximately 1 m. The tip can be located at any point of the lower part of the reactor, including the refractory piece and the chimney. The outlet is connected to a cyclone separator, with d50 ∼5 μ m for the typical flow rates in the experiments reported in this work.
Particles exiting the cyclone were collected in a Berner-type low pressure cascade impactor (BLPI, Hauke LPI 25/0,018/2) or, alternatively, deposited on a TEM grid by means of the impaction section described above. Because the sample flow rate was lower than the nominal value of the BLPI (controlled by an internal critical orifice), a suitable amount of filtered air was admitted through a T-fitting. The preimpactor and the orifice in the impaction device were modified (i.e., their i.d. was reduced) with respect to those used with the AQPS probe and according to the changes in volumetric flow and absolute pressure. The gas/particles residence time inside the probe and gas cooling rate have been estimated to be ∼ 0.25 s and 105 K/s, respectively.
Thermophoretic Sampling Probe
Thermophoretic sampling has been widely used in studies of particle formation in flames (mostly soot, but also inorganic compounds as silica or titania—see, e.g., CitationDobbins and Megaridis 1987; CitationKöylü et al. 1997), with the advantage of an almost constant collection efficiency over a sufficiently broad size range. It is usually based on the rapid immersion of a cold plate (e.g., a TEM grid) into the flame, where particles are collected by thermophoresis. Hence, this might seem a method suitable for the study of fine-particle formation in postcombustion gases. Two difficulties arise, however, when trying to apply this technique to the EFR postcombustion gases in which condensable species appear in significant amounts:
| 1. | the temperature gradient may promote condensation/ nucleation of these species, thus altering the sample properties, | ||||
| 2. | the low particle concentration imposes relatively long collection times (of the order of seconds), and a cooling system is required to avoid probe damage. | ||||
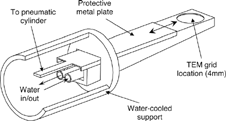
As a first attempt to perform thermophoretic sampling in this facility and application, the probe shown in has been constructed. It consists of a water-cooled, polished metal surface in which a circular (4 mm in diameter), ∼ 0.1-mm deep cavity is engraved; a TEM grid is located in this space, fixed with two, tiny drops of silver paint. A metal plate protects the grid, which is only exposed to the sampled atmosphere when the plate is removed by a pneumatic cylinder, and the sampling time is regulated by an electromagnetic valve. A 1 m long water jacket supports this assembly and allows for positioning of the probe tip parallel to the flow at any point along the lower part of the EFR.
As with the AQPS probe, several types of TEM grid films were tested. In this case, the polymer films were rapidly burnt (for exposures even shorter than 1 s), and hence discarded. The carbon film avoided this problem, and was finally employed (in continuous, i.e., nonlacy form).
Sample Analysis
The BLPI substrates were weighed before and after each experiment, and the corresponding particle size distribution (PSD) obtained by difference. The deposits were analysed by a SEM (JEOL JSM 6400) equipped with an X-ray energy-dispersive spectrometer (XEDS). A TEM-XEDS (JEM-2000FXII) system was used to analyse the morphology and composition of the particles collected on TEM grids by means of the different probes. In this case, chemical composition was restricted by the detector only to be used for elements with atomic number, Z, >12.
BRIEF DESCRIPTION OF THE FINE PARTICLE FORMATION PROCESS IN ORUJILLO COMBUSTION
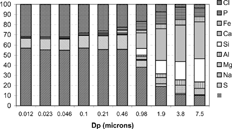
Because the aim of this work is to analyse and compare the results obtained by sampling in a stream of combustion products, it is deemed necessary to briefly describe the previous knowledge on this system. Orujillo, a residue of the olive oil production process whose extensive exploitation as fuel is recent, is burned along the EFR in realistic combustion conditions. The fuel properties have been reported elsewhere (CitationJiménez and Ballester 2005a), and indicate that orujillo is a good example of a broad group of biomasses (regarding, e.g., its alkali content). shows the elemental-mass composition of the fine particles emitted in pulverized orujillo combustion at the EFR (combustion temperature 1300°C, exit oxygen concentration 5% vol in a dry basis (db)), as determined with the BLPI (CitationJiménez and Ballester 2005a). As a reference for comparison with other results, the average K:Cl:S (i.e., major elements) atomic ratio in the fine-particle mode is 54:30:13. KCl and K2SO4, in roughly the same mass proportion, account for most of the mass of fine particles. A single fine-particle mode was observed at ∼ 200 nm. These results are almost coincident with a previous study for straw combustion in an industrial boiler published by CitationChristensen and Livbjerg (1996). The authors concluded that all the potassium is in the gas phase at the furnace exit (and so, almost no fine particles are present at high temperature). K2SO4 nucleated at ∼ 800°C and KCl condensed over these nuclei at lower temperatures. Oxidation of SO2 was identified as the limiting step in the K2SO4 formation process from KCl (gas). Pure thermodynamic equilibrium considerations led CitationJiménez and Ballester (2004) to a very similar scheme for this process, although KOH was found to be the main potassium gas compound present at high temperatures, rather than KCl. Therefore, a few aspects seem to be clear from theory, and may serve as a framework in which the results obtained with the different probes can be interpreted:
| • | No solid potassium-containing species exist above 1300°C. | ||||
| • | KCl(g) or KOH(g) react in the subsequent cooling section first to form potassium sulphate, while KCl(s) appears at lower temperatures. | ||||
| • | K2SO4 or KCl cannot exist in solid state above their corresponding fusion temperatures ∼ 1060°C and ∼ 770°C, respectively (CitationPerry 1997), because their partial pressure in the EFR flue gases is very small. | ||||
RESULTS AND DISCUSSION
Experiments with the AQPS Probe
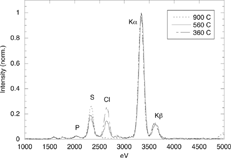
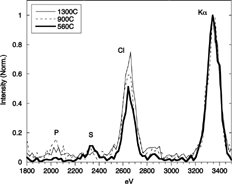
CitationJiménez and Ballester (2004) recently reported qualitative, experimental evidence of the formation sequence presented in the previous section by sampling the orujillo postcombustion gases with the AQPS probe at 1300, 900, and 560°C (and later extended to 360°C). shows the evolution of the particle composition (TEM-XEDS spectra). At 900°C a clear mode of K2SO4 particles appeared, while Cl was detected only in the particles sampled at lower temperatures, which is attributed to KCl condensation.
FIG. 5 TEM-XEDS analyses of samples collected with the AQPS probe at 900°C, 560°C, and 360°C burning orujillo. α and β refer to the Kα and Kβ lines of the potassium spectrum, respectively. Curves have been normalized by the height of the potassium (Kα) peak.
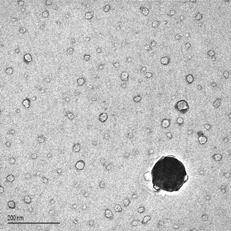
shows a TEM image of the typical particles collected with this probe when sampling is performed at ∼ 1300°C. High contrast is necessary to distinguish the ultrafine, almost electron-transparent particles seen in this picture; a dense, opaque particle corresponding to the lower tail of the distribution of coarse particles is also included for visual comparison. presents the spectrum obtained when an area ∼1 μm2 is analyzed in which only the first kind of particle is visible. K and Si are the only noticeable peaks in this spectrum. The Si peak is due to the XEDS detector internal fluorescence (CitationWilliams and Carter 1996), and appears as a consequence of a large (∼ 7500 counts) Cu signal (from the TEM parts and/or the TEM grid) at ∼ 8040 eV. The most significant aspect in the spectrum is the absence of any other peak, in particular that of chlorine at 2.62 keV.
Such results can help to ascertain the form in which potassium is bound at high temperatures. As it has been pointed out above, both KOH and KCl have been proposed by different authors, but no experimental evidence has been reported in this respect. According to the calibration of the TEM-XEDS detector with bulk KCl crystals, if KCl were present in the sample, the Cl peak height should have been ∼ 0.7 of the K peak (cf. , shown later). suggests, instead, that KOH accounts for the potassium present, and that the particles shown are formed by nucleation because of the drastic gas cooling near the tip of the probe. Very similar results have been obtained in experiments with other biomass types and combustion conditions (CitationJiménez and Ballester 2005b).
FIG. 13 TEM-XEDS group analysis of the particles collected at the outlet of the dilution probe when sampling at 1300, 900, and 560°C.
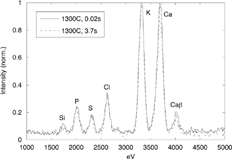
A detailed description of the evolution of condensable species and particles inside the probe would be required for identifying or discarding errors associated to sampling artifacts. However, no way to achieve such knowledge was found through theoretical or experimental approaches. As an alternative, some additional tests were carried out by modifying the configuration of the sampling system. Tubes of different lengths (0.1 to 9 m) were inserted between the cyclone outlet and the impaction device when sampling at 1300 and 900°C in order to modify the gas + particles residence time from aspiration (probe inlet) to collection. The delays introduced by these tubes are approximately 0.02, 0.07, 0.11, 0.18, 0.26, 0.63, and 3.7 s. The use of longer residence times was thought to potentially result in modifications of the particle properties and should be avoided when using the probe. However, this was precisely the effect sought with this test series, which provided results in order to gain further insight into the behavior of the AQPS probe.
shows two equivalent macroscopic (∼20 × 20 μm2) analyses of samples aspired at 1300°C with delays of 0.02 and 3.7 s. No appreciable variation is observed between both spectra, which indicates that the sample did not undergone any significant transformation between analyses. The Ca peak has been used as a reference in the TEM analysis because this element is contained in the dense, fragmentation-generated particles and therefore, it is not affected by nucleation/condensation processes. It should be noted that the fraction of Ca in the fine-particle mode finally generated is negligible (). The K, Cl, and S peaks, observed in the analyses included in , are due to the presence of sparse, amorphous 0.5–1 μm-in-diameter particles, in the samples. These particles were not observed in previous experiments under the same conditions as those reported in CitationJiménez and Ballester (2004). The particles are thought to be caused by re-entrainment of particles composed of K2SO4 and KCl, which had been deposited on the probe or cyclone walls in previous tests at lower temperatures: intensive work with this probe had been performed with orujillo and other biomasses. Nevertheless, the signal associated with these elements is even lower than that for Ca, which demonstrates the negligible importance of this spurious mode.
FIG. 8 Macroscopic (∼20 × 20 μm2) analysis of the samples collected, near the probe and 3.7 s after it at 1300°C.
Two phenomena of minor relevance have been observed in the associated TEM images, which are omitted for brevity:
| • | The amount of ultrafine particles decreases as the delay in collection increases, and totally disappear for the longest sampling line (3.7 s). Little, if any, coagulation/aggregation is observed in the remaining ultrafine particles along the sample series (mean corpuscle size varies from ∼ 14 nm just after the probe to ∼ 20 nm 0.18 s later). Therefore, the observed disappearance is attributed to particle losses by diffusion to the tube walls, which could, in fact, explain the slight increase in mean diameter. | ||||
| • | At the exit of the longest tube (3.7 s), a few disperse and long KCl crystals are observed. The global TEM analysis of this sample (included in ), shows their minor global importance because the Cl signal is not noticeably enhanced with respect to the 0.02 s delay. If KOH is assumed to be the K containing species in both the gas phase and the ultrafine particles, these KCl crystals might be formed by heterogeneous reaction of the KOH particles with HCl. | ||||
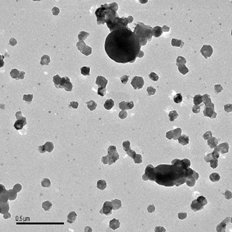
Similar results were obtained when sampling at 900°C with different collection delays. shows the particles collected at this temperature with a delay of 0.02 s. The finest particles (∼ 15 nm) contain potassium and, probably, elements with Z < 12 (see ) without any detectable Cl or S. No significant morphological changes are observed when the length of the sampling line is increased, apart from the practical disappearance of the ultrafine particles in the longer delays.
FIG. 7 TEM-XEDS analysis of the ultrafine particles collected at 1300°C and 900°C. The energies associated to chlorine and sulphur (Kα lines) are indicated for reference.
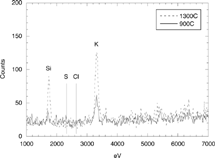
FIG. 9 TEM image of a sample collected with the AQPS at 900°C and after a delay of 0.02 s from sampling to impaction on the TEM grid.
It can be concluded, therefore, that all the potassium vapors are suddenly transformed to very small nuclei near the tip of the probe, leaving no K for subsequent reaction in the short or long term with S or Cl. The fine (∼ 150 nm) particles existing at 900°C are not affected by long residence times inside the sampling system, which further confirms that all the relevant processes take place in the first sections of the AQPS probe.
Sampling Experiments with the Dilution Probe
Several tests were performed with the dilution probe described above (DR ∼ 1:6), each using different experimental setups:
| • | the probe was connected to the BLPI and samples were collected at 900 and 560°C. | ||||
| • | the impaction section described above was used to collect particles at the outlet of the probe for study in the TEM-XEDS, and approximately the same sampling locations selected for the AQPS probe (i.e., at temperatures ∼ 1300, 900 and 560°C). | ||||
shows the elemental atomic composition for the different size bands of the particles collected with the BLPI when sampling at 900°C (a) and 560°C (b). K, Cl, and S account for over 98% of the atoms in all the cases (not including elements with Z < 10). The corresponding PSDs were essentially monomodal with mode peaks around 150 nm (a) and 200 nm (b). From this data, an approximate compound distribution versus size has been derived and shown in .
FIG. 10 Elemental atomic composition vs. size of the particles collected with the dilution probe at 900°C (a) and 560°C (b), determined with the BLPI and SEM analysis.
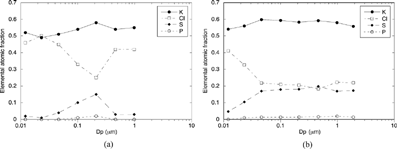
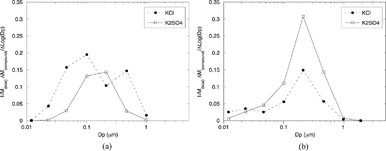
FIG. 11 Approximate KCl, K2SO4 mass distribution vs. particle size for samples collected with the dilution probe at 900°C (a) and 560°C (b), derived from the data of and the particle size distributions measured with the BLPI.
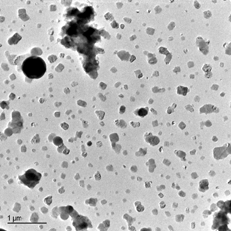
The particles collected with the dilution probe at 1300°C and impacted on the TEM grids constituted a homogeneous mode of crystalline corpuscles with a mean diameter of ∼ 150 nm; their cubic appearance (), as well as their group analysis (), clearly indicate that they are essentially composed of KCl. A similar mode of particles is found when the sampled gas temperature is ∼ 900°C. The corresponding TEM analysis is included in , which indicates that K and Cl account for most of the mass, although there occurs a small amount of S. The S/Cl ratio is further increased in the particles obtained by sampling at 560°C (). Their morphology is somewhat different as well, and presents a crystalline but less regular appearance.
These results can be interpreted in the framework of the existing knowledge on the “sampled system” summarized in a previous section:
| • | The existence of KCl in the samples collected at 1300°C and 900°C (–, ) must be entirely attributed to condensation or nucleation of inorganic vapor inside the probe because KCl cannot exist in condensed phase above its fusion temperature, i.e., ∼ 770°C (CitationPerry 1997). This is considered to be the main artifact introduced by the probe. | ||||
| • | Formation of K2SO4 inside the probe is, on the contrary, much more limited. The K2SO4 peak observed in is consistent, when considered separately, with the results obtained with the AQPS probe at 900°C. Analogously, the composition of the particles in BLPI stages 3–8 (∼ 50 nm to 2 μm) in is also similar to that shown in the TEM analysis of for the corresponding 560°C case. | ||||
| • | The gradual reduction of the Cl/S ratio in is consistent with these conclusions. The formation of K2SO4 in the flue gases detracts some gaseous K species, and therefore reduces its availability to form KCl. | ||||
| • | As mentioned above, the emitted fine particles are composed essentially of KCl and K2SO4 in the same mass proportion. Differently, the nucleation mode observed at 560°C () in the ultrafine range clearly cannot account for the difference in KCl and K2SO4 that is also noticeable in (KCl represents about half of the K2SO4 mass). This can be attributed to vapor deposition near the probe entrance, as suggested by CitationStrand et al. (2004) for another dilution probe design, or to enhanced fine-particle losses because of the higher diffusion/deposition rates of the finest mode. The lower inorganic vapor concentration at 560°C might also explain the smaller mean-particle size of the KCl particles formed when sampling at this temperature. | ||||
Preliminary Study with the Thermophoretic Sampling Probe
The tip of the thermophoretic probe was located at ∼ 900°C in the orujillo flue gases. The coated TEM grid was exposed to the gas + particles during 1 to 60 s in different tests. Water condensation was evident in the vicinity of the grid for the longest exposure, while too few particles were observed for the shortest one. Thus, the corresponding samples were discarded for these reasons.
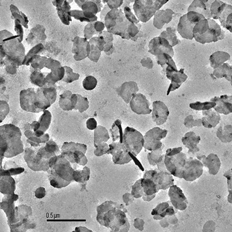
With an exposure time of 20 s, several types of spurious solids were detected. An almost continuum layer of particles like those of covered the grid, whose spectrum only displayed a peak associated to chlorine. In some cases, crystalline structures, apparently growing from the grid metal wires, were observed. HCl was discarded as the compound present because it is not solid at room temperature. Cupric chloride (copper is present in the grid) might be a possibility but the Cu peak in the spectrum cannot be adequately interpreted because of the spurious TEM signal, as explained above. Finally, sodium, which cannot be detected with this XEDS detector, is present in the flue gases in a minor proportion (CitationJiménez and Ballester 2005a), and the formation of NaCl in the probe boundary layer might explain these results. The presence of larger crystals near the grid wires is explained by enhanced vapor condensation because of the lower temperature of the wires. This presence is similar to the higher soot-deposition rates observed near the metal pieces in experiments with flames (see CitationDobbins and Megaridis 1987).
DISCUSSION
The performance of the three probes, which were tested for the sampling of aerosols in postcombustion gases was demonstrated to be very different. The lack of a detailed model on the aerosol formation process in this type of system prevents providing a definitive explanation for such differences. Nevertheless, an attempt to discuss the distinct behaviors achieved with these sampling methods, in the light of the available knowledge, is included in what follows.
The AQPS probe has been used in combustion studies, analogous to those presented here, with other biomasses with results similar to those included in the preceding sections (CitationJiménez and Ballester 2005b). Specifically, ultrafine particles have been found at high temperatures in all the cases (with particle sizes 10 to 15 nm), which further confirms that the artifact introduced by the probe is the nucleation of the vapors present in very small particles because of the sudden cooling near the probe inlet.
Furthermore, it is relevant to note that the high concentration of ∼ 150 nm, K2SO4 particles in the sampled gases at 900°C should enhance condensation to the detriment of nucleation. However, freshly nucleated particles are observed in the sample collected in this case (). Nucleation of the existing vapors as a result of the sampling process seems to be the only possible path for those vapors because gaseous K2SO4 cannot exist in considerable amounts: as soon as the sulphate is formed, it is converted to solid because of its very low saturation pressure. Also significant condensation of other K-containing compounds can be discarded by the analysis shown in .
In contrast, particles of KCl were formed by nucleation and condensation inside the dilution probe with sizes varying greatly with the sampling position. The formation of these KCl particles may be the consequence of different processes, depending on the vapor existing in the flue gases:
| • | Nucleation and condensation of KCl(g) appears as the most reasonable path if, as proposed by CitationChristensen and Livbjerg (1996), this species accounts for a major part of the vapor present. | ||||
| • | Instead, the experimental results reported here, as well as the thermodynamic equilibrium calculation of CitationJiménez and Ballester (2004), suggest that KOH(g) is the main K-containing vapor at high temperatures. In this case, homogeneous reaction with HCl(g) to form KCl, as well as its subsequent condensation/nucleation, is thought to be the dominant process. The alternative path of KOH(g) nucleation with subsequent KOH-HCl heterogeneous reaction has been shown to be slow: at gas-partial pressures similar to although a little lower than those existing in this probe, dispersed KCl crystals which were observed only when particle collection was delayed 3.7 s. The total residence time along the dilution probe was estimated to be, ∼ 0.25 s. | ||||
The different formation rates of KCl and K2SO4 inside the dilution probe can be explained if the only rate-limited step in the aerosol formation process is assumed to be the SO2 oxidation, as postulated by CitationChristensen and Livbjerg (1996) and experimentally confirmed by CitationJiménez and Ballester (2005c). Because such limitations do not seem to exist in the KCl formation route, this compound would be much more readily formed inside the probe once the temperature is reduced below its melting point at the N2-injection section.
The different gas-cooling rates at the entrance of each probe may explain their different behavior. Estimated rates are of the order of 108 and 105 K/s for the AQPS and the dilution probe, respectively. In the first place, an enhanced nucleation rate is expected in the AQPS probe because of the high supersaturation values achieved in sudden gas cooling. Lower supersaturation values are expected in the dilution probe, thus enabling condensation and greater particle diameters, as observed in the results presented above. In the second place, the conditioning of the sample afforded by the AQPS probe performs a more effective quenching of chemical reactions. The slower cooling rate of the dilution probe may provide more opportunities for KCl(g) to form from KOH in the gas phase; however, this cannot be estimated because of the lack of an expression for the associated reaction rates. In case the KCl(g) formation mechanism involves radicals, the reaction rate would be further reduced in the AQPS probe because of the enhancement of radical destruction on the probe walls at low pressures, which can be as relevant as the thermal quenching (CitationFristrom 1983).
Both probes, therefore, introduce significant sampling artifacts. Specifically, all potassium in the vapor state of the sampled gases is converted to solid in both probes. However, the radical differences found in the conversion route can make one probe preferable to the other. In particular, the spurious particles generated in the dilution probe have been shown to have characteristics comparable (in size and composition) to those actually existing in the flue gases, as probe is unable to freeze the properties of the particles at the sampling point. This makes the probe—at least, for the configuration and DR used here—unable to resolve the intermediate stages along the process of particle formation. The applicability to other systems should be carefully studied to determine whether actual existing particles and artifacts can be distinguished.
An additional difficulty when using the dilution method in the presence of condensable vapors is that the artifacts introduced depend on the specific probe configuration and DR, as well as the temperature and nature of the sample (gas + vapors + particles) existing in the probing location. For example, CitationStrand et al. (2004) found significant differences in the gas-to-particle conversion results depending on the dilution ratio used—their probe being considerably different to the one used in this study. The apparent unpredictability of these effects pose serious difficulties in the interpretation of the results obtained because the relation between vapor species and the subsequent modifications in particle composition resulting from sampling artifacts are not, in principle, straightforward.
Assuming that the artifact introduced by the use of the aerodynamic quenching is essentially reduced to the conversion of vapors into very small particles, the applicability of the whole sampling system (AQPS probe + impaction + TEM) will depend on the sampled system and the research objectives. In the present case, for example, the clear differentiation between “spurious” and “real” particles allowed characterizing the particle formation process. In other cases, the size of the final emitted particles might be comparable to the spurious particles (say, <30 nm), thus fading the border size blurs the border between both types of particles and making difficult the interpretation of the results.
The combination of the described probe, or similar designs, with other measurement equipment (e.g., Differential Mobility Analyser, BLPI, etc.) might constitute a powerful tool for the investigation of aerosol formation in combustion systems because it might yield information on both condensed and vapor phases. The main obstacles in achieving this goal appear to be the need for sonic values at the probe entrance and for study under rough vacuum conditions. In this respect, the works of CitationSeto et al. (1997) and CitationSeol et al. (2000) with low-pressure DMAs are encouraging.
Because of the limited scope of the tests performed with the thermophoretic probe, it is difficult to provide a definitive diagnostic for the possibilities of its use in similar types of systems. Nevertheless, there seems to be a fundamental difficulty in using this sampling strategy: particle capture is achieved in a thermophoretic probe by using a cold surface to create a temperature gradient, which at the same time leads to nucleation/condensation of inorganic vapors. Designs and operating temperatures of the probe different to those used here might enable the use of this technique in similar types of applications, but their definition does not appear to be an easy task. Specifically, it would involve finding conditions in which the thermophoretic drift is active and, at the same time, minimize modifications due to of chemical reactions, nucleation, or condensation across the thermal boundary layer.
CONCLUSIONS
Three different sampling systems have been used to obtain samples of the particles formed in biomass postcombustion products, which are based on aerodynamic quenching, dilution, and thermophoresis, respectively. The corresponding artifacts have been identified and discussed.
In all the tests performed, the use of the AQPS probe resulted in the formation of very fine particles (∼ 15 nm) by nucleation of the inorganic vapors. Longer residence times inside the probe did not led to significant changes in the samples collected, except for the depletion of these nuclei that probably occurred by diffusion to the tube walls; this suggests that aerodynamic quenching prevents further phase changes. The reproducibility of the mentioned artifact might enable the study of other vapor + particle systems. The use of the AQPS probe in combination with different analysis techniques is thought to be a valid strategy for the complete characterization of both phases.
Differently, the particles collected using a dilution probe (DR ∼ 1:6), even at temperatures as high as 1300°C are comparable in size and composition to the final emissions. In particular, the main artifact introduced by this probe has been the condensation of KCl, which results in high amounts of this compound at any temperature. Therefore, the use of this probe—at least, as it is used here—is judged inappropriate for the study of particle-formation processes in the presence of condensable species.
Finally, some tests were performed using a thermophoretic probe. This technique has been used successfully for the sampling of submicron particles (e.g., soot) in other types of systems, but no previous reference has been found in the literature regarding its application in the presence of condensable species. The results showed that this probe can introduce significant artifacts because of nucleation and/or condensation of vapors, which again results in particles and macroscopic crystalline structures whose composition could not be exactly determined.
The complexity and variety of the effects observed in the tests reported here demonstrate that particle sampling in high temperature, vapor-containing systems involve significant difficulties and require careful interpretation. According to the results reported here, the AQPS probe presents a number of advantages over the other methods. The reproducibility of the artifacts and the size of the spurious particles—that, nevertheless, could finally limit the range of systems studied—make spurious and “real” particles easier to distinguish. Also, a closer relation between vapors and nucleated particles should be found with this probe in which chemical reactions are efficiently quenched by the very fast reduction in the temperature of the sample.
The tests performed using the AQPS probe support KOH as the main K-containing gaseous species at high temperatures, rather than KCl. This result, on the other hand, illustrates how this probe can be used to address the study of the properties of the vapors existing at the sampling point.
REFERENCES
- Bowman , C. T. 1977 . “ Probe Measurements in flames ” . In Experimental Diagnostics in Gas Phase Combustion Systems Edited by: Zinn , B. T. Vol. 53 , pp. 1 – 24 . Progress in Astronautics and Aeronautics
- Christensen , K. A. 1995 . The Formation of Submicron Particles from the Combustion of Straw , Lyngby : Technical University of Denmark . PhD Thesis, Department of Chemical Engineering
- Christensen , K. A. and Livbjerg , H. 1996 . A Field Study of Submicron Particles from the Combustion of Straw . Aerosol Sci. Technol. , 25 : 185 – 199 .
- Christensen , K. A. and Livbjerg , H. 2000 . A Plug Flow Model for Chemical Reactions and Aerosol Nucleation and Growth in an Alkali-Containing Flue Gas . Aerosol Sci. Technol. , 33 : 470 – 489 . [CROSSREF]
- Christensen , K. A. , Stenholm , M. and Livbjerg , H. 1998 . The Formation of Submicron Aerosol Particles, HCl and SO2 in Straw-Fired Boilers . J. Aerosol Sci. , 29 ( 4 ) : 421 – 444 . [CSA] [CROSSREF]
- Colkett , M. B. III , Chiapetta , L. , Guile , R. N. , Zabielski , M. F. and Seery , D. J. 1982 . Measurements; Diagnostics. Internal Aerodynamics of Gas Sampling Probes . Combustion and Flame. , 44 : 3 – 14 . [CSA] [CROSSREF]
- Davis , S. B. , Gale , K. G. , Wendt , J. O. L. and Linak , W. P. 1998 . Multicomponent Coagulation and Condensation of Toxic Metals in Combustors . Proceedings of the Combustion Institute. , 27 : 1785 – 1791 . [CSA]
- Dobbins , R. A. and Megaridis , C. M. 1987 . Morphology of Flame-Generated Soot as Determined by Thermophoretic Sampling . Langmuir. , 3 : 254 – 259 . [CROSSREF]
- Flagan , R. C. and Friedlander , S. K. 1978 . “ Particle Formation in Pulverized Coal Combustion: A Review ” . In Recent Developments in Aerosol Science , Edited by: Shaw , D. T. pp. 25 – 59 . New York : John Wiley .
- Fristrom , R. M. 1983 . Comments on Quenching Mechanisms in the Microprobe Sampling of Flames . Combustion and Flame , 50 : 239 – 242 . [CROSSREF]
- Jiménez , S. and Ballester , J. 2004 . Formation and Emission of Submicronic Particles From Pulverized Olive Residue (Orujillo) Combustion . Aerosol Sci. Technol. , 38 : 707 – 723 . [CROSSREF]
- Jiménez , S. and Ballester , J. 2005a . Effect of Co-Firing on the Properties of Submicron Aerosols from Biomass Combustion . Proceedings of the Combustion Institute , 30 : 2965 – 2973 . [CROSSREF]
- Jiménez , S. and Ballester , J. 2005b . Particulate Matter Formation and Emission in the Combustion of Different Pulverized Biomasses . Accepted for publication in Combustion Sci. Technol ,
- Jiménez , S. and Ballester , J. 2005c . Influence of Operating Conditions and the Role of Sulphur in the Formation of Aerosols from Biomass Combustion . Combustion and Flame. , 140 : 346 – 358 . [CROSSREF]
- Johansson , L. S. , Tullin , C. , Leckner , B. and Sjövall , P. 2003 . Particle Emission from Biomass Combustion in Small Combustors . Biomass and Bioenergy , 25 : 435 – 446 . [CROSSREF]
- Köylü , Ü. Ö. , McEnally , C. S. , Rosner , D. E. and Pfefferle , L. D. 1997 . Simultaneous Measurements of Soot Volume Fraction and Particle Size/Microstructure in Flames Using a Thermophoretic Sampling Technique . Combustion and Flame , 4 : 494 – 507 .
- Lind , T. , Valmari , T. , Kauppinen , E. , Maenhaut , W. and Huggins , F. 1998 . “ Ash Formation and Heavy Metal Transformations During Fluidised Bed Combustion of Biomass ” . In Series Thermal Biomass Utilization , vol. 3 , Austria : Graz . BIOS ed.
- McElroy , M. W. , Carr , R. C. , Ensor , D. S. and Markowski , G. R. 1982 . Size Distribution of Fine particles From Coal Combustion . Science , 215, 4528 : 13 – 19 .
- Neville , M. and Sarofim , A. F. 1982 . The Stratified Composition of Inorganic Submicron Particles Produced During Coal Combustion . Proc. Comb. Inst. , 19 : 1441 – 1449 .
- Nielsen , M. T. , Livbjerg , H. , Fogh , C. L. , Jensen , J. N. , Simonsen , P. , Lund , C. , Poulsen , K. and Sander , B. 2002 . Formation and Emission of Fine Particles from Two Coal-Fired Power Plants . Combustion Sci. Technol. , 174 : 79 – 113 .
- Perry's Chemical Engineers' Handbook . 1997 . , 7th ed. , Edited by: Green , D. W. McGraw-Hill .
- Quann , R. J. , Neville , M. and Sarofim , A. F. 1990 . A Laboratory Study of the Effect of Coal Selection of Combustion Generated Submicron Particles . Combustion Sci. Technol. , 74 : 245 – 265 .
- Sarofim , A. F. , Howard , J. B. and Padia , A. S . 1977 . The Physical Transformation of the Mineral Matter in Pulverized Coal Under Simulated Combustion Conditions . Combustion Sci. Technol. , 16 : 187 – 204 .
- Seol , K. S. , Tsutatani , Y. , Camata , R. P. , Yabumoto , J. , Isomura , S. , Okada , Y. , Okuyama , K. and Takeuchi , K. 2000 . A Differential Mobility Analyzer and a Faraday Cup Electrometer for Operation at 200-930 Pa Pressure . J. Aerosol Sci. , 12 : 1389 – 1395 . [CROSSREF]
- Seto , T. , Nakamoto , T. , Okuyama , K. , Adachi , M. , Kuga , Y. and Takeuchi , K. 1997 . Size Distribution Measurement of Nanometer-Sized Aerosol Particles Using DMA Under Low-Pressure Conditions . J. Aerosol Sci. , 2 : 193 – 206 . [CROSSREF]
- Strand , M. , Bohgard , M. , Swietlicki , E. and Sanati , M. 2003 . Method for Characterization of Fly Ash Particles at High Temperatures . Proc. Euro. Aerosol. Conf. , : p. 1321 Madrid, 2003
- Strand , M. , Bohgard , M. , Swietlicki , E. , Gharibi , A. and Sanati , M. 2004 . Laboratory and Field Test of a Sampling Method for Characterisation of Combustion Aerosols at High Temperatures . Aerosol Sci. Technol. , 38 : 757 – 765 . [CSA] [CROSSREF]
- Valmari , T. , Lind , T. M. , Kauppinen , E. I. , Sfiris , G. , Nilsson , K. and Maenhaut , W. 1999 . Field Study on Ash Behavior During Circulating Fluidized-Bed Combustion of Biomass. Point 1. Ash Formation . Energy & Fuels. , 13 : 379 – 389 . [CSA] [CROSSREF]
- Williams , D. B. and Carter , C. B. 1996 . Transmission Electron Microscopy. A Textbook for Materials Science , New York : Plenum Press .