The optical properties of the light-absorbing, carbonaceous substance often called “soot,” “black carbon,” or “carbon black" have been the subject of some debate. These properties are necessary to model how aerosols affect climate, and our review is targeted specifically for that application. We recommend the term light-absorbing carbon to avoid conflict with operationally based definitions. Absorptive properties depend on molecular form, particularly the size of sp 2-bonded clusters. Freshly-generated particles should be represented as aggregates, and their absorption is like that of particles small relative to the wavelength. Previous compendia have yielded a wide range of values for both refractive indices and absorption cross section. The absorptive properties of light-absorbing carbon are not as variable as is commonly believed. Our tabulation suggests a mass-normalized absorption cross section of 7.5 ± 1.2 m2/g at 550 nm for uncoated particles. We recommend a narrow range of refractive indices for strongly-absorbing carbon particles, of which the highest is 1.95–0.79i. Our refractive indices are consistent with most measurements reported in the literature, and values used in present-day climate modeling are in error. Realistic refractive indices underpredict measured absorption by about 30% when used with common theories for spherical particles or aggregates. Field programs since about 1970 have measured quantities relevant to light absorption, but have only recently made enough measurements to isolate the light-absorbing carbonaceous component and determine its absorptive properties.
1. INTRODUCTION
Optical properties and radiative effects of atmospheric trace species may be predicted from their concentrations and chemical composition. This assumption is fundamental to modeling how anthropogenic activities affect climate. It was first used to model carbon dioxide (CitationHansen et al. 1981) and other greenhouse gases (Ramanathan et al. 1985). Later followed models of sulfate aerosols (CitationCharlson et al. 1991; CitationKiehl and Briegleb 1993), mineral dust (CitationTegen et al. 1996), and carbonaceous aerosols (CitationPenner et al. 1992; CitationChylek and Wong 1995; CitationHaywood and Shine 1995).
It has long been believed that carbonaceous particles could affect climate. Light-absorbing particles warm the atmosphere, counteracting cooling caused by particles such as sulfates that predominantly scatter light. The role of carbonaceous particles in determining whether aerosols warm or cool has stimulated a large body of research. The choice of optical properties is critical in calculating the effects of aerosols on radiative transfer, but these calculations have frequently relied on values from compilations more than two decades old.
The present article was motivated by two deficiencies in the literature. First, tabulations of optical properties should be updated to include recent measurements. This paper tabulates information on light-absorbing carbon (LAC) particles, especially the extensive combustion literature. Second, measured absorptive properties demonstrate variability that has not been represented in climate models. This variability needs to be explained, or fundamental uncertainties will remain in simulations of radiative transfer.
Writing this paper has proven an exhausting and humbling experience. It is clear that we could have rewritten it indefinitely to accommodate new results. Although we have limited the topics included here, we have included some discussions that may be somewhat foreign to atmospheric scientists. The remainder of this section discusses our approach to this review by identifying information required for climate research. Section 2 gives a history of examinations of light-absorbing carbon particles. Because this field has been fraught with inconsistencies in definition, we discuss nomenclature in Section 3. We review causes of variation in refractive indices of LAC (Section 4), methods of calculating mass absorption cross section (Section 5), measurement techniques (Section 6), measurements of absorption by freshly-generated particles (Section 7), and measurements of absorption in the atmosphere (Section 8). Finally, we recommend values for use in radiative-transfer modeling (Section 9).
1.1. Details: The Chemistry-Optics Paradigm
The use of transport models to calculate climatic effects of any species relies on two implicit assumptions:
| 1. | There is a predictable relationship between emission (generation of new particles) and spatially distributed atmospheric concentration. This relationship is governed by advection, chemical transformation, and removal. | ||||
| 2. | There is a predictable relationship between atmospheric concentration and its effects on radiative transfer. This relationship involves the optical properties of the species. | ||||
A model of radiative transfer requires knowledge of emissions and simulations of the two relationships above, all embedded in a characterization of the Earth system usually called a climate model. Emissions of carbonaceous particles are treated in other papers (CitationCooke et al. 1999; CitationLiousse et al. 1996; CitationBond et al. 2004). The transport models that perform calculation (1) above are numerous and are also described elsewhere (CitationPenner et al. 1998; CitationHaywood and Ramaswamy 1998; CitationMyhre et al. 1998; CitationKoch 2000; CitationJacobson 2001; CitationCooke et al. 2002; CitationChin et al. 2002; CitationChung and Seinfeld 2002).
The present paper focuses on the second assumption: the relationship between chemical constituents and radiative transfer. Predicting this relationship is a greater challenge for aerosols than for greenhouse gases, because scattering and absorption depend on the particles' physical form. For sulfate aerosols, size distribution and growth with relative humidity are important. Carbonaceous aerosols introduce new challenges. It is not clear how well the optics of these particles can be characterized by common measurements of their physical and chemical nature.
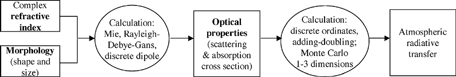
Climate forcing is most often defined as the change in net radiative flux at the tropopause attributable to a specific component. A positive forcing is an increase in flux, tending toward warming of the Earth-atmosphere system. Forcing is so called because it is an input to the system determined by factors outside it. shows how the change in radiative transfer is determined from atmospheric concentration of light-absorbing particles. Most climate modelers first assume physical properties (size, shape and state of mixing, categorized as morphology) and a refractive index, obtain scattering and absorption cross sections, and apply those properties to modeled concentrations. A few models of global climate have examined effects of differing morphology (CitationHaywood and Shine 1998; CitationChung and Seinfeld 2002) by comparing climate forcing calculated with different assumptions. Only one global model (CitationJacobson 2001) has simulated changing particle morphology within the model to account for changing optical properties. All models have assumed that particles are spherical, and have chosen single values for the refractive index. If underlying model assumptions are inappropriate, then scattering, absorption, and radiative forcing estimates will be incorrect. While our discussion will end with calculating scattering and absorption, it provides suggestions for improvements in climate models and has repercussions for calculation of radiative forcing.
The global information provided by satellite remote sensing may soon allow observationally based estimates of climate forcing. Estimates of radiative flux can be correlated with observed aerosol properties at the Earth's surface, and used to estimate aerosol forcing without invoking the relationship between chemistry and optics (e.g., CitationChristopher et al. 2000). However, interpretation of these results, as well as all historical or future projections of climate forcing by aerosols, will continue to rely on global simulations. Inversions used to infer aerosol optical depth from observed radiance also rely on assumptions about aerosol properties.
1.2. Why Another Review?
There is a need to understand optical properties of carbonaceous aerosols for both climate modeling and remote sensing applications. At a minimum, the following tasks are necessary: (1) Understand the variability in the refractive index of carbonaceous particles. (2) Identify critical aspects of morphology that affect absorption and scattering. (3) Reduce these aspects to observable quantities that can be assessed with widespread atmospheric measurements. (4) Represent this information in climate models with efficient parameterizations.
The present article contributes to the first two tasks and provides information for the fourth. There are many tabulations of relevant properties for atmospheric particles (CitationHorvath 1993a; CitationLiousse et al. 1996; CitationFuller 1999), or combustion-generated particles (CitationMedalia and Richards 1972; CitationDobbins and Megaridis 1994; CitationSmyth and Shaddix 1996). In the combustion literature, a typical tabulation presents a range of measured refractive indices and discusses the resulting uncertainty in absorption; the authors might then select one value after criticizing the methods of some previous investigators. In the literature on atmospheric aerosols, reviews often collect a range of mass absorption cross sections and seek an explanation for such variability, perhaps based on particle mixing state, but without considering all possible refractive indices.
Missing from previous investigations has been a resolution of the reported variability. If variation in the refractive index is fundamentally inexplicable, then climate forcing will remain similarly uncertain, despite the ability to compute optics of torturous morphologies. If extremely complex morphologies are required to predict measured mass absorption cross sections, then the likelihood of representing these particles in global atmospheric models is questionable.
In this article, we provide a critical review of refractive indices and mass absorption cross sections of fresh LAC. We suggest explanations for the wide range of values that have appeared in the literature for pure, combustion-generated particles. We also discuss observed mass absorption cross sections and the prospects for predicting them in climate models. Most of the refractive indices used to model LAC have been appropriated from measurements made for other purposes, so we identify only those materials that are similar to atmospheric LAC. Finally, we make recommendations for the most appropriate values to use in climate studies.
1.3. Limits of this Review
Our discussion will not revisit some relevant presentations that appear elsewhere. First, we do not tabulate modeled estimates of total climate forcing. These are summarized by the Intergovernmental Panel on Climate Change (IPCC 2001), have recently been reviewed by CitationHaywood and Boucher (2000), and will also be reviewed for the IPCC assessment due in 2005.
Second, we do not attempt a comprehensive tabulation of field measurements of light absorption, except to note the scope of the measurements that have been made. We also restrict most of our discussion to absorption in the mid-visible (550 nm), although we briefly discuss absorption at other visible wavelengths.
Finally, the task of measuring total aerosol carbon is a simple one, although not immune to sampling artifacts (e.g., CitationCadle et al. 1983; CitationHuebert and Charlson 2000; CitationKirchstetter et al. 2001). The strongly light-absorbing component forms just a few percent of the carbonaceous aerosol, and separating it from the remainder of the atmospheric aerosol is difficult. Methods for identifying this component include exposing the sample to high temperatures (e.g., CitationChow et al. 1993; CitationBirch and Cary 1996), washing the sample with solvents such as benzene (CitationGundel et al. 1984) or hydrogen peroxide (CitationOgren et al. 1983), or interpreting Raman spectroscopy (CitationRosen et al. 1978; CitationDippel et al. 1999). The separation of light-absorbing carbon by heating the particles to different temperatures is an area of active research. We review neither this method nor its uncertainties. Without delving into the peculiarities and methods of thermally-measured elemental carbon, we point out that this quantity may have a poor relationship with light absorption (CitationHuffman 1996; CitationReid et al. 1998; CitationMartins et al. 1998), and that combined thermal and optical techniques may not perfectly separate the strongly absorbing component (CitationMayol-Bracero et al. 2002a; CitationYu et al. 2002).
Errors in measuring the quantity of light-absorbing carbon in a sample affect the estimate of light absorption per mass (e.g., CitationCarrico et al. 2003) and could alter conclusions about whether absorption can be predicted from mass measurements. We will only compare absorption and mass of LAC when mass determination does not rely on thermal methods. This decision eliminates a large number of atmospheric measurements from consideration. While such comparisons can be important, they do not advance the purposes of this review.
2. HISTORY: HOW DID WE GET HERE?
Much is known about the optics of particles. The solution to Maxwell's equations for scattering and absorption by spherical particles was presented by CitationMie (1908), and treatments of the problem appeared decades earlier (CitationLogan 1965). Mie theory has been corroborated by measurements in the intervening years. Books by CitationVan de Hulst (1957), CitationKerker (1969), and CitationBohren and Huffman (1983) give extensive theoretical and practical discussions. A critical review by CitationSorensen (2001) covers optics of agglomerated spheres, commonly assumed as the structure of freshly generated LAC. The present article is not a substitute for such fundamental work. The reader is particularly referred to Section 14.2 in CitationBohren and Huffman (1983) for discussions and cautions regarding theories that have been applied to atmospheric aerosols.
shows that the study of light absorption by carbonaceous particles is not a new field, either. (Here we provide only some of the earliest references in each field.) The 1940s brought the excitement of X-ray analysis and the ability to compare the structures of carbon black and graphite (CitationBiscoe and Warren 1942). Atmospheric aerosols were observed with electron microscopes in the mid-1950s; heating was used to drive off volatile carbon compounds (Cartwright et al. 1955).
TABLE 1 Discussions that contribute to understanding of absorption by LAC
Changes in radiative transfer due to particulate light absorption were first estimated around 1970 (CitationCharlson and Pilat 1969; CitationSchneider 1972). During the 1970s, few researchers believed there was much absorption in atmospheric aerosols. Most researchers believed that the light-absorbing material in urban atmospheres did not travel very far from their source. Models of the atmospheric aerosol either ignored absorbing particles (CitationToon and Pollack 1976) or assumed that they occurred only in urban aerosols (CitationShettle and Fenn 1979). There was simply not enough information to create a more detailed view of atmospheric aerosols.
There had been a long history of measurements of aerosol absorption, particularly those known as “soiling index” and “British Smoke Shade” (e.g., CitationWaldram 1945; CitationRoach 1961). However, the first systematic measurements of aerosol absorption were made in the early 1970s at the University of Mainz (CitationFischer 1970) and at the University of Washington (CitationLin et al. 1973). Some of the first measurements of absorption due to mineral dust were made by groups at White Sands, New Mexico (CitationLindberg 1975) and Leningrad, Soviet Union (CitationKondratyev et al. 1973).
The energy crisis of the 1970s led to increasing use of diesel vehicles, and extensive work on soot formation and measurement ensued. Soot formation in idealized flames and heat transfer by soot in boilers were also hot topics in combustion research (CitationMcLean et al. 1981; CitationSeeker et al. 1981), resulting in many measurements of pure soot. Concurrently, a series of field experiments investigated the light-absorbing component of atmospheric aerosol in Denver, Colorado (CitationWolff et al. 1981). A 1980 conference at General Motors produced a pair of books on combustion soot and atmospheric elemental carbon, a partnership that was regrettably neglected for some years.
The 1980s saw the advent of real-time instruments for measuring light absorption, including photoacoustic (CitationJapar and Szkarlat 1981) and filter-based (CitationHansen et al. 1982) methods. The comparative ease of measuring absorption led to a desire to understand the relationship between absorption and mass of light-absorbing carbon. If the relationship were simple, the more rapid optical methods could be used to measure both diesel emissions (regulated by mass) and atmospheric concentrations. At the same time, detailed aerosol-climate studies appeared under the guise of studies on nuclear winter (CitationTurco et al. 1983; CitationNelson 1989; CitationPenner and Molenkamp 1989). These investigations raised questions complementary to the information required by automobile manufacturers: given a mass emission rate of aerosol from conflagrations following nuclear war, how much light would be absorbed by that aerosol, and how would climate change?
Despite a long history of using absorption as a surrogate for mass, significant difficulties in establishing the relationship between the two still exist. Determining the relationship for atmospheric aerosol requires extracting the light-absorbing component from a complex mixture. Methods for accomplishing that task were not available much before 1980, and are open to question even today. Mueller and Appel had used thermal methods to extract light-absorbing material in the Los Angeles ACHEX study during the 1970s (CitationHidy 1980), but it wasn't until the 1978 Denver Brown Cloud study that a single atmospheric field program measured both absorption and thermally measured elemental carbon (CitationGroblicki et al. 1981). Even the early Aerosol Characterization Experiments (CitationQuinn et al. 1998; CitationRaes et al. 2000) under the umbrella of the International Global Atmospheric Chemistry organization did not provide both absorption and mass measurements. Monitoring programs that did make such paired measurements, such as the Interagency Monitoring of Protected Visual Environments (CitationMalm et al. 1994) gave useful information on air quality trends and the nature of pollutants, but did not explain the observed magnitude of absorption.
In the mid-1990s, simple estimates of forcing by light-absorbing carbonaceous particles were developed (CitationHaywood and Shine 1995). These estimates were low (+0.1 W m−2 for fossil-fuel emissions) compared with those of greenhouse gases (+2.45 W m−2). Three-dimensional transport models of carbonaceous aerosols were developed a few years later, and gave somewhat higher estimates (∼ +0.2 W m−2 for fossil-fuel emissions; CitationHaywood et al. 1997; CitationHaywood and Ramaswamy 1998; CitationPenner et al. 1998; CitationMyhre et al. 1998; CitationCooke et al. 1999; CitationKoch 2000; CitationChung and Seinfeld 2002). While these studies acknowledged uncertainties in the optical properties of the aerosols, only a few propagated these uncertainties into estimates of radiative forcing (CitationWang 2004).
Mixing between carbonaceous aerosols and other negligibly-absorbing substances (discussed in Section 5) might increase absorption by a factor of two, and this increase was not included in most estimates of radiative forcing (CitationAckerman and Toon 1981; CitationChylek et al. 1995; CitationHaywood and Shine 1995). It was also known that open biomass burning emitted about the same quantity of LAC as fossil-fuel burning (CitationLiousse et al. 1996; CitationBond et al. 2004). However, CitationJacobson (2001) drew new attention to the particles' contribution to climate forcing when he combined both total global emission and considerations of mixing state to obtain a high radiative forcing estimate of +0.54 W m−2.
Around the same time, measurement campaigns identified large concentrations of both absorbing and negligibly-absorbing atmospheric aerosols (CitationNovakov et al. 1997; CitationQuinn and Bates 2003), and measured how they alter the Earth's radiative balance (CitationSatheesh and Ramanthan 2000). Other simulations examined more complex changes caused by these particles, such as changes in cloud cover (CitationAckerman et al. 2000; CitationRamanathan et al. 2001; CitationMenon et al. 2002).
Recent years have seen proposals by CitationHansen et al. (2000, Citation(2001) and CitationJacobson (2002) that reducing emissions of black carbon might ameliorate global warming, and disagreements about whether carbonaceous aerosols cause warming at all (CitationPenner et al. 2003). Uncertainties in optical properties of these particles prevent assessing some of these ideas.
3. NOMENCLATURE: WHAT SHALL WE CALL IT?
The strongly light-absorbing component known to climate modelers as “black carbon” has had a variety of different names, of which soot is probably the most common. The remaining less-absorbing carbonaceous aerosol is loosely called “organic carbon,” a catchall term whose remedy is outside the scope of this review. We list some common terminology in this section, first discussing terms relevant to composition, and then nomenclature describing absorption and mixing state.
3.1. Strongly-Absorbing Carbon
According to chemists, truly elemental carbon has three forms: graphite, diamond, and C-60 (buckminsterfullerene or buckyballs). Although fullerenes may contribute to growth of LAC particles (CitationJohnson et al. 2002), they are relatively rare compared to other forms and will not be discussed further in this review. Graphite consists of sp 2-bonded carbon in planar layers, and diamond contains sp 3-bonded carbon in crystalline form. Neither of these pure forms is found in the atmosphere (or aerosol scientists would be considerably wealthier). An array of names has represented the carbon that exhibits extensive arrays of sp 2 bonds.
Elemental carbon, as used in atmospheric chemistry, usually identifies carbon that does not volatilize below a certain temperature, usually about 550°C. This term is an operational definition based on the stability of carbon at elevated temperatures (CitationHuntzicker et al. 1982; CitationChow et al. 1993; CitationBirch and Cary 1996). A more precise name for this substance is refractory carbon. The fraction identified as elemental carbon under this method depends on the heating conditions (CitationSchmid et al. 2001). The component that does not dissolve in hydrogen peroxide (CitationOgren et al. 1983) has also been called elemental carbon. For diesel exhaust, there is a strong correlation between light absorption and the refractory carbon content (CitationScherrer et al. 1981; CitationJapar et al. 1981; CitationSzkarlat et al. 1983), but this relationship has not been tested for all carbon particles. For example, the relationship between absorption and refractory carbon content for particles from benzene flames varies with pyrolysis temperature (CitationLee 1983).
Graphitic carbon refers to the molecular state of the carbon. This label is accurate to describe strongly absorbing carbon particles because the conjugation of unsaturated bonds results in light absorption (see Section 4); it is inaccurate because planes in light-absorbing carbonaceous particles are confined to spherical surfaces rather than infinite in extent as in graphite (see Sections 5 and 7). Carbonaceous material has also been called graphitic if its Raman spectrum is like that of graphite (CitationRosen et al. 1978). The response of Raman spectroscopy also depends on crystallite size and particle surface area (CitationDippel et al. 1999), and many complex aromatic molecules exhibit similar spectra.
The term soot is used by the Intergovernmental Panel on Climate Change to denote any light-absorbing, combustion-generated aerosols (CitationIPCC 1996). Combustion researchers often classify all combustion-generated carbonaceous aerosol as soot. Because soot is a vague term that may include any dark-appearing, carbon-containing compound generated in combustion (CitationWatson and Valberg 2001), CitationCachier (1998) recommends the term black carbon. The term smoke, an early name for the same aerosols, abounds in literature of the early 20th century (e.g., CitationPopplewell 1901).
Black carbon is probably the most widely used term for light-absorbing carbonaceous aerosols among climate modelers. The term implies carbonaceous aerosols that have strong absorption across a wide spectrum of visible wavelengths. Some instruments such as the aethalometer (CitationHansen et al. 1984) report concentrations of black carbon based on light attenuation. The reported value is the mass of strongly light-absorbing carbon that would absorb as much light as the sample.
Because the present article examines the nature and causes of light absorption, we will use the term “light-absorbing carbon,” abbreviated LAC, which has been suggested by CitationMalm et al. (1994). We deliberately avoid names that have been used for some other purpose; such a choice might perpetuate conflicts in an already splintered field. The words “graphitic" and “elemental” imply identification of the molecular carbon structure that is inappropriate. The word “black” has come to be associated with measurements by filter-based optical methods, which frequently assume a particular wavelength dependence and absorption per unit mass. Thus, we decline to use any of these terms.
Other work has identified forms of carbon that have weak light absorption with strong wavelength dependence (CitationMillikan 1961; CitationBond 2001; CitationSato et al. 2003; CitationKirchstetter et al. 2004). Although this type of carbon may affect climate, we deliberately do not address it here, so that we can focus on the strongly-absorbing carbonaceous particles. We also recognize that “strongly light-absorbing carbon” is a more descriptive term for the material we examine here, but the resulting acronym is not as tasteful. (We apologize to the Stanford Linear Accelerator Center for this admittedly arbitrary judgment.)
3.2. Other Forms of Light-Absorbing Carbon
Some substances are not associated with atmospheric research, but their study has contributed to the understanding of atmospheric carbon.
Carbon black is used to make pigments and ink and to reinforce automobile tires. Its properties, including optical properties, depend on the manufacturing process, which involves burning either natural gas or oil under very controlled conditions. Early work on scattering and absorption by black particles examined this substance.
Amorphous carbon is a solid that has no long-range crystalline order, composed of a mixture of sp 2 and sp 3 bonds. The International Union of Pure and Applied Chemistry excludes material with sp 2-bonded clusters greater than 1 nm in extent (approximately seven aromatic rings). It is usually generated by vapor deposition and may contain hydrogen and nitrogen.
Coal is geologically processed vegetable matter. Its ranks or grades range from lignite to anthracite. Exposure to high pressures and elevated temperatures on geologic time scales results in increased aromatic content. As this change occurs, the material appears more black (Citationvan Krevelen 1981).
Graphite is one of the pure forms of elemental carbon. As we discuss in Section 7.1.3, both the macroscopic shape and the crystalline structure of graphite differ from that of LAC. The carbon particles of interest to this review may differ from graphite in their optical properties and density.
Tar has a wide variety of meanings; the term often indicates non-black viscid combustion residue of high molecular weight. However, in research on solid-fuel combustion, tar can mean any condensable product ejected from solid matter.
There is also a rich literature on the topic of black carbon in sediments (e.g., CitationKuhlbusch 1995; CitationClark 1997; CitationMasiello and Druffel 1998; CitationSchmidt and Noack 2000; Nguyen et al. 2003) It is comforting, but not reassuring, to note that the atmospheric community's difficulties in separating LAC from other types of carbon are repeated in sediment research (and probably elsewhere), although with different analytical techniques.
3.3. Scattering and Absorption
The term absorption coefficient is commonly used to describe the absolute magnitude of atmospheric absorption. This quantity has units of inverse length, or cross section per volume of air. It is often given the symbol b in atmospheric literature, with subscripts indicating the nature and source of the extinction, e.g., b ap is the absorption coefficient due to particles. The symbol σ has also been suggested. In the radiative transfer literature, the coefficients are given Greek symbols such as β (extinction), κ (absorption), or σ (scattering).
It is often desirable to normalize scattering and absorption cross sections to the mass of particles, and the terminology describing this normalized value is not consistent. The units are often cross section of absorption or scattering per mass of material (m2/g), and α, σ, k, B, E, “mass absorption coefficient,” and “mass absorption efficiency" have all appeared as nomenclature. The term “efficiency" is more appropriate to identify the ratio between optical and geometric cross sections, when the result is properly dimensionless. We suggest the more descriptive term “mass absorption cross section” (MAC), which does not conflict with other definitions. This term may be criticized as a new entry into an already crowded field; we choose it because it is unambiguous, not because it is satisfying. The modifier “mass” indicates that the absorption cross section is referenced or normalized to the mass of the particle.
Another quantity of interest is the single-scattering albedo, or scattering divided by extinction (absorption plus scattering). If single-scattering albedo is close to one, extinction results primarily from scattering. Lower values—even as high as 0.85—indicate that the aerosol has significant absorption and may result in positive forcing (CitationHaywood and Shine 1995).
3.4. Morphology
Several terms have been used to describe the distribution of absorbing and negligibly-absorbing material within particles, as illustrated in . In each of these models, the properties of the particle ensemble are obtained by summing the properties of each type of particle separately.
FIG. 2 Idealized relationships between absorbing and nonabsorbing material. (a) External mixture: a heterogeneous population of internally homogeneous particles. (b) volume averaged mixture: a homogeneous population of internally homogeneous particles. (c) Heterogeneous particle composition and population. Both (b) and (c) have been called “internal mixtures”.
The term external mixture implies a heterogeneous population of homogeneous particles (), none of which has acquired other material since its formation. The term internal mixture is used inconsistently. It can be used to describe any occurrence of multiple species in the same particle, but it is an incomplete description of the mixing that may affect absorption, as we will discuss in Section 5. Internal mixtures may describe a homogeneous population of homogeneous particles, where the strongly absorbing material is perfectly mixed with other material at the molecular level (); we suggest that the term “volume mixture” is more descriptive.
An internal mixture may also refer to a particle that is internally heterogeneous (), with absorbing and negligibly absorbing material distributed unevenly throughout each individual particle. An absorbing core surrounded by a shell of negligibly-absorbing material is one way, but not the only way, to visualize particles that are internally heterogeneous. Descriptions such as “shell-and-core” imply a concentricity that may not exist; we favor the term “encapsulated,” initiated by CitationFuller et al. (1999), as most descriptive of the likely mixing state of these particles.
4. Refractive Index: From Molecular Structure to Bulk Optics
In this section, we review research on the relationship between molecular form and complex refractive index of light-absorbing carbon, discussing the material properties that govern light absorption. Measurements leading to inferred (and prevalent) values of the refractive index are discussed in Section 7.
The interaction between a material and incident radiation can be expressed as a function of either the material's complex refractive index (m = n − ik) or its square, the complex dielectric function (ϵ), as long as the material is not magnetic. In this review, we have adopted the convention of writing the imaginary part of the refractive index as a negative number, implying that time dependence is expressed as exp(iω t). Equations to calculate scattering and absorption by particles are often presented as functions of refractive index. While light absorption is closely related to the imaginary part of refractive index (k), it is also affected by the real part (n). Use of either m or ϵ ssumes that a single average property represents the material's nanoscale electronic and molecular variations.
4.1. Carbon Bonding
Chemical and optical properties of carbonaceous material are governed by the molecular form. In diamond, the s-orbital and the three p-orbitals are hybridized into a symmetric set of four tetrahedrally directed bonds known as sp 3 bonds. In graphite, three of the valence electrons are found in hybrid sp 2 orbitals that combine the s-orbital and two p-orbitals; these lie in a plane with an angle of 120° between them. The fourth valence electron is in a π-orbital normal to the plane and, not participating in bonds, is loosely held.
Carbonaceous particles with a high fraction of graphitic bonds are different from other atmospheric aerosols. The stability engendered by the aromatic bonds makes the material nearly inert in the atmosphere; it resists oxidation at atmospheric temperatures, and it is insoluble in water and many other solvents. The free movement of π -electrons makes this substance one of the only non-metallic compounds with high electrical and thermal conductivity. The energy levels of these loosely-held electrons are closely spaced, so the material absorbs electromagnetic radiation across a broad spectrum. Unlike pure graphite, flame-generated carbon contains sp 3 bonds in addition to sp 2 bonds, and it also includes hydrogen and oxygen (CitationAkhter et al. 1985a). In this respect, flame-generated carbon and atmospheric LAC are similar to amorphous carbon and coal, which also contain both sp 3 and sp 2 bonds.
4.2. Explaining the Refractive Index
Some studies have used physical principles, such as the predicted density of electronic states, to link refractive index with the molecular structure of carbon (CitationXanthakis 2000; CitationFanchini and Tagliaferro 2001). More common than this computationally-intensive process is a hybrid of theory and empirical data. A model for the expected interaction with light (reflectance or absorption) is chosen based on physical principles. Then, model parameters (including the refractive index) are adjusted until predictions match measurements.
4.2.1. Elemental Composition
Attempts to explain the absorptive properties of carbon by using elemental composition, mainly H/C (hydrogen-to-carbon) ratio, span several decades. CitationMillikan (1961) reported changes in the wavelength dependence of flame-generated carbon with H/C ratio. CitationMedalia and Richards (1972) suggested that values of k exhibit a linear relationship with H/C ratio for carbon black. CitationDalzell and Sarofim (1969) found that the refractive index of soot has no relationship with fuel H/C ratio, but did not investigate the H/C ratio of the soot. CitationHabib and Vervisch (1988) found that k did decrease with H/C ratio. CitationVaglieco et al. (1990) provided a physical explanation for variations with H/C ratio: hydrogen atoms “act as traps for the electrons, removing them from the π valence band.” The H/C ratio of flame-generated particles tends to be higher than that of carbon black (CitationMedalia and Rivin 1982). CitationCachier et al. (1989) measured the H/C ratio of soot as 0.15 ± 0.05, and of ambient aerosol as 0.20–1.50.
This explanation is simplistic; the H/C ratio is only the beginning of a predictive relationship. CitationFelske et al. (1984) dismiss such approaches as being “without theoretical basis.” Changes in this ratio may be informative, but they do not uniquely identify the molecular form of the carbon. Other theories account for molecular form more directly.
4.2.2. Linear Oscillators
The dispersion equationsFootnote 1 (e.g., CitationDitchburn 1976; CitationBorn and Wolf 1981) represent the classical Lorentz-Drude approach to electromagnetic radiation. Electrons are treated as linear oscillators that interact with radiation, and optical properties of a material are obtained by summing the interactions of the electrons it contains. The complex refractive index (m) as a function of frequency is related to properties of the j electrons:
CitationStull and Plass (1960) introduced the dispersion equations to model the refractive index of carbon, estimating parameters from the data of CitationSenftleben and Benedict (1918). CitationBoynton et al. (1968) and CitationDalzell and Sarofim (1969) were among the first to apply the theory to carbon in flames. Subsequent studies provided different measurements and interpretations (CitationLee and Tien 1981; CitationBen Hamadi et al. 1987; CitationHabib and Vervisch 1988; CitationChang and Charalampopoulos 1990). Most commonly, three types of electrons are considered: (Equation1) sp 2-bonded electrons, (2) π electrons, and (3) free conduction electrons, which have a resonant frequency of zero. Electrons in the σ shell absorb at ultraviolet wavelengths and do not affect visible absorption (CitationLee and Tien 1981).
The dispersion equations predict the wavelength dependence of the refractive index, as shown in using parameters from two studies. This model predicts a minimum in k at 450 nm. Depending on the parameters chosen, π electrons affect predicted absorption most; the number of free conduction electrons is much lower and they have little effect. Absorption increases and its spectral dependence decreases when the density of π -electrons increases, or when their damping constant decreases.
FIG. 3 Imaginary refractive index predicted by the dispersion model with the parameters of CitationLee and Tien (1981) and CitationDalzell and Sarofim (1969). The figure differs from that of Lee and tien because it is evaluated at 300 K instead of flame temperature. See the text (Section 4.2.2, 7.2.2, and 7.6) for caveats on the use of the dispersion model to represent flame generated carbon.
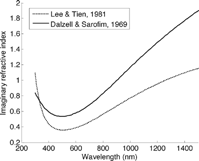
An advantage of the dispersion model is its plausible physical explanation for how materials interact with light. A disadvantage is a lack of predictive capability. For non-crystalline substances, the dispersion model is considered empirical, not exact (CitationStagg and Charalampopoulos 1993). Disagreement about values of n, ω res , and g d for each type of electron results in discrepancies in predicted refractive index (CitationFletcher et al. 1997), as shown in . The parameters are estimated for carbon particles by fitting the model to measurements of extinction or scattering at several wavelengths. Observations are often insufficient to constrain the parameters, and the fitted values do not agree between studies. A second disadvantage is the possible inadequacy of the dispersion equations to represent LAC. Graphite may contain only the three electron types listed above, but LAC has a more complex structure. Finally, the model predicts features that are not observed in atmospheric LAC, such as the 450-nm minimum in k and the large magnitude of n.
4.2.3. Medium-Range Order and the Optical Gap
Band-gap theory was first used by CitationTauc et al. (1966) to explain the absorption behavior of germanium. Formally, the theory follows from the oscillator theory of interaction between a material and radiation. The optical gap is the energy required for an electron to reach the first excited state: the difference between the highest-energy occupied molecular orbital and the lowest-energy unoccupied molecular orbital. Photons of higher energy may cause electronic transitions; photons with lower energy cannot cause transitions and are not absorbed. The optical-gap concept has been used to explain the electronic behavior of carbon containing many different types of electrons. The theory has been applied to flame-generated carbon (CitationMinutolo et al. 1996), amorphous carbon (CitationMcKenzie et al. 1983; CitationRobertson 1991), and emissions from burning of solid fuel (CitationBond 2001).
The absorption coefficient α follows the relationship
The structure of amorphous carbon on the scale of several atoms is known as medium-range order (as opposed to order in an entire crystal or on an atomic scale). Both theory and research indicate that electronic properties are controlled by this medium-range order—specifically, the number of sp 2-bonded rings (CitationRobertson 1991) that are adjacent or clustered together. The optical gap for benzene, with one ring, corresponds to a wavelength of about 200 nm. As adjacent rings are added, the optical gap decreases and photons of lower energy or longer wavelength can be absorbed. For material with many adjacent aromatic rings, the optical gap approaches zero. CitationPlatt (1949) explained this shift theoretically by postulating a classical wavefunction directed around the perimeter of adjacent rings. As the perimeter's length increased, the energies of this wavefunction would become more closely spaced. CitationCoulson et al. (1959) also demonstrated this concept with a computational method.
The finding that the extent of sp 2-islands governs the optical or electronic properties is an important one, and has been confirmed in several clever and detailed experiments (CitationChhowalla et al. 2000; CitationChen and Zhao 2000; CitationChoi et al. 2001). Increasing island size decreases the optical gap and increases absorption. The optical gap affects the real part of the refractive index as well (CitationBouzerar et al. 2001).
The sp 2-bonded clusters are surrounded by sp 3-bonded carbon. Unlike optical properties, structural properties of amorphous carbon are controlled by these sp 3-containing boundaries. Manufacture of amorphous carbon has capitalized on the ability to control optical and physical properties separately by manipulating the molecular form. For example, a decrease in sp 2 bonding and an increase in optical gap can be obtained by adding hydrogen to the formation environment (CitationJäger et al. 1999).
The transformation between carbon with primarily sp 3 bonds and that with sp 2 bonds is often called graphitization, and is promoted by elevated temperatures. This transition has been studied to understand how coal changes when it is heated (e.g., CitationOberlin 1984) and to transform amorphous carbon (e.g., CitationSattel et al. 1997). It is also important in determining the optical properties of flame-generated carbon particles.
A variant of graphitization occurs as particles pass through flames, with non-graphitic particles observed low in the flames (CitationWersborg 1975; CitationSaito et al. 1991; Citationd'Alessio et al. 1992; CitationDobbins et al. 1994). These particles may appear yellow to brown. Sooting, when these tarry precursor particles transform to a more graphitic substance, appears to occur rapidly (Citationvan der Wal 1996). The particles become even more like graphite as they remain in the flame longer (CitationMuñoz and Charalampopoulos 1998). The dominant mechanism of soot formation might affect the molecular form of the material, and hence its optical properties. For discussions of soot formation mechanisms, we suggest the standard work of CitationGlassman (1988); reviews by CitationHaynes and Wagner (1981) and CitationKennedy (1997) on formation kinetics; CitationSmith (1981) on formation in diesel engines, or CitationLahaye and Ehrburger-Dolle (1994) on carbon black formation.
4.2.4. Final Comments on Structure
The role of sp 2 bonds and cluster size explains why H/C ratio is a reasonable but imperfect predictor of absorption. Hydrogen is a likely partner for carbon with sp 3 bonds, but sp 2-bonded carbon has no such partners. LAC, however, is chemically complex, and hydrogen content is not a perfect proxy for the number of non-carbon constituents or sp 2 bonds. Even if the fraction of sp 2 bonds were known, chemical composition is not uniquely correlated with cluster size,
Only processes that affect sp 2 cluster size or other absorbing structures can change the absorptive properties. At atmospheric temperatures, addition to or destruction of these stable clusters proceeds slowly, although changes in optical properties may occur (CitationGelencser et al. 2003). Cluster size can change at elevated temperatures even if overall chemical composition does not (CitationChhowalla et al. 2000). The refractive index is most likely to be established or altered in two environments: formation during combustion, and measurement of elemental carbon by thermal analysis techniques.
5. MORPHOLOGY: FROM BULK OPTICS TO ABSORPTION BY PARTICLES
Absorption and scattering cross sections are common measures of particles' interaction with light. While mass absorption cross section, or MAC, is not the only measure of interest, it serves as one convenient proxy for the relationship between radiative transfer and the aerosol mass represented in models. In this section, we discuss the relationship between absorption and scattering cross sections and a particle's chemical composition (represented by refractive index), shape, and size.
Mie theory predicts absorption and scattering by spherical particles (Citationvan de Hulst 1957; CitationKerker 1969; CitationBohren and Huffman 1983). Other theories have been discussed for non-spherical particles (e.g., CitationJones 1979; CitationBerry and Percival 1986; CitationNelson 1989; CitationMountain and Mulholland 1988; CitationChen et al. 1991; CitationDobbins and Megaridis 1991; CitationKöylü and Faeth 1994; CitationFuller 1994; Mackowski et al. 1994; CitationFarias et al. 1995; CitationMishchenko et al. 1996; CitationSorensen 2001; to name just a few). We do not review these theories, except to determine the knowledge of the refractive index, size and shape, and single-particle homogeneity required to model absorption and scattering.
For this section only, we will present results as volume absorption cross sections—that is, cross section per particle volume, rather than per particle mass. Density does not enter into calculations of absorption and scattering cross sections, and is determined with unrelated measurements. When normalized to volume instead of mass, absorption cross section depends only on size and refractive index. For volumetric absorption cross section, we adopt the somewhat awkward units of m2/cm3. MAC is easily obtained by dividing by an appropriate density.
5.1. Spherical, Homogeneous Particles
The equations presented by Gustav Mie predict absorption and scattering from the complex refractive index and size of the particles and the wavelength of incident light. The solutions to the electromagnetic equations in spherical coordinates are formulated in terms of the non-dimensional parameter x = π d/λ, where d is the particle diameter.Footnote 2 They contain series expansions and are usually calculated with computer codes provided by CitationDave (1970) or CitationBohren and Huffman (1983, BHMie). A listing of the available codes, including those by Warren Wiscombe, is presently at http://atol.ucsd.edu/∼pflatau/scatlib/scatterlib.htm
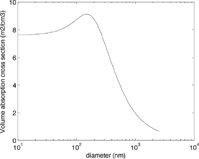
shows the type of calculation results provided in several papers (CitationBergstrom 1973; CitationRoessler 1982; CitationHorvath 1993a; CitationMartins et al. 1998; CitationFuller et al. 1999). Cross section is plotted versus particle diameter for one complex refractive index and wavelength; sometimes results for a few different refractive indices are presented. Common features include constant MAC for very small particles (diameters below about 100 nm), uniformly-decreasing MAC for larger particles (diameters above about 300 nm), and a higher peak in between. The small-particle and large-particle behavior can be understood geometrically; if light can penetrate to the center of the particle, the entire particle mass participates in absorption. If it cannot, then only the particle's skin contributes to absorption, and MAC decreases roughly as d −1. The peak between the two has no such geometric interpretation, but follows from the Mie solutions.
FIG. 4 Volume absorption cross section versus diameter. Refractive index is 1.56–0.47i from CitationDalzell and Sarofim (1969), wavelength is 550 nm. These results are often divided by the particles' material density and presented as mass absorption cross section. (We favor other refractive indices to represent pure LAC; see Section 7.)
For particles small relative to the wavelength, an approximation provides simple equations for the scattering and absorption cross sections (C) per particle volume (v) in units of inverse length (Citationvan de Hulst 1957; CitationKerker 1969; CitationBohren and Huffman 1983):
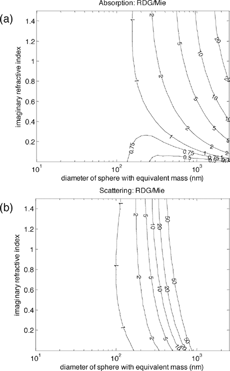
, not limited to small particles, shows how volume absorption cross section varies with diameter and refractive index. Imaginary and real parts of the refractive index are varied separately in and , respectively. is equivalent to a horizontal cross section of one of these contour plots. Apparent in are the constant absorption cross section for small particles and the decrease at larger diameters. The intermediate peaks also appear, and the figure confirms that there is a relationship between imaginary refractive index and absorption for small particles. It also shows that absorption depends mainly on size for particles larger than about 150–200 nm.
FIG. 5 Contour plots of volume absorption cross section at a wavelength of 550 nm, as a function of (a) diameter and imaginary refractive index, assuming a real refractive index of 1.55; and (b) diameter and real refractive index, assuming an imaginary refractive index of 0.55. These refractive indices are similar to those measured for LAC (see ). The calculations are for a narrow polydispersion of particles (geometric standard deviation of 1.1). Choosing a polydispersion eliminates some of the Mie “wiggles that are observed in true monodispersions but are likely never found in atmospheric aerosol.
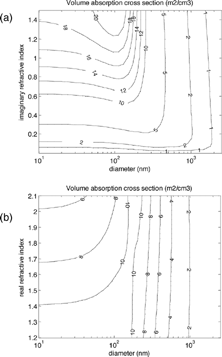
shows that real refractive index also changes absorption, because it affects the amount of light that can enter a particle. For small particles, an increase in real refractive index is associated with a decrease in absorption. For large particles with non-negligible absorption, all the light that enters the particle is absorbed and the absorption limit is a weak function of the real and imaginary refractive indices (CitationKerker 1969; CitationBergstrom 1973; CitationBohren and Huffman 1983).
demonstrates that the real and imaginary refractive indices are both important in calculating absorption. To be sure, real and imaginary refractive indices do not vary separately and should never be so treated; both depend on the material's molecular structure.
5.2. Aggregates
When first emitted, LAC particles are agglomerates of primary spherules. The structure of these aggregates can be described by relationships that are termed “mass-fractal,” and the optics are discussed by several authors (CitationDobbins and Megaridis 1991; CitationRogak et al. 1993; CitationKöylü and Faeth 1994; CitationSorensen 2001). Scattering by these aggregate particles is not like that of spherical particles, and corresponds more closely to that exhibited by flame-generated particles (CitationKöylü and Faeth 1994; CitationChoi et al. 1995). To summarize the theory, an aggregate is described by a relationship between N (the number of aggregated primary particles), and a characteristic size, R g (the radius of gyration):
The value of D f appears to vary slightly with combustion or generation conditions (CitationRoessler and Faxvog 1981; CitationSchnaiter et al. 2003). For freshly generated soot, D f is usually around 1.8 for both measured (CitationKöylü and Faeth 1994; CitationNyeki and Colbeck 1995; CitationSorensen and Feke 1996; CitationLee et al. 2002a) and simulated (CitationMountain and Mulholland 1988) agglomerates. This value of D f also corresponds to many aggregate particles in the urban atmosphere (CitationKatrinak et al. 1993). Values of D f for carbon black generally lie between the expected values for a disk and a sphere (CitationMedalia and Heckman 1969). While the fractal dimensions of fresh particles are fairly well known, the same particles have not been as thoroughly characterized after residence in the atmosphere. The particles collapse from lacy structures to more compact ones when they are wetted (CitationHallett et al. 1989; CitationColbeck et al. 1990; CitationRamachandran and Reist 1995; CitationNyeki and Colbeck 1995) or after aging (CitationSchnaiter et al. 2003), a change that corresponds to an increase in fractal dimension. Collapse affects both absorption and scattering, and is one explanation for a decrease in absorption with atmospheric lifetime (CitationLiousse et al. 1993).
The primary spherule size, d, also depends on particle generation: it lies between 20-50 nm for many soots (e.g., CitationKöylü and Faeth 1992; CitationClague et al. 1999; CitationLee et al. 2002a; Hu and Koylu 2004) but is just a few nanometers for spark-generated soot (CitationWentzel et al. 2003). Some particle diameters assumed in climate models (e.g., Citationd'Almeida et al. 1991) are similar to those of primary spherules, not entire particles.
The simplest method of predicting absorption and scattering for aggregates is the Rayleigh-Debye-Gans (RDG) theory, which assumes that multiple scattering and interactions between particles are negligible. For an aggregate of N component spherules, the theory can be summarized as follows: (1) Scattering and absorption by aggregates can be represented as functions of scattering and absorption of a single spherule; (2) Aggregate absorption is N times the sum of absorption by a spherule; (3) Aggregate scattering scales approximately as N 2 times the sum of scattering by spherules. Further details can be found elsewhere (CitationDobbins and Megaridis 1991; CitationKöylü and Faeth 1994; CitationSorensen 2001); the paper by CitationSorensen (2001) provides an admirable review of the state of knowledge. RDG theory predicts that particles with D f < 2 (such as freshly-generated combustion soot) absorb about the same as and scatter more than spheres of equivalent mass, particularly when aggregates contain a large number of spherules.
Another method, more accurate but computationally intensive, involves calculating the electromagnetic field considering the spherule interactions, and integrating around the aggregate to determine scattering (e.g., CitationJones 1979; CitationFuller 1994, and references therein). By comparing RDG theory with more exact calculations, several authors (CitationDrolen and Tien 1987; CitationNelson 1989; CitationFuller 1994; CitationFarias et al. 1995; CitationMackowski 1995; CitationFarias et al. 1996; CitationSorensen 2001) suggest that it reasonably approximates scattering and absorption of combustion-generated particles at visible wavelengths. CitationFarias et al. (1996) found that errors in scattering predicted by RDG are lower than 30% for common values of refractive index and size.
Other reports disagree with the finding that RDG estimates absorption well. CitationMackowski (1995) showed that the RDG simplification could underpredict absorption at infrared wavelengths. CitationFuller et al. (1995) calculated that the interaction between neighboring spherules could increase absorption by about 30% above the sum-of-spheres approximation for a small, compact group of spherules. CitationIskander et al. (1991) showed that this increase could range from zero to 50%, depending on the number and size of primary spherules.
shows the ratio between RDG and Mie theory for particles of equivalent solid volume or equivalent mass. (This ratio is useful for relating scattering and absorption cross sections to particle mass, but a different relationship is needed when particle size is measured by mobility methods.) If a particle's solid volume is lower than that of a 100-nm sphere, then calculations assuming spherical particles give reasonable estimates of scattering and absorption. For larger particles, the spherical-particle assumption can greatly underpredict both absorption and scattering. Because aggregate absorption is the sum of absorption by small particles, the small-particle limit for light absorption applies even to large particles, and the lower MAC observed in for large particles no longer applies. As an aggregate collapses and becomes more like a sphere, the absorption decreases.
FIG. 6 Ratio between predictions of Rayleigh-Debye-Gans aggregate theory and Mie theory for two particles of equivalent mass. Where no contours appear, ratio is constant. Assumptions: real refractive index 1.55, primary spherule diameter 25 nm, wavelength 550 nm.
The RDG formalism may not be appropriate for collapsed atmospheric particles with D f > 2, but it should be used to interpret measurements on suspended particles in flames, whence many inferences of refractive index originate. In particular, the wavelength dependence of scattering may be affected by the particles' form.
5.3. Addition of Negligibly Absorbing Material
A particle that absorbs light strongly at the beginning of its atmospheric lifetime may acquire negligibly absorbing compounds when vapors condense on it or when it coagulates with other particles. Absorption is predicted to increase as a result of this mixing (CitationKattawar and Hood 1976; CitationAckerman and Toon 1981; CitationChylek and Hallett 1992; CitationChylek et al. 1995; CitationHaywood and Ramaswamy 1998; CitationJacobson 2000). Our investigation of this issue, originally intended for inclusion here, evolved to extend beyond the review nature of this paper and will be provided elsewhere (CitationBond et al. 2005). Here, we review only the basic ideas and some cautions. The terminology used in this section is defined in Section 3.
Absorption calculated for a particle ensemble depends on how the mixing between strongly-absorbing and negligibly absorbing material is represented (CitationAckerman and Toon 1981; CitationChylek and Hallett 1992; CitationChylek et al. 1995; CitationHaywood and Ramaswamy 1998; CitationJacobson 2000). For example, CitationJacobson (2000) calculated that radiative forcing by LAC was lowest in an external mixture (+0.27 W/m2), twice as high in a more realistic shell-and-core model (+0.54 W/m2), and highest in a volume mixture (+0.78 W/m2).
The volume-mixture idealization is one of the most common methods of representing mixing, but it is one of the poorest. Indices of refraction are produced by weighting the real and imaginary parts of each component's refractive index by the volume fraction, and Mie calculations are performed using these calculated refractive indices. This method is presently used in most, but not all, climate models that report forcing by internally-mixed particles (e.g., CitationHaywood and Shine 1995; CitationHaywood and Ramaswamy 1998; CitationMyhre et al. 1998; CitationChung and Seinfeld 2002). CitationJacobson (2000) pointed out that the volume-mixture model is physically inconsistent for small absorbing carbon particles, which do not dissolve. Section 4 suggests another inconsistency: distributing absorbing material evenly throughout a particle would disrupt the medium-range order that governs absorption. A more detailed treatment (CitationBond et al. 2005) suggests that much of the enhanced absorption due to volume mixing is artificial and would not be replicated by real particles.
Other mixing rules have been suggested to provide effective refractive indices of mixed material (CitationHeller 1965; CitationGraham 1974; CitationBohren and Huffman 1983). The Bruggeman approximation may be best for void-containing soot pellets (CitationFelske et al. 1984), while the Maxwell Garnett approximation might be used for small black particles suspended in water (CitationLesins et al. 2002).
Although perfect mixing of LAC and negligibly absorbing material within a particle is implausible, heterogeneously-mixed particles are likely. CitationAckerman and Toon (1981) described a likely situation: absorbing carbonaceous particles, often called cores or inclusions, surrounded by shells or coatings of negligibly absorbing material. Natural mechanisms that cause coatings include ionic compounds condensing on LAC particles and promoting water uptake (CitationRedemann et al. 2001), and organic compounds with low vapor pressures condensing on these particles. Model results predict that most LAC is mixed with other substances within 5 days of emission (CitationJacobson 2001). Detailed examinations of ambient particles demonstrate that such mixed particles are common (e.g., CitationOkada 1983; CitationLiousse et al. 1993; CitationPósfai et al. 1999; CitationNaoe and Okada 2001; CitationHeintzenberg et al. 2002; CitationWhiteaker et al. 2002).
Laboratory studies have shown that absorption can be enhanced by 35% when particles are coated (CitationSchnaiter et al. 2003). Very high enhancements require particular conditions, such as a very small inclusion located exactly at the center of a large particle (CitationFuller 1995; CitationFuller et al.; 1999). Some enhancement of absorption is likely, but extreme enhancement is unlikely. More specific guidelines regarding absorption enhancement are presented elsewhere (CitationBond et al. 2005).
5.4. Summary and Cautions
Particle size and not imaginary refractive index primarily determines MAC for spherical LAC particles with diameters greater than about 100–150 nm. Particle size is always important in determining scattering. If large particles behave optically as spheres, then accurate size measurements may be sufficient to represent their scattering and absorption. For smaller spherical particles, and for aggregates of such smaller particles, both real and imaginary parts of the refractive index must be known.
For aggregates, the particle size and fractal dimension D f govern whether particle diameter or refractive index dominates absorption. However, the quantities of large (> 150 nm), spherical LAC particles in the atmosphere are not usually measured. Exceptions include size-resolved measurements of elemental carbon (CitationKleeman et al. 1999; CitationDillner et al. 2001) and density measurements that may indicate the number of fluffy particles (CitationMcMurry et al. 2002).
Finally, we suggest caution in using the concept of the effective refractive index for atmospheric aerosol. The effective refractive index is a value that causes a particular theory to agree with measured scattering, absorption, or other observations. Many combinations of morphology and refractive index can lead to the same MAC, as noted over 20 years ago (CitationAckerman and Toon 1981). Scattering and absorption are affected by mass fraction of LAC, particle size or fractal dimension, and coating. Any of these may change while the particles reside in the atmosphere, resulting in different effective values at different locations. Mixing models (CitationMallet et al. 2004), or black carbon fractions (CitationFiebig et al. 2002) that agree with optical properties of the aerosol at one location may not be valid at other locations, and the resulting predictions of scattering and absorption may be in error. Investigators in astrophysics, attempting the inverse task of identifying interstellar matter from its extinction, have concluded that the range of possible solutions precludes identifying the form or content of the material (CitationMichel et al. 1999). Fortunately, atmospheric researchers have the option of capturing the material and measuring its properties with other methods.
6. TOOLBOX: MEASUREMENT METHODS
Instruments used to measure light absorption by particles in the atmosphere are discussed elsewhere (CitationBohren and Huffman 1983; CitationHorvath 1993a; CitationHeintzenberg et al. 1997). These descriptions are not repeated here. We limit our discussion to two topics: first, developments that occurred after earlier reviews, and second, assumptions in each measurement method that affect interpretation of the results.
For atmospheric aerosol, light absorption is a small fraction of the total extinction. Measuring absorption has proven more difficult than measuring scattering, because unlike scattered light the absorbed photons have disappeared and cannot be sensed. We separate the discussion of measurements into those that are direct and those that are remote. The meaning of these terms varies among scientific fields. We define direct methods as those that measure changes in transmission or temperature due to absorption, and remote methods as those that measure backscattering or forward scattering and use theories to infer the aerosol properties.
No method of measuring absorption is specific to LAC particles. In contrast to gas measurements, which can select a particular absorption feature or line unique to a particular gas,Footnote 3 these measurements are sensitive to any particle that absorbs. Many measurements are made at a single wavelength, frequently 550 nm, while others cover just a few wavelengths. Extrapolation beyond the measured wavelengths requires assumptions about the refractive index and particle nature. Instruments are developing to cover a wider range of wavelengths.
It is also important to remember that absorption is not a proxy for light-absorbing carbon mass. Many investigators assume a constant ratio between absorption and LAC mass. That practice is only valid when the particles to be measured always have the same absorptive properties as the particles originally used to determine the ratio. The present review, and the field studies summarized in Section 8, suggest that the mass absorption cross section is not constant.
6.1. Direct Measurement Methods
Direct measurement methods can measure either radiation transmitted through a layer of particles, or the change in temperature caused by absorption. summarizes the most common direct techniques, including filter-based measurement methods that are equivalent in principle but yield somewhat different responses to absorption.
TABLE 2 Direct methods of measuring absorption by particles
6.1.1. Filter-Based Measurements
The integrating plate, sphere, and sandwich as well as the laser transmission method are laboratory instruments that determine absorption by aerosols collected on filters. The term integrating refers to the fact that these methods collect or integrate the scattered light, so that absorption alone should reduce transmitted light. The aethalometer and particle soot absorption photometer continuously measure absorption using the same principle as the integrating plate; because of their rapid time response, they have been used at ground sites or on aircraft. Some models measure at multiple wavelengths (seven for the aethalometer, three for the particle soot absorption photometer).
Except for carefully designed integrating spheres, most of these techniques suffer from errors, because aerosol scattering does affect transmitted light despite the instrumental design (CitationHitzenberger 1993; CitationHorvath 1993b; CitationPetzold et al. 1997; CitationBond et al. 1999). Also, absorption by particles collected on a filter is increased above that of the same particles in suspension, because multiple scattering by the filter allows more than one chance for a photon to be absorbed. The enhancement depends on the filter type because of differences in filter scattering and particle embedding (CitationBond et al. 1999; CitationArnott et al. 2005). summarizes some of these enhancement factors for different filters.
TABLE 3 Correction factors for absorbing particles collected on filter
Finally, a new development measures both reflectance at multiple angles and transmittance for particles on a filter. The technique, known as multi-angle absorption photometry, may correct for some of the artifacts associated with other filter-based measurements (CitationPetzold et al. 2005).
6.1.2. Flux Divergence
Transmitted and scattered radiation can also be measured for an aerosol layer in the atmosphere. A layer's absorption is determined by measuring the net solar flux above and below a layer (CitationPilewskie et al. 2003). The method is actually quite old, dating to the 1940s. Both broad-band (CitationBush and Valero 2002) and discrete-band (CitationHignett et al. 1999; CitationPilewskie et al. 2003; CitationWendling et al. 2002) instruments have been used. Total absorption can be obtained by measurements alone, but calculation of the atmospheric absorption coefficient, or absorption per atmospheric volume, requires a radiative transfer model (CitationBergstrom et al. 2003). This method requires clear skies, aerosol layers that are stable with respect to time and space, large aerosol depths, and an underlying surface of uniform albedo (CitationPilewskie et al. 2003).
6.3.1. Photoacoustic and Calorimetry
Two instruments measure a change in temperature resulting from absorption of light and redistribution of energy. These are the photoacoustic method (CitationTerhune and Anderson 1977; CitationFoot 1979; CitationAdams et al. 1988; CitationArnott et al. 1997) and the calorimeter (CitationHänel and Hildebrant 1989). The photoacoustic technique reacts to the amount of light actually absorbed, but relies on converting that absorption to a change in pressure.
In the past, these instruments have not been used widely, in part because they are more complex than filter-based measurements (CitationMoosmuller et al. 1998), and in part because of their high detection limits. Recent advances have allowed these instruments to measure absorption at atmospheric concentrations, and they are presently very promising because they disturb the aerosol less than filter-based measurements. These instruments also respond to gaseous absorption; in fact, the ability to calibrate with absorbing gases is one of their advantages.
6.1.4. Difference (Extinction Minus Scattering)
Subtracting scattering from total extinction (e.g., CitationLewis and Dzubay 1986) to obtain absorption has a long history as well. Compared to other techniques, such as filter-based collection and photoacoustic, this method requires the least perturbation of the aerosol, and thus has been used as a reference method in calibration (CitationHorvath 1993b; CitationBond et al. 1999; CitationSheridan et al. 2005). However, the method uses a small difference of two large numbers; even corrections for angular truncation required to adjust scattering measurements by nephelometers (CitationAnderson and Ogren 1998) might be of the same magnitude as the absorption. Further, at atmospheric concentrations, a long extinction path is required to obtain sufficient sensitivity, and the sample volume needed is much greater than that needed for scattering measurements. Thus, extinction and scattering may be measured on air volumes containing different aerosol.
The cavity-ring-down instrument has recently been applied to extinction by atmospheric aerosol (CitationStrawa et al. 2002). This instrument uses a pulsed laser beam that travels a number of times between two mirrors on both sides of a sensing volume. The path length can be made large enough to accurately determine extinction, and scattering is determined simultaneously. This instrument eliminates some of the sensitivity issues of the difference method, and can be mounted in an airplane.
6.1.5. Instrument Comparisons
Measurement techniques have been assessed by comparing the instruments' response to the same aerosol. The first study took place over 20 years ago (CitationGerber and Hindmann 1982), and there has been a series of comparisons since that time (CitationEdwards et al. 1983; CitationClarke et al. 1987; CitationFoot and Kilby 1988; CitationHansen and McMurry 1990; CitationCampbell et al. 1995; CitationBond et al. 1999; CitationHitzenberger et al. 1999; CitationWeingartner et al. 2003; CitationSheridan et al. 2005.) Other evaluations have occurred in connection with specific field programs (e.g., CitationReid et al. 1998; CitationLavanchy et al. 1999). The studies generally show some degree of scatter between the methods, never demonstrating perfect correlation. Each method has advantages, and the question of which measurement should be considered the reference invariably arises.
6.2. Remote Measurements
Remote measurements infer absorption from scattered atmospheric radiationFootnote 4 , and both space and surface observations have been used for this purpose (CitationKaufman et al. 2001; CitationKaufman et al. 2002a). Satellite instruments measure reflection from the atmosphere and surface, while transmitted and downward-scattered radiation are measured at surface sites. Neither measurement can determine the MAC or refractive index of light-absorbing particles without additional measurements of chemical composition.
Remote methods invert the equation of radiative transfer to determine aerosol optical depth and, sometimes, aerosol size; they are known as inversion techniques for that reason. The procedure cannot retrieve a full description of the aerosol, including quantity, size distribution and refractive index. Instead, the assumption is made that a fixed set of properties is associated with each type of aerosol, such as urban pollution or desert dust. This assumed description is termed an aerosol model, and is implicit even when the inversion accounts for mixed or regionally specific aerosol types. Inversion accuracy is limited by variation in aerosol properties; a constant set of aerosol properties cannot represent any location.
As long as the aerosol model defines the complex refractive index, the technique does not measure absorption, but identifies a hypothetical aerosol consistent with the measured radiance. The earliest aerosol models used in satellite retrievals assumed spherical particles with no absorption (Gordon et al. 1983; Gordon et al. 1997). Other models assigned distinct properties to aerosol with different origins (CitationShettle and Fenn 1976). Current inversions of satellite data use an approach in which results of radiative transfer calculations with specific aerosol models are performed for various optical depths, solar angles and satellite observing angles, and saved in a look-up table. The table is then used to infer the aerosol present by obtaining the best fit of the observed satellite radiances to combinations of values in the table (CitationKaufman and Tanré 1998).
6.2.1. Satellite Measurements
Early satellite instruments measured radiance at only one wavelength band, and thus retrieved only one unknown variable: optical depth (CitationHusar et al. 1997). More recently, multi-wavelength satellite measurements have identified the location and amount of absorbing particles using a method known as the TOMS technique (Gordon et al. 1997; CitationTorres et al. 1998). TOMS (Total Ozone Mapping Spectrometer) was designed to measure ozone at ultraviolet wavelengths, but a feature that could not be explained by ozone absorption was found to indicate the presence of absorbing aerosols. Several research groups have applied the TOMS technique to data from other satellites to identify the location of absorbing particles. Quantifying the amount of aerosol absorption by this technique has proven difficult (CitationQuijano et al. 2000; CitationMahowald and Dufresne 2004), and its accuracy has not been established (CitationTorres et al. 2005).
The wavelength dependence of the optical depth can assist in determining whether the aerosol is composed of absorbing small particles (assumed to contain LAC), absorbing large particles (assumed to contain dust), negligibly absorbing small particles (sulfates) and negligibly absorbing large particles (sea salt) (CitationHigurashi and Nakijima 2002; CitationHsu et al. 2004). Recently, CitationHsu et al. (2004) combined the TOMS technique with detailed information about surface reflectance and were able to infer different absorptive properties for dust from different deserts. Similar studies may examine sources of LAC in the near future.Other techniques envisioned to measure absorption directly have been proposed (CitationKaufman et al. 2002b) but are not yet realized.
6.2.2. Surface-Based Measurements
The most extensive remote surface measurements come from the AERONET network (e.g., Holben et al. 2000). Directly-transmitted beam and skylight radiance at specific wavelengths are used to estimate the optical depth and single-scattering albedo. Again, the technique involves a suite of aerosol models combined with radiative-transfer calculations. While comparison with other measurements is difficult because the single-scattering albedo is derived for the entire atmospheric layer, the results have been shown to be consistent with likely spectrally dependent properties of LAC and dust (CitationDubovik et al. 2002). There are hundreds of operating sites around the world (see the AERONET web site http://aeronet.gsfc.nasa.gov for details).
7. MEASURED OPTICAL PROPERTIES OF FLAME-GENERATED CARBON
In this section, we discuss the measurements that have provided the optical properties and inferred refractive indices of flame-generated carbon. This section most directly addresses the tasks outlined in the introduction, although the material presented in earlier sections was required in order to conduct the following discussion. We tabulate LAC refractive indices and absorption cross sections, seeking explanations for variations in these properties. Our goal is to determine the appropriate optical properties for atmospheric LAC.
7.1. Structure and Morphology of Absorbing Materials
To the best of current knowledge, strongly absorbing carbon particles in the atmosphere are produced exclusively by combustion of carbon-based fuels. Major sources include diesel engines, accidental and intentional burning of biomass, and coal combustion in industrial and domestic applications (CitationBond et al. 2004). Ideally, optical properties used for atmospheric LAC would be determined from particles emitted from these types of burning. In fact, refractive indices have been derived from measurements of coal, graphite, carbon black, and particulate carbon from spark discharge and laboratory flames. Which of these materials are most like atmospheric LAC? We should choose their optical properties for use in climate models.
Indicators that can suggest whether two materials are similar include elemental content, molecular structure, size, and shape. As discussed in Section 3, elemental content does not uniquely indicate the medium-range order that governs absorption. However, differences in elemental content might suggest that molecular structures, and hence absorptive properties, are not the same.
7.1.1. Carbon Black and Other Controlled Flame Generation
Nearly three decades of research on the microstructure of carbon black (neatly reviewed by CitationHeckman 1964) resulted in enough confidence in its form that it was selected as a standard for electron microscopy (CitationHeidenreich et al. 1968). The form is an onion-like structure with an outside armor of layered graphitic platelets and an inner core of amorphous material. The platelet layers are not parallel as they are in graphite, but disordered and wrinkled, a condition termed turbostratic (CitationBiscoe and Warren 1942).
Both carbon black and LAC are formed when carbon-containing fuels decompose at high temperatures, and both are aggregates. For that reason, carbon black is probably similar to LAC, although material vaguely called “soot” may also contain both negligibly-absorbing carbon and ash (CitationWatson and Valberg 2001). There is a wide variety of carbon black formation processes (CitationMarsh et al. 1971), used to manufacture material with specific physical and optical properties (CitationDonoian and Medalia 1967; CitationMedalia and Heckman 1969). Investigators who use carbon black as a surrogate for LAC would do well to identify the type examined. However, CitationPalotás et al. (1996b) reported that at least one type of carbon black is structurally similar to diesel soot. CitationMedalia and Richards (1972) found that particle size could account for most of the variability in absorption.
The nature of a carbon particle depends on the pathway it has followed through the flame region and the exhaust. For example, temperatures as low as 500°C can affect the structure of partially aromatized amorphous carbon (CitationJäger et al. 1998; CitationCelzard et al. 2000; CitationChhowalla et al. 2000). Higher temperatures affect both structural and optical properties. These changes may be kinetically limited, and the final molecular form depends not only on maximum temperature but also on the residence time at each temperature.
Quenching, or cooling from flame temperature, stops the reactions that affect composition and optical properties. LAC from common combustion is quenched more slowly than carbon black (CitationClague et al. 1999). Spark-discharge soot is quenched even more rapidly than carbon black; it is disordered (CitationWentzel et al. 2003) and differs from diesel soot (CitationKirchner et al. 2003), probably because ordered graphitic layers did not have time to form. More orderly carbon black (CitationPalotás et al. 1996a) or diesel soot (CitationIshiguro et al. 1991) results from either oxidation or elevated temperatures (CitationSmith 1984; CitationBuseck et al. 1987; CitationJäger et al. 1998).
Other reactive compounds may be incorporated into the particles. Even different lubricating oils may affect the structure of LAC in a diesel engine (CitationDonnet et al. 1997). Inorganic materials may be present as well (CitationWatson and Valberg 2001; CitationLee et al. 2002b). The impurities may adsorb to the particle surface, possibly affecting the refractive index (CitationChang and Charalampopoulos 1990). They may also be incorporated in the molecular structure of LAC itself (CitationAkhter et al. 1985a, Citation1985b; CitationJäger et al. 1999). Commercial carbon blacks are formed in controlled environments, and contain less hydrogen and oxygen than soot from oil furnaces (CitationMedalia and Rivin 1982) or diesel soot (CitationClague et al. 1999). However, CitationClague et al. (1999) found little difference between diesel soot and carbon black using several advanced techniques.
Because many aspects of the formation environment affect material properties, carbon black and flame-generated particles should be most like atmospheric LAC when the formation conditions are similar to those in widely practiced combustion. Much of the literature on optical properties of soot (e.g., CitationDalzell and Sarofim 1969; CitationLee and Tien 1981; CitationChang and Charalampopoulos 1990; CitationKöylü and Faeth 1994; CitationWu et al. 1997; CitationMulholland and Choi 1998) has examined particles formed in flames with simple structures, such as diffusion flames. The fuels used are more pure, and the combustion is more controlled, than those in widespread sources such as diesel engines and burning of solid-fuels. Carbon black and laboratory soot may differ from LAC formed in a less pure environment with a different time-temperature history.
Both combustion soot and carbon black might contain material that would not be classified as LAC. Refractive indices of this mixed material would be lower than those of pure LAC. However, reported hydrogen contents are usually low, suggesting a high LAC content (CitationDalzell and Sarofim 1969; CitationWu et al. 1997). At least one type of carbon black is nearly 100% elemental carbon, as measured by thermal-optical methods (CitationSchauer et al. 2003). Some of the soot and carbon black measured in studies of optical properties is probably similar to pure, flame-generated LAC. However, these substances may differ from the impure, less-carefully generated LAC in the atmosphere.
7.1.2. Amorphous Carbon
As discussed previously, amorphous carbon covers a wide range of materials, with varying optical gaps controlled by sp2-cluster size (e.g., CitationSmith 1984; CitationRobertson 1991). The formation of this material is tightly controlled, and it is created in thin films rather than in aggregates of particles. Research on amorphous carbon helps to understand flame-generated carbonaceous material, but it is not like atmospheric LAC.
7.1.3. Graphite and Coal
Coal contains a high fraction of sp3 bonds as well as nitrogen and oxygen bridges between carbon atoms (e.g., CitationSmith et al. 1993). Like amorphous carbon films that become progressively graphitized as temperature rises, coals exhibit a continuum. If they have been exposed to higher temperatures and pressures, they have more graphitic bonds. These coals also have higher heating values, and are called “high-rank” in coal-science terms. Some optical properties depend linearly on H/C ratio (CitationErgun and McCartney 1960) or aromatic fraction (CitationWhite et al. 1987). Graphite and coal are both planar; graphite, containing exclusively sp2 bonds, lies at the most aromatized end of the coal continuum.
The electronic properties of particles emitted from diesel soot, which comprises a large fraction of atmospheric LAC, may lie along the continuum between high-rank coal and graphite. CitationJiang et al. (2002) reported that the conductivity of diesel soot was greater than that of high-rank coal, and that 13C-NMR results positioned it between coal and graphite.
There are at least two differences between graphite and carbon black. First, the interplanar distance in graphite is smaller than that in carbon black, a difference that disappears with increasing temperature (CitationFranklin 1951). Second, the platelets surrounding a particle have finite extent, and their optical properties may differ from those of planes with nearly infinite extent. CitationHeckman (1964) summarized literature indicating that these platelets become more like graphite, as particles increased in size. Such a change would affect optical properties, as discussed in Section 4. In support of this hypothesis, the much smaller spherules formed by spark-discharge appear less absorbing than diesel-generated particles (CitationSchnaiter et al. 2003).
Examining graphite and coal may be instructive in terms of understanding the optical properties of light-absorbing carbon. However, these materials differ too greatly even from carbon black to be taken as representative of atmospheric LAC. The difference appears in molecular structure, electronic properties, and form.
7.2. Inferred Refractive Indices
We now revisit the literature on inferred refractive indices, with the aim of determining which are most representative of atmospheric LAC. We emphasize that refractive indices are always inferred by assuming a theory and applying it to optical measurements. tabulates inferred values of refractive index, showing a bewildering range. For comparability, the real part n appears to vary from that of water to that of diamond; the imaginary part k varies from that of negligibly absorbing material to that of graphite.
TABLE 4 Published values of LAC refractive index
Although previous reviews on atmospheric aerosol have reported a wide range of refractive indices available for LAC, most of these tabulated values are traceable to only a few original studies. contains all the primary references incorporated into other frequently cited tabulations (CitationTwitty and Weinman 1971; CitationMedalia and Richards 1972; CitationDobbins and Megaridis 1991; CitationHorvath 1993a; CitationFuller et al. 1999), as well as the secondary references cited by these authors. also contains some values, mostly from combustion-oriented journals, that are not frequently cited in atmospheric literature.
Inferences that appear to generate new refractive index values are separated in . For example, CitationMedalia and Richards (1972) extrapolated data given by CitationErgun and McCartney (1960) to a different H/C ratio, and CitationHorvath (1993a) postulated data for particles containing 50% and 75% air based on measurements given by CitationJanzen (1979), but these values do not constitute new information. We have deliberately excluded values inferred from mass cross sections of absorption and scattering (e.g., CitationSchnaiter et al. 2003) because we use those values later in a closure comparison. We have eliminated inferences (e.g., CitationVaglieco et al. 1990; CitationKöylü and Faeth 1994) that rely on values drawn from earlier publications (CitationLee and Tien 1981; CitationDalzell and Sarofim 1969, respectively).
Before discussing the data in the table, we will review some cautions on the two most common methods of measuring optical properties of LAC. While many other methods can measure optical properties (e.g., CitationPalik 1991), the two we discuss are the most common for highly-absorbing material that cannot be deposited from the gas phase.
7.2.1. Cautions: Measuring Optical Properties of Compressed Solids
Optical properties of a bulk solid may be determined from reflectance at different angles or from ellipsometry, using the phase difference between the incident and reflected light. Accurate determination of the refractive index requires a specularFootnote 5 surface, and for that reason coal is polished before measurement. Flame-generated carbon is compressed into pellets to obtain such a surface. This procedure leads to two major uncertainties in the inferred refractive indices.
First, the refractive index that should be used in Mie or RDG theory is that of void-free LAC. CitationFelske et al. (1984) reported that pellets contain about 18% void, even after they were compressed at 280 MPa. They emphasized that the void fraction affecting optical measurements is near the pellet's surface. They pointed out that the Bruggeman approximation is more appropriate for compressed soot pellets than Maxwell Garnett approximation, but they also found that the inferred refractive index did not depend greatly on the chosen mixing rule.
The second problem is less tractable. Voids in the surface of a compressed solid are about as large as the wavelength of visible light. The surface is specular for infrared light but not for visible light (CitationJanzen 1979; CitationFelske et al. 1984). CitationStagg and Charalampopoulos (1991) found that inverting polarization measurements can correct for this problem, but that both real and refractive indices can be underestimated without correction. CitationMullins and Williams (1987) also estimated that accounting for surface roughness would increase the inferred value of k.
In summary, refractive indices inferred from reflectance should be considered suspect at visible wavelengths unless great care is taken. Both real and imaginary parts appear to be biased low if not corrected for void fraction or surface roughness.
7.2.2. Cautions: Measuring Optical Properties of Suspended Particles
Scattering and absorption by suspended particles has also been used to infer the refractive index. Studies have measured extinction alone or angular scattering of polarized light (e.g., CitationDobbins et al. 1983). The particles may be suspended within a flame (e.g., CitationChippett and Gray 1978), above a flame (CitationKöylü and Faeth 1994), or in a medium after collection (CitationJanzen 1979; CitationMullins and Williams 1987). The refractive index is inferred by obtaining a best fit to either Mie or RDG theory. Particle size distributions affect this determination and are usually estimated from microscopy (e.g., CitationChang and Charalamopopoulos 1990) or changes in transmitted light with wavelength (e.g., CitationLee and Tien 1981). CitationCharalampopoulos and Shu (2002) showed that ignoring polydispersity of the primary spherules could lead to slight underestimates and overestimates of the real and imaginary refractive indices, respectively.
As discussed in Section 5, the scattering behavior of aggregates differs from that of spheres, and inferences based on assuming spherical particles may be incorrect. If the particles are actually agglomerates, the effective refractive index obtained from scattering measurements is probably that of a void-containing particle (CitationDobbins et al. 1983). Particles early in the flame are thought to consist of polyaromatic hydrocarbons (CitationWersborg 1975; CitationVaglieco et al. 1990; CitationMinutolo et al. 1994), and the assumption of spherical particles may be justified. These young particles differ from the chain agglomerates that leave the flame. They may form part of the atmospheric aerosol, but their optical properties are not like those of the material currently classified as LAC.
shows that absorption cross section alone is insufficient to constrain refractive index. The same is true of extinction cross section; for example, 75-nm particles in air with m = 1.5 − 0.6i and m = 1.86 − 0.75i have similar cross sections. Obtaining enough data to fit theory to measurements requires either multiple wavelengths or multiple angles. One must be particularly cautious when inferences have assumed spherical particles. Mie theory represents neither the angular nor the spectral dependence of scattering by aggregates.
Inverting multi-wavelength measurements requires further constraints on the spectral dependence of the refractive index. Assumed relationships have included a constant refractive index (CitationJanzen 1979; CitationChippett and Gray 1978), dispersion equations (CitationLee and Tien 1981; CitationHabib and Vervisch 1988; discussed in Chapter 3), or Kramers-Kronig relations (CitationChang and Charalampopoulos 1990). The Kramers-Kronig relations are exact, but measurements over a wavelength range broad enough to support the calculation are frequently not made. Results constrained by the dispersion equations should be interpreted with caution for refractive indices at visible wavelengths. Data points at infrared wavelengths may strongly influence the fitted parameters, while a minimum in k at visible wavelengths is predetermined by the electron resonant frequencies, which are not treated as adjustable. CitationBoynton et al. (1968) reported difficulty matching dispersion theory to measured data across the entire visible and infrared range. Adjustments to dispersion constants proposed by CitationHabib and Vervisch (1988) are also based on infrared data.
7.2.3. Analysis of Refractive Indices
We now return to with the intent of constraining values to use for atmospheric LAC. We examine the variation further by plotting the real part n against the imaginary part k in . In the following discussion, the year associated with each reference will be followed by the number used to mark the relevant point in . The refractive indices of graphite are unlike those of the other materials, and we use only one point to represent the two values in .
FIG. 7 Refractive indices from . Numbers on each data point refer to reference numbers in the table. Curve marked “void fraction” assumes that LAC has a single refractive index and that variation can be expressed by the Bruggeman effective-medium theory. Curve marked “graphitization" shows variation in refractive index of carbon containing different sp2-bond content. See text in Section 7.2.3 for further explanation.
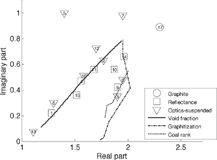
We now hypothesize that strongly absorbing carbon with a single refractive index exists, and that some of the variation in reported refractive indices results from void fractions in the material. If our hypothesis is correct, n and k should vary together. Void fractions affect both compressed solids (CitationGraham 1974; CitationFelske et al. 1984) and suspended particles (CitationDobbins et al. 1983) that are erroneously assumed to be spherical. We assume that the voids are air (n = 1.0, k = 0.0), and that the refractive index of LAC with little to no void is 1.95–0.79i. This refractive index corresponds to the properties of amorphous carbon heated to 750°C; at this temperature, the band gap has decreased to zero (CitationSmith et al. 1984). We chose this value instead of the refractive index of graphite, in which plane layers are more closely spaced. We solve the Bruggeman relationship at different void fractions to obtain the effective refractive index of LAC mixed with voids. The calculated relationship between n and k is marked “void fraction,” and corresponds to most of the data.
We do not assert that 1.95–0.79i is the refractive index of void-free carbon at 550 nm. It could represent carbon with voids, since it is very difficult to obtain void-free material. Alternatively, these values of n and k could both be too high; shows that most of the inferred refractive indices are lower. The value 1.95–0.79i merely provides agreement with many of the measurements. Other values that lie along the upper portion of the line could also be consistent with these measurements and are listed in . We consider this the likely range of refractive indices for LAC.
TABLE 5 Refractive indices that lie along the upper void-fraction line in , for 550 μm
According to the discussion in Section 4, increasing sp 2 cluster size also affects the imaginary refractive index strongly and the real refractive index slightly. Characteristics of this process are a rapid, steady increase in k, and a slight increase in n. As mentioned previously, some studies of in-flame soot measure particles before they aggregate. If graphitization and aggregation occur simultaneously, lower values of k may be expected for the particles that are early in the flame (CitationSaito et al. 1991). Among others, CitationSmith et al. (1984) and CitationSattel (1997) have verified this continuum of optics and structure.
The curved line in , marked “graphitization,” represents the changes in refractive index as sp2-clusters grow. This curve shows the measured change in n and k for a film of amorphous carbon heated from 250° to 750°C (CitationSmith 1984). CitationErgun and McCartney (1960) report values for coals of increasing rank that fall on a nearly identical line, labeled “coal rank.” Values that lie closer to the graphitization curve than to the void-fraction line are those of CitationChippett and Gray (1978, #3), CitationLee and Tien (1981, #8), CitationMullins and Williams (1987, #9), CitationMarley et al. (2001, #10) and CitationSenftleben and Benedict (1918, #14). Of these, #8 and #14 might be expected to be partially graphitized—#8 because it is taken low in an unusual flame, that of a burning solid, and #14 because the rapid quenching during arc-discharge does not result in fully graphitized carbon. The material studied in #3 and #9 should represent graphitized, aggregate carbon. Mysteriously, we were unable to reproduce the inferred refractive indices in these two studies given the data presented in the papers. Partially-graphitized carbon cannot represent these two data points, but they are not consistent with the data presented in the papers, either. The material in #10 (candle soot) may also differ from atmospheric LAC, particularly since quenching during collection may stop the reaction, but we do not have evidence to support that hypothesis.
One extreme outlier in (1.3–1i) is that of Erickson et al. (1964, #5). These authors admitted that their value was only consistent with the results of their angular-scattering technique. Other values closer to the void-fraction line can be derived from their data, including 1.8–0.8i and 1.9–0.75i. In the interest of fairness, we also re-analyzed the results of Janzen (1979, #7), whose results lie much closer to our void-fraction line. These data are consistent with a range from 1.5–0.91i to 2–1i, so this author could have just as easily produced a value that did not agree with the void-fraction line. It is interesting that the CitationJanzen (1979) paper criticizes earlier work, particularly that of CitationDalzell and Sarofim (1969), and is frequently used as support for disregarding reflectance-based measurements. Yet the extinction measurements of CitationJanzen (1979) are taken at a narrow range of wavelengths, and can no more constrain the real and imaginary refractive indices than do other studies.
Two points lie just above the void-fraction line, suggesting that a higher value for solid LAC might be appropriate. These data are those of CitationPluchino et al. (1980, #12) and CitationJanzen (1979, #7), and they correspond to measurements on fairly large carbon-black spheres (8 μ m and 75 nm, respectively). These spheres are much larger than the spherules that make up flame-generated carbon; as spherule size increases, the platelets forming the outer shell become more perfect and graphitized (CitationHeckman 1964). These large particles might have a higher imaginary refractive index than normal component spherules. While we appreciate the elegance of the approach in #12, these particles may overestimate the value of k for atmospheric LAC.
To summarize, some of the reported variation in refractive index is consistent with partial graphitization. Most of the variation is consistent with the idea that some of the material has voids, suggesting that a constant refractive index could represent solid carbon emitted from flames. We have not yet identified the point corresponding to void-free LAC, nor matched it with a density. Both of these tasks will be necessary in order to obtain a relationship between scattering and absorption and material mass.
7.3. Ensemble Properties: Absorption
In this section, we discuss the mass absorption cross section for freshly generated LAC. We assume that the ability to predict scattering and absorption by fresh combustion aerosol is a prerequisite to calculating optics of more complex, mixed aerosol. We wish to exclude the effects of atmospheric processing and mixing with negligibly absorbing substances, as well as possible problems in interpreting thermal-optical measurements intended to isolate LAC. We exclude ambient aerosol other than that sampled from traffic tunnels, because it could contain a large fraction of non-combustion aerosol.
summarizes absorption cross sections of LAC measured at or near combustion sources. Measured material includes laboratory aerosol, diesel engines (including traffic tunnels), and carbon black. The latter part of the table includes values cited in other reviews (CitationHorvath 1993a; CitationLiousse et al. 1996) that are not measurements. These include values calculated from Mie theory, secondary references and ambient aerosol. Reports that provide extinction (absorption plus scattering) rather than absorption alone are excluded. The values tabulated in these two reviews average about 9.5 m2/g. As shown in , there is no clear mode, but most of the values lie between 5 and 13 m2/g.
FIG. 8 Comparison of mass absorption cross sections tabulated (a) in previsous reviews, showing large variability, and (b) in this review for near-source measurements, with much smaller variability. Legend in Figure 8a: (1) Traceable to value reported. (2) Not traceable to value reported.
TABLE 6 Published values of LAC refractive index: original inferences, derived values and secondary references
We make two corrections: (Equation1) We adjust all measurements to a wavelength of 550 nm, assuming that cross section varies inversely with wavelength; this assumes that all particles examined are small relative to the wavelength. (2) We adjust absorption measured on filters according to for the specific filter medium. Concurrent measurements of scattering are not usually available, and the correction for scattering is not applied. As we discuss below, the scattering cross section is about 25% of the absorption cross section, so that the overestimate of absorption due to scattering artifacts should be less than 10%.
summarizes data on MAC taken at and near sources, including aerosol from diesel engines and in traffic tunnels. Data for this figure exclude values of MAC that rely on normalization by thermal-optical methods, as well as ambient measurements that may represent substantially changed aerosol. In many cases, the two constraints are similar, because ambient measurements contain other material and require some kind of estimate of LAC fraction.
Given the previously reported wide range of MAC, the data are surprisingly consistent. Of 21 measurements, 16 lie between 6.3–8.6 m2/g. The lowest value (CitationJapar and Killinger 1979) was an early effort by a group that went on to produce much higher values, some of which are listed in this tabulation. While we have no reason to doubt their measurement, it is interesting that their later papers never cite it. The highest outlier (CitationTruex and Anderson 1979) also results from the earliest days of work with photoacoustic measurements.
We suggest that there is a consistent value of MAC: about 7.5 ± 1.2 m2/g. The average is that of 17 measurements that exclude the lowest and highest values, and the two measurements that reported absorption cross section of particles in liquid. The uncertainty range is one standard deviation of those measurements. (The average including the liquid-suspended particles is 7.3 m2/g.) Our statement contrasts with other reports that suggest a bewildering and inexplicable array of mass absorption cross sections, but it applies only to freshly generated LAC.
A commonly cited value for MAC is 10 m2/g. That value is more than two standard deviations away from our central estimate and does not appear in our corrected tabulation. Many reports of 10 m2/g were secondary references traceable to the paper by CitationDonoian and Medalia (1967). That paper reported measurements of carbon black suspended in water, where the absorption cross section would be much greater than in air (30–60% for reasonable refractive indices of LAC). Measurements uncorrected for enhancement by filter media also provide values of 10 m2/g (CitationHorvath et al. 1993b; CitationPetzold et al. 1997; CitationBond et al. 1999; CitationFuller et al. 1999). A large body of literature suggests that some ambient aerosol has mass absorption cross sections greater than 7.5 m2/g. contains some of these values. We do not refute these measurements, but we do emphasize that available measurements on fresh combustion aerosol do not exhibit MAC of that magnitude.
A full exploration of the higher values of MAC for atmospheric aerosol is beyond the scope of this review. We suggest at least two plausible explanations, and both probably contribute. First, there may be anomalies in the thermal-optical measurements used to normalize absorption to elemental carbon mass. Uncertainties in thermal-optical measurements are widely acknowledged, and have been identified as a reason for apparently high absorption (CitationMartins et al. 1998). Second, particle coatings may increase the absorption cross section, as discussed in Section 4. When LAC and negligibly-absorbing material are emitted from the same combustion process, the form is probably that of an absorbing core surrounded by a negligibly absorbing (or less absorbing) shell. LAC is formed in high-temperature regions of the flame, where semi-volatile organic material can neither persist nor condense. As the exhaust gas cools, semi-volatile material condenses on available surfaces, including LAC. Further mixing occurs in the atmosphere: coagulation with other particles, coating by condensation, or coating after evaporation of a water droplet that contains LAC and other substances.
7.4. Ensemble Properties: Single-Scattering Albedo
While absorption cross section per unit mass is almost constant for the particle sizes emitted from combustion, scattering per mass depends more on particle size. Therefore, a consistent value of mass scattering cross section for LAC particles is not expected. Because MAC is relatively constant, single-scattering albedo (scattering divided by absorption plus scattering) might also change. For combustion aerosol, total scattering is not frequently measured, but lists a few values. They are surprisingly consistent, suggesting a single-scattering albedo of about 0.2–0.3.
TABLE 7 Measured values of single-scattering alberdo from fresh combustion aerosol
These values of single-scattering albedo are higher than those predicted by Mie theory. Even 100-nm particles should have a single-scattering albedo of about 0.1, and ω0 would be much lower for smaller particles. The spherical-particle assumption is unable to match scattering by these particles because they are aggregates. CitationSorensen (2001) points out that the single-scattering albedo is quite sensitive to the radius of the primary spherules, and reaches an asymptote with regard to the number of spherules in the aggregate. This asymptote matches measured ω0 for primary particle diameters of 20–30 nm, in good agreement with observations.
7.5. Closure
Section 4 discussed theories—both Mie and aggregate—that predict scattering and absorption from the refractive index when particle size is known. We have estimated refractive index (Section 7.2.3) and should be able to combine it with an appropriate theory to match the separately-constrained absorption cross section (Section 7.3).
We assume that LAC does not lie along the graphitization line; rather, it consists of flame-generated particles that have become as graphitic as the flame can make them. We surmise that the refractive index must lie along the void-fraction line in , although we admit that we do not know precisely where. It is possible to imagine conditions under which partially graphitized material would be emitted, but we investigate the more strongly absorbing carbon here. We also assume that Rayleigh-Debye-Gans (RDG) theory represents fresh LAC better than Mie theory. As long as the primary spherules are small (relative to the wavelength of light below about 80 nm), spherule size does not affect absorption. Observed spherule sizes are below that size. Thus, the MAC of any aggregate particle can be represented with that of some small particle, say 50 nm. Finally, we assume that the density of the spherules is 1.8 g/cm3 (CitationMullins and Williams 1987; CitationWu et al. 1997; CitationPark et al. 2004).
examines whether any plausible void-free refractive indices are consistent with measured MAC. The figure shows the mass absorption cross section of spherical 50-nm particles (or aggregates of such spherules). While only the imaginary refractive index (k) is shown on the x-axis, the real refractive index also varies according to the void-fraction line in . We do not know exactly which point along this line corresponds to void-free LAC, so we examine all of them as candidates. The figure also shows MAC along two additional lines based on the higher and lower values of refractive index in . A constant density of 1.8 g/cm3 is used in the calculations; the figure would look different if we were examining how MAC depended on voids in the material.
FIG. 9 Mass absorption cross sections m2/g for fresh LAC: measured values from (shaded region, with central estimate marked by a thick line), and calculated values using the best guess of refractive index for fully-graphitized carbon; see . Central curve shows “void-fraction” line in (approximately 1.8–0.74i); higher and lower curves are similar “void-fraction” lines assuming that pure LAC has refractive indices of 2 − 1i and 1.96 − 0.66i. The latter two values are not expected to represent atmospheric LAC, as discussed in text. Assumed density is 1.8 g /cm3.
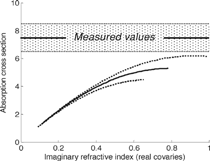
The band representing measured MAC is also shown in . The prediction and the measurements do not overlap. The discrepancy is about 30%, with measured MAC greater than calculated. A discrepancy of 30% in absorption cross section may not be important in some disciplines, but would be significant in determining climate forcing. also demonstrates why attempts to calculate high MAC using realistic refractive indices have been unsuccessful. None of the predictions using plausible refractive indices overlap the measurements. One might try to increase calculated MAC by choosing higher values of k, but the corresponding increase in n (demonstrated in ) has an offsetting effect. The result is an asymptote at high n and k.
What are possible reasons for this discrepancy? There could be some uncertainty in the refractive index, which might allow the upper bound of the uncertain predictions to overlap the lower bound of the uncertain measurements. However, the refractive index would have to be far outside the range of any of the values tabulated here.
Perhaps Mie theory is better than RDG for this type of particle, but it results in a smaller MAC for combustion-sized particles (∼ 100 nm), not a greater one. Or perhaps the primary spherules lie in the region of peak absorption cross section, but primary spherules of this size (100–200 nm diameter) are not observed in combustion particles. Finally, our density of 1.8 g/cm3 could be too high, particularly if gaseous adsorption does not measure the same volume that interacts with light. A density of about 1.35 g/cm3 would be required to bring the high end of the calculated curves into agreement with the measurements. Such a major discrepancy does not seem realistic.
Despite our attempts to choose only freshly generated LAC, our collection of MAC could be drawn from coated particles for which absorption is enhanced. An enhancement of 30% would require either very thick coatings compared to those expected from fresh LAC or very large cores, as shown in a more detailed investigation (CitationBond et al. 2005). The particles could contain material other than carbon because they form in impure environments. Surface impurities could change the chemical nature and reduce the imaginary refractive index as discussed in Section 3. In that case, predictions would exhibit an even greater discrepancy with measurement. Oxygenation of the surface could reduce the real refractive index, as shown by CitationStagg and Charalampopoulos (1993) for amorphous carbon. A decrease of about 25%—less than that observed by CitationStagg and Charalampopoulos (1993)—would increase MAC by 30%. But such a change is less likely for turbostratic particles than for a glassy, shiny carbon.
As discussed in Section 5, CitationIskander et al. (1991) showed that interactions between spherules increase the MAC above that of RDG theory by factors up to 1.5; CitationFuller et al. (1999) estimated that this increase would be limited to about 30%. Although we consider this interactional enhancement a likely explanation for the 30% discrepancy (as did Iskander et al.), this result has yet to be demonstrated with measurements.
Our purpose here is to identify, as completely as possible, the parameters required to represent climate forcing of LAC. We have identified a range of possible refractive indices consistent with the varied measurements; this range provides an upper limit for calculated MAC. We have bounded the mass absorption cross section of fresh LAC. Finally, we have confirmed that measured MAC is greater by about 30% than prediction by simple theories (either Mie or RDG), and that variability in either refractive index or MAC is unlikely to account for the discrepancy.
CitationFuller et al. (1999) also identified several possible reasons for this discrepancy. Our work differs from theirs because we focus less on detailed calculations and more on systematically identifying the properties of pure, void-free LAC. The most plausible explanations for this discrepancy, about 30%, are (Equation1) interactions between particles or (2) density estimates that are too high. These possibilities should be confirmed with measurements. There might be other explanations for the 30% discrepancy between predicted and measured MAC. Clearly, the nature of these interesting little particles is not fully understood.
7.6. Beyond 550 nm
Our review, including the synthesis in , , and , concentrated on identifying optical properties at the mid-visible wavelength of 550 nm. This limitation provided focus needed to conduct the review, but properties at a single wavelength are insufficient for climate modeling. Here, we address the possibility of extending these results to visible wavelengths (400–700 nm) for fully graphitized carbon that lies along the void-fraction line in .
We propose that a constant refractive index for LAC at visible wavelengths is consistent with available data. This notion requires some support, which we will discuss below. We emphasize that this assumption is valid at visible wavelengths, and not ultraviolet or infrared wavelengths. Electronic transitions at ultraviolet wavelengths result in narrow peaks in absorption (e.g., CitationSakata et al. 1995). Both real and imaginary refractive indices are greater and non-constant at infrared wavelengths (e.g., CitationDalzell and Sarofim 1969; CitationFoster and Howarth 1968; CitationJäger et al. 1998).
Refractive index estimates based on reflectance measurements (e.g., CitationSenftleben and Benedict 1918) often show little spectral dependence. (Here, we express the relationship with wavelength as λ n ; for example, λ−1 is an inverse dependence on wavelength). CitationStagg and Charalampopoulos (1993) provided data for wavelengths from 400-700 nm; the wavelength dependence of n and k are approximately λ0.2 and λ−0.1, respectively. The data of CitationMarley et al. (2001) suggest λ 0.05 and λ0.1 for n and k respectively. These are essentially constant within measurement error. Band-gap theory suggests a constant imaginary refractive index as an asymptote for large sp2 clusters. Refractive indices of carbonaceous material exhibit large and sometimes rapid variations from ultraviolet to infrared; if n and k for LAC are constant across visible wavelengths, it is a fortuitous consequence of the narrow wavelength range.
The frequent observation of λ−1 dependence for aerosol absorption (e.g., CitationBergstrom et al. 2002; CitationSchnaiter et al. 2003; CitationKirchstetter et al. 2004) also supports the notion of a constant refractive index, assuming that the particles observed were small relative to the wavelength. If the real refractive index had a wavelength dependence of even λ−0.1, absorption would vary as λ−0.84. The same wavelength dependence for imaginary refractive index would result in absorption varying as λ−1.1. Perhaps these deviations from λ−1 do exist, but they are buried in the measurement uncertainties. There are limited data on the wavelength dependence of either refractive index or absorption; we can say only that they appear consistent with a constant refractive index at visible wavelengths.
Some measurements do suggest that LAC has a varying imaginary refractive index. These are scattering measurements in flames (e.g., CitationChang and Charalampopoulos 1990) and inferences from the dispersion model (e.g., CitationDalzell and Sarofim 1969; CitationLee and Tien 1981; CitationHabib and Vervisch 1988; of this review). The spectral dependence inferred from scattering measurements is greatly affected by the assumption of spherical particles. As discussed previously, the visible spectral dependence predicted by the dispersion model is questionable. In fact, measured data in the references cited are equally consistent with a constant refractive index throughout visible wavelengths.
Our statements about wavelength dependence apply only to LAC, not graphite or amorphous carbon. Some measurements of combustion-generated aerosol (CitationBond 2001; CitationKirchstetter et al. 2004), and spark-generated carbon (CitationSchnaiter et al. 2003) have demonstrated that the imaginary refractive index may also depend on wavelength. This material also exhibits weak light absorption (CitationBond 2001), and is not the material we are calling LAC. We predict that it lies along the graphitization line in .
7.7. A Return to History
The history of refractive index values tabulated by CitationShettle and Fenn (1976, Citation(1979) is worth special mention. These are by far the most prevalent values for use in climate modeling, and have been incorporated into widely-cited literature, including a book by Citationd'Almeida et al. (1991), and the Optical Properties of Aerosols and Clouds (OPAC) program (CitationHess et al. 1998). The original work by CitationShettle and Fenn (1976) averaged values from an earlier review by CitationTwitty and Weinman (1971). In turn, the averaged data are taken from CitationMcCartney et al. (1965), who measured three coals, and CitationSenftleben and Benedict (1918), who reported soot generated from an arc lamp. The review does not incorporate most of the findings on soot in the combustion literature, and indeed was written before most of that work was available. The precision of both n and k provided in OPAC values (three decimal places) is unwarranted, given this history. The OPAC value of 1.74–0.44i is drawn from incompletely graphitized carbon and has a lower value of k than most soot.
The value of CitationSenftleben and Benedict (1918, 1.96–0.66i) is also used to model LAC. Again, their material lies along the graphitization line in and is not LAC. Of the data used in climate models of previous years, only the data tabulated by CitationNilsson (1979) result from combustion soot, citing the data of CitationDalzell and Sarofim (1969) without correction for void fraction.
The optical and physical data for LAC propagated by Citationd'Almeida et al. (1991) have some interesting properties. Along with an imaginary refractive index that is too low, these authors recommend: (Equation1) a particle size that is far too small (23 nm is the approximate size of primary spherules, not aggregates); (2) a geometric standard deviation that is somewhat too large (2.0); and (3) a density that is far too low (a density of 1.0 is never observed; CitationFuller et al. (1999) tabulate measurements indicating densities of about 1.8 g/cm3). Despite returning to the string of citations that led to Citationd'Almeida et al. (1991), we have been unable to unearth the sources of these values. When compared with measured values, each of the individual assumptions above may lead to an error of 50–75% in calculated properties that affect climate forcing.
Somewhat surprisingly, this combination of parameters yields a mass absorption cross section and single-scattering albedo that is close to the values we recommend. We speculate that, lacking recommendations from the measurement community, modelers simply selected values that match the absorptive properties of LAC, as suggested by CitationPenner et al. (1998). Because aggregate particles have less absorption per mass than large spherical particles, models had to use very small sizes in order to represent strong absorption. (Assuming such a large number of particles per mass probably has implications for modeling how particles affect clouds.) These very small particles do not scatter much light, so a long lognormal tail provided a few large, scattering particles. The low value of imaginary refractive index yielded too little absorption per mass, and there was no method of accounting for the discrepancy between prediction and measured values. Those omissions were offset by very low particle densities. The parameters chosen for LAC were reasonable as long as they were treated in isolation. However, better estimates of radiative forcing require representing interactions with other particles. In such endeavors, reliance on offsetting errors is unwise.
Climate models must predict absorption and scattering by particles based on the mass of substance present, unless there is a change in the paradigm of representing optics as a function of chemistry. The refractive index is, at best, a convenience (although the discussion here may call that into question!). It should not be selected in isolation from other parameters, and modeled scattering and absorption should be compared with measurements.
7.8. What's Missing?
We began this section by asking which materials were representative of the LAC found in the atmosphere. In seeking closure between measured absorption cross section and theory based on particle characteristics, we used the most-graphitized material in . Comparing light-absorbing properties and single-scattering albedos, particles emitted from diesel engines do appear similar to materials from controlled combustion, which tend to lie along the void-fraction line.
A similar confirmation has not been made for the remaining major sources of atmospheric LAC. Does carbon from all sources have identical optical properties? Less-graphitized carbon occurs within flames (e.g., CitationSaito et al. 1991; Citationd'Alessio et al. 1992; Citationvan der Wal 1996), and could have a lower absorption cross section than LAC. Optical properties of carbon particles from many combustion sources have not been investigated.
Although we have scarcely discussed wavelength dependence of absorption, limiting our focus to interactions with 550-nm light, absorption by incompletely graphitized carbon would have stronger wavelength dependence, as discussed by CitationBond (2001). Such material may be present in the atmosphere (e.g., CitationKirchstetter et al. 2004) and should be investigated.
It would be an unsatisfying review that reached so near to a conclusion and failed to give values and procedures to replace those we have criticized. Recommendations for representing LAC particles in climate models are contained in Section 9. We have separated these recommendations from the lengthier discussion here, for the convenience of those who are concerned about model inputs and not all that enthralled with the details.
8. MEASURED LIGHT ABSORPTION OF ATMOSPHERIC LAC
Relating the properties of freshly emitted LAC particles to the properties of the particles in the atmosphere is critical to determining their climatic effects. If particles change immediately or shortly after emission, fresh-particle properties cannot be used to represent all LAC particles. Plausible physical changes that affect scattering and absorption were discussed in Section 5.2.
An obvious procedure for investigating whether such changes occur is to examine important properties in ambient aerosol, compare them with those of fresh aerosol, and assess any differences in the shape, mixing or other properties. Such a comparison should indicate whether physical properties change in the atmosphere, and how those changes affect absorption and scattering. We had hoped to undertake such an evaluation with the tabulation given in this section, but as we will discuss, this task is not yet possible with the present body of measurements.
8.1. Measurement Tabulation
There have been hundreds of reported measurements of absorption by carbonaceous particles in the atmosphere. CitationWaggoner et al. (1981) discuss the measurements made up to that time, with emphasis on those made by a group at the University of Washington. CitationHorvath (1993a) provides a general discussion of absorption measurements and methods, including many European measurements. CitationPenner and Novakov (1998) discuss some early LAC measurements in the context of the series of conferences entitled Carbonaceous Particles in the Atmosphere, which began in 1978. Reid et al. (2004) recently reviewed the absorptive properties of biomass burning emissions.
lists field programs that contain information about absorption by atmospheric aerosols. Until about 1978, aerosol measurements were limited to broad-band solar absorption by a column of aerosols. There were very few absorption measurements in field programs during the 1980s. Intensive aerosol characterization experiments began in about 1990. The variety of instruments used during each program increased, as researchers came to appreciate the need for coordinated measurements of many aerosol properties. For example, the intensive field campaign known as ACE-Asia (CitationHuebert et al. 2003) consisted of measurements made on two ships, four aircraft, several satellites, and ground stations in three different countries.
TABLE 8 Atmospheric field programs that have measured quantities relevant to LAC or absorption
8.2. Limitations of Atmospheric Measurements
The measurements listed in have produced valuable data and have established light absorption by carbonaceous particles as an important factor in radiative transfer. Yet despite the large number of field programs, information needed to assess possible changes in MAC is lacking. Often LAC mass is not determined at the same time as the absorption, a situation that has only recently begun to improve. Remote measurements cannot estimate the mass of LAC particles in the column or determine MAC. These measurements establish the existence of atmospheric aerosol absorption, and sometimes its magnitude, but not the radiative properties of LAC alone. Meticulous inversions of remote data have estimated concentrations and MAC that are consistent with measured radiance (CitationSchuster et al. 2005), but even these rely on assumptions about the refractive index and particle mixing.
Much effort has been spent on establishing the absorption properties of the aerosols in the atmosphere. However, the distinction between the effective optical properties of the atmospheric aerosol, and the optical properties of the LAC itself, is an important one. Knowledge about radiative properties of LAC itself is quite limited, and this quantity is needed to determine how changes in emissions will affect climate. This critique also applies to measurements of the single-scattering albedo, which is strongly affected by LAC. While crucial for determining the sign of radiative forcing by aerosols (CitationHaywood and Shine 1995; CitationHansen et al. 1997) and useful in determining causes of absorption (CitationBergstrom et al. 2002; CitationDubovik et al. 2002), measuring the single-scattering albedo does not foster a link between emissions of LAC and its effect on radiation.
Some programs have provided the measurements needed to estimate atmospheric MAC by measuring both absorption and LAC. However, there have been difficulties in interpreting the results as representative of particular regions, source categories, or particle transformation. The variability observed in atmospheric MAC is greater than that of freshly emitted LAC particles. For example, the Aerosol Characterization Experiment conducted near the coast of Asia (ACE-Asia) reported MAC for carbonaceous particles varying from 5 to 25 m2/g (CitationHuebert et al. 2003; CitationQuinn et al. 2004). This range is not much different from that given by CitationLiousse et al (1993): 6 to 20 m2/g. This variability could be caused by different sources, atmospheric transformation or measurement uncertainties. For example, dust absorption may have affected determinations of LAC absorption during ACE Asia (CitationBergstrom et al. 2004).
Throughout this section, we have raised concerns regarding the inability to assess absorption by LAC using atmospheric measurements. Following are some limitations of past measurement campaigns that should be addressed in the future:
| 1. | All techniques, including remote sensing, measure absorption due to all particles, not just LAC particles. | ||||
| 2. | If absorption is normalized to LAC mass, the absorption and mass measurements may be made on different samples. | ||||
| 3. | When absorption is normalized to LAC mass, there is considerable uncertainty in the thermal-optical methods that are commonly used. This is a major uncertainty, one that prevents understanding whether the range in atmospheric MAC values is real or an artifact of the measurements. | ||||
| 4. | Even if the absorption and mass determinations are perfect, the morphology of the particles is usually unknown and often unknowable. Even detailed electron microscopy focuses on a small fraction of the particles. This lack of information prevents correlations between observed MAC and particle should form. | ||||
8.3. Outlook
Modeling scattering and absorption by LAC will improve if significant differences in important properties can be attributed to specific conditions, such as different combustion sources or atmospheric processing. In our view, it is not possible to assess the accuracy of the present tabulation of MAC in the atmosphere. To be sure, we can estimate a range for MAC, which varies by about a factor of four (5–20 m2/g). However, lack of a reliable method for measuring LAC mass prevents firm conclusions about whether this variability is real. For example, CitationCarrico et al. (2003) calculated MAC using the same absorption measurement and three different methods of measuring LAC mass, resulting in values of 5.3, 9.5, and 18 m2/g. CitationMartins et al. (1998) pointed out that extremely high values of MAC were associated with potassium, which affects the thermal-optical method. While ours may seem to be an overly negative outlook, we believe that the current focus on resolving some of these issues will rapidly counter the present uncertainty. Current research addresses the accuracy of thermal measurements and improved methods of measuring absorption.
Despite our extensive caveats, the available data do allow a few inferences. First, a lower bound of about 5 m2/g for submicron MAC particles is observed. The particle collapse observed by CitationSchnaiter et al. (2003), even after just two hours, reduced absorption by just over 20%, corresponding to a change from our fresh value of 7.5 m2/g to about 5.8 m2/g. CitationClarke et al. (2004) reported values of 6–8 m2/g by normalizing absorption to the non-volatile fraction of the aerosol. We suggest that the lowest values of 5 m2/g are collapsed but uncoated. They may also represent large agglomerates formed in the thick plumes of open biomass burning, as suggested by CitationLiousse et al. (1993). These low values are less commonly observed than other values, and we suggest that the uncoated aerosol does not remain in that state for very long.
Observations of absorption above the fresh value of 7.5 m2/g are far more common than low values (e.g., CitationQuinn et al. 2004). Ambient values of MAC in polluted regions tend to exhibit a mode around 9–12 m2/g. Perhaps not coincidentally, a spherical particle with our suggested refractive indices, with an absorption enhancement of about 80% due to coating (CitationBond et al. 2005), results in MAC of about 11 m2/g—in the range of the most prevalent observed atmospheric MAC.
What of the additional 30%, postulated due to spherule interaction, that we had to invoke to match the fresh MAC? CitationFuller et al. (1999) showed that this interactional enhancement does not occur when the LAC particle is encapsulated. Absorption by aggregates without coatings is about 30% higher than that of uncoated spherical particles; absorption by coated particles is about 80% higher than that of uncoated particles. The coated particles that exist after some atmospheric lifetime have absorption about 50% higher than the particles emitted as aggregates.
9. RECOMMENDATIONS
We summarize here our recommended approach to representing LAC in climate models. As in the review, our approach is skewed toward representing freshly generated LAC, but we also suggest some methods of representing the material later in its lifetime. In providing these recommendations, we maintain the caveats that have been sprinkled liberally throughout this document. This is particularly true of our concerns about whether the material that has been studied is similar to that in the atmosphere.
9.1. Choice 1: Absorption and Scattering
Mass absorption cross section. As outlined in Section 7.3, we suggest a value of 7.5 ± 1.2 m2/g for the MAC of fresh light-absorbing carbon. During a particle's lifetime, this value may increase due to coating and decrease due to particle coagulation and aggregate collapse, as discussed next.
Mixing/coating. A preliminary estimate of the enhancement due to coating by negligibly absorbing aerosol is a factor of 1.5. This value is supported by some observational evidence and by some theoretical investigations.
Single-scattering albedo. Values of 0.20–0.30, with a central value of about 0.25, appear supportable for fresh aerosol, as shown in . These values will increase after emission.
Backscattering fraction. We did not discuss this value previously, but the only published values we know of are those of CitationSchnaiter et al. (2003): 0.16 to 0.18.
Wavelength dependence. Absorption cross section may be assumed to depend inversely on wavelength throughout the visible spectrum. We make no statements about the nature of absorption at ultraviolet and infrared wavelengths.
The values given here are sufficient to calculate one-dimensional climate forcing by fresh aerosol, as discussed in Section 9.4.
9.2. Choice 2: Optical Model
For those who wish to calculate scattering and absorption of fresh LAC, this section suggests a simple approach. These particles are not spherical, and it is possible to implement Rayleigh-Debye-Gans theory in a modeling framework or lookup table. We recommend the review by CitationSorensen (2001) as a primer; he tabulates the equations neatly. However, even though RDG theory is better than Mie theory, it underpredicts absorption by about 30%. More precise calculations would not be computationally efficient enough for climate models. It is probably better to use a constant absorption cross section and single-scatter albedo for unmixed particles, and apply the parameters described below after the particles have mixed. This recommendation should be revisited as more measured absorptive properties of combustion aerosol become available.
Refractive index. The value commonly used by climate modelers (m = 1.74−0.44i at 550 nm) represents none of the possible refractive indices and should be retired. It should be assumed that most LAC lies on the void-fraction line in and . Our best guess is that the high values in the table are most promising, but shows that the values are not distinguishable in terms of absorptive properties.
Density. The density of LAC has been measured as 1.7–1.9 g/cm3 and the use of 1.0 g/cm3 should be abandoned.
Aging. While Mie theory should be not used for fresh aerosol, Mie-based equations that represent encapsulated, concentric (shell-and-core) particles may be used for aged, coated aerosol along with the higher refractive indices in . These will yield MAC values similar to those observed in the atmosphere. Other guiding relationships on coating versus enhancement will be forthcoming (CitationBond et al. 2005).
Wavelength dependence. Refractive index may be assumed to be constant throughout the visible spectrum. We make no statements about its nature at ultraviolet and infrared wavelengths, but we emphasize that it is likely to be much different.
9.3. Cautions
All modelers should beware of blindly choosing particle sizes and applying Mie theory. The same is true of volume-mixing of refractive indices. This procedure is not physical and is discouraged.
Sensitivity studies on the optical models can be performed more rapidly than sensitivity studies on the global models of radiative transfer. These may suffice to inform modelers when results are very sensitive to the chosen input parameters. At the very least, values of mass absorption cross section and single-scattering albedo should be given for each modeling study that estimates climate forcing.
9.4. Simple Climate Forcing
The much-quoted forcing equation of CitationChylek and Wong (1995) can be modified to provide a direct forcing in watts per gram, if the mass absorption cross section, single-scattering albedo, and backscatter fraction are known. We term this modified equation the “simple forcing efficiency” (SFE):
9.5. Suggested Reading
Although we have examined several references in preparing this review, some have been particularly instructive. The review of aggregates by CitationSorensen (2001), mentioned previously, is both readable and comprehensive. CitationHeckman's 1964 review of carbon black microstructure is highly recommended as a reminder of what has been known and apparently forgotten. The paper by CitationFuller et al. (1999) is a good overview of absorption that can and cannot be explained with calculation. And of course, the delightful book by CitationBohren and Huffman (1983) should be consulted regularly.
Acknowledgments
We are grateful to Craig Bohren for critical comments on both content and language. We also thank James Barnard, Antony Clarke, Stephen Warren, Otmar Schmid, and an anonymous reviewer for very helpful suggestions.
TCB is grateful for the support of the U.S. Environmental Protection Agency under STAR grant RD-83108501, and of the National Aeronautics and Space Administration's Earth Observing System under grant NNG04GL91G. She is indebted to Timothy Bates and Patricia Quinn at the NOAA Pacific Marine Environmental Laboratories, and Theodore Anderson at the University of Washington, who tolerated episodic diversions into old literature when she should have been doing other work. She appreciates the able assistance of Vedran Coralic at UIUC in gathering references. TCB also acknowledges enlightening discussions with Stephen Warren and Warren Wiscombe regarding the optics of particles suspended in liquid.
RWB gratefully acknowledges support provided by NASA's Earth Observing System Interdisciplinary Science (EOS-IDS) Program, by NASA's Radiation Sciences Program, and by the Aerosol-Climate Interaction Program of NOAA's Office of Global Programs. He also appreciates the indulgence of his colleagues Peter Pilewskie and Phil Russell.
Notes
1So called because they represent the variation of refractive index with wavelength, the reason for “dispersion” of white light passing through a prism.
2The parameter k = 2 π /λ is also frequently used in these equations. We do not use it in this review in order to avoid confusion with the imaginary refractive index.
3Aerosol absorption can even affect gas measurements. In fact, the TOMS technique discussed below was developed because aerosol absorption interfered with ozone measurements. The aerosol from the eruption of Pinatubo was detected because it interfered with stratospheric gas measurements.
a Incorporates both manufacturer's correction and additional calibration.
b Unpublished data taken during calibration study described in CitationBond et al. 1999.
4We refer to measurements in the solar spectrum. LAC does not dominate absorption at infrared wavelengths, although other particles do contribute to it.
a Paper assumed to contain data from conference proceedings paper by CitationHorvath (1993) citation.
5“Specular” means that the parallel component of the reflectivity equals the square of the perpendicular component of the reflectivity for light incident at 45 o . When this condition is violated, the material does not obey Fresnel theory used to infer the refractive index.
(a) Used values of five carbon blacks corresponding to smallest particles. Value often cited (9.68 m2/g) is for one carbon black, Monarch 71.
(b) CitationHorvath (1993) cites Wolff, 1980, which is not given in the reference list. We assume that this was a conference proceeding or personal communication regarding the Denver Brown Cloud study, for which MAC measurements are given by CitationGroblicki et al (1981). We also assume that Horvath's citation of 12.7 m2/g was a preliminary value produced in this study.
(c) See note for Adjustment 3.
REFERENCES
- Ackerman , A. S. , Toon , O. B. , Stevens , D. E. , Heymsfield , A. J. , Ramanthan , V. and Welton , E. J. 2000 . Reduction of Tropical Cloudiness by Soot . Science , 288 : 1042 – 1047 . [CSA]
- Ackerman , T. P. and Toon , O. B. 1981 . Absorption of Visible Radiation in Atmosphere Containing Mixtures of Absorbing and Nonabsorbing Particles . Appl. Opt. , 20 ( 20 ) : 3661 – 3667 . [CSA]
- Ackerman , T. P. and Valero , F. P. J. 1984 . The Vertical Structure of Arctic Haze as Determined from Airborne Net-Flux Radiometer Measurements . Geophys. Res. Let. , 11 : 469 – 472 . [CSA]
- Adams , K. M. 1988 . Real-time in situ Measurements of Atmospheric Optical Absorption in the Visible via Photoacoustic Spectroscopy. 1: Evaluation of Photoacoustic Cells . Appl. Opt. , 27 ( 19 ) : 4052 – 4057 . [CSA]
- Adams , K. M. , Davis , L. I. , Japar , S. M. and Filey , D. R. 1990 . Measurement of Atmospheric Elemental Carbon: Real-Time Data for Los Angeles During Summer 1987 . Atmos. Env. , 24A ( 3 ) : 597 – 604 . [CSA]
- Akhter , M. S. , Chughtai , A. R. and Smith , D. M. 1985a . The Structure of Hexane Soot I: Spectroscopic Studies . Appl. Spectrosc. , 39 ( 1 ) : 143 – 153 . [CROSSREF] [CSA]
- Akhter , M. S. , Chughtai , A. R. and Smith , D. M. 1985b . The Structure of Hexane Soot II: Extraction Studies . Appl. Spectrosc. , 39 ( 1 ) : 154 – 167 . [CROSSREF] [CSA]
- Anderson , T. L. and Ogren , J. A. 1998 . Determining Aerosol Radiative Properties using the Tsi 3563 Integrating Nephelometer . Aerosol Sci. Technol. , 29 : 57 – 69 . [CSA]
- Andreae , M. O. , Fishman , J. and Lindesay , J. 1996 . The Southern Tropical Atlantic Region Experiment (STARE): Transport and Atmospheric Chemistry near the Equatoral Atlantic (TRACE A) and Southern African Fire-Atmosphere Research Initiative (SAFARI): An introduction . J. Geophys. Res. , 101 : 23519 – 23520 . [CROSSREF] [CSA]
- Ansmann , A. , Wandinger , U. and Wiedensohler , A. 2002 . Lindenberg Aerosol Characterization Experiment 1998 (LACE 98) Overview . J. Geophys. Res. , 107 ( D21 ) : 8120 doi: 10.1029/2000JD00023[CROSSREF] [CSA]
- Arnott , W. P. , Moosmuller , H. and Rogers , C. F. 1997 . Photoacoustic Spectrometer for Measuring Light Absorption by Aerosol: Instrument Description . Atmos. Env. , 33 ( 17 ) : 2845 – 2852 . [CSA]
- Arnott , W. P. , Hamasha , K. , Moosmüller , H. , Sheridan , P. J. and Ogren , J. A. 2005 . Towards Aerosol Light-Absorption Measurements with a 7-Wavelength Aethalometer: Evaluation with a Photoacoustic Instrument and 3-Wavelength Nephelometer . Aerosol Sci. Technol. , 39 : 17 – 29 . [CROSSREF] [CSA]
- Artaxo , P. , Martins , J. V. , Yamasoe , M. A. , Procópio , A. S. , Pauliquevis , T. M. , Andreae , M. O. , Guyon , P. , Gatti , L. V. and Leal , A. M. C. 2002 . Physical and Chemical Properties of Aerosols in the Wet and Dry Seasons in Rondônia, Amazonia . J. Geophys. Res. , 107 ( D20 ) : 8081 doi: 10.1029/2001JD000666[CROSSREF] [CSA]
- Ballach , J. , Hitzenberger , R. , Schultz , E. and Jaeshcke , W. 2001 . Development of an Improved Optical Transmission Technique for Black Carbon (BC) Analysis . Atmos. Env. , 35 : 2089 – 2100 . [CROSSREF] [CSA]
- Batten , C. E. 1985 . Spectral Optical Constants of Soots from Polarized Angular Reflectance Measurements . Appl. Opt. , 24 ( 8 ) : 1193 – 1199 . [CSA]
- Ben Hamadi , M. , Vervisch , P. and Coppalle , A. 1987 . Radiation Properties of Soot from Premixed Flat Flames . Comb. Flame , 68 : 57 – 67 . [CROSSREF] [CSA]
- Bergstrom , R. W. 1972 . Predictions of the Spectral Absorption and Extinction Coefficients of an Urban Air Pollution Aerosol Model . Atmos. Env. , 6 : 247 – 258 . [CROSSREF] [CSA]
- Bergstrom , R. W. 1973 . Extinction and Absorption Coefficients of the Atmospheric Aerosol as a Function of Particle Size . Contr. Atmos. Phys. , 46 : 223 – 234 . [CSA]
- Bergstrom , R. W. , Pilewskie , P. , Pommier , J. , Rabbette , M. , Russell , P. B. , Schmid , B. , Redemann , J. , Higurashi , A. , Nakajima , T. and Quinn , P. K. 2004 . Spectral Absorption of Solar Radiation by Aerosols During ACE-Asia . J. Geophys. Res. , 109 ( D19S15 ) doi: 10.1029/2003JD004467[CSA]
- Bergstrom , R. W. , Pilewskie , P. , Schmid , B. and Russell , P. B. 2003 . Estimates of the Spectral Aerosol Single Scatter Albedo and Aerosol Radiative Effects During SAFARI 2000 . J. Geophys. Res. , 108 ( D13 ) : 8474 doi: 10.1029/2002JD02435[CROSSREF] [CSA]
- Bergstrom , R. W. , Russell , P. B. and Hignett , P. 2002 . The Wavelength Dependence of Black Carbon Particles: Predictions and Results from the Tarfox Experiment and Implications for the Aerosol Single Scattering Albedo . J. Atmos. Sci. , 59 : 567 – 577 . [CROSSREF] [CSA]
- Berry , M. V. and Percival , I. C. 1986 . Optics of Fractal Clusters Such as Smoke . Opt. Acta , 33 ( 5 ) : 577 – 592 . [CSA]
- Birch , M. E. and Cary , R. A. 1996 . Elemental Carbon-Based Method for Monitoring Occupational Exposures to Particulate Diesel Exhaust . Aerosol Sci. Technol. , 25 : 221 – 241 . [CSA]
- Biscoe , J. and Warren , B. E. 1942 . An x-Ray Study of Carbon Black . J. Appl. Phys. , 13 : 364 – 371 . [CROSSREF] [CSA]
- Bodhaine , B. A. , Dutton , E. G. and DeLuisi , J. J. 1984 . Surface Aerosol Measurements at Barrow During AGASP . Geophys. Res. Let. , 11 : 377 – 380 . [CSA]
- Bohren , C. F. and Huffman , D. R. 1983 . Absorption and scattering of light by small particles , New York : John Wiley and Sons .
- Bond , T. C. 2001 . Spectral Dependence of Visible Light Absorption by Carbonaceous Particles Emitted from Coal Combustion . Geophys. Res. Let. , 28 ( 21 ) : 4075 – 4078 . [CROSSREF] [CSA]
- Bond , T. C. , Anderson , T. L. and Campbell , D. 1999 . Calibration and Intercomparison of Filter-Based Measurements of Visible Light Absorption by Aerosols . Aerosol Sci. Technol. , 30 : 582 – 600 . [CROSSREF] [CSA]
- Bond , T. C. , Streets , D. G. , Yarber , K. F. , Nelson , S. M. , Woo , J.-H. and Klimont , Z. 2004 . A Technology-Based Global Inventory of Black and Organic Carbon Emissions from Combustion . J. Geophys. Res. , 109 : D14203 doi: 10.1029/2003JD003697[CROSSREF] [CSA]
- Bond , T. C. , Habib , G. and Bergstrom , R. W. 2005 . Limitations in the Enhancement of Visible Light Absorption Due to Mixing State Manuscript in Preparation
- Born , M. and Wolf , E. 1959 . Principles of Optics , New York : Pergamon Press .
- Bouzerar , R. , Amory , C. , Zeinert , A. , Benlahsen , M. , Racine , B. , Durand-Drouhin , O. and Clin , M. 2001 . Optical Properties of Amorphous Hydrogenated Carbon Thin Films . J. Non-Cryst. Solid , 281 : 171 – 180 . [CROSSREF] [CSA]
- Boynton , F. P. , Ludwig , C. B. and Thomson , A. 1968 . Spectral Emissivity of Carbon Particle Clouds in Rocket Exhausts . AIAA Journ. , 6 ( 5 ) : 865 – 871 . [CSA]
- Bruce , C. W. , Stromberg , T. F. , Gurton , K. P. and Mozer , J. B. 1991 . Trans-Spectral Absorption and Scattering of Electromagnetic Radiation by Diesel Soot . Appl. Opt. , 30 ( 12 ) : 1537 – 1546 . [CSA]
- Buseck , P. R. , Bo-Jun , H. and Keller , L. P. 1987 . Electron Microscope Investigations of the Structures of Annealed Carbons . Energy Fuels , 1 : 105 – 110 . [CROSSREF] [CSA]
- Bush , B. C. and Valero , F. P. J. 2002 . Spectral Aerosol Radiative Forcing at the Surface During the Indian Ocean Experiment (INDOEX) . J. Geophys. Res. , 107 ( D19 ) : 8003 doi: 10.1029/2000JD000020[CROSSREF] [CSA]
- Cachier , H. 1998 . “ Carbonaceous combustion aerosols ” . In Atmospheric Particles , Edited by: Harrison , R. M. and VanGrieken , R. 295 – 348 . New York : John Wiley & Sons Ltd. .
- Cachier , H. , Brémond , M.-P. and Buat-Ménard , P. 1989 . Thermal Separation of Soot Carbon . Aerosol Sci. Technol. , 10 : 358 – 364 . [CSA]
- Cadle , S. H. , Groblicki , P. J. and Mulawa , P. A. 1983 . Problems in the Sampling and Analysis of Carbon Particulate . Atmos. Env. , 17 ( 3 ) : 593 – 600 . [CROSSREF] [CSA]
- Campbell , D. , Copeland , S. and Cahill , T. 1995 . Measurement of Aerosol Absorption Coefficient from Teflon Filters Using Integrating Plate and Integrating Sphere Techniques . Aerosol Sci. Technol. , 22 : 287 – 292 . [CSA]
- Carrico , C. M. , Bergin , M. H. , Xu , J. , Baumann , K. and Maring , H. 2003 . Urban Aerosol Radiative Properties: Measurements During the 1999 Atlanta Supersite Experiment . J. Geophys. Res. , 108 ( D7 ) [CROSSREF] [CSA]
- Carter , J. G. , Huebner , R. H. , Hamm , R. N. and Birkhoff , R. D. 1965 . Optical Properties of Graphite in the Region 1100 to 3000 A . Phys. Rev. , 137 ( 2A ) : A639 – A641 . [CROSSREF] [CSA]
- Cartwright , J. , Nagelschmidt , G. and Skidmore , J. W. 1956 . The Study of Air Pollution with the Electron Microscope . Q. J. Roy. Met. Soc. , 82 : 82 – 86 . [CSA]
- Celzard , A. , Marêché , J. F. , Payot , F. , Bégin , D. and Furdin , G. 2000 . Electrical Conductivity of Anthracites as a Function of Heat Treatment Temperature . Carbon , 38 : 1207 – 1215 . [CROSSREF] [CSA]
- Chang , H. and Charalampopoulos , T. T. 1990 . Determination of the Wavelength Dependence of Refractive Indices of Flame Soot . Proc. R. Soc. Lond. A , 430 : 577 – 591 . [CSA]
- Charalampopoulos , T. T. and Shu , G. 2002 . Effects of Polydispersity of Chainlike Aggregates On Light-Scattering Properties and Data Inversion . Appl. Opt. , 41 ( 4 ) : 723 – 733 . [CSA]
- Charlson , R. J. , Langner , J. , Rodhe , H. , Leovy , C. B. and Warren , S. G. 1991 . Perturbation of the Northern Hemisphere Radiative Balance by Backscattering from Anthropogenic Sulfate Aerosols . Tellus , 43AB ( 4 ) : 152 – 163 . [CSA]
- Charlson , R. J. and Pilat , M. J. 1969 . Climate: The Influence of Aerosols . J. Appl. Met. , 8 ( 6 ) : 1001 – 1002 . [CROSSREF] [CSA]
- Chen , H. Y. , Iskander , M. and Penner , J. E. 1991 . Empirical Formula for Optical Absorption by Fractal Aerosol Agglomerates . Appl. Opt. , 30 ( 12 ) : 1547 – 1551 . [CSA]
- Chen , Z. Y. and Zhao , J. P. 2000 . Optical Constants of Tetrahedral Amorphous Films in the Infrared Region and at a Wavelength of 633 nm . J. Appl. Phys. , 87 ( 9 ) : 4268 – 4273 . [CROSSREF] [CSA]
- Chhowalla , M. , Ferrari , A. C. , Robertson , J. and Amaratunga , G. A. J. 2000 . Evolution of sp 2 Bonding with Deposition Temperature in Tetrahedral Amorphous Carbon Studied by Raman Spectroscopy . Appl. Phys. Let. , 76 ( 11 ) : 1419 – 1421 . [CROSSREF] [CSA]
- Chin , M. , Ginoux , P. , Kinne , S. , Torres , O. , Holben , B. N. , Duncan , B. N. , Martin , R. V. , Logan , J. A. , Higurashi , A. and Nakajima , T. 2002 . Tropospheric Optical Thickness from the Gocart Model and Comparisons with Satellite and Sun Photometer Measurements . J. Atm. Sci. , 59 : 461 – 483 . [CROSSREF] [CSA]
- Chippett , S. and Gray , W. A. 1978 . The Size and Optical Properties of Soot Particles . Comb. Flame , 31 : 149 – 159 . [CROSSREF] [CSA]
- Choi , M. Y. , Mulholland , G. W. , Hamins , A. and Kashiwagi , T. 1995 . Comparisons of the Soot Volume Fraction Using Gravimetric and Light Extinction Techniques . Comb. Flame , 102 : 161 – 169 . [CROSSREF] [CSA]
- Choi , S. , Lee , K.-R. , Oh , S.-B. and Lee , S. 2001 . Wide Wavelength-Range Optical Studies of Hydrogenated Amorphous Carbon Films: from 700 nm to 10 μm . Applied Surface Science , 169–170 : 217 – 222 . [CSA]
- Chow , J. C. , Watson , J. G. , Pritchett , L. C. , Pierson , W. R. , Frazier , C. A. and Purcell , R. G. 1993 . The Dri Thermal/Optical Reflectance Carbon Analysis System: Description, Evaluation and Applications in U.S. Air Quality Studies . Atmos. Env. , 27A ( 8 ) : 1185 – 1201 . [CSA]
- Christopher , S. A. , Chou , J. , Zhang , J. , Li , X. , Berendes , T. A. and Welch , R. M. 2000 . Shortwave Direct Radiative Forcing of Biomass Burning Aerosols Estimated Using Virs and Ceres Data . Geophys. Res. Let. , 27 ( 15 ) : 2197 – 2200 . [CROSSREF] [CSA]
- Christopher , S. A. , Kliche , D. V. , Chou , J. and Welch , R. M. 1996 . First Estimates of the Radiative Forcing of Aerosols Generated from Biomass Burning Using Satellite Data . J. Geophys. Res. , 101 ( D16 ) doi: 10.1029/96JD02161[CROSSREF] [CSA]
- Chung , S. H. and Seinfeld , J. H. 2002 . Global Distribution and Climate Forcing of Carbonaceous Aerosols . J. Geophys. Res. , 107 ( D19 ) : 4407 – 4441 . [CROSSREF] [CSA]
- Chylek , P. and Hallett , J. 1992 . Enhanced Absorption of Solar Radiation by Cloud Droplets Containing Soot Particles in Their Surface . Quarterly Journal of the Royal Meteorology Society , 118 : 167 [CROSSREF] [CSA]
- Chylek , P. , Videen , G. , Ngo , D. , Pinnick , R. G. and Klett , J. D. 1995 . Effect of Black Carbon on the Optical Properties and Climate Forcing of Sulfate Aerosols . J. Geophys. Res. , 100 ( D8 ) : 16325 – 16332 . [CROSSREF] [CSA]
- Chylek , P. and Wong , J. 1995 . Effect of Absorbing Aerosols on Global Radiation Budget . Geophys. Res. Let. , 22 ( 8 ) : 929 – 931 . [CROSSREF] [CSA]
- Clague , A. D. H. , Donnet , J. B. , Wang , T. K. and Peng , J. C. M. 1999 . A Comparison of Diesel Engine Soot with Carbon Black . Carbon , 37 : 1553 – 1565 . [CROSSREF] [CSA]
- Clark , J. S. 1997 . Sediment Records of Biomass Burning and Global Change. , NATO ASI series Berlin : Springer .
- Clarke , A. D. 1982 . Integrating Sandwich: A New Method of Measurement of the Light Absorption Coefficient for Atmospheric Particles . Appl. Opt. , 21 ( 16 ) : 3011 – 3020 . [CSA]
- Clarke , A. D. 1989 . Aerosol Light Absorption by Soot in Remote Environments . Aerosol Sci. Technol. , 10 : 161 – 171 . [CSA]
- Clarke , A. D. and Noone , K. J. 1985 . Soot in the Arctic Snowpack: A Cause for Perturbations in Radiative Transfer . Atmos. Env. , 19 ( 12 ) : 2045 – 2053 . [CROSSREF] [CSA]
- Clarke , A. D. , Noone , K. J. , Heintzenberg , J. , Warren , S. G. and Covert , D. S. 1987 . Aerosol Light Absorption Measurement Techniques: Analysis and Intercomparison . Atmos. Env. , 21 ( 6 ) : 1455 – 1465 . [CSA]
- Clarke , A. D. , Shinozuka , Y. , Kapustin , V. N. , Howell , S. , Huebert , B. , Doherty , S. , Anderson , T. , Covert , D. , Anderson , J. , Hua , X. , Moore , K. G. II , McNaughton , C. , Carmichael , G. and Weber , R. 2004 . Size Distributions and Mixtures of Dust and Black Carbon Aerosol in Asian Outflow: Physiochemistry and Optical Properties . J. Geophys. Res. , 109 ( D15S09 ) doi: 10.1029/2003JD004378[CSA]
- Colbeck , I. , Appleby , L. , Hardman , E. J. and Harrison , R. M. 1990 . The Optical Properties and Morphology of Cloud-Processed Carbonaceous Smoke . J. Aerosol Sci , 21 ( 4 ) : 527 – 538 . [CROSSREF] [CSA]
- Colbeck , I. , Atkinson , B. and Johar , Y. 1997 . The Morphology and Optical Properties of Soot Produced by Different Fuels . J. Aerosol Sci , 28 ( 5 ) : 715 – 723 . [CROSSREF] [CSA]
- Colbeck , I. , Hardman , E. J. and Harrison , R. M. 1989 . Optical and Dynamical Properties of Fractal Clusters of Carbonaceous Smoke . J. Aerosol Sci , 20 ( 7 ) : 765 – 774 . [CROSSREF] [CSA]
- Collins , W. D. , Rasch , P. J. , Eaton , B. E. , Khattatov , B. V. , Lamarque , J. F. and Zender , C. S. 2001 . Simulating Aerosols Using a Chemical Transport Model with Assimilation of Satellite Aerosol Retrievals: Methodology for INDOEX . J. Geophys. Res. , 106 ( D7 ) : 7313 – 7336 . [CROSSREF] [CSA]
- Cooke , W. F. , Liousse , C. , Cachier , H. and Feichter , J. 1999 . Construction of a 1° × 1° Fossil Fuel Emission Data Set for Carbonaceous Aerosol and Implementation and Radiative Impact in the ECHAM4 Model . J. Geophys. Res. , 104 ( D18 ) : 22137 – 22162 . [CROSSREF] [CSA]
- Cooke , W. F. , Ramaswamy , V. and Kasibhatla , P. 2002 . A General Circulation Model Study of the Global Carbonaceous Aerosol Distribution . J. Geophys. Res. , 107 ( D16 ) doi: 10.1029/2001JD001274[CROSSREF] [CSA]
- Coulson , C. A. , Schaad , L. J. and Burnelle , L. 1959 . “ Benzene to Graphite—the Change in Electronic Energy Levels ” . In Third conference on carbon , 27 – 35 . London : Pergamon Press .
- D'Alessio , A. D. , D'Anna , A. , D'Orsi , A. , Minutolo , P. , Barbella , R. and Ciajolo , A. 1992 . “ Precursor Formation and Soot Inception in Premixed Ethylene Flames ” . In Twenty-fourth Symposium (International) on Combustion , 973 – 980 . Combustion Institute .
- d'Almeida , G. A. , Koepke , P. and Shettle , E. P. 1991 . Atmospheric Aerosols: Global Climatology and Radiative Characteristics , Hampton, VA : A. Deepak Publishing .
- Dalzell , W. H. and Sarofim , A. F. 1969 . Optical Constants of Soot and Their Application to Heat-Flux Calculations . J. Heat Trans. , 91 : 100 – 104 . [CSA]
- Dave , J. V. 1970 . Coefficients of the Legendre and Fourier Series for the Scattering Functions of Spherical Particles . Appl. Opt. , 9 ( 8 ) : 1888 – 1896 . [CSA]
- Dillner , A. M. , Stein , C. , Larson , S. M. and Hitzenberger , R. 2001 . Measuring the Mass Extinction Efficiency of Elemental Carbon in Rural Aerosol . Aerosol Sci. Technol. , 35 : 1009 – 1021 . [CROSSREF] [CSA]
- Dippel , B. , Jander , H. and Heintzenberg , J. 1999 . NIR FT Raman Spectroscopic Study of Flame Soot . Phys. Chem. Chem. Phys. , 1 : 4707 – 4712 . [CROSSREF] [CSA]
- Ditchburn , R. W. 1976 . Light , 3rd ed. , London : Academic Press .
- Dobbins , R. A. and Megaridis , C. M. 1991 . Absorption and Scattering of Light by Polydisperse Aggregates . Appl. Opt. , 30 ( 33 ) : 4747 – 4754 . [CSA]
- Dobbins , R. A. , Santoro , R. J. and Semerjian , H. G. 1983 . Interpretation of Optical Measurement of Soot in Flames . Prog. Aero. Astro. , 92 : 208 – 237 . [CSA]
- Dobbins , R. A. and Subramaniasivam , H. 1994 . “ Soot Precursor Particles in Flames ” . In Soot Formation in Combustion: Mechanisms and Models , Edited by: Bockhorn , H. 290 – 301 . Heidelberg : Springer-Verlag .
- Donnet , J. B. , Peng , C. H. , Wang , T. K. and Clague , A. D. H. 1997 . Scanning Tunneling Microscopy of Lubricating Oil Soots . Carbon , 35 ( 6 ) : 853 – 864 . [CROSSREF] [CSA]
- Donoian , H. C. and Medalia , A. I. 1967 . Scattering and Absorption of Light by Carbon Black . J. Paint Technol. , 39 ( 515 ) : 716 – 728 . [CSA]
- Draine , B. T. and Lee , H. M. 1984 . Optical Properties of Interstellar Graphite and Silicate Grains . The Astrophysical Journal , 285 : 89 – 108 . [CROSSREF] [CSA]
- Drolen , B. L. and Tien , C. L. 1987 . Absorption and Scattering of Agglomerated Soot Particulate . J. Quant. Spectrosc. Radiat. Transfer , 37 ( 5 ) : 433 – 448 . [CROSSREF] [CSA]
- Dubovik , O. , Holben , B. , Eck , T. F. , Smirnov , A. , Kaufman , Y. J. , King , M. D. , Tanre , D. and Slutsker , I. 2002 . Variability of Absorption and Optical Properties of Key Aerosol Types Observed in Worldwide Locations . J. Atm. Sci. , 59 ( 3 ) : 590 – 608 . [CROSSREF] [CSA]
- Eck , T. F. 2004 . Variability of Biomass Burning Aerosol Optical Characteristics in Southern Africa During SAFARI 2000 Dry Season Campaign and a Comparison of Single Scattering Albedo Estimates from Radiometric Measurements . J. Geophys. Res. , 108 ( D13 ) : 8500 10.1029/2002JD002321[CROSSREF] [CSA]
- Eck , T. F. , Holben , B. N. , Ward , D. E. , Dubovik , O. , Reid , J. S. , Smirnov , A. , Mukelabai , M. M. , Hsu , N. C. , O'Neil , N. T. and Slusker , I. 2001 . Characterization of the Optical Properties of Biomass Burning Aerosol in Zambia During the 1997 ZIBBEE Field Campaign . J. Geophys. Res. , 106 ( 106 ) : 3425 – 3448 . [CROSSREF] [CSA]
- Edwards , J. D. , Ogren , J. A. , Weiss , R. E. and Charlson , R. J. 1983 . Particulate Air Pollutants: A Comparison of British “Smoke” with Optical Absorption Coefficient and Elemental Carbon Concentration . Atmos. Env. , 17 ( 11 ) : 2337 – 2341 . [CROSSREF] [CSA]
- Ergun , S. and McCartney , J. T. 1960 . Reflectance of Coals, Graphite and Diamond . Fuel , 6 : 449 – 453 . [CSA]
- Erickson , W. D. , Williams , G. C. and Hottel , H. C. 1964 . Light Scattering Measurements on Soot in a Benzene-Air Flame . Comb. Flame , 8 : 127 – 132 . [CROSSREF] [CSA]
- Fanchini , G. and Tagliaferro , A. 2001 . Localisation and Density of States in Amorphous Carbon-Based Alloys . Diamond Relat. Mater. , 10 : 191 – 199 . [CROSSREF] [CSA]
- Farias , T. L. , Carvalho , M. G. , Koylu , U. O. and Faeth , G. M. 1995 . Computational Evaluation of Approximate Rayleigh-Debye-Gans/Fractal Aggregate Theory for the Absorption and Scattering Properties of Soot . J. Heat Trans. , 117 : 152 – 159 . [CSA]
- Farias , T. L. , Köylü , Ü. Ö. and Carvalho , M. G. 1996 . Range of Validity of the Rayleigh-Debye-Gans Theory for Optics of Fractal Aggregates . Appl. Opt. , 35 ( 33 ) : 6560 – 6567 . [CSA]
- Felske , J. D. , Charampopoulos , T. T. and Hura , H. S. 1984 . Determination of the Refractive Indices of Soot Particles from the Reflectivities of Compressed Soot Pellets . Comb. Sci. Tech. , 37 : 263 – 284 . [CSA]
- Fiebig , M. , Petzold , A. , Wandinger , U. , Wendisch , M. , Kiemle , C. , Stifter , A. , Ebert , M. , Rother , T. and Leiterer , U. 2002 . Optical Closure for an Aerosol Column: Method, Accuracy, and Inferable Properties Applied to a Biomass-Burning Aerosol and Its Radiative Forcing . J. Geophys. Res. , 107 ( D21 ) : 8130 doi: 10.1029/2000JD000192[CROSSREF] [CSA]
- Fischer , K. 1970 . Measurements of Absorption of Visible Radiation by Aerosol Particles . Contr. Atmos. Phys. , 43 : 244 – 254 . [CSA]
- Fletcher , T. H. , Ma , J. , Rigby , J. R. , Brown , A. L. and Webb , B. W. 1997 . Soot in Coal Combustion Systems . Prog. Energy Combust. Sci. , 23 : 283 – 301 . [CROSSREF] [CSA]
- Foot , J. S. 1979 . Spectrophone Measurements of the Absorption of Solar Radiation by Aerosol . Q. J. Roy. Met. Soc. , 105 : 275 – 283 . [CROSSREF] [CSA]
- Foot , J. S. and Kilby , C. G. 1988 . Absorption of Light by Aerosol Particles and an Intercomparison of Techniques and Spectral Observations . Atmos. Env. , 23 ( 2 ) : 489 – 495 . [CSA]
- Formenti , P. , Andreae , M. O. , Andreae , T. W. , Ichoku , C. , Schebeske , G. , Kettle , J. , Maenhaut , W. , Cafmeyer , J. , Ptasinsky , J. , Karnieli , A. and Lelieveld , J. 2001 . Physical and Chemical Characteristics of Aerosols Over the Negev Desert (Israel) During Summer 1996 . J. Geophys. Res. , 106 ( D5 ) doi: 10.1029/2000JD90055[CROSSREF] [CSA]
- Formenti , P. , Boucher , O. , Reiner , T. , Sprung , D. , Andreae , M. O. , Wendish , M. , Wex , H. , Kindred , D. , Tzortziou , M. , Vasaras , A. and Zerefos , C. 2002 . STAARTE-MED 1998 Summer Airborne Measurements Over the Aegean Sea:2. Aerosol Scattering and Absorption, and Radiative Calculations . J. Geophys. Res. , 107 ( D21 ) doi: 10.1029/2001JD001536[CSA]
- Foster , P. J. and Howarth , C. R. 1968 . Optical Constants of Carbons and Coals in the Infrared . Carbon , 6 : 719 – 729 . [CROSSREF] [CSA]
- Franklin , R. E. 1951 . The Structure of Graphitic Carbons . Acta Cryst. , 4 : 253 – 261 . [CROSSREF] [CSA]
- Fuller , K. A. 1993 . Scattering of Light by Coated Spheres . Opt. Lett. , 18 ( 4 ) : 257 – 259 . [CSA]
- Fuller , K. A. 1994 . Scattering and Absorption Cross Sections of Compounded Spheres. I. Theory for External Aggregation . J. Opt. Soc. Am. , 11 ( 12 ) : 3251 – 3260 . [CSA]
- Fuller , K. A. 1995 . Scattering and Absorption Cross Sections of Compounded Spheres. II. Calculations For External Aggregation . J. Opt. Soc. Am. , 11 ( 12 ) : 881 – 892 . [CSA]
- Fuller , K. A. , Malm , W. C. and Kreidenweis , S. M. 1999 . Effects of Mixing on Extinction By Carbonaceous Particles . J. Geophys. Res. , 104 ( D13 ) : 15941 – 15954 . [CSA]
- Gelencser , A. , Hoffer , A. , Kiss , G. , Tombácsz , E. , Kurdi , R. and Bencze , L. 2003 . In-Situ Formation of Light-Absorbing Organic Matter in Cloud Water . J. Atm. Chem. , 45 : 25 – 33 . [CROSSREF] [CSA]
- Gerber , H. E. and Hindman , E. E. 1982 . Light Absorption by Aerosol Particles: First International Workshop . Appl. Opt. , 21 ( 3 ) : 370 [CSA]
- Gilbert , L. A. 1962 . Refractive Indices and Absorption Coefficients of Coal in Bulk Measured in the Range 6000 to 2400 Å by a Polarized Light Technique . Fuel , 41 : 351 – 358 . [CSA]
- Glassman , I. 1988 . “ Soot Formation in Combustion Products ” . In Twenty-Second Symposium (International) on Combustion , 295 – 311 . Combustion Institute .
- Graham , S. C. 1974 . The Refractive Indices of Isolated and of Aggregated Soot Particles . Comb. Sci. Tech. , 9 : 159 – 163 . [CSA]
- Grams , G. W. , Blifford , I. H. Jr. , Schuster , B. G. and DeLuisi , J. S. 1972 . Complex Index of Refraction of Airborne Fly Ash Determined by Laser Radar and Collection of Particles at 13 km . J. Atm. Sci. , 29 ( 5 ) : 900 – 905 . [CROSSREF] [CSA]
- Greenaway , D. L. , Harbeke , G. , Bassani , F. and Tosatti , E. 1969 . Anisotropy of the Optical Constants and the Band Structure of Graphite . Phys. Rev. , 178 ( 3 ) : 1340 – 1348 . [CROSSREF] [CSA]
- Groblicki , P. J. , Wolff , G. T. and Countess , R. J. 1981 . Visibility-Reducing Species in the Denver “Brown Cloud”– I. Relationships Between Extinction and Chemical Composition . Atmos. Env. , 15 ( 12 ) : 2473 – 2484 . [CROSSREF] [CSA]
- Gundel , L. A. , Dod , R. L. , Rosen , H. and Novakov , T. 1984 . The Relationship Between Optical Attenuation and Black Carbon Concentration for Ambient and Source Particles . Sci. Total Env. , 26 : 197 – 202 . [CROSSREF] [CSA]
- Habib , Z. G. and Vervisch , P. 1988 . on the Refractive Index of Soot at Flame Temperature . Comb. Sci. Tech. , 59 : 261 – 274 . [CSA]
- Hallett , J. , Hudson , J. G. and Rogers , C. F. 1989 . Characterization of Combustion Aerosols for Haze and Cloud Formation . Aerosol Sci. Technol. , 10 : 70 – 83 . [CSA]
- Hänel . 1987 . Radiation Budget of the Boundary Layer. Part II: Simultaneous Measurement of Mean Solar Volume Absorption and Extinction Coefficients of Particles . Beitr. Phys. Atmosph. , 60 ( 2 ) : 241 – 247 . [CSA]
- Hänel , G. and Hillenbrand , C. 1989 . Calorimetric Measurement of Optical Absorption . Appl. Opt. , 28 ( 3 ) : 510 – 516 . [CSA]
- Hansen , A. D. A. and McMurry , P. H. 1990 . An Intercomparison of Measurements of Aerosol Elemental Carbon During the 1986 Carbonaceous Species Method Comparison Study . J. Air and Waste Manage. Assoc. , 40 ( 6 ) : 894 – 895 . [CSA]
- Hansen , A. D. A. and Rosen , H. 1984 . Vertical Distributions of Particulate Carbon, Sulfur, and Bromine in the Arctic Haze and Comparison with Ground-Level Measurements at Barrow, Alaska . Geophys. Res. Let. , 11 : 381 [CSA]
- Hansen , A. D. A. , Rosen , H. and Novakov , T. 1982 . Real-Time Measurement of the Absorption Coefficient of Aerosol Particles . Appl. Opt. , 21 ( 17 ) : 3060 – 3062 . [CSA]
- Hansen , A. D. A. , Rosen , H. and Novakov , T. 1984 . The Aethalometer—An Instrument for the Real-Time Measurement of Optical Absorption by Aerosol Particles . Sci. Total Env. , 36 : 191 – 196 . [CROSSREF] [CSA]
- Hansen , J. , Johnson , D. , Lacis , A. , Lebedeff , S. , Lee , P. , Rind , D. and Russell , G. 1981 . Climate Impact of Increasing Atmospheric Carbon Dioxide . Science , 213 : 957 – 966 . [CSA]
- Hansen , J. , Sato , M. and Ruedy , R. 1997 . Radiative Forcing and Climate Response . J. Geophys. Res. , 102 ( D6 ) : 6831 – 6864 . [CROSSREF] [CSA]
- Hansen , J. E. and Sato , M. 2001 . Trends of Measured Climate Forcing Agents . Proc. Natl. Acad. Sci. , 98 ( 26 ) : 14778 – 14783 . [CROSSREF] [CSA]
- Hansen , J. E. , Sato , M. , Ruedy , R. , Lacis , A. and Oinas , V. 2000 . Global Warming in the Twenty-First Century: An Alternative Scenario . Proc. Natl. Acad. Sci. , 97 ( 18 ) : 9875 – 9880 . [CROSSREF] [CSA]
- Haynes , B. S. and Wagner , H. G. 1981 . Soot Formation . Prog. Energy Combust. Sci. , 7 : 229 – 273 . [CROSSREF] [CSA]
- Haywood , J. and Boucher , O. 2000 . Estimates of the Direct and Indirect Radiative Forcing Due to Tropospheric Aerosols: A Review . Rev. Geophys. , 38 ( 4 ) : 513 – 543 . [CROSSREF] [CSA]
- Haywood , J. M. and Ramaswamy , V. 1998 . Global Sensitivity Studies of the Direct Radiative Forcing Due to Anthropogenic Sulfate and Black Carbon Aerosols . J. Geophys. Res. , 103 ( D6 ) : 6043 – 6058 . [CROSSREF] [CSA]
- Haywood , J. M. , Roberts , D. L. , Slingo , A. , Edwards , J. M. and Shine , K. P. 1997 . General Circulation Model Calculations of the Direct Radiative Forcing by Anthropogenic Sulfate and Fossil-Fuel Soot Aerosol . J. Clim. , 10 ( 7 ) : 1562 – 1577 . [CROSSREF] [CSA]
- Haywood , J. M. and Shine , K. P. 1995 . The Effect of Anthropogenic Sulfate and Soot Aerosol on the Clear Sky Planetary Radiation Budget . Geophys. Res. Let. , 22 ( 5 ) : 603 – 606 . [CROSSREF] [CSA]
- Heckman , F. A. 1964 . Microstructure of Carbon Black . Rubber Chemistry and Technology , 37 : 1245 – 1298 . [CSA]
- Heckman , F. A. and Harling , D. F. 1966 . Progressive Oxidation of Selected Particles of Carbon Black: Further Evidence for a New Microstructural Model . Rubber Chemistry and Technology , 39 : 1 – 3 . [CSA]
- Heidenreich , R. D. , Hess , W. M. and Ban , L. L. 1968 . A Test Object and Criteria for High Resolution Electron Microscopy . J. Appl. Cryst. , 1 ( 1–19 ) [CSA]
- Heintzenberg , J. 1982 . Size-Segregated Measurements of Particulate Elemental Carbon and Aerosol Light Absorption at Remote Arctic Locations . Atmos. Env. , 16 ( 10 ) : 2461 – 2469 . [CROSSREF] [CSA]
- Heintzenberg , J. , Charlson , R. J. , Clarke , A. D. , Liousse , C. , Ramaswamy , V. , Shine , K. P. , Wendisch , M. and Helas , G. 1997 . Measurements and Modelling of Aerosol Single-Scattering Albedo: Progress, Problems and Prospects . Contr. Atmos. Phys. , 70 ( 4 ) : 249 – 263 . [CSA]
- Heintzenberg , J. and Meszaros , A. 1985 . Elemental Carbon, Sulfur and Metals in Aerosol Samples at a Hungarian Regional Air Pollution Station . Idojaras , 89 ( 6 ) : 313 – 319 . [CSA]
- Heintzenberg , J. , Okada , K. and Luo , B. P. 2002 . Distribution of Optical Properties Among Atmospheric Submicrometer Particles of Given Electrical Mobilities . J. Geophys. Res. , 107 ( D11 ) : 4107 doi: 10.1029/2001JD000372[CROSSREF] [CSA]
- Heller , W. 1965 . Remarks on Refractive Index Mixture Rules . J. Phys. Chem. , 69 ( 4 ) : 1123 – 1129 . [CSA]
- Henning , T. , Il'in , V. B. , Krivova , N. A. , Michel , B. and Voshchinnikov , N. V. 1999 . WWW Database of Optical Constants for Astronomy . Astron. Astrophys. Suppl , 136 : 405 – 406 . [CROSSREF] [CSA]
- Hess and Herd . 1993 . Carbon Black , Edited by: Donnet , J.-B. and Wang , M.-J. New York : Marcel Dekker .
- Hess , M. , Koepke , P. and Schult , I. 1998 . Optical Properties of Aerosols and Clouds: The Software Package OPAC . Bull. Am. Met. Soc. , 79 ( 5 ) : 831 – 844 . [CROSSREF] [CSA]
- Hidy , G. M. 1980 . the Character and Origins of Smog Aerosols: A Digest of Results from the California Aerosol Characterization Experiment (ACHEX) , New York : Wiley .
- Hignett , P. , Taylor , J. P. , Francis , P. N. and Glew , M. D. 1999 . Comparison of Observed and Modelled Direct Aerosol Forcing During TARFOX . J. Geophys. Res. , 104 : 2279 – 2287 . [CROSSREF] [CSA]
- Higurashi , A. and Nakajima , T. 2002 . Detection of Aerosol Types Over the East China Sea Near Japan from Four-Channel Satellite Data . Geophys. Res. Let. , 29 ( 17 ) : 1836 [CROSSREF] [CSA]
- Hitzenberger . 1993 . Absorption Measurements with an Integrating Plate Photometer– Calibration and Error Analysis . Aerosol Sci. Technol. , 18 ( 1 ) : 70 – 84 . [CSA]
- Hitzenberger , R. , Dusek , U. and Berner , A. 1996 . Black Carbon Measurements Using an Integrating Sphere . J. Geophys. Res. , 101 ( D14 ) : 19601 – 19606 . [CROSSREF] [CSA]
- Hitzenberger , R. , Jennings , S. G. , Larson , S. M. , Dillner , A. , Cachier , H. , Galambos , Z. , Rouc , A. and Spain , T. G. 1999 . Intercomparison of Measurement Methods for Black Carbon Aerosols . Atmos. Env. , 33 : 2823 – 2833 . [CROSSREF] [CSA]
- Holben , B. N. , Eck , T. F. , Slutsker , I. , Tanre , D. , Buis , J. P. , Setzer , A. , Vermote , E. , Reagan , J. A. , Kaufman , Y. J. , Nakajima , T. , Lavenu , F. , Jankowiak , I. and Smirnov , A. 1998 . AERONET- a Federated Instrument Network and Data Archive for Aerosol Characterization . Remote Sens. Environ. , 66 ( 1 ) : 1 – 16 . [CROSSREF] [CSA]
- Horvath , H. 1993a . Atmospheric Light Absorption: A Review . Atmos. Env. , 27A ( 3 ) : 293 – 317 . [CSA]
- Horvath , H. 1993b . Comparison of Measurements of Aerosol Optical Absorption by Filter Collection and a Transmissometric Method . Atmos. Env. , 27A ( 3 ) : 319 – 325 . [CSA]
- Horvath , H. and Metzig , G. 1990 . Experimental Determination of the Accuracy of Light Absorption Measurement with the Integrating Plate Method . J. Aerosol Sci , 21 : 525 – 528 . [CROSSREF] [CSA]
- Hsu , N. C. , Tsay , S.-C. , King , M. D. and Herman , J. R. 2004 . Aerosol Properties Over Bright-Reflecting Source Regions . IEEE Trans. Geosci. Remote Sens. , 42 ( 3 ) : 557 – 569 . [CROSSREF] [CSA]
- Huebert , B. J. , Bates , T. , Russell , P. B. , Shi , G. , Kim , Y. J. , Kawamura , K. , Carmichael , G. and Nakajima , T. 2003 . An overview of ACE-Asia: Strategies for Quantifying the Relationships between Asian Aerosols and Their Climatic Impacts . J. Geophys. Res. , 108 ( D23 ) : 8633 doi: 10.1029/2003JD003550[CROSSREF] [CSA]
- Huebert , B. J. and Charlson , R. J. 2000 . Uncertainties in Data on Organic Aerosols . Tellus , 52B : 1249 – 1255 . [CSA]
- Huffman , H. D. 1996 . The Reconstruction of Aerosol Light Absorption by Particle Measurements at Remote Sites: An Independent Analysis of Data from the IMPROVE Network—II . Atmos. Env. , 30 ( 1 ) : 85 – 99 . [CROSSREF] [CSA]
- Huntzicker , J. J. , Johnson , R. L. , Shah , J. J. and Cary , R. A. 1982 . “ Analysis of Organic and Elemental Carbon in Ambient Aerosols by a Thermal-Optical Method ” . In Particulate Carbon: Atmospheric Life Cycle , Edited by: Wolff , G. T. and Klimisch , R. L. 79 – 88 . New York : Plenum Press .
- Husar , R. B. , Prospero , J. M. and Stowe , L. L. 1997 . Characterization of Tropospheric Aerosols Over the Oceans with the Noaa Advanced Very High Resolution Radiometer Optical Thickness Operational Product . J. Geophys. Res. , 102 ( D14 ) : 16889 – 16909 . [CROSSREF] [CSA]
- IPCC (Intergovernmental Panel on Climate Change) . 1996 . Climate change 1995: The science of climate change , Cambridge : University Press .
- Ishiguro , T. , Suzuki , N. , Fujitani , Y. and Morimoto , H. 1991 . Microstructural Changes of Diesel Soot During Oxidation . Comb. Flame , 85 : 1 – 6 . [CSA]
- Iskander , M. F. , Chen , H. Y. and Penner , J. E. 1991 . Resonance Optical Absorption by Fractal Agglomerates of Smoke Aerosols . Atmos. Env. , 25A ( 11 ) : 2563 – 2569 . [CSA]
- Jacobson , M. Z. 2000 . A Physically-Based Treatment of Elemental Carbon Optics: Implications for Global Direct Forcing of Aerosols . Geophys. Res. Let. , 27 ( 2 ) : 217 – 220 . [CROSSREF] [CSA]
- Jacobson , M. Z. 2001 . Strong Radiative Heating Due to the Mixing State of Black Carbon in Atmospheric Aerosols . Nature , 409 : 695 – 697 . [PUBMED] [INFOTRIEVE] [CROSSREF] [CSA]
- Jacobson , M. Z. 2002 . Control of Fossil–Fuel Particulate Black Carbon and Organic Matter, Possibly the Most Effective Method of Slowing Global Warming . J. Geophys. Res. , 107 ( D19 ) doi: 10.1029/2001JD001376[CSA]
- Jaenicke . 1988 . “ Properties of atmospheric aerosols ” . In Landold-Bornstein's Numerical Data & Functional Relationships in Science and technology vol 4b , p. 417
- Jäger , C. , Mutsche , H. and Henning , T. 1998 . Optical Properties of Carbonaceous Dust Analogues . Astron. Astrophys. , 332 : 291 – 299 . [CSA]
- Jäger , C. , Henning , T. , Schlögl , R. and Spillecke , O. 1999 . Spectral Properties of Carbon Black . J. Non-Cryst. Solid , 258 : 161 – 179 . [CROSSREF] [CSA]
- Janzen , J. 1979 . The Refractive Index of Carbon Black . J. Colloid Interface Sci. , 69 ( 3 ) : 436 – 447 . [CROSSREF] [CSA]
- Japar , S. M. , Brachaczek , W. W. , Gorse , R. A. Jr. , Norbeck , J. M. and Pierson , W. R. 1986 . The Contribution of Elemental Carbon to the Optical Properties of Rural Atmospheric Aerosols . Atmos. Env. , 20 ( 6 ) : 1281 – 1289 . [CROSSREF] [CSA]
- Japar , S. M. and Killinger , D. K. 1979 . Photoacoustic and Absorption Spectrum of Airborne Carbon Particulate Using a Tunable Dye Laser . Chem. Phys. Let. , 66 ( 1 ) : 207 – 209 . [CROSSREF] [CSA]
- Japar , S. M. and Szkarlat , A. C. 1981 . Measurement of Diesel Vehicle Exhaust Particulate Using Photoacoustic Spectroscopy . Comb. Sci. Tech. , 24 : 215 – 219 . [CSA]
- Japar , S. M. , Szkarlat , A. C. and Gorse , R. A. Jr. 1981 . Optical Properties of Particulate Emissions from On-Road Vehicles . Atmos. Env. , 15 ( 10/11 ) : 2063 – 2070 . [CSA]
- Japar , S. M. , Szkarlat , A. C. and Pierson , W. R. 1984 . The Determination of the Optical Properties of Airborne Particle Emissions from Diesel Vehicles . Sci. Total Env. , 36 : 121 – 130 . [CROSSREF] [CSA]
- Jennings , S. G. and Pinnick , R. G. 1980 . Relationships Between Visible Extinction, Absorption and Mass Concentration of Carbonaceous Smokes . Atmos. Env. , 14 : 1123 – 1129 . [CROSSREF] [CSA]
- Jiang , Y. J. , Solum , M. S. , Pugmire , R. J. , Grant , D. M. , Schobert , H. H. and Pappano , P. J. 2002 . A New Method for Measuring the Graphite Content of Anthracite Coals and Soots . Energy Fuels , 16 : 1296 – 1300 . [CROSSREF] [CSA]
- Johnson , M. P. , Donnet , J. B. , Wang , T. K. , Wang , C. C. , Locke , R. W. , Brinson , B. E. and Marriott , T. 2002 . A Dynamic Continuum of Nanostructured Carbons in the Combustion Furnace . Carbon , 40 : 189 – 194 . [CROSSREF] [CSA]
- Jones , A. R. 1979 . Electromagnetic Wave Scattering by Assemblies of Particles in the Rayleigh Approximation . Proc. R. Soc. Lond. A , 366 : 111 – 127 . [CSA]
- Kahn , R. , Banerjee , P. and McDonald , D. 2001 . Sensitivity of Multiangle Imaging to Natural Mixtures of Aerosols Over Ocean . J. Geophys. Res. , 106 ( D16 ) : 18219 – 18238 . [CROSSREF] [CSA]
- Katrinak , K. A. , Rez , P. A. , Perkes , P. R. and Buseck , P. R. 1993 . Fractal Geometry of Carbonaceous Aggregates from an Urban Aerosol . Environ. Sci. Technol. , 27 : 539 – 547 . [CROSSREF] [CSA]
- Kattawar , G. W. and Hood , D. A. 1976 . Electromagnetic Scattering from a Spherical Polydispersion of Coated Spheres . Appl. Opt. , 15 ( 8 ) : 1996 – 1999 . [CSA]
- Kaufman and Tanré , Y. J. D. Algorithm for Remote Sensing of Tropospheric Aerosol from MODIS , 1998, Product ID MOD04. Available at http://modis-atmos.gsfc.nasa.gov/MOD04_L2/)
- Kaufman , Y. , Tanré , D. and Boucher , O. 2002a . A Satellite View of Aerosols in the Climate System . Nature , 219 : 215 – 223 . [CROSSREF] [CSA]
- Kaufman , Y. J. , Hobbs , P. V. , Kirchhoff , V. W. J. H. , Artaxo , P. , Remer , L. A. , Holben , B. N. , King , M. D. , Ward , D. E. , Prins , E. M. , Longo , K. M. , Mattos , L. F. , Nobre , C. A. , Spinhirne , J. D. , Ji , Q. , Thompson , A. M. , Gleason , J. F. , Christopher , S. A. and Tsay , S.-C. 1998 . Smoke, clouds, and radiation—Brazil (SCAR-B) experiment . J. Geophys. Res. , 103 ( D24 ) : 31783 – 31808 . [CROSSREF] [CSA]
- Kaufman , Y. J. , Martins , J. V. , Remer , L. A. , Schoeberl , M. R. and Yamasoe , M. A. 2002b . Satellite Retrieval of Aerosol Absorption Over The Oceans Using Sunglint . Geophys. Res. Let. , 29 ( 19 ) : 1928 [CROSSREF] [CSA]
- Kaufman , Y. J. , Tanre , D. , Dubovik , O. , Karnieli , A. and Remer , L. A. 2001 . Absorption of Sunlight By Dust as Inferred from Satellite and Ground-Based Remote Sensing . Geophys. Res. Let. , 28 : 1479 – 1483 . [CROSSREF] [CSA]
- Kennedy , I. M. 1997 . Models of Soot Formation and Oxidation . Prog. Energy Combust. Sci. , 23 : 95 – 132 . [CROSSREF] [CSA]
- Kerker , M. 1969 . the Scattering of Light and other Electromagnetic Radiation , New York : Academic Press .
- Kiehl , J. T. and Briegleb , B. P. 1993 . The Relative Roles of Sulfate Aerosols and Greenhouse Gases in Climate Forcing . Science , 260 : 311 – 314 . [CSA]
- Kirchner , U. , Vogt , R. , Natzeck , C. and Goschnick , J. 2003 . Single Particle MS, SNMS, XPS and FTIR Spectroscopic Analysis of Soot Particles During the AIDA Campaign . J. Aerosol Sci , 34 : 1323 – 1346 . [CROSSREF] [CSA]
- Kirchstetter , T. W. , Corrigan , C. E. and Novakov , T. 2001 . Laboratory and Field Investigation of the Adsorption of Gaseous Organic Compounds Onto Quartz Filters . Atmos. Env. , 35 ( 9 ) : 1663 – 1671 . [CROSSREF] [CSA]
- Kirchstetter , T. W. , Novakov , T. and Hobbs , P. V. 2004 . Evidence That the Spectral Dependence of Light Absorption by Aerosols is Affected by Organic Carbon . J. Geophys. Res. , 109 : D21208 doi: 10.1029/2004JD004999[CROSSREF] [CSA]
- Kleeman , M. J. , Schauer , J. J. and Cass , G. R. 1999 . Size and Composition Distribution of Fine Particulate Matter Emitted from Wood Burning, Meat Charbroiling, and Cigarettes . Environ. Sci. Technol. , 33 : 3516 – 3523 . [CROSSREF] [CSA]
- Koch , D. 2000 . Transport and Direct Radiative Forcing of Carbonaceous and Sulfate Aerosols in the GISS GCM . J. Geophys. Res. , 106 ( D17 ) : 20311 – 20332 . [CROSSREF] [CSA]
- Kondratyev , K. Y. , Vasilyev , O. B. , Grishenhkin , V. S. , Prokofyev , M. A. , Chapursky , L. S. and Ivlev , L. S. 1973 . Spectral Radiative Flux Divergence in the Troposphere . Proceedings of the International Radiation Symposium, Radiation Commission of the International Association of Meteorology and Physics, H.J.B. 1973 . Sendai, , Japan : Meteorological Institute of the University of Munich, West Germany .
- Kondratyev , K. Y. , Welch , R. M. , Vasilyev , O. B. , Zhvalev , V. F. , Ivlev , L. S. and Radionov , V. F. 1979 . Calculations of Free Atmospheric Shortwave Spectral Characteristics Over the Desert . Tellus , 31 : 13 – 27 . [CSA]
- Köylü , U. O. and Faeth , G. M. 1992 . Structure of Overfire Soot in Buoyant Turbulent Diffusion Flames at Long Residence Times . J. Heat Trans. , 89 : 140 – 156 . [CSA]
- Köylü , U. O. and Faeth , G. M. 1994 . Optical Properties of Overfire Soot in Buoyant Turbulent Diffusion Flames at Long Residence Times . J. Heat Trans. , 116 : 152 – 159 . [CSA]
- Kuhlbusch , T. A. J. 1995 . Method for Determining Black Carbon in Residues of Vegetation Fires . Environ. Sci. Technol. , 29 ( 2695–2702 ) [CSA]
- Lahaye , J. and Ehrburger-Dolle , F. 1994 . Mechanisms of Carbon Black Formation: Correlation with the Morphology of Aggregates . Carbon , 32 ( 7 ) : 1319 – 1324 . [CROSSREF] [CSA]
- Lavanchy , V. M. H. , Gäggeler , H. W. , Nyeki , S. and Baltensperger , U. 1999 . Elemental Carbon (EC) and Black Carbon (Bc) Measurements with a Thermal Method and an Aethalometer at the High-Alpine Research Station Jungfraujoch . Atmos. Env. , 33 : 2759 – 2769 . [CROSSREF] [CSA]
- Lee , K. O. , Cole , R. , Sekar , R. , Choi , M. Y. , Kang , J. S. , Bae , C. S. and Shin , H. D. 2002a . Morphological Investigation of the Microstructure, Dimensions, and Fractal Geometry of Diesel Particulates . Proceedings of the Combustion Institute . 2002a . Vol. 29 , pp. 647 – 653 .
- Lee , K.-T. 1983 . Generation of Soot Particles and Studies of Factors Controlling Soot Light Absorption: , 162 p. Seattle, Washington : University of Washington . Ph.D.
- Lee , S. C. and Tien , C. L. 1981 . Optical Constants of Soot in Hydrocarbon Flames . Eighteenth Symposium (International) on Combustion , : 1159 – 1166 . [CSA]
- Lee , T. H. , Yao , N. , Chen , T. J. and Hsu , W. K. 2002b . Fullerene-Like Carbon Particles in Petrol Soot . Carbon , 40 : 2263 – 2279 . [CROSSREF] [CSA]
- Lesins , G. , Chylek , P. and Lohmann , U. 2002 . A Study of Internal and External Mixing Scenarios and Its Effect on Aerosol Optical Properties and Direct Radiative Forcing . J. Geophys. Res. , 107 ( D10 ) : 4094 [CROSSREF] [CSA]
- Lewis , C. W. and Dzubay , T. G. 1986 . Measurement of Light Absorption Extinction in Denver . Aerosol Sci. Technol. , 5 : 325 – 326 . [CSA]
- Liley , J. B. , Baumgardner , D. , Kondo , Y. , Kita , K. , Blake , D. R. , Koike , M. , Machida , T. , Takegawa , N. , Kawakami , S. , Shirai , T. and Ogawa , T. 2002 . Black Carbon in Aerosol During BIBLE B . J. Geophys. Res. , 108 ( D3 ) : 8399 doi: 10.1029/2001JD000845[CROSSREF] [CSA]
- Lin , C.-I. , Baker , M. and Charlson , R. J. 1973 . Absorption Coefficient of Atmospheric Aerosol: A Method for Measurement . Appl. Opt. , 12 ( 6 ) : 1356 – 1363 . [CSA]
- Lindberg , J. D. 1975 . The Composition and Optical Absorption Coefficient of Atmospheric Particulate Matter . Opt. Quant. Electronics , 7 : 131 – 139 . [CROSSREF] [CSA]
- Liousse , C. , Cachier , H. and Jennings , S. G. 1993 . Optical and Thermal Measurements of Black Carbon Aerosol Content in Different Environments . Atmos. Env. , 27A ( 8 ) : 1203 – 1211 . [CSA]
- Liousse , C. , Penner , J. E. , Chuang , C. , Walton , J. J. and Eddleman , H. 1996 . A Global Three-Dimensional Study of Carbonaceous Aerosols . J. Geophys. Res. , 101 ( D14 ) : 19411 – 19432 . [CROSSREF] [CSA]
- Logan , N. A. 1965 . Survey of Some Early Studies of the Scattering of Plane Waves by a Sphere . Proc. IEEE , 53 : 773 – 785 . [CSA]
- Mackowski , D. W. 1995 . Electrostatics Analysis of Radiative Absorption by Sphere Clusters in the Rayleigh Limit: Application to Soot Particles . Appl. Opt. , 34 ( 18 ) : 3535 – 3545 . [CSA]
- Magi , B. , Hobbs , P. V. , Kirchstetter , T. W. , Novakov , T. , Hegg , D. A. , Gao , S. , Redemann , J. and Schmid , B. 2005 . Aerosol Properties and Chemical Apportionment of Aerosol Optical Depth at Locations off the United States East Coast in July and August 2001 . J. Atmos. Sci. , 62 ( 4 ) : 919 – 933 . [CROSSREF] [CSA]
- Mahowald , N. M. and Dufresne , J. L. 2004 . Sensitivity of TOMS Aerosol Index to Boundary Layer Height: Implications for Detection of Mineral Aerosol Sources . Geophys. Res. Let. , 31 : 03103 doi: 10.1029/2003GL018865[CROSSREF] [CSA]
- Mallet , M. , Roger , J. C. , Despiau , S. , Putaud , J. P. and Dubovik , O. 2004 . A Study of the Mixing State of Black Carbon in Urban Zone . J. Geophys. Res. , 109 : D04202 doi: 10.1029/2003JD003940[CROSSREF] [CSA]
- Malm , W. C. , Sisler , J. F. , Huffman , D. , Eldred , R. A. and Cahill , T. A. 1994 . Spatial and Seasonal Trends in Particle Concentration and Optical Extinction in the United States . J. Geophys. Res. , 99 ( D1 ) : 1347 – 1370 . [CSA]
- Marley , N. A. , Gaffney , J. S. , Baird , J. C. , Blazer , C. A. , Drayton , P. J. and Frederick , J. E. 2001 . An Empirical Method for the Determination of the Complex Refractive Index of Size-Fractionated Atmospheric Aerosols for Radiative Transfer Calculations . Aerosol Sci. Technol. , 34 : 535 – 549 . [CSA]
- Marsh , P. A. , Voet , A. , Mullens , T. J. and Price , L. D. 1971 . Quantitative Micrography of Carbon Black Microstructure . Carbon , 9 : 797 – 805 . [CROSSREF] [CSA]
- Martins , J. V. , Artaxo , P. , Liousse , C. , Cachier , H. , Kaufman , Y. and Fattori , A. P. 1996 . “ Size Distribution, Elemental Composition, Carbon Measurements and Calculated Optical Properties of Biomass Burning Aerosol Particles During the SCAR-C Experiment ” . In Global Biomass Burning , Edited by: Fiocco , G. 74 – 77 . Cambridge : MIT Press .
- Martins , J. V. , Artaxo , P. , Liousse , C. , Reid , J. S. , Hobbs , P. V. and Kaufman , Y. 1998 . Effects of Black Carbon Content, Particle Size, and Mixing on Light Absorption by Aerosol from Biomass Burning in Brazil . J. Geophys. Res. , 103 ( D24 ) : 32041 – 32050 . [CROSSREF] [CSA]
- Masiello , C. A. and Druffel , E. R. M. 1998 . Black Carbon in Deep-Sea Sediments . Science , 280 : 1911 – 1913 . [PUBMED] [INFOTRIEVE] [CROSSREF] [CSA]
- Mayol-Bracero , O. L. , Gabriel , R. , Andreae , M. O. , Kirchstetter , T. W. , Novakov , T. , Ogren , J. , Sheridan , P. and Streets , D. G. 2002b . Carbonaceous Aerosols Over the Indian Ocean During the Indian Ocean Experiment (INDOEX): Chemical Characterization, Optical Properties, and Probable Sources . J. Geophys. Res. , 107 ( D19 ) : 8030 [CROSSREF] [CSA]
- Mayol-Bracero , O. L. , Guyon , P. , Graham , B. , Roberts , G. , Andreae , M. O. , Decesari , S. , Facchini , M. C. , Fuzzi , S. and Artaxo , P. 2002a . Water–Soluble Organic Compounds in Biomass Burning Aerosols Over Amazonia. 2. Apportionment of the Chemical Composition and Importance of the Polyacidic Fraction . J. Geophys. Res. , 107 ( D20 ) : 8091 doi: 10.1029/2001JD000522[CROSSREF] [CSA]
- McCartney , J. T. , Yasinsky , J. B. and Ergun , S. 1965 . Optical Constants of Coals by Reflectance Measurements in the Ultra-Violet and Visible Spectrum . Fuel , 44 : 349 – 354 . [CSA]
- McKenzie , D. R. , McPhedran , R. C. , Savvides , N. and Botten , L. C. 1983 . Properties and Structure of Amorphous Hydrogenated Carbon Films . Philos. Mag. B , 48 ( 4 ) : 341 – 364 . [CSA]
- McLean , W. J. , Hardesty , D. R. and Pohl , J. H. 1981 . “ Direct Observations of Devolatilizing Pulverized Coal Particles in a Combustion Environment ” . In Eighteenth Symposium (International) on Combustion , 1239 – 1248 . Combustion Institute .
- McMurry , P. H. , Wang , X. , Park , K. and Ehara , K. 2002 . The Relationship Between Mass and Mobility For Atmospheric Particles: A New Technique for Measuring Particle Density . Aerosol Sci. Technol. , 36 : 227 – 238 . [CROSSREF] [CSA]
- Medalia , A. I. and Heckman , F. A. 1969 . Morphology of Aggregates—II. Size and Shape Factors of Carbon Black Aggregates from Electron Microscopy . Carbon , 7 : 569 – 582 . [CROSSREF] [CSA]
- Medalia , A. I. and Richards , L. W. 1972 . Tinting Strength of Carbon Black . J. Colloid Interface Sci. , 40 ( 2 ) : 233 – 252 . [CROSSREF] [CSA]
- Medalia , A. I. and Rivin , D. 1982 . Particulate Carbon and Other Components of Soot and Carbon Black . Carbon , 20 ( 6 ) : 481 – 492 . [CROSSREF] [CSA]
- Menon , S. , Hansen , J. and Nazarenko , L. 2002 . Climate Effects of Black Carbon Aerosols in China and India . Science , 297 : 2250 – 2253 . [PUBMED] [INFOTRIEVE] [CROSSREF] [CSA]
- Michel , B. , Henning , T. , Jäger , C. and Kreibig , U. 1999 . Optical Extinction by Spherical Carbonaceous Particles . Carbon , 37 : 391 – 400 . [CROSSREF] [CSA]
- Mie , G. 1908 . Beiträge Zur Optik Trüber Medien, Speziell Kolloidaler Metallösungen . Annalen der Physik , 25 ( 3 ) : 377 – 445 . [CSA]
- Millikan , R. C. 1961 . Optical Properties of Soot . J. Opt. Soc. Am. , 51 : 698 – 699 . [CSA]
- Minutolo , P. , Gambi , G. and D'Alessio , A. 1996 . “ The Optical Band Gap Model in the Interpretation of the UV-Visible Absorption Spectra of Rich Premixed Flames ” . In Twenty-Sixth Symposium (International) on Combustion , 951 – 957 . Combustion Institute .
- Minutolo , P. , Gambi , G. , D'Alessio , A. and D'Anna , A. 1994 . Optical and Spectroscopic Characterization of Rich Premixed Flames Across the Soot Formation Threshhold . Comb. Sci. Tech. , 101 : 311 – 325 . [CSA]
- Mishchenko , M. I. , Travis , L. D. and Mackowski , D. W. 1996 . T-Matrix Computations of Light Scattering by Nonspherical Particles: A Review . J. Quant. Spectrosc. Radiat. Transfer , 55 ( 5 ) : 535 – 575 . [CROSSREF] [CSA]
- Moosmuller , H. , Arnott , W. P. , Rogers , C. F. , Chow , J. C. and Frazier , C. A. 1998 . Photoacoustic and Filter Measurements Related to Aerosol Light Absorption During the Northern Front Range Air Quality Study (Colorado 1996/1997) . J. Geophys. Res. , 103 ( D21 ) : 28149 – 28157 . doi: 10.1029/2003JD003409[CROSSREF] [CSA]
- Mountain , R. D. and Mulholland , G. W. 1988 . Light Scattering from Simulated Smoke Agglomerates . Langmuir , 4 : 1321 – 1326 . [CROSSREF] [CSA]
- Mulholland , G. W. and Choi , M. Y. 1998 . “ Measurement of the Mass-Specific Extinction Coefficient for Acetylene and Ethene Smoke Using the Large Agglomerate Optics Facility ” . In Twenty-Seventh Symposium (International) on Combustion , 1515 – 1522 . Combustion Institute .
- Mullins , J. and Williams , A. 1987 . The Optical Properties of Soot: A Comparison Between Experimental and Theoretical Values . Fuel , 66 : 277 – 280 . [CROSSREF] [CSA]
- Muñoz , R. H. and Charalampopoulos , T. T. 1998 . “ Evolution of Compositional and Structural Properties of Soot in Premixed Alkane Flames ” . In Twenty-Seventh Symposium (International) on Combustion , 1471 – 1479 . Combustion Institute .
- Myhre , G. , Stordal , F. , Restad , K. and Isaksen , I. S. A. 1998 . Estimation of the Direct Radiative Forcing due to Sulfate and Soot Aerosols . Tellus , 50B ( 5 ) : 463 – 477 . [CSA]
- Nakajima , T. , Sekiguchi , M. , Takemura , T. , Uno , I. , Higurashi , A. , Kim , D. , Sohn , B. J. , Oh , S.-N. , Nakajima , T. Y. , Ohta , S. , Okada , I. , Takamura , T. and Kawamoto , K. 2003 . Significance of Direct and Indirect Radiative Forcings of Aerosols in the East China Sea Region . J. Geophys. Res. , 108 ( D23 ) : 8658 doi: 10.1029/2002JD003261[CROSSREF] [CSA]
- Naoe , H. and Okada , K. 2001 . Mixing Properties of Submicrometer Aerosol Particles in the Urban Atmosphere–with Regard to Soot Particles . Atmos. Env. , 2001 ( 35 ) : 5765 – 5772 . [CROSSREF] [CSA]
- Nelson , J. 1989 . Fractality of Sooty Smoke: Implications for the Severity of Nuclear Winter . Nature , 339 : 611 – 613 . [CROSSREF] [CSA]
- Nguyen , T. H. , Brown , R. A. and Ball , W. P. 2004 . An Evaluation of Thermal Resistance as a Measure of Black Carbon Content in Diesel Soot, Wood Char, and Sediment . Organic Geochem. , 35 ( 3 ) : 217 – 234 . Submitted to[CROSSREF] [CSA]
- Nilsson , B. 1979 . Meteorological Influence on Aerosol Extinction in the 0.2–40 um Wavelength Range . Appl. Opt. , 18 ( 20 ) : 3457 – 3473 . [CSA]
- Novakov , T. , Hegg , D. A. and Hobbs , P. V. 1997 . Airborne Measurements of Carbonaceous Aerosols on the East Coast of the United States . J. Geophys. Res. , 102 ( D25 ) : 30023 – 30330 . [CROSSREF] [CSA]
- Nyeki , S. and Colbeck , I. 1995 . Fractal Dimension Analysis of in-situ, Restructured Carbonaceous Aggregates . Aerosol Sci. Technol. , 23 : 109 – 120 . [CSA]
- Oberlin , A. 1984 . Carbonization and Graphitization . Carbon , 22 ( 6 ) : 521 – 541 . [CROSSREF] [CSA]
- Ogren , J. A. and Charlson , R. J. 1983 . Elemental Carbon in the Atmosphere: Cycle and Lifetime . Tellus , 35B : 241 – 254 . [CSA]
- Okada , K. 1983 . Nature of Individual Hygroscopic Particles in the Urban Atmosphere . Journal of the Meteorological Society of Japan , 61 ( 5 ) : 727 – 736 . [CSA]
- Ouimette , J. R. and Flagan , R. C. 1982 . The Extinction Coefficient of Multicomponent Aerosols . Atmos. Env. , 16 ( 10 ) : 2405 – 2419 . [CROSSREF] [CSA]
- Palik , E. D. , ed. 1991 . Handbook of Optical Constants of Solids II , Boston : Harcourt Brace Jovanovich .
- Palotás , Á. B. , Rainey , L. C. , Felderman , C. J. , Sarofim , A. F. and Sande , J. B. V. 1996b . Soot Morphology: An Application of Image Analysis in High-Resolution Transmission Electron Microscopy . Microscopy Research and Technique , 33 : 266 – 278 . [CROSSREF] [CSA]
- Palotás , Á. B. , Rainey , L. C. , Sarofim , A. F. , Sande , J. B. V. and Ciambelli , P. 1996a . Effect of Oxidation on the Microstructure of Carbon Blacks . Energy Fuels , 10 : 254 – 259 . [CROSSREF] [CSA]
- Park , K. , Kittelson , D. B. , Zachariah , M. R. and McMurry , P. H. 2004 . Measurement of Inherent Material Density of Nanoparticle Agglomerates . Journal of Nanoparticle Research , 6 ( 267–272 ) [CSA]
- Patterson , E. M. , Duckworth , R. M. , Wyman , C. M. , Powell , E. A. and Gooch , J. W. 1991 . Measurements of the Optical Properties of the Smoke Emissions from Plastics, Hydrocarbons, and Other Urban Fuels for Nuclear Winter Studies . Atmos. Env. , 25 ( 11 ) : 2539 – 2552 . [CSA]
- Patterson , E. M. , McMahon , C. K. and Ward , D. E. 1986 . Absorption Properties and Graphitic Carbon Emission Factors of Forest Fire Aerosols . Geophys. Res. Let. , 13 ( 1 ) : 129 – 132 . [CSA]
- Penner , J. E. , Chuang , C. C. and Grant , K. 1998 . Climate Forcing by Carbonaceous and Sulfate Aerosols . Clim. Dyn. , 14 ( 12 ) : 839 – 851 . [CROSSREF] [CSA]
- Penner , J. E. , Dickinson , R. E. and O'Neill , C. A. 1992 . Effects of Aerosol from Biomass Burning on the Global Radiation Budget . Science , 256 ( 5062 ) : 1432 – 1434 . [CSA]
- Penner , J. E. and Molenkamp , C. R. 1989 . Predicting the Consequences of Nuclear War: Precipitation Scavenging of Smoke . Aerosol Sci. Technol. , 10 : 51 – 62 . [CSA]
- Penner , J. E. and Novakov , T. 1998 . Carbonaceous Particles in the Atmosphere: A Historical Perspective to the Fifth International Conference on Carbonaceous Particles in the Atmosphere . J. Geophys. Res. , 101 : 28149 – 28157 . [CSA]
- Penner , J. E. , Zhang , S. Y. and Chuang , C. C. 2003 . Soot and Smoke Aerosol May Not Warm Climate . J. Geophys. Res. , 108 ( D21 ) : 4657 doi: 10.1029/2003JD003409[CROSSREF] [CSA]
- Petzold , A. , Kopp , C. and Niessner , R. 1997 . The Dependence of the Specific Attenuation Cross-Section On Black Carbon Mass Fraction and Particle Size . Atmos. Env. , 31 ( 5 ) : 661 – 672 . [CROSSREF] [CSA]
- Petzold , A. , Schloesser , H. , Sheridan , P. J. , Arnott , W. P. , Ogren , J. A. and Virkkula , A. 2005 . Evaluation of Multiangle Absorption Photometry for Measuring Aerosol Light Absorption . Aerosol Sci. Technol. , 39 : 40 – 51 . [CROSSREF] [CSA]
- Pilewskie , P. , Pommier , J. , Bergstrom , R. , Gore , W. , Howard , S. , Rabbette , M. , Schmid , B. , Hobbs , P. V. and Tsay , S. C. 2003 . Solar Spectral Radiative Forcing During the Southern African Regional Science Initiative . J. Geophys. Res. , 108 ( D13 ) [CROSSREF] [CSA]
- Pilewskie , P. and Valero , F. P. J. 1992 . Radiative Effects of the Smoke Clouds from the Kuwait Oil Fires . J. Geophys. Res. , 97 ( D13 ) [CSA]
- Platt , J. R. 1949 . Classification of Spectra of Cata-Condensed Hydrocarbons . J. Chem. Phys. , 17 ( 5 ) : 484 – 495 . [CROSSREF] [CSA]
- Pluchino , A. B. , Goldberg , S. S. , Dowling , J. M. and Randall , C. M. 1980 . Refractive-Index Measurements of Single Micron-Sized Carbon Particles . Appl. Opt. , 19 ( 19 ) : 3370 – 3372 . [CSA]
- Popplewell , W. C. 1901 . The Prevention of Smoke , London : Scott, Greenwood and Co. .
- Pósfai , M. , Anderson , J. R. , Buseck , P. R. and Sievering , H. 1999 . Soot and Sulfate Particles in the Remote Marine Troposphere . J. Geophys. Res. , 104 ( D17 ) : 21685 – 21693 . [CROSSREF] [CSA]
- Powell , E. A. and Zinn , B. T. 1983 . In situ Measurement of the Complex Refractive Index of Combustion Generated Particles . Prog. Aero. Astro. , 92 : 238 – 251 . [CSA]
- Quijano , A. L. , Sokolik , I. N. and Toon , O. B. 2000 . Influence of the Aerosol Vertical Distribution on the Retrievals of Aerosol from Satellite Radiance Measurements . Geophys. Res. Let. , 27 ( 21 ) doi: 10.1029/1999GL011235[CSA]
- Quinn , P. K. and Bates , T. S. 2003 . North American, Asian, and Indian Haze: Similar Regional Impacts on Climate? . Geophys. Res. Let. , 30 ( 11 ) : 1555 [CROSSREF] [CSA]
- Quinn , P. K. , Coffman , D. J. , Bates , T. S. , Welton , E. J. , Covert , D. S. , Miller , T. L. , Johnson , J. E. , Maria , S. , Russell , L. , Arimoto , R. , Carrico , C. M. , Rood , M. J. and Anderson , J. 2004 . Aerosol Optical Properties Measured on Board the Ronald H. Brown During Ace-Asia as a Function of Aerosol Chemical Composition and Source Region . J. Geophys. Res. , 109 : D19S01 doi: 10.1029/2003JD004010[CROSSREF] [CSA]
- Quinn , P. K. , Coffman , D. J. , Kapustin , V. N. , Bates , T. S. and Covert , D. S. 1998 . Aerosol Optics in the Marine Boundary Layer During Ace-1 and the Underlying Chemical and Physical Aerosol Properties . J. Geophys. Res. , 103 : 16547 – 16563 . [CROSSREF] [CSA]
- Raes , F. , Bates , T. , McGovern , F. and Liedekerke , M. v. 2000 . the 2Nd Aerosol Characterization Experiment (Ace-2): General Overview and Main Results . Tellus , 52B : 111 – 125 . [CSA]
- Ramachandran , G. and Reist , P. C. 1995 . Characterization of Morphological Changes in Agglomerates Subject to Condensation and Evaporation using Multiple Fractal Dimensions . Aerosol Sci. Technol. , 23 : 431 – 442 . [CSA]
- Ramanathan , V. , Crutzen , P. J. , Kiehl , J. T. and Rosenfeld , D. 2001 . Aerosols, Climate and the Hydrological Cycle . Science , 294 : 2119 – 2124 . [CROSSREF] [CSA]
- Redemann , J. , Russell , P. B. and Hamill , P. 2001 . Dependence of Aerosol Light Absorption and Single-Scattering Albedo on Ambient Relative Humidity for Sulfate Aerosols with Black Carbon Cores . J. Geophys. Res. , 106 ( D21 ) : 27485 – 27495 . [CROSSREF] [CSA]
- Reid , J. S. , Hobbs , P. V. , Liousse , C. , Martins , J. v. , Weiss , R. E. and Eck , T. F. 1998 . Comparisons of Techniques for Measuring Shortwave Absorption and Black Carbon Content of Aerosols from Biomass Burning in Brazil . J. Geophys. Res. , 103 ( D24 ) : 32031 – 32040 . [CROSSREF] [CSA]
- Roach , W. T. 1961 . Absorption of Solar Radiation by Water Vapor and CO2 in a Cloudless Atmosphere . Q. J. Roy. Met. Soc. , 87 : 364 – 373 . [CSA]
- Robertson , J. 1991 . Hard Amorphous (Diamond-Like) Carbons . Prog. Solid St. Chem. , 21 : 199 – 333 . [CROSSREF] [CSA]
- Robinson , G. D. 1970 . “ Some Meteorological Aspects of Radiation and Radiation Measurement ” . In Advances in Geophysics , Edited by: Lansberg , H. E. 285 – 306 . New York : Academic Press .
- Roessler , D. M. and Faxvog , F. R. 1979 . Optoacoustic Measurement of Optical Absorption in Acetylene Smoke . J. Opt. Soc. Am. , 69 ( 12 ) : 1699 – 1705 . [CSA]
- Roessler , D. M. and Faxvog , F. R. 1980 . Photoacoustic Determination of Optical Absorption to Extinction Ratio in Aerosols . Appl. Opt. , 19 ( 4 ) : 578 – 581 . [CSA]
- Roessler , D. M. and Faxvog , F. R. 1981 . Changes in Diesel Particulates with Engine Air/Fuel Ratio . Comb. Sci. Tech. , 26 : 225 – 231 . [CSA]
- Roessler , D. M. 1982 . Diesel Particle Mass Concentration by Optical Techniques . Appl. Opt. , 21 ( 22 ) : 4077 – 4086 . [CSA]
- Roessler , D. M. 1984 . Photoacoustic Insights on Diesel Exhaust Particles . Atmos. Env. , 23 ( 8 ) : 1148 – 1155 . [CSA]
- Rogak , S. N. , Flagan , R. C. and Nguyen , H. V. 1993 . The Mobility and Structure of Aerosol Agglomerates . Aerosol Sci. Technol. , 18 : 25 – 47 . [CSA]
- Röhl , R. , McClenny , W. A. and Palmer , R. A. 1982 . Photoacoustic Determination of Optical Properties of Aerosol Particles Collected on Filters: Development of a Method Taking Into Account Substrate Reflectivity . Appl. Opt. , 21 ( 3 ) : 375 – 381 . [CSA]
- Rosen , H. and Hansen , A. D. A. 1984 . Role of Combustion-Generated Carbon Particles in the Absorption of Solar Radiation in the Arctic Haze . Geophys. Res. Let. , 11 ( 5 ) : 461 – 464 . [CSA]
- Rosen , H. , Hansen , A. D. A. , Gundel , L. and Novakov , T. 1978 . Identification of the Optically Absorbing Component in Urban Aerosols . Appl. Opt. , 17 ( 24 ) : 3859 – 3861 . [CSA]
- Rosen , H. and Novakov , T. 1983 . Optical Transmission Through Aerosol Deposits on Diffusely Reflective Filters: A Method for Measuring the Absorbing Component of Aerosol Particles . Appl. Opt. , 22 ( 9 ) : 1265 – 1267 . [CSA]
- Russell , P. B. and Heintzenberg , J. 2000 . An Overview of the ACE-2 Clear Sky Column Closure Experiment (CLEARCOLUMN) . Tellus , B52 : 463 – 483 . [CSA]
- Russell , P. B. , Hobbs , P. V. and Stowe , L. L. 1999 . Aerosol Properties and Radiative Effects in the United States Mid-Atlantic Haze Plume: An Overview of the Tropospheric Aerosol Radiative Forcing Observational Experiment (TARFOX) . J. Geophys. Res. , 104 : 2213 – 2222 . [CROSSREF] [CSA]
- Russell , P. B. and Livingston , J. M. 1984 . Slant-Lidar Aerosol Extinction Measurements and Their Relation to Measured and Calculated Albedo Changes . J. Clim. Appl. Met. , 23 : 1204 – 1221 . [CROSSREF] [CSA]
- Russell , P. B. , Livingston , J. M. and Uthe , E. E. 1979 . Aerosol-Induced Albedo Change: Measurement and Modeling of an Incident . J. Atm. Sci. , 36 : 1587 – 1608 . [CROSSREF] [CSA]
- Saathoff , H. , Moehler , O. , Schurath , U. , Kamm , S. , Dippel , B. and Mihelcic , D. 2003 . The AIDA Soot Aerosol Campaign 1999 . J. Aerosol Sci , 34 : 1277 – 1296 . [CROSSREF] [CSA]
- Sadler , M. , Charlson , R. J. , Rosen , H. and Novakov , T. 1981 . An Intercomparison of the Integrating Plate and the Laser Transmission Methods for Determination of Aerosol Absorption Coefficients . Atmos. Env. , 15 ( 7 ) : 1265 – 1268 . [CROSSREF] [CSA]
- Saito , K. , Gordon , A. S. , Williams , F. A. and Stickle , W. F. 1991 . A Study of the Early History of Soot Formation in Various Hydrocarbon Diffusion Flames . Comb. Sci. Tech. , 80 : 103 – 119 . [CSA]
- Sakata , A. , Wada , S. , Tokunaga , A. T. and Narisawa , T. 1995 . Comparison of the Absorption Curves of Soots, Pitch Samples and Qccs to the Interstellar Extinction Curve . Planet. Space Sci. , 43 ( 10/11 ) : 1223 – 1226 . [CROSSREF] [CSA]
- Santoro , R. J. , Semerjian , H. G. and Dobbins , R. A. 1983 . Soot Particle Measurements in Diffusion Flames . Comb. Flame , 51 : 203 – 218 . [CROSSREF] [CSA]
- Satheesh , S. K. and Ramanthan , V. 2000 . Large Differences in Tropical Aerosol Forcing at the Top of the Atmosphere and Earth'S Surface . Nature , 405 : 60 – 63 . [PUBMED] [INFOTRIEVE] [CROSSREF] [CSA]
- Sato , M. , Hansen , J. , Koch , D. , Lacis , A. , Ruedy , R. , Dubovik , O. , Holben , B. , Chin , M. and Novakov , T. 2003 . Global Atmospheric Black Carbon Inferred from AERONET . Proc. Natl. Acad. Sci. , 100 ( 11 ) : 6319 – 6324 . [PUBMED] [INFOTRIEVE] [CROSSREF] [CSA]
- Sattel , S. , Robertson , J. and Ehrhardt , H. 1997 . Effects of Deposition Temperature on the Properties of Hydrogenated Tetrahedral Amorphous Carbon . J. Appl. Phys. , 82 ( 9 ) : 4566 – 4576 . [CROSSREF] [CSA]
- Schauer , J. J. , Mader , B. T. , DeMinter , J. T. , Heidemann , G. , Bae , M. S. , Seinfeld , J. H. , Flagan , R. C. , Cary , R. A. , Smith , D. , Huebert , B. J. , Bertram , T. , Howell , S. , Quinn , P. , Bates , T. , Turpin , B. , Lim , H. J. , Yu , J. and Yang , H. 2003 . ACE-Asia Intercomparison of a Thermal-Optical Method for the Determination of Particle-Phase Organic and Elemental Carbon . Environ. Sci. Technol. , 37 ( 5 ) : 993 – 1001 . [PUBMED] [INFOTRIEVE] [CROSSREF] [CSA]
- Scherrer , H. C. , Kittelson , D. B. and Dolan , D. F. 1981 . Light Absorption Measurements of Diesel Particulate Matter: . SAE Paper 810181[CSA]
- Schmid , H. , Laskus , L. , Abraham , H. J. , Baltensperger , U. , Lavanchy , V. , Bizjak , M. , Burba , P. , Cachier , H. , Crow , D. , Chow , J. , Gnauk , T. , Even , A. , Brink , H. M. t. , Giesen , K.-P. , Hitzenberger , R. , Huegelin , C. , Maenhaut , W. , Pio , C. , Carvalho , A. , Putaud , J.-P. , Toom-Sauntry , D. and Puxbaum , H. 2001 . Results of the “Carbon Conference” International Aerosol Carbon Round Robin Test Stage I . Atmos. Env. , 35 : 2111 – 2121 . [CROSSREF] [CSA]
- Schmidt , M. W. I. and Noack , A. G. 2000 . Black Carbon in Soils and Sediments: Analysis, Distribution, Implications, and Current Challenges . Glob. Biogeochem. Cyc. , 14 ( 3 ) : 777 – 793 . [CROSSREF] [CSA]
- Schnaiter , M. , Horvath , H. , Möhler , O. , Naumann , K.-H. , Saathoff , H. and Schöck , O. 2003 . UV-VIS-NIR Spectral Optical Properties of Soot and Soot-Containing Aerosols . J. Aerosol Sci , 34 ( 10 ) : 1421 – 1444 . [CROSSREF] [CSA]
- Schneider , S. H. 1972 . Atmospheric Particles and Climate: Can We Evaluate the Impact of Man's Activities? . Quat. Res. , 2 ( 3 ) : 425 – 435 . [CROSSREF] [CSA]
- Schuster , G. L. , Dubovik , O. , Holben , B. N. and Clothiaux , E. E. 2005 . Inferring Black Carbon Content and Specific Absorption from Aerosol Robotic Network (AERONET) Aerosol Retrievals . J. Geophys. Res. , 110 : D10S17 doi: 10.1029/2004JD004548[CROSSREF] [CSA]
- Sciare , J. , Cachier , H. , Oikonomou , K. , Ausset , P. , Sarda-Est'eve , R. and Mihalopoulos , N. 2003 . Characterization of Carbonaceous Aerosols During the MINOS Campaign in Crete . Atmos. Chem. Phys. Discuss. , 3 : 3373 – 3410 . [CSA]
- Seeker , W. R. , Samuelsen , G. S. , Heap , M. P. and Trolinger , J. D. 1981 . “ The Thermal Decomposition of Pulverized Coal Particles ” . In Eighteenth Symposium (International) on Combustion , 1213 – 1226 . Combustion Institute .
- Senftleben , H. and Benedict , E. 1918 . Über die Optischen Konstanten Und die Strahlungsgesetze der Kohle . Annalen der Physik , 54 : 65 – 78 . [CSA]
- Sheridan , P. J. , Arnott , W. P. , Ogren , J. A. , Andrews , E. , Atkinson , D. B. , Covert , D. S. , Moosmüller , H. , Petzold , A. , Schmid , B. , Strawa , A. W. , Varma , R. and Virkkula , A. 2005 . The Reno Aerosol Optics Study: An Evaluation of Aerosol Absorption Measurement Methods . Aerosol Sci. Technol. , 39 : 1 – 16 . [CROSSREF] [CSA]
- Sheridan , P. J. and Ogren , J. A. 1999 . Observations of the Vertical and Regional Variability of Aerosol Optical Properties Over Central and Eastern North America . J. Geophys. Res. , 104 ( D14 ) : 16793 – 16805 . [CROSSREF] [CSA]
- Shettle , E. P. and Fenn , R. W. 1976 . Models of the Atmospheric Aerosols and their Optical Properties . AGARD Conference Proceedings, No. 183 AGARD-CP–183 . 1976 .
- Shettle , E. P. and Fenn , R. W. 1979 . Models for the Aerosols of the Lower Atmosphere and the Effects of Humidity Variations on their Optical Properties: Air Force Geophysics Lab. , Hanscom AFB, MA : Optical Physics Division Environmental Research Paper .
- Smith , F. W. 1984 . Optical Constants of a Hydrogenated Amorphous Carbon Film . J. Appl. Phys. , 55 ( 3 ) : 764 – 771 . [CROSSREF] [CSA]
- Smith , K. L. , Smoot , L. D. and Fletcher , T. H. 1993 . “ Coal Characteristics, Structure, and Reaction Rates ” . In Fundamentals of Coal Combustion , Edited by: Smoot , L. D. 131 – 237 . Amsterdam : Elsevier .
- Smith , O. I. 1981 . Fundamentals of Soot Formation in Flames with Application to Diesel Engine Particulate Emissions . Prog. Energy Combust. Sci. , 7 : 275 – 291 . [CROSSREF] [CSA]
- Smyth , K. C. and Shaddix , C. R. 1996 . The Elusive History of m = 1.57–0.56i for the Refractive Index of Soot . Comb. Flame , 107 : 314 – 320 . [CROSSREF] [CSA]
- Sorensen , C. M. 2001 . Light Scattering by Fractal Aggregates: A Review . Aerosol Sci. Technol. , 35 : 648 – 687 . [CROSSREF] [CSA]
- Sorensen , C. M. and Feke , G. D. 1996 . The Morphology of Macroscopic Soot . Aerosol Sci. Technol. , 25 : 328 – 337 . [CSA]
- Soulen , P. F. , King , M. D. , Tsay , S.-C. , Arnold , G. T. and Li , J. Y. 2000 . Airborne Spectral Measurements of Surface-Atmosphere Anisotropy During the Scar-A, Kuwait Oil Fire, and TARFOX Experiments . J. Geophys. Res. , 105 ( D8 ) doi: 10.1029/1999JD901115[CROSSREF] [CSA]
- Stagg , B. J. and Charalampopoulos , T. T. 1991 . Surface-Roughness Effects on the Determination of Optical Properties of Materials by the Reflection Method . Appl. Opt. , 30 ( 28 ) : 4113 – 4118 . [CSA]
- Stagg , B. J. and Charalampopoulous , T. T. 1993 . Refractive Indices of Pyrolytic Graphite, Amorphous Carbon, and Flame Soot in the Temperature Range 25° to 600°C . Comb. Flame , 94 : 381 – 386 . [CSA]
- Strawa , A. W. , Castaneda , R. , Owano , T. , Baer , P. S. and Paldus , B. A. 2002 . The Measurement of Aerosol Optical Properties Using Continuous Wave Cavity Ring-Down Techniques . J. Atm. Ocean. Tech. , 20 ( 4 ) : 454 – 465 . [CSA]
- Stull , V. R. and Plass , G. N. 1960 . Emissivity of Dispersed Carbon Particles . J. Opt. Soc. Am. , 50 ( 2 ) : 121 – 129 . [CSA]
- Swap , R. J. , Annegarn , H. J. , Suttles , J. T. , King , M. D. , Platnick , S. , Privette , J. L. and Scholes , R. J. 2003 . Africa Burning: A Thematic Analysis of the Southern African Regional Science Initiative (SAFARI 2000) . J. Geophys. Res. , 108 : 8465 doi: 10.1029/2003JD003747[CROSSREF] [CSA]
- Szkarlat , A. C. and Japar , S. M. 1981 . Light Absorption by Airborne Aerosols: Comparison of Integrating Plate and Spectrophone Techniques . Appl. Opt. , 20 ( 7 ) : 1151 – 1155 . [CSA]
- Szkarlat , A. C. and Japar , S. M. 1983 . Optical and Chemical Properties of Particle Emissions from on-Road Vehicles . JAPCA , 33 ( 6 ) : 592 – 597 . [CSA]
- Taft , E. A. and Philipp , H. R. 1965 . Optical Properties of Graphite . Phys. Rev. , 138 ( 1A ) : A197 – A202 . [CROSSREF] [CSA]
- Tauc , J. , Grigorovici , R. and Vancu , A. 1966 . Optical Properties and Electronic Structure of Amorphous Germanium . Phys. Stat. Sol. , 15 : 627 – 637 . [CSA]
- Tegen , I. , Lacis , A. A. and Fung , I. 1996 . The Influence on Climate Forcing of Mineral Aerosols from Disturbed Soils . Nature , 380 : 419 – 422 . [CROSSREF] [CSA]
- Terhune , R. W. and Anderson , J. E. 1977 . Spectrophone Measurements of the Absorption of Visible Light By Aerosols in the Atmosphere . Opt. Lett. , 1 ( 2 ) : 70 – 72 . [CSA]
- Theye , M.-L. and Paret , V. 2002 . Spatial Organization of the sp2-Hybridized Carbon Atoms and Electronic Density of States of Hydrogenated Amorphous Carbon Films . Carbon , 40 : 1153 – 1166 . [CROSSREF] [CSA]
- Tien , C. L. and Lee , S. C. 1982 . Flame Radiation . Prog. Energy Combust. Sci. , 8 : 41 – 59 . [CROSSREF] [CSA]
- Toon , O. B. and Pollack , J. B. 1976 . A Global Average Model of Atmospheric Aerosol for Radiative Transfer Calculations . J. Appl. Met. , 15 : 226 – 246 . [CROSSREF] [CSA]
- Torres , O. , Bhartia , P. K. , Herman , J. R. , Ahmad , Z. and Gleason , J. 1998 . Derivation of Aerosol Properties from Satellite Measurements of Backscattered Ultraviolet Radiation: Theoretical Basis . J. Geophys. Res. , 103 ( D14 ) : 17,099 – 17,110 . [CROSSREF] [CSA]
- Torres , O. , Bhartia , P. K. , Sinyuk , A. , Welton , E. J. and Holben , B. 2005 . Total Ozone Mapping Spectrometer Measurements of Aerosol Absorption from Space: Comparison to SAFARI 2000 Ground-Based Observations . J. Geophys. Res. , 110 : D10S18 doi: 10.1029/2004JD004611[CROSSREF] [CSA]
- Truex , T. J. and Anderson , J. E. 1979 . Mass Monitoring of Carbonaceous Aerosols with a Spectrophone . Atmos. Env. , 13 : 507 – 509 . [CROSSREF] [CSA]
- Tsu , R. , González , J. and Hernández , I. 1978 . Observation of Splitting of the E2g Mode and Two-Phonon Spectrum in Graphites . Solid State Communications , 27 : 507 – 510 . [CROSSREF] [CSA]
- Turco , R. P. , Toon , O. B. , Ackerman , T. P. , Pollack , J. B. and Sagan , C. 1983 . Nuclear Winter: Global Consequences of Multiple Nuclear Explosions . Science , 222 ( 4630 ) : 1283 – 1292 . [CSA]
- Twitty , J. T. and Weinman , J. A. 1971 . Radiative Properties of Carbonaceous Aerosols . J. Appl. Met. , 10 : 725 – 731 . [CROSSREF] [CSA]
- Twomey , S. 1977 . The Influence of Pollution on the Shortwave Albedo of Clouds . J. Atm. Sci. , 34 : 1149 – 1152 . [CROSSREF] [CSA]
- Vaglieco , B. M. , Beretta , F. and D'Alessio , A. 1990 . In situ Evaluation of the Soot Refractive Index in the uv-Visible from the Measurement of the Scattering and Extinction Coefficients in Rich Flames . Comb. Flame , 79 : 259 – 271 . [CROSSREF] [CSA]
- van , d e and Hulst , H. C. 1957 . Light Scattering by Small Particles , Dover Publications, Inc. : New York .
- Van , d er and Wal , R. L. 1996 . Onset of Carbonization: Spatial Location via Simultaneous LIF-LII and Characterization via TEM . Comb. Sci. Tech. , 118 : 343 – 360 . [CSA]
- van Krevelen , D. W. 1958 . Nature , 181 : 640 [CSA]
- van Krevelen , D. W. 1981 . Coal: Typology, Chemistry, Physics, Constitution, , 3rd ed. , Amsterdom : Elsevier Science .
- Waggoner , A. P. , Weiss , R. E. , Ahlquist , N. C. , Covert , D. S. , Will , S. and Charlson , R. J. 1981 . Optical Characteristics of Atmospheric Aerosols . Atmos. Env. , 15 ( 10/11 ) : 1891 – 1909 . [CSA]
- Waldram , J. M. 1945 . Measurements of the Photometric Properties of the Upper Atmosphere . Q. J. Roy. Met. Soc. , 71 : 319 – 336 . [CSA]
- Wang , C. 2004 . A Modeling Study on the Climate Impacts of Black Carbon Aerosols . J. Geophys. Res. , 109 : D03106 doi: 10.1029/2003JD004084[CROSSREF] [CSA]
- Watson , A. Y. and Valberg , P. A. 2001 . Carbon Black and Soot: Two Different Substances . American Industrial Hygiene Association Journal , 62 : 218 – 228 . [PUBMED] [INFOTRIEVE] [CSA]
- Weingartner , E. , Saathof , H. , Schnaiter , M. , Streit , N. , Bitnar , B. and Baltensperger , U. 2003 . Absorption of Light by Soot Particles: Determination of the Absorption Coefficient by Means of Aethalometers . J. Aerosol Sci , 34 : 1445 – 1463 . 14537 – 14540 . [CROSSREF] [CSA]
- Weiss , R. E. and Hobbs , P. V. 1992 . Optical Extinction Properties of Smoke from the Kuwait Oil Fires . J. Geophys. Res. , 97 ( D13 ) doi: 10.1029/92JD01374[CSA]
- Weiss , R. E. and Waggoner , A. P. 1984 . “ Aerosol Optical Absorption: Accuracy of Filter Measurement by Comparison with In-Situ Extinction ” . In Aerosols: Science, Technology, and Industrial Applications of Airborne Particles , Edited by: Liu , B. Y. H. , Pui , D. Y. H. and Fissan , H. J. 397 – 400 . New York : Elsevier Science .
- Wendling , P. , Stifter , A. , Mayer , B. , Fiebig , M. , Kiemle , C. , Flentje , H. , Wendisch , M. , Armbruster , W. , Leiterer , U. , Hoyningen-Huene , W. v. and Petzold , A. 2002 . Aerosol-Radiation Interaction in the Cloudless Atmosphere During LACE 98–2. Aerosol-Induced Solar Irradiance Changes Determined from Airborne Pyranometer Measurements and Calculations . J. Geophys. Res. , 107 ( D21 ) doi: 10.1029/2000JD000288[CROSSREF] [CSA]
- Wentzel , M. , Gorzawski , H. , Naumann , K.-H. , Saathoff , H. and Weinbruch , S. 2003 . Transmission Electron Microscopical and Aerosol Dynamical Characterization of Soot Aerosols . J. Aerosol Sci. , 34 : 1347 – 1370 . [CROSSREF] [CSA]
- Wersborg , B. L. , Fox , L. K. and Howard , J. B. 1975 . Soot Concentration and Absorption Coefficient in a Low-Pressure Flame . Comb. Flame , 24 : 1 – 10 . [CROSSREF] [CSA]
- Wex , H. , Neusüß , C. , Wendisch , M. , Stratmann , F. , Koziar , C. , Keil , A. , Wiedensohler , A. and Ebert , M. 2002 . Particle Scattering, Backscattering, and Absorption Coefficients: An in situ Closure and Sensitivity Study . J. Geophys. Res. , 107 ( D21 ) : 8122 doi: 10.1029/2000JD000234[CROSSREF] [CSA]
- White , C. M. , Perry , M. B. , Schmidt , C. E. and Douglas , L. J. 1987 . Relationship Between Refractive Indices and Other Properties of Coal Hydrogenation Distillates . Energy Fuels , 1 : 99 – 105 . [CROSSREF] [CSA]
- Whiteaker , J. A. , Suess , D. T. and Prather , K. A. 2002 . Effects of Meteorological Conditions on Aerosol Composition and Mixing State in Bakersfield, CA . Environ. Sci. Technol. , 36 : 2345 – 2353 . [PUBMED] [INFOTRIEVE] [CROSSREF] [CSA]
- Wolff , G. T. , Countess , R. J. , Groblicki , P. J. , Ferman , M. A. , Cadle , S. H. and Muhlbaier , J. L. 1981 . Visibility-Reducing Species in the Denver “Brown Cloud”–II. Sources and Temporal Patterns . Atmos. Env. , 15 ( 12 ) : 2485 – 2502 . [CROSSREF] [CSA]
- Wu , J.-S. , Krishnan , S. S. and Faeth , G. M. 1997 . Refractive Indices at Visible Wavelengths of Soot Emitted from Buoyant Turbulent Diffusion Flames . J. Heat Trans. , 119 : 230 – 237 . [CSA]
- Xanthakis , J. P. 2000 . Effects of Short–Range Order on the Electronic Structure and Optical Properties of Amorphous Carbon . Diamond Relat. Mater. , 9 : 1369 – 1373 . [CROSSREF] [CSA]
- Yu , J. Z. , Xu , J. and Yang , H. 2002 . Charring Characteristics of Atmospheric Organic Particulate Matter in Thermal Analysis . Environ. Sci. Technol. , 36 : 754 – 761 . [PUBMED] [INFOTRIEVE] [CROSSREF] [CSA]