The design and experimental characterization of a condensation nucleus counter (CNC) is presented. The counter produces supersaturation by means of fast volume-controlled adiabatic expansion. The aerosol number concentration is derived from observing scattered laser light in the forward direction under a solid angle between 1.1° and 4.4° over the full annular sector. The number concentration is derived by application of Mie theory from the characteristic pattern in the temporal evolution of the detected signal during the droplet growth process. The equation for calculation of the aerosol number density by this method is presented. Theoretical considerations for the smallest aerosol particles that can be activated indicate a lower size cut-off between 2.5 and 3.0 nm. Model calculations of the expected Mie scatter signal during expansion agree very well with the experimental observations. The Expansion-CNC can be operated fully automated under computer (PC) control in 10-second sample cycles. For characterization it is compared with a TSI 3025A Ultrafine-CPC (TSI UCPC) for measurements of monodisperse sodium chloride and sulfuric acid aerosol particles, indicating good agreement between the two counters down to particle sizes as low as 3.5 nm under laboratory conditions. In addition, ambient aerosol measurements in urban air show excellent agreement with simultaneous TSI UCPC measurements for particle number concentrations ranging from roughly 50 cm− 3 to 130000 cm− 3.
1. INTRODUCTION
The study of ultrafine aerosol particles (with particle diameters below 0.1 μ m) is currently of major interest in many scientific fields. Aerosol particles can affect human health through inhalation with the ultrafine particles being potentially the most dangerous (CitationNel 2005). Nucleation events (the formation of ultrafine atmospheric particles from gaseous precursors) can lead to an increase in the number concentration of cloud condensation nuclei and therefore global climate models require reliable parameterizations of nucleation processes (CitationKulmala et al. 2004). During smog events in urban polluted areas fine and ultrafine particles can be photochemically produced. Thus for research as well as monitoring purposes the development of fully automated, robust, sensitive and possibly inexpensive instrumentation is desirable.
The principle of counting aerosol particles after condensational growth by use of an adiabatic expansion has been invented more than a century ago by J. Aitken and P. J. Coulier (for a historical review see CitationSpurny 2000). Several generations of Condensation Nucleus Counters based on this principle have been developed since (CitationMcMurry 2000, and references therein).
One class of condensation nucleus counters (CNC) determines particle number concentration by comparison of scattered or attenuated monochromatic light signals with the Mie theory. CitationWagner (1985) studied particle formation processes by observing simultaneously the scattered and the transmitted light flux of a parallel monochromatic beam of light in an expansion chamber (Constant-Angle Mie Scattering Method, CAMS Method). The scattered light flux is monitored as a function of time at a selectable scattering angle. Since the scattered light is attenuated to a certain degree due to light extinction, it has to be normalized to the transmitted light flux. This method allows the quantitative measurement of particle size and number concentration during the condensation process by comparison with Mie theory. The CAMS method needs no external calibration and can be regarded as an absolute method.
CitationLiu et al. (1982) and CitationSzymanski and Wagner (1990) describe a size-analyzing counter (SANC) that uses both the CAMS method and the observation of the light attenuation for absolute aerosol number concentration measurements. Comparing the scattered light signal with the Mie theory gives information about the size of the aerosol population at any time. By knowing the size of the droplets the aerosol number concentration can be calculated from the attenuated light signal.
CitationLovejoy and Hanson (1995) detect the light of a laser beam attenuated by growing aerosol particles. The data recorded after adiabatic expansion shows a step structure that results from interference between the phase-shifted light that has passed through the particles and the light that has not interacted with the particles. By comparison with the Mie theory the size of the aerosol particles can be determined at each step and from that information together with the signal height the aerosol number density can be calculated.
CitationHolländer et al. (2002) present a Kelvin spectrometer that operates at supersaturations of a few percent. The droplet size during the growth process is determined from the ratio of scattered laser light signal under 30° forward direction and the attenuated light signal. The number concentration can be calculated from the size and optical thickness of the aerosol population.
Here we present design, characterization and sample measurements of an automated expansion-type condensation nucleus counter (Expansion-CNC) that is demonstrated to provide accurate measurements of the ultrafine particle number concentration. The Expansion-CNC can be operated with various condensing liquids without the necessity of redesigning the instrument which could provide information on particle surface properties. However, the results we show here include only water as the condensing fluid because of its relevance for atmospheric condensation processes.
2. THEORY
Condensation Processes
The Expansion-CNC produces supersaturation by means of fast adiabatic expansion. The temperature inside the measurement chamber after expansion (T 2) can be calculated according to:
FIG. 1 Dependence of Saturation ratio after expansion with temperature before expansion according to Equations (Equation1) and (Equation2) (solid curve). Kelvin diameter versus temperature before expansion for a given saturation ratio according to Equation (Equation3) (dotted curve) for water.
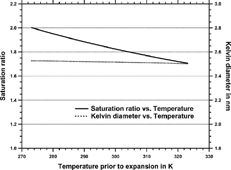
The results from show that variations in room temperature might have an influence on the detection limit. However, these variations are small and the detection limit is expected to be below 3 nm for all indicated temperatures for hydrophilic particles.
Calculation of the Particle Number Concentration
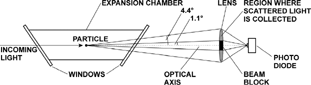
The principle of scattered light detection with the Expansion-CNC is shown in . A particle that is illuminated by laser light scatters light in all directions but only the part that is scattered in the forward direction between 1.1° and 4.4° is detected. The light is collected over the full annular sector by a lens and is focused onto a photo diode detector. The direct beam and the light between 0° and 1.1° are blocked. This is the major difference between our Expansion-CNC and the SANC method of CitationSzymanski and Wagner (1990) that detects scattered light under a selectable angle. The instrument of CitationLovejoy and Hanson (1995) has in principle a similar set-up but it measures the light attenuation. However, the attenuated light differs only very little from the incident light and therefore it is difficult to detect small aerosol concentrations quantitatively. Nevertheless, it is necessary to discuss light attenuation briefly in order to obtain an expression for the aerosol number concentration calculated for the Expansion-CNC method.
FIG. 2 Schematic diagram of scattered light detection with the Expansion-CNC. Particles inside an expansion chamber scatter incoming laser light. The direct light beam and the scattered light in the forward direction between 0° and 1.1° are blocked. Scattered light that hits the annular area of a lens between 1.1° and 4.4° (shaded area) is collected and focused onto a photo diode detector.
The light transmitted by the aerosol cloud inside the expansion chamber, when the beam stop is removed, can be calculated by Beer's law:
C SCA gives the effective area of a particle for scattering light and can be calculated by Mie theory (e.g., with Fortran program given in CitationBohren and Huffman 1983). The scattering cross section is defined as the product of the dimensionless scattering efficiency factor Q SCA and the geometrical area of the particle:
The difference between the incident light I 0 and the transmitted light I ext is the intensity of the light scattered in all directions when absorption is neglected. If this value is multiplied by the ratio of the partial scattering cross section and the total scattering cross section, the light intensity I that is detected according to (shaded area of the lens) is
This equation shows that the scattered light signal depends directly on the particle number concentration. The unknown variable is the partial scattering cross section that depends only on the particle size because the wavelength and particle refractive index can be regarded as constant. Note that the partial scattering cross section can vary over orders of magnitude for different particles sizes. This means that only very little light is scattered for small particles, whereas the scattered light intensity becomes much higher for large particles for a certain number concentration.
3. EXPERIMENTAL
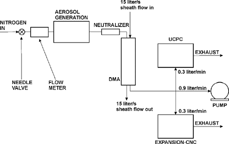
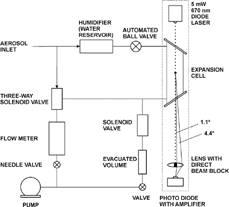
The experimental set-up of the Expansion-CNC is shown in . The aerosol is humidified by passing over a water reservoir and is then brought into the expansion cell where it is isolated by closing the automated ball valve and switching the three-way valve. Instead of water also other condensable liquids like butanol or Fluorinert can be used resulting in different activation radii. The expansion cell consists of a cylindrical stainless steel chamber (2.5 cm i.d., 10 cm long) with Pyrex windows at both ends. The windows are mounted at Brewster's angle to minimize reflection losses and to reduce the amount of spurious light reaching the detector when no particles are present. The gas is rapidly expanded by opening a solenoid valve that separates the expansion chamber from a small evacuated volume. The aerosol particles start taking up water vapor and grow to small water droplets. Provided that the expansion is fast enough all particles with sizes above the detection limit are activated simultaneously. The maximum size they achieve depends on the particle number concentration, the amount of water vapor present, the degree of supersaturation and on the heat transfer rate from the walls. The growing aerosol particles are illuminated by a diode laser of 670 nm and the Mie scattered light is measured in the forward direction between 1.1° and 4.4° relative to the center of the expansion chamber with a photo diode as described above. When the measurement period is finished the ball valve and the three-way valve are opened and the solenoid valve is shut again. In order to achieve a quasi-continuous flow the three-way solenoid valve is used to bypass the chamber during the measurement. The flow rate is measured with a flow meter and can be adjusted with a needle valve. Usually the instrument is operated at a flow rate of 0.3 liter/min and sample rates of the instrument are typically 0.1 Hz. Faster operation is not recommended because it takes some time to exchange the sampled air inside the expansion cell completely. The instrument operates fully automated controlled by a PC which also records the data. A Labview program controls the ball valves, the solenoid valves, the data acquisition and performs the calculation of the aerosol number density from the detected signals.
FIG. 3 Diagram of the Expansion-CNC. The dotted rectangle depicts the optical part of the instrument that is usually covered by a light-absorbing box.
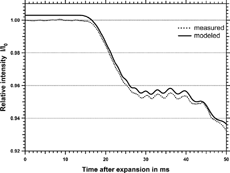
In the temporal evolution of the photo diode output of an exemplary measurement is plotted against the time after expansion. The scattered light signal shows a series of minima and maxima. Pairs of maxima and minima are designated as “steps” in the following. These steps arise from interferences between the phase-shifted light that has passed the water droplets and the light which did not impinge onto the particles (CitationBohren and Huffman 1983). The first step occurs approximately 4 ms after expansion because by then the droplets have grown to a well defined size. While the droplets grow with time their scattering cross section increases as prescribed by Mie theory. The steps can be compared with the curve in where the scattering light intensity is calculated as a function of particle size according to Equation (Equation11). The theoretical curve also shows the same step structures as the experimental curve. Therefore the droplet size can be derived together with the corresponding partial scattering cross section at each step of the experimental curve. This can be done by simply taking the maxima (or the minima) of the recorded detector signal time series. However, this method leads to some uncertainties because of a fine substructure that is superimposed on the experimental and theoretical data. This substructure arises from higher order resonances. CitationSzymanski, Pohl, and Wagner (1982) verified the existence of such partial-wave resonances by light scattering experiments in an expansion chamber. In section 4 an example for this ripple structure is given as well. Besides the ripple structure from these resonances also noise from fluctuations in the laser intensity and from the electronics influences the measured signal. Therefore the measured signal is smoothed by a running average algorithm during data acquisition. Smoothing the signal results in a decrease of the maximum and an increase of the minimum at a step. For this reason the average of the maximum and the minimum signal at a step is taken as the representative value rather than the extreme values themselves. This procedure is also applied for the theoretical curve (the maximum and the minimum of the third step are indicated in exemplarily). The values for the first four steps are given in . These values were calculated with a modified Fortran program from CitationBohren and Huffman (1983) for pure water droplets. The assumption of pure water droplets is justified since the volume fraction of a particle initially 0.1 μ m in size is only 0.0125% when the particle has grown to a droplet with a diameter of 2 μ m. The calculation of the aerosol number density is done on-line in real time by the data acquisition computer program following Equation (Equation11):
FIG. 4 Comparison of experimental (a) and theoretical (b) scattered light intensity. The vertical lines indicate the maximum and the minimum of the third step, respectively.
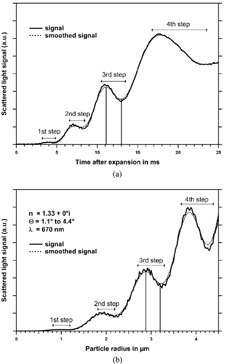
TABLE 1 Radius range at which a step can be observed and the corresponding average total and partial scattering cross section
The detection of the steps can be done in two different ways. The first one is to monitor the exact times after starting the expansion at which the steps occur and to assume that these times are constant (i.e., aerosol populations are always growing at the same rate). In our experiments this method was applicable for stable particle number concentrations and constant ambient conditions. However, when conditions are unstable the aerosol population might grow faster or slower. Especially when particle sizes are close to the Kelvin diameter the largest deviations concerning the growth pace are expected. In this case the operator must readjust the time values at which the step is detected in order to get correct results. Another way is the detection of the steps by numerical methods. This can be used when particle number concentration exceeds a certain limit (> 1000 cm−3). Then the signal shows well-defined steps which are reliably detected. Note also that the assumption of a constant solid angle between the point where the laser light is scattered and the lens that collects the light induces a slight error. The solid angle changes with the position where the laser lights interacts with a particle from a range of 0.8° to 3.3° at the beginning of the expansion chamber to a range of 1.6° to 6.5° at its end. However, the error for the first four steps when calculating the partial scattering cross section is in any case smaller than 5%.
The incident light intensity I 0 has to be determined before the measurement starts and needs to be checked from time to time during operation. This is done with the same detector by removing the beam block when no aerosol is present inside the expansion chamber. Since the detector reaches saturation when exposed directly to the laser beam a neutral density filter is placed in the light path additionally. This procedure was performed before every measurement series in order to account for aging effects of the laser diode and the photo diode detector. For long term monitoring purposes a suitable subroutine could be included in the Labview program to periodically perform this task.
We compared the Expansion-CNC with a TSI UCPC in order to calibrate and characterize the Expansion-CNC. The TSI UCPC works with butanol as the condensing fluid and has a lower cut-off diameter d 50 of 3 nm. The experimental set-up for the inter-comparison experiments is shown in . The measurements were carried out with sodium chloride and with sulfuric acid particles generated by suitable laboratory devices. For example the sodium chloride particles were generated in a tube furnace by heating NaCl in an N2-carrier gas stream. The NaCl was vaporized at temperatures larger than 600°C adopting a method described by CitationScheibel and Porstendörfer (1983). Sulfuric acid particles were generated with a homogenous nucleation aerosol generator (CitationMiddlebrook, Thomson, and Murphy 1997). It consists of a heated aluminum block with a small glass reservoir inside containing liquid sulfuric acid. The sulfuric acid is vaporized into an N2-carrier gas stream at temperatures between 80°C and 120°C. Since the resulting aerosol number distributions are dependent on the flow rate and on the temperature of the aerosol generators the particles were experimentally classified with a nano-DMA (TSI 3085, TSI Inc.) to obtain monodisperse particles with sizes between 3.5 and 60 nm. Both, the TSI UCPC and the Expansion-CNC work with flows of 0.3 liter/min. To reduce diffusion losses to the wall and tubing system when measuring particles with sizes below 10 nm an additional pump was used to increase the flow rate to a total flow of 1.5 liter/min.
4. RESULTS AND DISCUSSION
Comparison of Measured and Modeled Signal Intensities
From it can be seen that a non-linear relationship exists between the particle radius and the time elapsed after expansion. This implies the particles are growing more slowly in radius the larger they become. CitationLovejoy and Hanson (1995) used the following equation to model particle growth in their CNC:
FIG. 6 Comparison of measured and modeled curve for two different number concentrations. Modeled curve is calculated for monodisperse particles.
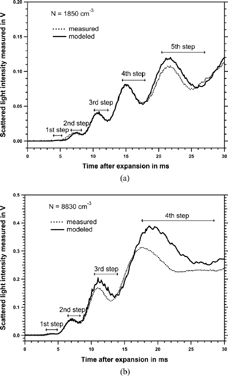
shows the comparison between model and experiment for a concentration of 8830 cm−3 measured by the TSI UCPC. Here the first step can clearly be detected whereas the two curves start deviating from each other already at the time of the third step. This demonstrates that at higher concentrations only the first and the second step give correct number concentrations. The reason for the deviation beginning at lower sizes for high concentrations in comparison to low concentrations is due to the effect that in the first case a larger amount of latent heat is released for the same particle size. An additional limitation might be the finite water vapor supply inside the expansion chamber resulting in an inhomogeneous growth process for a particle population at larger sizes.
These results show that each step gives useful results only within certain concentration ranges. The results for the measurement ranges are summarized in , showing that the overall range for the accessible particle number concentrations is approximately 50 to 130000 cm−3. A suitable look-up table in the data acquisition software can accommodate for these caveats.
TABLE 2 Concentration ranges that can be measured with a certain step
Multiple scattering corrections are neglected in the calculation of the number density. This is justified by the fact that the maximum optical densities that can be calculated from Equation (Equation9) with the average total scattering cross sections from and the maximum concentration values from do not exceed the value of τ = 0.1. Nevertheless, the Expansion-CNC is capable to measure higher concentrations of approximately 200000 cm−3 with the first step but then multiple scattering corrections have to be applied. Corrections for transmittance measurements can be found for example in CitationWind and Szymanski (2002).
Another comparison between model and experiment was carried out for an extinction measurement with the Expansion-CNC. When the beam block is removed the aerosol number density can be calculated according to Equation (Equation4) as described by CitationLovejoy and Hanson (1995). In the attenuated light signal is shown against time after expansion (solid curve). The observed step again results from interference of the phase-shifted light that has passed through the particles and light that has not reached the particles. But also a fine ripple structure from higher order resonances within the spherical particles is observed. The measured curve is in satisfactory agreement with the modeled curve (dotted line) derived from Equation (Equation13). However, these extinction measurements are not carried out routinely because of the difficulties that arise when measuring small particle number concentrations as mentioned before.
Determination of the Lower Size Cut-Off
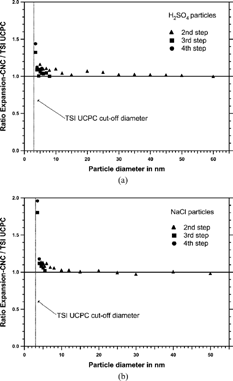
shows the inter-comparison of the counting efficiency for ultrafine particles measured with the TSI UCPC and the Expansion-CNC. From the appropriate intensity step was chosen dependent on the particle concentration range measured. Particles with sizes larger than 4.5 nm could be generated with sufficiently high concentrations (approximately 5000 cm−3) and the second step in the Mie response curve was used for the comparison. Smaller particles could only be generated with concentrations less than 800 cm−3 due to diffusion losses; therefore the third and the fourth step had to be used. In the comparison for sulfuric acid and sodium chloride particles is shown. The uncertainties are between 10% for the largest particle sizes (50 nm and 60 nm, respectively) and 15% for the smallest particle size (3.5 nm). The sampling efficiency is unity within the error limits for all sizes larger than 4 nm. In both cases at 3.5 nm the concentration observed with the Expansion-CNC becomes significantly larger than the TSI UCPC measurement, indicating that the d 50 of the Expansion-CNC might be even lower than for the TSI UCPC and/or diffusion losses within the Expansion-CNC are smaller.
Ambient Air Measurement
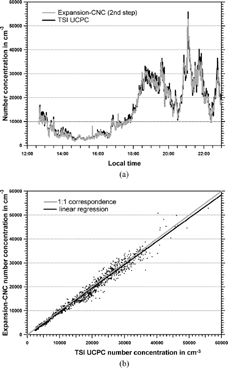
In order to test whether the Expansion-CNC is suitable as field instrument particle concentrations in ambient urban air were measured with the TSI UCPC and the Expansion-CNC. These measurements were conducted in Mainz, Germany, on December 12, 2003. The inter-comparison shown in the time series of shows good agreement over a wide range of ambient polydisperse aerosol concentrations. In the same data is shown as a scatter plot for the TSI UCPC and the Expansion-CNC results. Linear regression yields a linear correlation coefficient of 0.99. The 1:1 correspondence line is shown for reference as well. Differences between the two instruments are typically smaller than 5%. However, the inter-comparison between the TSI UCPC and the Expansion-CNC for ambient aerosol particles depends on the composition of the particles. The TSI UCPC counts single particles, a method which is independent of the composition, whereas the Expansion-CNC detects the amount of total scattered light. This latter method is dependent on the refractive index of the particles. Although in most cases the amount of condensed water on the particles exceeds the amount of the initial particle, the refractive index might have an influence in some cases especially for absorbing particles. This effect has not been studied here but is discussed elsewhere (CitationWriedt and Schuh 2002; CitationWind et al. 2004).
5. SUMMARY AND CONCLUSIONS
A condensation nucleus counter based on adiabatic expansion and measurement of the intensity of forward-scattered laser light has been designed and characterized by laboratory experiments and compared with a TSI 3025A UCPC. The characteristics of the Expansion-CNC are summarized in . The counter works at a flow rate of 0.3 liter/min with a quasi-continuous flow. A major advantage of the newly developed instrument is that it can operate with water as the condensing fluid in contrast to most commercially available CNCs. The measurement of the number concentration with the Expansion-CNC is based on detection of the forward-scattered light and can be calculated by Mie theory. In this respect it constitutes an absolute measurement. However, when compared to the concentrations measured by the TSI UCPC the values of the Expansion-CNC deviate by a constant factor independent of particle number densities. This is probably due to intricacies inherent in the scattered light detection. From comparison with the TSI UCPC and from modeling results it was found that for different number concentrations the appropriate intensity step (i.e., time interval after expansion) has to be used for calculation of the aerosol number concentration. For high concentrations (approximately more than 1000 cm−3) only the first two intensity steps give reliable results, for low concentrations (less than 1000 cm−3) the third and the fourth step have to be used. Further development is aimed at improving the software algorithm in order to find the appropriate step for every measurement cycle automatically. The inter-comparison with the TSI UCPC showed a nearly perfect agreement for sulfuric acid and sodium chloride particles in a size range between 4 and 60 nm. For both aerosol types the sampling efficiency of the Expansion-CNC increases significantly at 3.5 nm. This is indicating that the lower size cut-off of the Expansion-CNC is probably lower than the TSI UCPC cut-off. Another reason for the increased detection efficiency at low particle sizes might be smaller diffusion losses in the Expansion-CNC. Diffusion losses are expected to be small since the length of tubing is held as short as possible. Nevertheless, further experiments in the particle size range between 2 and 4 nm need to be carried out as well as studies on the influence of the condensing fluid used.
TABLE 3 Summarized characteristics of the Expansion-CNC
The Expansion-CNC presented here is a robust, low-cost instrument that can be used in the future for laboratory measurements of ion-induced nucleation, combustion exhaust, as well as for routine monitoring of the ambient aerosol concentration.
APPENDIX A
List of Terms and Symbols
| C SCA | = |
Total scattering cross section |
| C SCA,p | = |
Partial scattering cross section |
| C SCA,p,i | = |
Partial scattering cross section at step i |
| b | = |
Growth factor for single exponential growth |
| d K | = |
Kelvin diameter |
| d 50 | = |
Lower size cut-off |
| E | = |
Expansion ratio |
| G | = |
Correction factor |
| i | = |
step index |
| i 1, i 2 | = |
Mie intensity functions |
| I | = |
Light intensity measured at the detector |
| I 0 | = |
Incident light intensity |
| I i | = |
Light intensity measured at the detector |
| I ext | = |
Attenuated light intensity |
| L | = |
Length of the expansion chamber |
| M | = |
Molecular weight |
| n | = |
Complex refractive index |
| N | = |
Particle number density |
| N i | = |
Particle number density at step i |
| p s | = |
Saturation vapor pressure |
| Q SCA | = |
Scattering efficiency factor |
| Q SCA,p | = |
Partial scattering efficiency factor |
| r | = |
Partial radius |
| r ∞ | = |
Maximum radius for single exponential growth |
| R* | = |
Universal gas constant |
| S | = |
Saturation ratio |
| t | = |
Time |
| t 0 | = |
Time at which single exponential growth starts |
| T i | = |
Temperature before expansion |
| T 2 | = |
Temperature after expansion |
Greek Symbols
| δ | = |
Tolman's correction for the surface tension |
| κ | = |
Adiabatic ratio |
| λ | = |
Wavelength |
| Θ | = |
Scattering angle |
| σ | = |
Surface tension |
| ρ | = |
Density |
| τ | = |
Optical density |
Acknowledgments
We thank Edward R. Lovejoy for introduction to the NOAA Aeronomy Laboratory's Expansion-CNC, discussion and comprehensive help. Financial support by the University of Mainz and the Max-Planck-Institute for Chemistry is gratefully acknowledged.
REFERENCES
- Bohren , C. F. and Huffman , D. R. 1983 . Absorption and Scattering of Light by Small Particles , New York : John Wiley & Sons .
- Holländer , W. , Dunkhorst , W. , Lödding , H. and Windt , H. 2002 . Theoretical Simulation and Experimental Characterization of an Expansion-Type Kelvin Spectrometer with Intrinsic Calibration . J. Atmos. and Oceanic Tech. , 19 : 1811 – 1825 . [CSA]
- Kulmala , M. , Vehkamäki , H. , Petäjä , T. , Dal Maso , M. , Lauri , A. , Kerminen , V. -M. , Birmili , W. and Mc Murry , P. H. 2004 . Formation and Growth Rates of Ultrafine Atmospheric Particles: A Review of Observations . J. Aerosol Sci. , 35 : 143 – 176 . [CROSSREF] [CSA]
- Liu , B. Y. H. , Pui , D. Y. H. , McKenzie , R. L. , Agarwal , J. K. , Jaenicke , R. , Pohl , F. G. , Preining , O. , Reischl , G. , Szymanski , W. and Wagner , P. E. 1982 . Intercomparison of Different “Absolute” Instruments for Measurement of Aerosol Number Concentration . J. Aerosol Sci. , 13 : 429 – 450 . [CROSSREF] [CSA]
- Lovejoy , E. R. and Hanson , D. R. 1995 . Measurement of the Kinetics of Reactive Uptake by Submicron Sulfuric Acid Particles . J. Phys. Chem. , 99 : 2080 – 2087 . [CROSSREF] [CSA]
- McMurry , P. H. 2000 . The History of Condensation Nucleus Counters . Aerosol Sci. Technol. , 33 : 297 – 322 . [CROSSREF] [CSA]
- Middlebrook , A. M. , Thomson , D. S. and Murphy , D. M. 1997 . On the Purity of Laboratory-Generated Sulfuric Acid Droplets and Ambient Particles studied by Laser Mass Spectrometry . Aerosol Sci. Technol. , 27 : 293 – 307 . [CSA]
- Nel , A. 2005 . Air Pollution-Related Illness: Effects of Particles . Science , 308 : 804 – 806 . [PUBMED] [INFOTRIEVE] [CROSSREF] [CSA]
- Rogers , R. R. and Yau , M. K. 1989 . A Short Course in Cloud Physics, , 3rd ed. , Oxford : Pergamon Press .
- Scheibel , H. G. and Porstendörfer , J. 1983 . Generation of Monodisperse Ag- and NaCl-Aerosols with Particle Diameters between 2 and 300 nm . J. Aerosol Sci. , 14 : 113 – 126 . [CROSSREF] [CSA]
- Scheibel , H. G. and Porstendörfer , J. 1986 . Counting Efficiency and Detection Limit of Condensation Nuclei Counters for Submicrometer Aerosols, I. Theoretical Evaluation of the Influence of Heterogeneous Nucleation and Wall Losses . J. Colloid Interf. Sci. , 109 : 261 – 274 . [CROSSREF] [CSA]
- Spurny , K. R. 2000 . Atmospheric Condensation Nuclei P. J. Coulier 1875 and J. Aitken 1880 (Historical Review) . Aerosol Sci. Technol. , 32 : 243 – 248 . [CROSSREF] [CSA]
- Szymanski , W. , Pohl , F. G. and Wagner , P. E. 1982 . Observation of Resonances for Elastic Light Scattering by Spherical Particles . J. Colloid Interf. Sci. , 85 : 289 – 301 . [CROSSREF] [CSA]
- Szymanski , W. W. and Wagner , P. E. 1990 . Absolute Aerosol Number Concentration Measurement by Simultaneous Observation of Extinction and Scattered Light . J. Aerosol Sci. , 21 : 441 – 451 . [CROSSREF] [CSA]
- Tolman , R. C. 1948 . Consideration of the Gibbs Theory of Surface Tension . J. Chem. Phys. , 16 : 758 – 774 . [CROSSREF] [CSA]
- Tolman , R. C. 1949a . The Superficial Density of Matter at a Liquid-Vapor Boundary . J. Chem. Phys. , 17 : 118 – 127 . [CROSSREF] [CSA]
- Tolman , R. C. 1949b . The Effect of Droplet Size on Surface Tension . J. Chem. Phys. , 17 : 333 – 337 . [CROSSREF] [CSA]
- Van , d e and Hulst , H. C. 1957 . Light Scattering by Small Particles , New York : John Wiley & Sons .
- Wagner , P. E. 1985 . A Constant-Angle Mie Scattering Method (CAMS) for Investigation of Particle Formation Processes . J. Colloid Interf. Sci. , 105 : 456 – 467 . [CROSSREF] [CSA]
- Wind , L. and Szymanski , W. W. 2002 . Quantification of scattering corrections to the Beer-Lambert law for transmittance measurements in turbid media . Meas. Sci. Technol. , 13 : 270 – 275 . [CROSSREF] [CSA]
- Wind , L. , Hofer , L. , Nagy , A. , Winkler , P. , Vrtala , A. and Szymanski , W. W. 2004 . Light Scatterig from Dropplets with Inclusions and the Impact on Optical Measurement of Aerosols . J. Aerosol Sci. , 35 : 1173 – 1188 . [CROSSREF] [CSA]
- Wriedt , T. and Schuh , R. 2002 . The Inclusion-Concentration Measurement of Suspension Droplets Based on Monte Carlo ray Tracing . Meas. Sci. Technol. , 13 : 276 – 279 . [CROSSREF] [CSA]