Radiocarbon ( 14 C) measurements showed substantial levels of biogenic carbon, 52 to 89%, in PM2.5 samples collected near Tampa, Florida, during May 3–22, 2002. Nighttime biogenic percentages tended to be higher than daytime percentages. The average PM2.5 biogenic carbon concentration was 2.4 μ g m − 3 . The 14 C (and carbon mass concentration) results were highly reproducible, based on duplicate analyses of samples from collocated samplers. The work includes a first-time treatment of the potential for distortion of the 14C results by organic artifact during sampling, and a re-consideration of the impact on present-day 14 C results of the mid-twentieth century “bomb” effect. Neither was found to have a significant impact on the 14 C results. Concurrent organic and elemental carbon measurements were used to provide estimates of secondary organic aerosol (SOA) in the samples. The results of this study closely resemble those found in other summertime studies near Nashville, Tennesse (1999) and near Houston, Texas (2000) with regard to the joint importance and connection of biogenic PM2.5 and SOA in the Southeastern U.S. during summertime.
INTRODUCTION
Radiocarbon (14C) measurements are increasingly becoming used as a source apportionment technique for ambient aerosol. The ability of these measurements to separately quantify a sample's fossil-fuel-related and non-fossil-fuel-related carbon (the latter referred to as “biogenic” carbon in this article), and the fact that organic matter (carbon and its associated elements) generally accounts for a large fraction of PM2.5, should make these measurements a powerful tool in developing effective regulatory measures for the control of PM2.5. An additional attractive feature is that, as a nuclear property, the 14C label is completely impervious to atmospheric chemical changes in the organic material.
While use of the 14C technique for source apportionment of ambient aerosol is hardly new (CitationClayton et al. 1955) the last few years has seen a greatly increased interest in applying it. Recent PM10 work includes 14C measurements on samples collected across three seasons at a remote mountain site in Tennessee (CitationTanner et al. 2004), and during summer in Zurich, Switzerland (CitationSzidat et al. 2004a and 2004b), where the methodology was applied separately to the organic carbon (OC) and elemental carbon (EC) components, as well as the water-soluble and insoluble components of OC. CitationEndo et al. (2004) reported 14C results on samples in four size-fractions ranging from > 7 to < 1.1 μm aerodynamic dia. collected during spring in Tokyo, Japan. Results for summer PM2.5 samples collected in Houston, Texas (CitationLemire et al. 2002), Nashville, Tennessee (CitationLewis et al. 2004), Yosemite National Park, California (CitationBench and Herckes 2004), and in Seattle, Washington, and five rural sites across the northern U.S. (CitationFallon and Bench 2004) are also newly available.
The present work is the third in a series of field studies conducted by our laboratory, the primary goal being to survey the 14C content of PM2.5 at different geographical locations in the U.S., particularly at times other than the residential heating season (CitationLemire et al. 2002; CitationLewis et al. 2004). These two studies, as well as many of the others previously listed, have shown a sizable contribution from biogenic carbon. Consequently a special feature of the present work is an examination of two effects that might cause the measured 14C amounts to be artificially high: (1) an investigation of the potential for organic sampling artifact to distort the 14C results, through development of a theoretical model and its application to the present field measurements; and (2) a re-consideration of the impact of atmospheric nuclear weapons testing during the mid-twentieth century on interpreting present-day 14C measurements. An additional emphasis of the present work is the reproducibility of the 14C measurements, which we regard as having been insufficiently examined in previous studies.
EXPERIMENTAL
Sampling
Ambient air samples were collected at the Bay Region Aerosol Characterization Experiment (BRACE) Tower Dairy suburban/rural site, located in a pasture at 3762 South 70th Street, Tampa, Florida (27.9125ˆ N, 82.3765ˆ W). Two satellite views of the site and nearby region are shown in , generated from Google Earth software (http://earth.google.com). The site is 10 km southeast of the Tampa city center and 3 km east of Hillsborough Bay, a larger body of water south of East Bay and connected to it. A temporary fence isolated the sampling equipment from cattle that also occupied the pasture. Ambient sampling occurred during May 3–22, 2002. Day and night samples were collected for 11.5-h durations beginning at 8 am and 8 pm (Eastern Daylight Time) each day. Sample collection was performed with two collocated Model 310 Universal Air Samplers (MSP, Inc., Minneapolis, MN), which sampled at 285 1 min−1 onto 90-mm dia. quartz-fiber filters using a virtual impactor (270/15 flow ratio) to separate the sampled aerosol into a PM2.5 fraction and a “coarse” particle fraction composed mostly of particles larger than 2.5 μm dia. Only the PM2.5 samples were subsequently analyzed in this study. For seven of the sampling periods two filters were placed in the filter holder instead of the usual single filter. This front/backup filter combination was intended to provide data that could be used to estimate the effect of positive organic artifact. All filter details (preparation, handling, transport to and from the field, and storage) were the same as described previously (CitationLewis et al. 2004).
Samples of new-growth leaves were collected in the vicinity of the sampling site. These samples were intended to provide a consistency check of the 14C content of living material from the local sampling environment in comparison to the Northern Hemisphere atmospheric 14CO2 level for 2002. Details of the handling and storage of these samples were the same as described previously (CitationLewis et al. 2004).
Nine samples of Tampa-area gasoline (all three octane grades) were obtained from three different-brand service stations, and two samples of diesel fuel were obtained from an additional two different-brand stations. Gasohol (approximately 10% vol. ethanol, likely to be biogenic) identified as such was not advertised for sale in the Tampa area.
Laboratory Analyses
Measurements of OC and EC on 1.4 cm2 aliquots from the quartz filters were performed by Sunset Laboratory (Hillsborough, NC) using the NIOSH 5040 method of thermo-optical analysis (CitationBirch and Cary 1996). No samples showed any presence of carbonate, so total carbon (TC) was equivalent to the sum of OC and EC. The latter two quantities are not fundamental entities, but are operationally defined by the method.
Information from the Florida Department of Environmental Protection (CitationState of Florida 2003; CitationCostello 2005) indicates that in May, 2002, the consumption of gasohol relative to gasoline was only 0.05%, with biodiesel at a similarly low level. Consequently no pMC measurements on samples of fuel from the Tampa area were performed to confirm the strong expectation that 14C would be absent from the fuels. Analysis of the Tampa conventional gasoline and diesel samples using GC/FID/MS methodology showed no presence of ethanol or any other possible biogenic additive, as expected. Methyl tertiary butyl ether (MTBE) was found in amounts ranging from 0.1 to 3 wt. %. Since MTBE is fossil-fuel-derived however, this additive does not contribute any 14C to the motor vehicle fuels.
Ambient air and leaf samples were submitted to the University of Arizona—National Science Foundation Accelerator Mass Spectrometry (AMS) facility for 14C analysis (CitationDonahue et al. 1990). Prior to sample submission the following sample preparation steps were performed. Upon their receipt from the field the leaf samples were baked at 190ˆC for 5 min to stabilize them with respect to biological decomposition. For the ambient air samples, to decrease the filter blank a 75.7-mm dia. circle was punched from within the 81.0-mm dia. aerosol deposit area, and the outer ring was discarded.
Results reported by the Arizona AMS facility are in terms of “fraction modern (fM)” carbon, equivalent to
The results, as supplied, include correction for 13C fractionation using δ13C measurements on each sample. Except for expressing the fM results in terms of percent Modern Carbon (pMC = l00 · fM) to be consistent with the notation used in previous articles in this series (CitationLemire et al. 2002; CitationLewis et al. 2004) the results as supplied by the AMS facility were used without further modification. Radiocarbon results are also frequently reported in a Δ14C representation (CitationStuiver and Polach 1977), but which is less directly related to the purposes of this study than the fM representation.
RESULTS AND DISCUSSION
Reproducibility
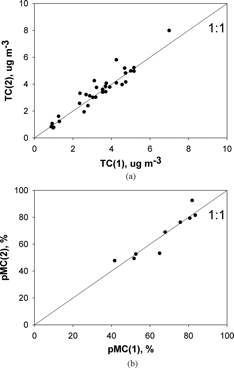
shows the degree of reproducibility of the measurements for both total carbon and pMC data from the ambient filters. Because the data are from two collocated samplers the comparison includes uncertainty from both sampler operation and analytical procedure. The two types of corrections described in the section that follows have not been applied to the data in the figure, since they affect both samplers equally.
Corrections
Positive Organic Artifact
It is well known that quantitative filter collection of ambient organic aerosol is not simple, due to volatility and gas adsorption issues. CitationTurpin et al. (2000) have summarized the long history of experimental and theoretical attempts to relate the measured carbon mass collected on a filter to the true concentration of aerosol carbon as it exists in the atmosphere prior to collection. In fact there can be substantial differences between the measured and true carbon masses. Since pMC is an associated property of carbon mass the same issues obviously carry over to the relationship of measured versus true pMC. To our knowledge however there has never been either a model proposed or experiments performed to investigate the impact of these issues on the pMC of atmospheric aerosol, even though there is no reason a priori to assume the magnitude of the effect to be any less important for pMC than for carbon mass. Instead previously reported pMC results have been generated from the simplest possible sampling arrangement: a single filter with at most an upstream particle size selection device. In the present study an initial attempt has been made to provide some quantitative information on this question where none presently exists.
Two competing effects occurring during sampling can cause the amount of (blank-corrected) carbon measured on a collection filter to differ from the corresponding amount of carbon in atmospheric aerosol: positive organic artifact (adsorption of gaseous carbonaceous species by the filter) and negative organic artifact (loss of semivolatile carbonaceous species from the filter). In the absence of an upstream denuder that would remove gas-phase carbon-containing compounds from the airstream—as is the case in the present study—CitationTurpin et al. (2000) conclude that positive artifact is expected to dominate any artifactual effects during sample collection. For our purposes we make the simplifying assumption that only positive artifact may be occurring. In this case a front/backup filter pair is useful for estimating the magnitude of positive artifact formation, not only for mass but also how it affects pMC. With the further simplifying assumption that any adsorbed gas is in equilibrium between the front and backup filters, so that the adsorbed amount is identical for the two filters, we obtain
Obtaining the pMCa analogue of Equation (Equation3) begins by expressing the pMC measured on the front filter (pMCF) as a carbon mass-weighted sum of the pMC contributions from atmospheric aerosol, adsorbed gases, and the filter blank:
Combining Equations (Equation2), (Equation3), and (Equation4) and solving for pMCa we obtain
To evaluate Equations (Equation3) and (Equation5) a filter pair arrangement was used during 7 of the 25 ambient sample collection periods. The individual measurements, and their means and standard deviations, are given in . The table shows that in all cases mF > mB, as expected, but also that pMCF > pMCB. Thus from Equation (Equation5) the artifact correction term is always positive, i.e., pMC measured on only a single filter underestimates the true pMC of the atmospheric aerosol. However as shown in the last column of the magnitude of the underestimate, while variable, is quite small: mean ± standard deviation = (2.8 ± 2.2) %. This is in marked contrast to the approximately 24% positive artifact correction to the carbon mass concentration (). Although Equations (Equation3) and (Equation5) avoid any need for explicit knowledge of the mass and pMC properties of the filter blank they were measured as a quality control step, and the results are also given in the table.
TABLE 1 Measurements of carbon mass and pMC for front, backup and blank filters, and calculated correction term for positive artifact effect
“Bomb” Effect
The correction for the inflation of 14C levels due to atmospheric nuclear weapons testing during the 1950s and 1960s is of a different nature than the positive artifact correction discussed above. Whereas the artifact correction attempts to account for otherwise incorrect pMC measurements resulting from inadequacies in the sampling methodology, the “bomb” correction instead relates to proper interpretation of pMC results presumed to be correctly measured. Such interpretation is conveniently expressed in terms of the environmentally relevant quantity, “percent biogenic carbon (pBC),” which for an unknown sample can be defined as
gives pMC results for the four different leaf samples collected near the sampling site during May, 2002. Because of the photosynthetic origin of plant growth the pMC of new-growth leaves should mirror the pMC of atmospheric CO2 at the time the growth occurs. The latter number, from pMC measurements for Northern Hemisphere atmospheric CO2 in Spring 2002 (CitationLevin and Kromer 2004), is 107%. This is in good agreement with the average of the results in (106.8 ± 2.1 (sd))%. The same direct connection is expected for any other short-lived vegetation (e.g., grasses, crops), including its combustion products. It is important to note that SOA resulting from precursor vegetative emissions, whatever the age of the vegetation, will also reflect the 14C signature of atmospheric CO2 at the time of emission. Thus in either of these two situations pMC bio = 107% is appropriate for the year 2002.
TABLE 2 Percent modern carbon measurements on different types of leaves
For aerosol from the combustion of long-lived vegetation (e.g., trees) the situation is more subtle. Generally, since varying 14C levels in post-1950 atmospheric CO2 have been incorporated into the tree material in a time-dependent manner, a weighted average of 14C levels over the tree's lifetime would be required (CitationLewis et al. 2004). However this complication is largely avoided in this study because of the particular circumstance that the sampling was done outside the residential heating season—the month of May in Florida. In this case the more likely origins for biomass combustion impact on air quality are wildfires or prescribed forest burning, in which foliar mass is preferentially combusted relative to bole and branch wood. Since foliage (whether deciduous or coniferous) is generally less than several years old, and because partial combustion of bole and branch wood means that only the outer (younger and lower pMC) layers are combusted, both factors in this situation act to bias the numerical value for pMCbio toward the then-current level of 14C in atmospheric CO2. These details were overlooked in the previous description of this effect, which was also for a non-heating season situation (CitationLewis et al. 2004). Thus for all three cases (the combustion of both short- and long-lived vegetation, and biogenic SOA from vegetative emissions) pMCbio = 107% is a good estimate for use in Equation (Equation6).
Corrected Results for PM2.5 Ambient Samples
contains the principal results of this study. The entries for atmospheric total carbon mass concentration (ma) and the OC portion of the OC/EC ratios have been corrected for positive organic artifact according to Equation (Equation3) using mB = 2.5 ± 0.5 μg cm−2 from . The uncertainty in mB is a minor contributor to ma and OC uncertainties. The quite large uncertainties shown for OC/EC are almost entirely due to the measurement uncertainty in EC. The entries for atmospheric total carbon pMC (pMCa) include the additive term + 2.8% from for the average pMC positive artifact correction, following Equation (Equation5). The uncertainty in pMCa is nearly all from the uncertainty in the artifact correction (2.2%), since the basic pMC measurement uncertainty averaged only 0.6%. The last column uses a numerical value of 107% for pMC bio (Equation (Equation6)), as justified in the previous section.
TABLE 3 PM2.5 total carbon concentration, organic to elemental carbon ratio, and percent modern carbon and percent biogenic carbon. nm = not measured. Day = 0800-1930. Night = 2000-0730
Biogenic Carbon Concentration
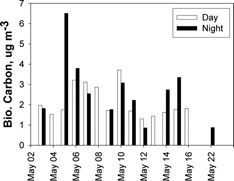
shows the time series of PM2.5 “biogenic carbon” concentration—the product of ma and pBCa from . The result for the May 5 night sample is strikingly larger than those for the remaining samples, a consequence of both ma and pBCa factors being large for this sample. The result appears reliable since during this period both ma and pBCa were also measured for the corresponding sample from the collocated sampler and found to be in good agreement (the highest point in and ). A transient local biomass burning event is a possible, but unproven, explanation for this unusual sample.
The biogenic carbon concentration average and standard deviation for all the sample results shown in is 2.4 ± 1.2 μg m−3 (2.2 ± 0.9 μg m−3, omitting the May 5 night sample). To the extent that this quantity can be associated with a (non-fossil-fuel-related) regional carbon background, it poses a potential problem for attainment of the current 15 μg m−3 annual PM2.5 U.S. National Ambient Air Quality Standard (NAAQS). The problem is all the more apparent when the additional mass of hydrogen, nitrogen, and oxygen that is typically associated with carbon in ambient aerosol is taken into account, which introduces a multiplier of 1.6–2.1 (CitationTurpin and Lim 2001).
Diurnal Variations
Using the data paired t-tests were used to compare total carbon mass and pBC characteristics of day samples with those of night samples, pairing each day with the following night. For carbon mass, day versus night concentrations were indistinguishable, regardless of whether the May 5 night sample is included (p = 0.9223 or 0.2728). For pBC, night sample percentages are statistically significantly larger (more biogenic) than for day samples, again regardless of whether the May 5 night sample is included (p = 0.0105 or 0.0240). No inference is implied as to whether these diurnal patterns exist outside this specific time period and location.
SOA and pMC
As shown by CitationTurpin and Huntzicker (1991)—and frequently applied by others since then—the ratio of OC to EC can be used to give a simple estimate of the amount of SOA in a sample. Expressing SOA as a fraction of the sample's total carbon TC the Turpin/Huntzicker result can be written as
The difficulty in using Equation (Equation7) quantitatively is in making an appropriate choice for the numerical value of (OC/EC)P. The two complications are (1) OC and EC are operationally defined rather than fundamental quantities, so that the OC/EC ratio can be significantly different (on the order of a factor of two) for different commonly used measurement methods (CitationChow et al. 2001); and (2) (OC/EC)P is inherently difficult to determine apart from the analytical measurement method issue. The discussion of these complications given by CitationLewis et al. (2004) suggests that (OC/EC)P = 5 is a plausible upper limit. Whatever the choice for (OC/EC)P however it can be readily seen from Equation (Equation7) that the SOA/TC fraction is a monotonically increasing function of OC/EC.
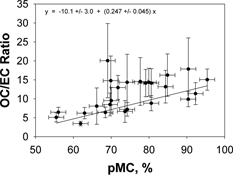
shows the pMC vs. OC/EC relationship for the data from . Most of the samples are above the (OC/EC)P threshold for SOA presence, even when that threshold is set at its plausible maximum (five). A weighted regression line, calculated from the REG procedure in SAS (SAS Institute, Inc., Cary, NC) is also shown. While there is substantial scatter the trend in the figure is that the greater a sample's OC/EC ratio—i.e., the greater its SOA/TC fraction—the greater the biogenic percentage of the sample. Using Equation (Equation7) with (OC/EC)P = 5, the range of measured OC/EC ratios imply that SOA/TC can be as large as 72%, or even larger if the actual (OC/EC)P threshold were lower. At the same time the radiocarbon-measured biogenic carbon percentages range as high as 89%. Within the context of the admittedly simple model given by Equation (Equation7) the conclusion is that a large portion of the carbon aerosol measured during the study must be SOA from biogenic VOC precursors. closely resembles those based on data collected in similar summertime field studies conducted in Houston, TX (CitationLemire et al. 2002), and Nashville, TN (CitationLewis et al. 2004), from which a similar conclusion was drawn for both studies.
CONCLUSIONS
The following are the principal findings from this study:
| • | A large fraction of PM2.5 total carbon measured near Tampa, FL, during May 2002 was biogenic: 14C measurements of pMC ranged from 55 to 95% (pBC, 52 to 89%). | ||||
| • | OC/EC ratios were consistent with SOA constituting a large fraction of PM2.5 carbon. | ||||
| • | Based on paired t-tests, pBC was statistically significantly larger for the night samples compared to the day samples, whereas TC was statistically indistinguishable between day and night samples. | ||||
| • | The average PM2.5 biogenic carbon concentration was 2.4 μg m−3, which may represent a largely uncontrollable regional carbon background in this area. | ||||
| • | The effect of positive organic artifact on the 14C results was small, causing an average 3% underestimate of the true atmospheric pMC. In contrast the artifact effect on total carbon concentrations was substantial, causing a 24% overestimate, on average. | ||||
| • | The results of this study closely resemble those found near Nashville, TN (summer 1999) and Houston, TX (summer 2000) regarding the importance of biogenic PM2.5 carbon and its relationship to SOA. | ||||
Acknowledgments
The United States Environmental Protection Agency through its Office of Research and Development managed and partially funded the research described here under Contract No. 68-D-00-206 to ManTech Environmental Technology, Inc. Funding was also provided by the Florida Department of Environmental Protection under Purchase Order No. S-3700-551920 to the University of Arizona. It has been subjected to Agency review and approved for publication.
We are grateful to EPA staff Leonard Stockburger for technical assistance throughout this project, Shelly Eberly for satellite map graphics and statistical assistance, and Bill Lonneman for fuel sample analysis; David Smith (Sunset Laboratory East) for OC/EC measurements; Alex Leonard (University of Arizona) for 14C measurements; Dwight Anderson (University of South Florida) for field sampling; Graham Bench (Lawrence Livermore National Laboratory) for identifying important differences for biomass combustion in residential heating versus wildfire settings; Bill Ellenson (ManTech Environmental Technology, Inc.—now Alion Science and Technology, Inc.) for contract management; and Tom Atkeson and Robert Stevens (Florida Department of Environmental Protection) for their funding support and encouragement.
Notes
1Multiply by 0.277 to convert to μg m−3.
2mB (pMCF − pMCB) / (mF − mB). See Equation (Equation5).
1Average uncertainty, ±10%.
2Corrected for positive organic artifact.
3(pMCa/107) · 100.
REFERENCES
- Bench , G. and Herckes , P. 2004 . Measurements of Contemporary and Fossil Carbon Contents of PM2.5 Aerosols: Results from Turtleback Dome, Yosemite National Park . Environ. Sci. Technol. , 38 ( 10 ) : 2424 – 2427 . [PUBMED] [CSA]
- Birch , M. E. and Cary , R. A. 1996 . Elemental Carbon-based Method for Monitoring Occupational Exposures to Particulate Diesel Exhaust . Aerosol Sci. Technol. , 25 : 221 – 241 . [CSA]
- Chow , J. C. , Watson , J. G. , Crow , D. , Lowenthal , D. H. and Merrifield , T. 2001 . Comparison of IMPROVE and NIOSH Carbon Measurements . Aerosol Sci. Technol. , 34 : 23 – 34 . [CSA]
- Clayton , G. D. , Arnold , J. R. and Patty , F. A. 1955 . Determination of Sources of Particulate Atmospheric Carbon . Science , 122 : 751 – 753 . [CSA]
- Costello , M. 2005 . Florida Department of Environmental Protection , private communication[CSA]
- Donahue , D. J. , Jull , A. J. T. and Toolin , L. J. 1990 . Radiocarbon Measurements at the University of Arizona AMS Facility . Nucl. Instr. and Meth. Phys. Res. , B52 : 224 – 228 . [CROSSREF] [CSA]
- Endo , M. , Yamamoto , N. , Yoshinaga , J. , Yanagisawa , Y. , Endo , O. , Goto , S. , Yoneda , M. , Shibata , Y. and Morita , M. 2004 . 14C Measurement for Size-Fractionated Airborne Particulate Matters . Atmos. Environ. , 38 ( 36 ) : 6263 – 6267 . [CROSSREF] [CSA]
- Fallon , S. and Bench , G. 2004 . “ IMPROVE Special Study: Hi-Vol Sampling for Carbon-14 Analysis of PM2.5 Aerosols ” . In Draft report , 94551 – 9900 . Livermore, California : Center for AMS, Lawrence Livermore National Laboratory .
- Lemire , K. R. , Allen , D. T. , Klouda , G. A. and Lewis , C. W. 2002 . Fine Particulate Matter Source Attribution for Southeast Texas using 14C/13C Ratios . J. Geophys. Res. , 107 ( D22 ) : 4613 1029/2002JD002339[CROSSREF] [CSA]
- Levin , I. and Kromer , B. 2004 . The Tropospheric 14CO2 Level in Mid-Latitudes of the Northern Hemisphere (1959–2003) . Radiocarbon , 46 ( 3 ) : 1261 – 1272 . [CSA]
- Lewis , C. W. , Klouda , G. A. and Ellenson , W. D. 2004 . Radiocarbon Measurement of the Biogenic Contribution to Summertime PM-2.5 Ambient Aerosol in Nashville, TN . Atmos. Environ. , 38 ( 35 ) : 6053 – 6061 . [CROSSREF] [CSA]
- State of Florida . 2003 . 2002 Florida Motor Gasoline and Diesel Fuel Report , Department of Environmental Protection . December 2003. http://www.dep.state.fl.us/energy/reports
- Stuiver , M. and Polach , H. A. 1977 . Discussion: Reporting of 14C Data . Radiocarbon , 19 ( 3 ) : 355 – 363 . [CSA]
- Szidat , S. , Jenk , T. M. , Gaggeler , H. W. , Synal , H.-A. , Fisseha , R. , Baltensperger , U. , Kalberer , M. , Samburova , V. , Reimann , S. , Kasper-Giebl , A. and Hajdas , I. 2004a . Radiocarbon (14C)-deduced Biogenic and Anthropogenic Contributions to Organic Carbon (OC) of Urban Aerosols from Zurich, Switzerland . Atmos. Environ. , 38 : 4035 – 4044 . [CROSSREF] [CSA]
- Szidat , S. , Jenk , T. M. , Gaggeler , H. W. , Synal , H.-A. , Fisseha , R. , Baltensperger , U. , Kalberer , M. , Samburova , V. , Wacker , L. , Sauer , M. , Schwikowski , M. and Hajdas , I. 2004b . Source Apportionment of Aerosols by C-14 Measurements in Different Carbonaceous Particle Fractions . Radiocarbon , 46 ( l ) : 475 – 484 . [CSA]
- Tanner , R. L. , Parkhurst , W. J. and McNichol , A. P. 2004 . Fossil Sources of Ambient Aerosol Carbon Based on 14C Measurements . Aerosol Sci. Technol. , 38 ( S1 ) : 133 – 139 . [CROSSREF] [CSA]
- Turpin , B. J. and Huntzicker , J. J. 1991 . Secondary Formation of Organic Aerosol in the Los Angeles Basin: A Descriptive Analysis of Organic and Elemental Carbon Concentrations . Atmos. Environ. , 25A : 207 – 215 . [CSA]
- Turpin , B. J. and Lim , H.-J. 2001 . Species Contribution to PM2.5 Mass Concentrations: Revisiting Common Assumptions for Estimating Organic Mass . Aerosol Sci. Technol. , 35 : 602 – 610 . [CSA]
- Turpin , B. J. , Saxena , P. and Andrews , E. 2000 . Measuring and Simulating Particulate Organics in the Atmosphere: Problems and Prospects . Atmos. Environ. , 34 ( 18 ) : 2983 – 3013 . [CROSSREF] [CSA]