With the advent of advanced real-time aerosol instrumentation, it has become possible to simultaneously measure individual particle mobility and vacuum aerodynamic diameters. This paper presents an experimental exploration of the effect of particle shape on the relationship between mobility and vacuum aerodynamic diameters. We make measurements on systems of three types: (1) Agglomerates of spheres, for which the density and the volume are known; (2) Ammonium sulfate, sodium chloride, succinic acid and lauric acid irregularly shaped particles of known density; and (3) Internally mixed particles, containing organics and ammonium sulfate, of unknown density and shape.
For agglomerates of spheres we observe and quantify alignment effects in the Differential Mobility Analyzer (DMA), an important consequence of which is that mobility diameter of aspherical particles can be a function of DMA operating voltages. We report the first measurements of the dynamic shape factors (DSFs) in free molecular regime. We report the first experimental determination of DSF for ammonium sulfate particles, for which we find DSF to increase from 1.03 to 1.07 as particle mobility diameter increases from 160 nm to 500 nm. Three types of NaCl particles were generated and characterized: nearly spherical particles with DSF of ∼ 1.02; cubic with DSF that increases from 1.06 to 1.17 as particle mobility diameter increases; and compact agglomerates with DSF 1.3–1.4. Organic particles were found to be nearly spherical. The data suggest that addition of organics to ammonium sulfate particles lowers their DSF.
INTRODUCTION
Advances in online aerosol measurement instrumentations have recently made it possible to measure simultaneously a number of aerosol attributes. Single Particle Mass Spectrometers (SPMS) and Aerodyne's Aerosol Mass Spectrometers (AMS) measure the vacuum aerodynamic diameter, d va , and composition of individual particles. Since in most cases the density and shape of the particles are unknown, it is not possible to extract from these measurements particle physical diameter or particle mass. A number of more elaborate measurement schemes have been devised to augment the SPMS and AMS measurements to allow more information to be extracted from the data (CitationBuzorius et al. 2002; CitationKatrib et al. 2005; CitationMurphy et al. 2004; CitationSlowik et al. 2004; CitationZelenyuk et al. 2005). Most notable is a scheme that uses differential mobility analyzers (DMA) to select a particle population with a narrow distribution of mobility diameters, d b , for measurement of d va and composition by the SPMS. Together, d va and d b can be used to extend our knowledge of the aerosol properties (CitationBuzorius et al. 2002; CitationKatrib et al. 2005; CitationSlowik et al. 2004; CitationZelenyuk et al. 2005). We have recently shown how this scheme can be applied to measure with high precision the density of spherical particles (CitationZelenyuk et al. 2005). However, the fact that the relationship between d va and d b is a complex function of particle morphology, which in most cases is unknown, suggests that the extension of this scheme to particles of irregular shapes must be done with caution (CitationDeCarlo et al. 2004).
As we show in this paper density, shape and alignment effects are all intertwined in the way they influence d va and d b , making it impossible to cleanly extract either density or shape without additional information or assumptions. Keeping this limitation in mind, we will explore several approaches one can take when utilizing the pair of measurements d va and d b for particles of unknown shapes. For example, it might be possible to use the mass spectral information to limit the range of possible densities and thus shapes. A very different approach may rely on the ratio of d va < eqid13 > d b , which is directly related to particle effective density, to aid in the classification of the mass spectral data without the need to assign to this ratio a definitive physical meaning. This approach only needs to demonstrate that for a given class of particles the ratio d va < eqid14 > d b is reproducible and that it can help distinguish between two aerosol classes whose mass spectra are too similar to be otherwise distinguishable in the classification process.
The need to understand the effect of asphericity on the measured aerodynamic and mobility diameters has become all the more important recently with the increased use of instruments that rely on low pressure aerodynamic lens as inlets. In these types of inlets aerosols are transported from the humid atmosphere into the vacuum resulting in a sudden and drastic reduction in relative humidity (RH). Hygroscopic particles that under normal ambient conditions are solution droplets may dry while transiting through the lens. The final velocity and therefore the apparent aerodynamic diameter reflect the overall history of the particle dynamics in the lens rather than the true particle size. There is no simple method to recover the original particle size from a measurement of its final velocity (CitationDeCarlo et al. 2004). While drying the particles prior to sampling avoids such ambiguities it means that a larger fraction of particles are aspherical, forcing us to face the reality of particle asphericity.
A recent publication by CitationDeCarlo et al. (2004) examines the potentials and limits of the information content of simultaneous measurements of d va and d b from a theoretical perspective. The present study is an experimental exploration of the effect of particle shape on the relationship between d va and d b . We present observations for a range of systems, starting with polystyrene latex (PSL) spheres agglomerates and ending with internally mixed particles of atmospheric relevance containing organics and sulfates.
The first section of the paper provides a brief summary of the theoretical framework to be applied for data analysis.
THEORETICAL BACKGROUND
a. The Dynamic Shape Factor from the Continuum to the Free Molecular Regime
We start with Stokes law extended to any flow regime with the addition of the Cunningham Slip Correction Factor C c (Kn) (CitationBaron and Willeke 2001):
In the present paper the DMA is operated at NTP and for the particle size range from 50 nm to 1000 nm Kn varies from 0.13 to 2.7, corresponding to the transition regime. The measurement of aerodynamic diameter in our Single Particle Mass Spectrometer takes place in the free molecular regime.
χ, the Dynamic Shape Factor (DSF) is defined as the ratio of the drag force on an aspherical particle to the drag force on a spherical particle with a volume equivalent diameter d ve (CitationHinds 1999):
A formulation of the drag force that was introduced by CitationDahneke (1973a) provides a smooth transition from the continuum to the free molecular regime. In Dahneke's formulation the Kn dependent χ is replaced by a constant, χ c , determined in the continuum regime, and in the slip correction factor d ve is replaced with an adjusted sphere diameter, d adj which can then be used to derive the shape factor at any Kn from the equation:
b. The Aerodynamic Diameter
It was shown (CitationHinds 1999) that particle aerodynamic diameter, d a , depends on χ according to Equation (Equation5):
Note also that the Reynolds number in the aerodynamic lens is smaller than ∼0.03 (CitationZhang et al. 2002). Since no alignment is expected for Reynolds numbers smaller than 0.1 (CitationHinds 1999) the DSFs we measure are dominated by random orientation.
c. The Mobility Diameter
The dependence of d b , the second diameter we measure, on the DSF is given by Equation (Equation7) (CitationDeCarlo et al. 2004, for example):
Since the mobility diameter is measured in the electric field of the DMA, particle alignment must be considered. When applying Equation (Equation7) to derive a DSF from a measured mobility diameter it is important to note that because of alignment in the DMA the notation χ t could be somewhat misleading. Given that in general the DSF one derives from a measured d b depends not only on Kn, but also on voltage, we use instead χ t,θ, an operationally defined DSF:
d. The Ratio d va /d b and the Average Dynamic Shape Factor, χ
Since under most circumstances particle shapes are irregular and d ve is unknown it is not possible to determine a DSF from the measurements of either of the particle diameters. The ratio d va /d b we measure can still be used to obtain information, albeit approximate, that relates to particle shape. Following CitationDeCarlo et al. (2004) we combine Equations (Equation6b) and (Equation8) to yield Equation (Equation9):
Empirically we find that is approximately equal to the average of χ
v
and χ
t,θ and is therefore a useful measure of particle shape. In the section on irregularly shaped particles we present a practical application of this approximation to derive
for a number of systems. For the majority of these systems we expect only minor effects due to alignment.
Note that because for most ambient measurements particle density is also unknown it is in general not possible to extract a definitive, even if approximate DSF. In the absence of additional information, the best that can be derived is a relationship between density and DSF and that over a limited range. In most cases the mass spectrum can be used to limit the range of densities and hence limit the range of possible DSFs.
When comparing the effect of shape on d va and d b it is important to note that asphericity causes the two to deviate from d ve in the opposite directions: d va decreases with increasing χ, while d b increases. Consequently the ratio d va /d b , which for spherical particles is proportional to particle density, is very sensitive to asphericity. For aspherical particles one of the customary ways to define an effective density is: ρ eff ≡ ρ0 d va /d b (CitationJimenez et al. 2003). There is a good reason to assume that ρ eff is reproducible for particles of the same size and composition, when measured under the similar conditions of pressure, temperature and RH, therefore, when added to the mass spectrum ρ eff may serve to help improve the classification process.
In the following sections we provide results and analysis of simultaneous measurements of d va and d b . We begin with simple system of PSL agglomerates and proceed to particles of atmospheric importance.
EXPERIMENTAL
A detailed description of simultaneous measurements of d va and d b using Single Particle Laser Ablation Time-of-flight mass spectrometer (SPLAT) and DMA is given in separate publication (CitationZelenyuk et al. 2005). Here we give a brief description only.
Particles of interest are generated by aerosolizing solutions of hygroscopic salts, organics and their binary mixtures or suspensions of PSL particles using an atomizer (TSI Inc., Model 3076) or nebulizer (Pelco, all-glass nebulizer). Exceptional care was taken to assure that particles are completely dried before they reach the DMA and that the measured effective density is not affected by residual water. In a typical experiment the aerosol flow was first dried by two diffusion dryers (TSI Inc., Model 3062), connected in series, and then diluted and further dried by mixing with dry compressed air at a 50:1 ratio in the large volume mixing/drying chamber. We estimate a final RH for the aerosol flow to be less than 0.1% RH. A test of particle dryness was conducted by adding a heated section of variable length to the sampling line to drive any residual water if present out. A comparison between heated and unheated runs shows no difference. Finally, it is important to note that all hygroscopic salts, organics and their binary mixtures in this study are known to effloresce, that is, they undergo phase transitions, shedding the solvent as they transform from droplets to anhydrous crystalline particles, at RHs significantly higher than what we estimate the final RH to be.
In several runs a second atomizer (TSI Inc., Model 3076) is used to generate internal calibrant particles by aerosolizing neat oil as described in details by CitationZelenyuk et al. (2005). The two aerosol flows are mixed in the mixing/drying chamber. The addition of internal calibrant was shown to improve the precision of the SPLAT/DMA.
The dried and mixed aerosol flow is sent to the DMA (TSI Inc., Model 3081) running with 5 lpm sheath flow and 0.5 lpm sample flow, to generate aerosol with a narrow distribution of mobility diameters. The DMA is equipped with a long classifier with center electrode that has an outer radius of 0.369 in. (0.937 cm) and is coaxial with the outer electrode, which has an inner radius of 0.772 in. (1.961 cm). The majority of the DMA output is sent to the CPC (TSI Inc., Model 3025A) for monitoring purposes and a small fraction is drawn into SPLAT for aerodynamic sizing. In some experiments the aerosol flow from the DMA is simultaneously sampled by SPLAT and collected onto copper Transmission Electron Microscopy (TEM) grids to obtain high vacuum images using Scanning Electron Microscope (SEM). Detailed description of the SEM setup and the Time Resolved Aerosol Collector (TRAC) was presented by CitationLaskin et al. (2005). When using the SEM to examine the particles great care was taken to find optimal conditions to avoid/minimize particle damage by the SEM electron beam.
A detailed description of SPLAT is given in CitationZelenyuk and Imre (2005a); here we give a brief description only. Particles enter the instrument through a 100-micron orifice into an aerodynamic lens inlet. The lens forms a very narrow low divergence particle beam and transmits the particles into the vacuum chamber with high efficiency. As the particles pass through the lens they acquire velocities that are function of their aerodynamic diameters. From the lens particles pass through two differentially pumped stages and enter the main chamber, which is equipped with two optical detection stages positioned 16 cm apart. In each optical detection stage a particle crosses a green laser beam and the scattered light it generates is collected by ellipsoidal reflector and detected by a photomultiplier. Each particle is detected twice, once at each stage, and the time of flight between the two optical detection stages is recorded. Measuring the time of flight of PSL particles (Duke Scientific, density 1.05 g cm− 3) of known diameter and density derives the time of flight to aerodynamic diameter calibration. Once detected and sized, particle composition is obtained by IR laser heating followed by UV laser ionization and time-of-flight mass spectroscopy. In the experiments described here the mass spectrometer is not used.
RESULTS AND DISCUSSION
1. The Dynamic Shape Factor of PSL Agglomerates
PSL agglomerates are an ideal model system for the study of the effect of particle shape on particle behavior. It is one of the few systems that can be reproducibly generated in the laboratory and for which shape, size, and most importantly the volume equivalent diameter can be easily controlled. A number of experimental studies of PSL agglomerates have been reported (CitationKousaka et al. 1996; CitationCheng et al. 1988, Citation1993; CitationScheuch and Heyder 1986; CitationAllen and Raabe 1985b; CitationHansson and Ahlberg 1985; CitationHorvath 1974, Citation1979; CitationHochrainer and Hänel 1975), all of which have been conducted in the continuum and transition regimes. To the best of our knowledge this study is the first report of the experimentally determined DSFs for these structures in the free molecular regime. Several theoretical investigations of PSL spheres agglomerates have also been reported (CitationCheng et al. 1988; CitationDahneke 1973a, Citation1973b, Citation1973c, Citation1982; CitationChan and Dahneke 1981).
Like many other quantifiable particle attributes, the DSF is a measure of particle behavior and not of particle intrinsic property, accordingly it depends on external conditions. In particular, χ is known to be dependent on Kn and on particle orientation, both of which play important roles in the present study.
The dependence of χ on Kn has been demonstrated by a number of experimental studies (CitationKousaka et al. 1996; CitationCheng et al. 1988; CitationScheuch and Heyder 1986; CitationHansson and Ahlberg 1985; CitationHorvath 1979; CitationHochrainer and Hänel 1975) and the theoretical formulation of the same was derived by CitationDahneke (1973a), Citation(1973b), Citation(1973c). In Dahnekes' formulation for the drag force the particle diameter is replaced with an adjusted diameter, d adj , a constant chosen to reproduce the drag force in the free molecular regime, and the dependence of χ on Kn becomes imbedded in C c (d adj ). In 1982 Dahneke demonstrated, in another theoretical study, the applicability of this formulation to straight chain aggregates of spheres and calculated adjusted diameters for chains of arbitrary length. Here we will be testing this formulation on a number of agglomerates.
Particle orientation is a second aspect of particle behavior that must be taken into consideration here. Particles can become aligned under certain flow conditions (CitationCheng et al. 1993; CitationHorvath 1974), or in electric fields (CitationKousaka et al. 1996; CitationCheng et al. 1988). Since the drag force on aspherical particles is in general a function of particle orientation, particle alignment can play an important role in determining the observed χ .
Particle alignment in the DMA has been reported by CitationKousaka et al. (1996) who found that at high fields (> 5000 V/cm) induced dipole can cause doublets and compact triplets of PSL spheres to align parallel to the field and at lower electric fields (< 2000 V/cm), when point charge dominates, particles tend to be aligned 45ˆ to the field. CitationKousaka et al. (1996) find that doublets of particles 150 nm and smaller do not become oriented parallel to the field even at electric fields higher than 5000 V/cm. CitationCheng et al. (1988) also observed electric field induced particle alignment in a Millikan apparatus while measuring the DSFs of doublets and compact triplets of PSL spheres.
The flow conditions in the aerodynamic lens are such that no particle alignment occurs, given that the Reynolds number in the lens is ∼ 0.03 or smaller (CitationZhang et al. 2002) and particle alignment does not occur at Reynolds numbers below 0.1 (CitationHinds 1999). But the fact that the drag force is orientation dependent implies that even here the observations will be effected by particle orientation. Transiting through the aerodynamic lens particles maintain their orientation and particles with unlike orientations may acquire slightly different velocities, which in turn contribute to the broadening of the d va distribution. Since no alignment is induced, the center of the d va distribution corresponds to the most probable random orientation and the width of the d va distribution reflects differences between the DSFs of particles of different orientations.
a. The Effect of Shape on Mobility Diameter (Doublets and Triplets)
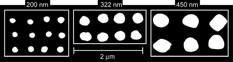
We begin with a presentation of the DMA data only, an example of which is shown in . In this DMA scan of aerosolized suspension of 233 nm PSL particles in addition to the singlets we observe peaks corresponding to doublets, triplets, and quadruplets, also visible are peaks corresponding to multiply charged particles as indicated. We have carried out similar measurements on PSL particles ranging in diameter from 51 nm to 404 nm. The observed mobility diameters are used to calculate the DMA DSFs, χ t,θ using Equation (Equation7).
FIG. 1 A mobility size distribution of aerosol produced by atomizing and drying a suspension containing 233 nm PSL spheres. Apparent in the distribution are singlets, doublets, triplets, and quadruplets. The peaks with mobility diameters smaller than the singlet correspond to multiply charged particles as marked. Also indicated are the volume equivalent diameters of the observed agglomerates. The upper scale provides the voltage in the DMA. To obtain electric field a gap of 1.024 cm between the two electrodes should be used.
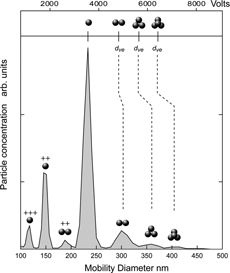
The derived χ t,θ for the doublets are presented in in the traditional plot of DSF as a function of Kn(d ve ). Since the pressure in these experiments is constant and the Knudsen number is varying due to change in particle size only, we provide the particle size scale along with the DMA voltage for the given size at the top of .
FIG. 2 A plot of the observed χ t,θ for doublets of PSL spheres as a function of Knudsen number. The solid lines marked parallel and random are based on CitationCheng et al. (1988) and show the calculated shape factors for the two orientations as a function of Kn. The line marked 45ˆ is based on CitationKousaka et al. (1996) and indicates the DSFs for the doublets oriented 45ˆ to the flow field as a function of Kn. The scales at the top indicate particle primary size and the DMA voltage. Three distinct regions are apparent: above 5000 V, between 2000 V and 5000 V, designated by shading, and below 2000 V. The error bars in this as well as all other figures are based on the standard deviation of multiple measurements.
The solid lines in depict the behavior of the DSFs for the indicated orientations as a function of Kn only. The solid lines for parallel and random orientations were adopted from CitationCheng et al. (1988). A third solid line exhibiting virtually no dependence on Kn is based on observations by CitationKousaka et al. (1996) and corresponds to particles oriented 45ˆ to the flow.
The plot is divided into three regions on the basis of voltage: V > 5000 V, 5000 V > V > 2000 V and V < 2000 V. The data suggest that above 5000 V doublets align parallel to the field and below 2000 V they tend to be oriented 45ˆ. Note also, that the doublets of the smallest particles (d 1 = 50 nm) approach the random orientation. Increasing Kn means that both, the DMA voltage and d 1 decrease. The two variables—lower voltage and smaller particles—work in consort to reorient particles from parallel to 45ˆ. Lower voltage lessens the induced dipole, and smaller particles require higher voltage to induce a dipole moment. As the induced dipole moment is diminished, the dominant effect becomes alignment due to point charge, which results in 45ˆ orientation. In we provide a summary of our measured χ t,θ for the doublets subdivided into three categories: parallel orientation, transition region and 45ˆ orientations and a comparison between our observed DSFs and those calculated on the basis of parameters given by CitationCheng et al. (1988) and CitationKousaka et al. (1996) for the parallel and 45ˆ orientations respectively. As the table shows our measured DSFs are in excellent agreement with the calculated values.
TABLE 1 Dynamic shape factors for doublets in the DMA
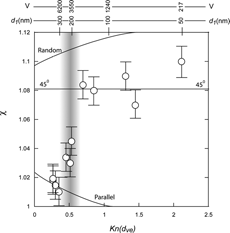
shows a plot of χ t,θ for the compact triplets, similar to for doublets. Here again the data indicate that at smaller Kn, i.e. for higher voltages and larger particles, the compact triplets are aligned parallel to the flow direction and at lower voltages the dominant orientation is 45ˆ. As we have seen in the case of the doublets the orientation of the smallest particles approaches the random orientation. is a summary of the observations for the compact triplets and a comparison between observed and predicted χ t,θ based on CitationHorvath (1974) and CitationCheng et al. (1988) for the parallel orientation and CitationKousaka et al. (1996) for the 45ˆ orientation, subdivided accordingly. Here again we find reasonably good agreement.
FIG. 3 A plot of the observed χ t,θ for triplets of PSL spheres as a function of Knudsen number. The solid lines marked parallel and random are based on CitationHorvath (1974) and show the calculated shape factors for the two orientations. The line marked 45ˆ is based on CitationKousaka et al. (1996) and indicates the DSFs for the triplets oriented 45ˆ to the flow field. All solid lines depict the dependence of the DSFs on Kn only. The scales at the top indicate particle primary size and the DMA voltage. Three distinct regions are apparent: above 5000 V, between 2000 V and 5000 V, designated by shading, and below 2000 V.
TABLE 2 Dynamic shape factors for triplets in the DMA
Shown in is a plot of χ t,θ for the doublets and the triplets as a function of voltage in the DMA measured at constant flow (5 lpm and 0.5 lpm for the sheath and aerosol flows respectively) for all particle sizes listed in and . provides very illustrative evidence of the effect of alignment on χ t,θ and hence on the observed mobility diameter. The relatively sharp transition from the parallel to 45ˆ and to the random orientation reflects the fact that voltage and particle size decrease simultaneously.
FIG. 4 A plot of the observed χ t,θ for doublets and triplets of PSL spheres as a function of DMA voltage obtained at constant flow rates. The smallest particles and hence at the lowest voltages particles are between the random and 45ˆ to the field. At slightly higher voltage they align 45ˆ to the field. The range between 2000 V and 5000 V is a transition region and above 5000 V the larger particles are aligned parallel to the flow field. The scale at the top indicates the mobility diameter for the corresponding voltage which is indicated on the bottom scale. (Data were taken at 5 lpm and 0.5 lpm sheath and aerosol flows respectively.)
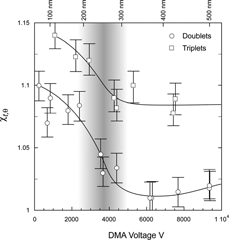
These data should not be interpreted to suggest that the doublets and compact triplets of all sizes are in the 45ˆ orientation below 2000 V and in the parallel orientation above 5000 V. The general rule that does emerge, on the basis of a number of additional measurements not shown here, is that doublets with primary particle diameter larger than 200 nm are in the parallel orientation for voltages above 6000 V. Similar general statements about the low voltage end cannot be made because as the voltage decreases it appears that particle alignment tends to be diminished.
Note that through the dependency on voltage the mobility diameter becomes dependent on the mode of DMA operation: in other words, changing the flow rate in the DMA can change the mobility diameter of aspherical particles for which alignment plays a role. This was illustrated in CitationKousaka et al. (1996), where the DSFs of same size doublets and triplets were measured at different voltages by changing the flow rates. Experiments in our laboratory on a number of particle sizes yield very similar results.
b. Dynamic Shape Factors in the Free Molecular Regime
The free molecular regime DSFs were derived by measuring the agglomerates d va using SPLAT and applying Equation (Equation6b). The fact that asphericity causes the aerodynamic diameter of the doublets to shift closer to that of the singlets makes it impossible to separate the weak doublets signal underneath the shoulder of the intense singlets peak. In contrast, asphericity causes the mobility diameter of the doublets to be larger than its volume equivalent diameter, making it easier to resolve it from the singlets. We take advantage of this last fact and use the DMA as a filter to isolate a single or a limited number of agglomerates for aerodynamic measurements by SPLAT. Fine tuning the DMA while observing changes in distributions of the observed d va helps in peak assignments for both instruments, making it possible to obtain DSFs for a number of higher agglomerates.
In we show a plot of the d va distribution of the doublets of 233 nm PSL particles obtained by setting the DMA to transmit particles of 305 nm mobility diameter to SPLAT as indicated by the arrow in the inset in . Despite the fact that the solution contains in addition to singlets and doublets many other agglomerates the only particles observed by SPLAT are the doublets. The simplicity and high signal to noise ratio of the doublets d va distribution is a consequence of using the DMA as a filter, making it possible to obtain very clean and easy to assign spectra.
FIG. 5 A plot of d va distribution of the doublets of 233 nm PSL spheres (solid line, shaded region). The inset shows the DMA scan and the arrow points to the mobility diameter that was selected for this measurement. For reference, also shown is the measured singlets d va distribution (dashed line). The line widths (FWHM) for the two distributions are indicated. The top scale indicates the volume equivalent diameter and the calculated (based on CitationCheng et al. (1988)) d va of the doublets oriented parallel and perpendicular to the flow direction. (The Y axis in this figure, as well as all other figures showing d va distributions is proportional to the number of particles detected).
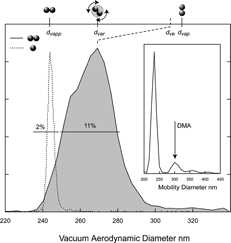
The d va distribution of the doublets peaks at 269 nm; together, the doublets known d ve of 293.6 nm and density of 1.05 g cm− 3 yield a free molecular regime DSF of 1.147 for the random orientation.
Returning to we note that both, line position and line shape contain significant information. For comparison, we show in the figure next to the doublets a measured d va distribution of the singlets. There is a clear and significant difference in the widths of the two distributions. Based on the singlets d va distribution alone we would expect the doublet line width to be slightly less than 3%. But as apparent from we observe a significantly wider distribution for the aspherical particles.
It is possible in this case to gain insight into the relationship between particle orientation and line width by calculating the d va of doublets in the two most extreme orientations: parallel and perpendicular. Once again we use values reported by CitationCheng et al. (1988), Citation(1991) (see ) for the orientation dependent DSFs, the associated adjusted diameters and Equation (Equation4), which together yield free molecular regime DSFs for the parallel and perpendicular orientation of 0.98 and 1.25 respectively. Translated to d va for the 233 nm doublets we obtain 314.6 nm and 235 nm for the parallel and perpendicular orientations respectively. These values are noted in , setting a scale for the upper and lower limits of the d va distribution.
TABLE 4 A comparison between calculated and observed dynamic shape factors for doublets, triplets, and quadruplets
We find, as a general rule, that asphericity increases the width of the d va distribution. In the case of the PSL particles this effect is dominated by orientation, but in the more general cases in addition to orientation one has to consider a distribution of particle shapes, each having the same mobility diameter but slightly different aerodynamic diameter. As we pointed out in an earlier publication (CitationZelenyuk et al. 2005) the correlation between particle asphericity and line shape provides in most cases a convenient and simple means to spot asphericity. We will return to discuss the shape of the d va distributions later in this paper.
To measure the DSFs of the other agglomerates the DMA is successively tuned to the appropriate mobility diameters and the vacuum aerodynamic sizes of the resultant aerosol are measured by SPLAT. By tuning the DMA and recording d va distributions it was possible to measure the d va and d b and derive DSFs for both regimes for a number of agglomerates that could not be directly measured on the basis of the DMA scan alone. To aid with peaks assignments simultaneously with the measurements of aerodynamic diameters particles were collected on TEM copper grids for analysis by microscopy. is a gallery of the microscopic images of some of the observed agglomerates.
FIG. 6 A gallery of micrographs of agglomerates of 302 nm PSL spheres: (a) doublet; (b) compact triplet; (c) compact quadruplet; (d) non-compact quadruplet; (e) Y-shaped quadruplet; (f) compact quintuplet; (g) flower shaped quintuplet; and (h) a large agglomerate with over 18 PSL spheres.
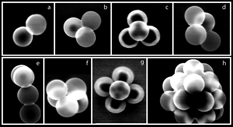
The data for all agglomerates we were able to observe and assign are summarized in , where we provide a listing of the specific agglomerates with their measured aerodynamic and mobility diameters and the derived DSFs for the DMA and for the free molecular regime. We have assigned all but one of the reported structures to be compact. Examples of the structures that make up the non-compact quadruplets in are shown in and . The data in indicate a general increase of both DSFs as a function of agglomerate size up to n = 6. For larger agglomerates the data suggest a relatively constant DSFs, remaining below χ = 1.26 for both regimes. This last observation is consistent with the DSFs for compact structures up to n = 23 reported by CitationStöber and Flachsbart (1969), none of which exceed 1.267.
TABLE 3 Summary of the dynamic shape factors of PSL spheres agglomerates in the free molecular regime and in the DMA
A comparison between our observed free molecular regime DSFs and those calculated using Equation (Equation4) is given in . We find perfect agreement between the calculated and observed values for the doublets. For the compact triplets and quadruplets the differences between the calculated free molecular regime DSFs of 1.15 and 1.12 and our measured DSF of 1.21 for both are rather large.
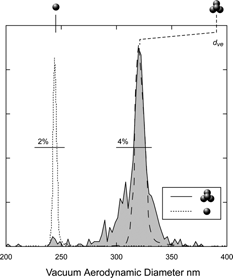
We note that the calculations are based on a formulation that was shown to apply to straight chains only and not to compact structures. The fact that the χ t,θ we derive for the transition regime of 1.14 is in good agreement with CitationHorvath (1974) indicates that the DMA was selecting the appropriate structure. CitationHorvath (1974) also finds for this structure an orientation independent DSF, which is a reflection of the special tetrahedral symmetry of the compact quadruplets. An orientation independent shape factor implies a narrower d va distribution for this structure. Indeed, shown in is a plot of the measured d va distribution for the 233 nm compact quadruplets, as the figure indicates, it has a line width of 4% (FWHM). For comparison we show the d va distribution for the singlet in short dashes and in long dashes the predicted line shape for the quadruplet assuming no broadening due to particle shape. The latter fits the central portion of the peak of the observed line shape for the quadruplet. Note however, that the line shape is complex and composed of a narrow central spike and a wider pedestal, which is most likely a reflection of the asphericity of the quadruplet and the fact that the DSF is slightly dependent on orientation.
FIG. 7 Plots of d va distributions of the compact quadruplets of 233 nm PSL spheres, (solid line, shaded region) and singlets (short dashed line). The line widths (FWHM) for the two distributions are indicated. Also shown (long dashed lines) is the expected line shape for 4 particles as derived on the basis of the singlet line shape. Note the wider than predicted pedestal. The top scale indicates the volume equivalent diameter of the quadruplets.
c. On the Relationship of d va and d b
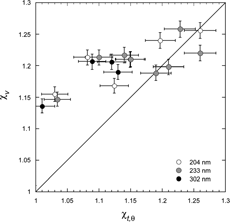
One of the goals of this study was to explore the effect of particle shape on the relationship between the two diameters, d va and d b . Because the density and the volume equivalent diameters of the agglomerates of PSL spheres are known, it is possible to independently derive χ t,θ from d b and χ v from d va . But agglomerates of PSL spheres are the exception, in most cases d ve is not known and the measurements of d va and d b are not sufficient to derive the DSFs without additional information or simplifying assumptions. We distinguish between two cases: one with unknown d ve but known ρ p , and other where neither is known. It was suggested by CitationDeCarlo et al. (2004) that for the former case it might be reasonable to use a simplifying approximation that sets χ v and χ t,θ to be equal. The present dataset of PSL spheres agglomerates for which χ v and χ t,θ are known provides a good test of the validity of this approximation. A comparison of the two DSFs is shown in in a plot of χ v vs. χ t,θ. For the smaller agglomerates, where alignment plays an important role, the differences between χ v and χ t,θ are significant, but for compact agglomerates these differences decrease with increasing agglomerate size.
FIG. 8 The relationship between the two DSFs, χ v and χ t,θ measured respectively by SPLAT at low pressure and with the DMA at 1 atmosphere. The majority of the data points represent particles that align either parallel or nearly parallel to the field in the DMA but are randomly oriented in SPLAT. The differences between χ v and χ t,θ are mostly a reflection of differences in orientation. Data suggest that alignment plays less of a role for larger compact agglomerates resulting in χ v ≅ χ t,θ.
In the section below, where we present results on systems of known density but of arbitrary shape, we will be forced to apply the approximation χ
v
= χ
t,θ = to enable us to extract a DSF from the measurements of d
va
and d
b
. But, before we proceed on to the more complex systems, for which there would be no way to evaluate the repercussions of such an approximation, it would be insightful to apply it to the PSL spheres agglomerates dataset and compare the calculated
with the measured χ
v
and χ
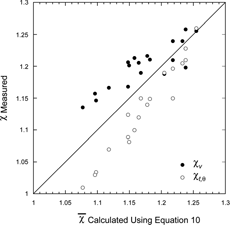
t,θ. Such a comparison is shown in in a plot of the two measured DSFs vs.
. Not surprisingly we find that
is approximately equal to the average of χ
v
and χ
t,θ and could therefore be a useful surrogate of particle asphericity. It is interesting to note that because
is influenced by alignment in the DMA it would in general be dependent on the flow in the DMA.
FIG. 9 The relationship between
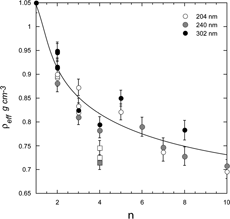
When neither density, nor shape is known, as is the case for most ambient particles, it is common to use the two diameters to derive ρ eff , defined above. Here again we use the data for PSL spheres agglomerates to calculate an “effective density,” which we present in in a plot of effective density as a function of agglomerate size. The decreasing effective density with agglomerate size is nothing more than a reflection of the increasing shape factor. The smooth line is only intended to guide the eye to the overall trend. The non-compact quadruplets clearly stand out in this plot. Since the shape factor for compact structures remains below 1.26 we would expect the effective density to remain above ∼ 0.7. Ascribing a physical interpretation to the effective density is clearly not straightforward. The true material density of each of the data points in is 1.05 g cm− 3, yet ρ eff varies from 0.69 g cm− 3 to 1.05 g cm− 3. While ρ eff may be significantly different from ρ p , it is a reproducible and measurable quantity that for particles of the same size and composition under the same conditions is expected to be constant.
FIG. 10 A plot of the calculated effective density, defined in the text, as a function of agglomerate size. The non-compact quadruplets are marked by squares, the rest of the points refer to compact agglomerates. The sizes of the primary particles are indicated.
The study of agglomerated PSL spheres has shown that the interpretation of measurements of irregularly shaped particles can be very complex. Because the effects of alignment and pressure are inescapable, in virtually every other system to extract information on particle size, shape and density from the combined measurements of d va and d b requires introduction of approximations, which obviously result in loss of accuracy.
In the next section we report on the simultaneous measurements of d va and d b of aspherical particles with irregular shapes. These measurements include ammonium sulfate, NaCl, succinic and lauric acids. We also present observations on internally mixed succinic acid with ammonium sulfate and lauric acid with ammonium sulfate.
2. Dynamic Shape Factors of Irregularly Shaped Particles
a. Ammonium Sulfate
The shape of ammonium sulfate particles has been mentioned in the literature a number of times, but the number of actual measurements of the shape of this atmospherically important particle system is rare. Microscopic images of ambient ammonium sulfate particles reveal mildly aspherical particles but with significant variability (CitationLi et al. 2003; CitationDick et al. 1998; CitationPerry et al. 1978). CitationDick et al. (1998) have applied DAWN-A (Dual Amplitude Weighted Nephelometer), a multi-angle single particle light scattering detector, in which amplitude fluctuations are used to discern between spherical and aspherical particles to ammonium sulfate particles. They, like others concluded that their laboratory generated ammonium sulfate particles were mildly aspherical, a conclusion consistent with their TEM analysis. CitationPerry et al. (1978) have also used multi-angle light scattering and microscopy and came to the same conclusion. CitationHuffman et al. (2005) measured particle beam divergence to derive a lift shape factor; a surrogate of the DSF, for laboratory generated 110 nm and 320 nm ammonium sulfate particles and found that the lift shape factor increases with particle size. In some cases, especially for modeling purposes, ammonium sulfate is assumed to be spherical (CitationKreidenweis et al. 2005).
Here we prepare ammonium sulfate by atomizing an aqueous solution of ammonium sulfate and drying the aerosol stream as described in the experimental section. The DMA is used to select an aerosol with a narrow distribution of mobility diameters, which is sent to SPLAT for aerodynamic sizing. Some of the runs include an internal calibrant as described in CitationZelenyuk et al. (2005) and in other runs the aerosol flow is split between SPLAT and TRAC for microscopic examination.
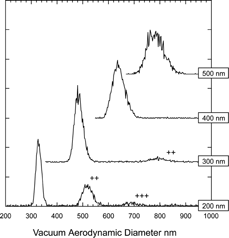
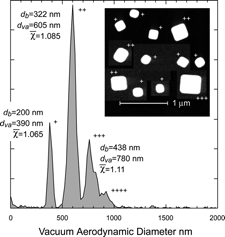
shows the measured d va distribution of DMA selected ammonium sulfate particles with mobility diameters of 200 nm, 300 nm, 400 nm, and 500 nm. Due to multiple charging other sizes are also apparent in the figure, these are labeled “++,” and “+++,” for doubly and triply charged particles respectively. As with most other cases of aspherical particles here as well we find that asphericity induces broadening of the d va distributions. In this case the distributions are 10–11% wide (FWHM), as compared with the typical 5% for the SPLAT/DMA system for spherical particles (CitationZelenyuk et al. 2005).
FIG. 11 Measured d va distributions of DMA selected ammonium sulfate particles. The mobility diameters are indicated on the right hand side. Multiply charged particles are labeled with “++” and “+++”.
The combined measurements of d
va
and d
b
are used to derive particle effective density defined in the text and to extract a DSF, using Equation (Equation10) into which we input the known bulk density of ammonium sulfate (1.77 g cm− 3) and the approximation χ
v
= χ
t,θ = . As an example, d
va
= 330 nm and d
b
= 200 nm yield ρ
eff
= 1.65 g cm− 3 and
= 1.04. To put the DSFs we derive for this system in a visual context we provide in a gallery of microscopic images of an assortment of ammonium sulfate particles of three different mobility diameters. We have attempted to perform a shape factor analysis of such images but found that the particle-to-particle variability and the low precision of the microscope prevented any coherent pattern from emerging.
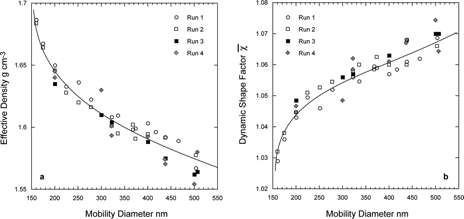
In and we present derived effective densities and DSFs for four separate runs of ammonium sulfate, two of which included internal calibrant and two in which particles were collected for microscopic analysis. The data indicate that the shape factors and effective densities of the ammonium sulfate particles prepared in our laboratory are systematically dependent on particle size. For smallest particles we find a DSF of 1.03 ± .01, but for larger particles an increasing shape factor is observed. It is interesting to note that the beam divergence measurements by CitationHuffman et al. (2005) also exhibit an increasing asphericity with increasing particle size.
FIG. 13 Size dependence of the derived effective densities (a), and DSFs (b), plotted as a function of ammonium sulfate particle mobility diameter. Shown are four separate runs, out of which runs 1 and 2 were carried out with pump oil as internal calibrant.
A measure of the experimental noise and run-to-run reproducibility of the derived shape factors can be obtained by fitting the data with a polynomial and calculating the residuals, δ, defined as δ = fit −
obs
. Statistical analysis of the residual produces a standard deviation of ± 0.003.
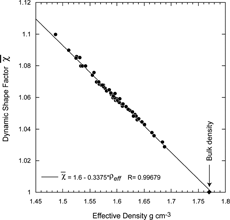
The relationship between the effective density and the calculated DSF is determined by Equation (Equation10). Nevertheless it is worth noting that is linearly related to ρ
eff
, as shown in . Moreover, when the fitted line is extrapolated to
= 1 it yields ρ
eff
= 1.778 g cm− 3, which is nearly the ammonium sulfate bulk density.
FIG. 14 A plot of the derived DSFs as a function of the effective density for ammonium sulfate particles of various sizes. The data can be fit with a straight line that approaches the bulk density (indicated with a diamond) when the DSF approaches 1.
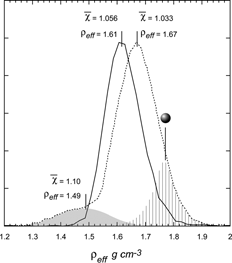
Finally, we do not want to leave the reader with the impression that ammonium sulfate particles of a given size have a definitive shape factor, which is independent of particle preparation and treatment. is a plot of two distributions of effective densities both of which correspond to 300 nm mobility diameter ammonium sulfate particles. The two distributions peak at slightly different effective densities and their average DSFs differ by 0.023. More importantly, close examination of the line shapes in reveals that one of the distributions is indicative of the presence of two types of 300 nm mobility diameter ammonium sulfate particles: one with an effective density of 1.67 and a shape factor of 1.033 and the other with an effective density of ∼ 1.49 and a shape factor of 1.10 (the second mode is indicated in the shaded area). The significantly larger shape factor associated with this mode may be suggestive of agglomeration; a phenomenon we have observed in a number of systems as discussed below. Also indicated in with the striped area, is the position and line shape expected for spherical ammonium sulfate, for reference.
FIG. 15 Derived effective density distributions of 300 nm mobility diameter ammonium sulfate particles from two separate runs, which illustrate the sensitivity of particle shape to preparation and treatment. The two distributions peak at slightly different effective densities and have different DSFs. The distribution labeled with dashed line shows that two significantly different types of ammonium sulfate particles were simultaneously produced. The striped area indicates the position of spherical ammonium sulfate particles.
b. Sodium Chloride
Like ammonium sulfate, the shape of NaCl particles has been discussed in a number of publications (CitationDick et al. 1998; CitationPerry et al. 1978; CitationKreidenweis et al. 2005). Sodium chloride particles are generally assumed to be cubic in shape with a DSF of 1.08, which is the calculated shape factor for ideal cubes (CitationHinds 1999). A number of investigators have directly measured the DSF for this system. CitationHorvath (1974) used a sedimentation apparatus and microscopy and found a DSF of 1.08 for cubic particles and 1.05 for cubes with rounded edges. CitationMikhailov et al. (2004) used hygroscopicity tandem differential mobility analyzer system complemented by TEM to derive DSF of 1.06 and 1.07 for 99 nm and 201 nm NaCl particles respectively and concluded that larger particles have slightly larger DSF. They also note that the DSF of the same aerosol can slightly change before and after deliquescence due to humidity induced microstructural rearrangements. CitationHuffman et al. (2005) used the reported measurements of particle beam divergence by CitationSchreiner et al. (1999) and CitationKatrib et al. (2005) to derive lift shape factors and found a strong dependence on particle size, increasing from 1.69 to 4.36 and 6.27 as particle size increases from 100 nm to a polydisperse aerosol with size ranges 190–480 nm and 300–850 nm respectively. The general conclusion is that NaCl particles generated by atomizing or nebulizing aqueous NaCl solution are nearly cubical with the smaller particles having more rounded edges and therefore smaller DSF. NaCl particles generated by condensation can be produced in a variety of shapes ranging from nearly spherical to complex agglomerates with DSF of up to 5 (CitationKrämer et al. 2000; CitationScheibel and Porstendörfer 1983; CitationKasper and Berner 1978).
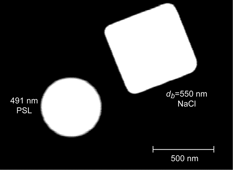
The measurement approach and data analysis for NaCl are the same as those used for ammonium sulfate above. A micrograph of a single 550 nm mobility diameter sodium chloride particle prepared in our laboratory is shown in . It is clearly consistent with the cubic structure generally assumed for this system. The second particle in the micrograph is a 491 nm PSL particle shown for scale.
FIG. 16 A micrograph of a single NaCl 550 nm mobility diameter particle. Also shown is a 491 nm PSL particle.
shows a measured d va distribution for 200 nm mobility diameter NaCl particles. Due to the presence of multiply charged particles, also apparent in the size distribution are particles with 322 nm, 437 nm, and 550 nm mobility diameter particles. Analysis of the data yields shape factors of 1.07, 1.09, and 1.11 for the singly, doubly and triply charged particles respectively. Simultaneously with the acquisition of these data particles were collected for microscopic analysis. Micrographs of a sampling of the collected particles are also presented in showing singly, doubly, and triply charged particles. The 550 nm particle shown in is a quadruply charged particle from the same run. As expected the general appearance suggests that the particles are cubic in shape. But a closer inspection shows that the corners of the singly charged particles are significantly rounded off as compared with those of the larger particles.
FIG. 17 The d va distribution of NaCl particles selected by the DMA to transmit 200 nm mobility diameter particles. Doubly, triply, and quadruply charged particles are marked. The mobility diameters, d va and derived DSFs are indicated for each peak. The inset shows a micrograph of representative particles collected simultaneously with the measurement of d va . Note that the corners of the smaller particles are significantly rounded off.
The DSFs we derive here are in excellent agreement with those reported by CitationHorvath (1974) and CitationMikhailov et al. (2004), despite the fact that their values correspond to the continuum and transition regimes, while the ones derived in the present study are an average of χ t,θ and χ v . The good agreement between all three studies is consistent with CitationHorvath (1974) finding of an orientation independent DSF for cubes. Moreover, the observed trend, of increasing DSF with increasing size is in general in good agreement with other reports.
It is interesting to compare the effect of symmetry on the d va distributions for compact quadruplets of PSL spheres and NaCl cubes. Both are expected by symmetry to have orientation independent DSFs, yet only the PSL quadruplet exhibits a narrow d va distribution. The difference stems from the fact that real NaCl particles are not identical ideal cubes; there is a significant variation in shape from one particle to the next. While it is true that within the DMA resolution the mobility diameter of each of the singly charged particles is 200 nm, the fact that their shapes differ, even if slightly, results in broadening of the d va distribution. That is because shape affects mobility and aerodynamic diameters quantitatively different. We find for NaCl, similar to ammonium sulfate, d va distributions widths to be 10–11% (FWHM).
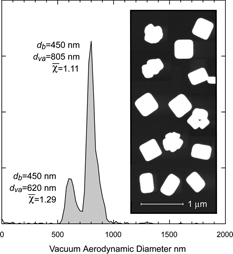
In we show the d va distribution of 450 nm mobility diameter NaCl particles. Two distinct modes are apparent with DSFs of 1.11 and 1.29, indicating the presence of two types of sodium chloride particles. Also shown in are micrographs of particles collected simultaneously with the measurements made by SPLAT. The micrographs, in agreement with d va distribution, show clearly the presence of two types of particles, cubes and agglomerates. We associate the peak with the larger shape factor with agglomerates and the second more intense peak with cubic NaCl particles. Note, however, that the shape factor for these cubic particles is slightly higher than 1.08.
FIG. 18 The d va distribution of NaCl particles selected by the DMA to transmit 450 nm mobility diameter particles. Two distinct modes with very different DSFs are evident. The higher shape factor peak was assigned to agglomerated NaCl particles. The inset shows micrographs of representative particles collected simultaneously with the measurement of d va , showing the presence of agglomerates. Note that the many of the cubic type NaCl particles are parallelepipeds.
and summarize the NaCl data in plots of effective density and DSF as a function of mobility diameter. Three distinct types of sodium chloride particles were observed: nearly spherical particles, cubes and agglomerates.
FIG. 19 Size dependence of the derived effective densities (a) and DSFs (b) plotted as a function of sodium chloride particle mobility diameter. Three distinct types of sodium chloride particles are evident: nearly spherical, cubic, and agglomerated. Micrographs of representative particles of each type are also shown.
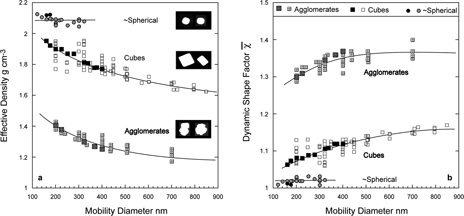
The derived DSF for the nearly spherical NaCl particles is 1.02 ± 0.01 and exhibits no dependence on particle size. However, this type of particles was observed only for the smaller mobility diameters (< 322 nm). Although we did not collect particles for microscopy when this type of particle was dominant, as the micrograph in shows we did observe that a significant number of the 200 nm mobility diameter particles were nearly spherical.
The cubic form of NaCl is the most prevalent. The derived DSF for this form of NaCl particles is size dependent, increasing from 1.06 to 1.17 as particle size increases from 200 nm to 800 nm. We note a significant spread in the DSFs of the smaller particles, which is not due to the reproducibility of measurements, but rather a result of run-to-run variations in generation and drying conditions that produce particles with a slightly different degree of roundness. This spread is therefore a reflection of the variability in particle shape. The filled squares represent a single run and provide an example of the trends observed under one set of conditions. The observed increase in DSF for the larger particles is consistent with particles with sharper corners and the presence of larger number of rectangular particles at larger sizes (see ).
NaCl agglomerates formed by condensation, with DSFs up to 5, indicative of highly irregular chain like structures, have been observed (CitationKrämer et al. 2000 and references therein). Following humidity induced microrearrangements these agglomerates become compact and their DSFs are reduced to below 1.5. In our system agglomerates are formed typically when the atomizer output flows directly into the mixing/drying chamber without prior drying with diffusion dryers. Mixing the aerosol flow with dry air at a ratio of 50:1 causes the RH to drop rapidly producing high number concentrations of smaller particles and the large volume of the mixing chamber provides the environment for coagulation. The micrographs in and many others like it are consistent with relatively compact irregularly shaped agglomerates with primary particle sizes ranging from 50–100 nm. Our derived DSFs for this form of NaCl are 1.3 to 1.4, consistent with compact agglomerates. These agglomerates are somewhat more distorted from sphericity than the compact agglomerates of PSL spheres (see ), which explains the slightly larger DSF.
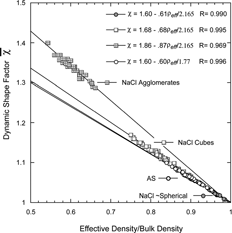
provides a second view of the three types of NaCl particles in a plot of the DSFs, as a function of the normalized effective density, (ρ eff /ρ p ). The data can be fit with three straight lines: one for each of the NaCl types, all approaching 1 as the DSF approaches 1. For comparison we have also included the ammonium sulfate data. The slope of the nearly spherical ammonium sulfate particles is virtually identical to that of the nearly spherical NaCl particles.
FIG. 20 A plot of derived DSFs as a function of normalized effective densities defined as ρ eff /ρ p for the three types of sodium chloride particles. The data segregate into three straight lines according to the particle types and all fitted lines approach 1 when the shape factor approaches 1. For comparison the ammonium sulfate data are also shown.
Which NaCl particle type is produced and with what exact DSF are functions of flow rates, solution concentration, and drying rates. More experiments are needed to establish a solid relationship between these variables and particle types and shapes. At this point our experience suggests that nearly spherical dry NaCl particles are produced under rapid drying conditions. Agglomerates of NaCl particles are produced in the mixing/drying chamber when particle number concentrations are high. Slow drying by diffusion dryers at lower particle number concentrations produces cubic NaCl.
c. Succinic Acid, Lauric Acid, and their Binary Mixtures with Ammonium Sulfate
Very little is known about the shape of particles containing organic compounds. We present here the results of measurements on particles composed of succinic acid (SA), lauric acid (LA), and of their binary mixtures with ammonium sulfate (AS:SA and AS:LA).
SA is a 4-carbon dicarboxylic acid with a crystal density of 1.56 g cm− 3 and LA is composed of a 12-carbon aliphatic chain with a carboxylic group at one end and a crystal density of 0.883 g cm− 3. SA particles were generated by aerosolizing either water (SA/W) or isopropyl (SA/IP) solutions. Internally mixed AS:SA particles were generated from water solutions. LA and AS:LA particles were generated by aerosolizing a methanol/water solutions.
shows a plot of the measured succinic acid effective density as a function of particle mobility diameter. The derived effective density for succinic acid particles is independent of size and its average value is 1.56 ± 0.02 g cm− 3, which is identical to that of the bulk. The average shape factor for this system is therefore 1.00, that fact, however, in and of itself does not prove that these particles are spherical, but only that they are very close to being spherical. We have previously demonstrated (CitationZelenyuk et al. 2005) that a more sensitive measure of sphericity is d va distribution line width. provides a summary of the measured effective densities and line widths for this system. The first entry in the table is for spherical particles, establishing for the present configuration a SPLAT/DMA sizing resolution of 4%. The line shape of the observed d va distribution of succinic acid particles is 7% and is independent of particle size, indicating that these particles are very mildly aspherical.
FIG. 21 Derived effective densities of succinic acid and succinic acid mixed with ammonium sulfate as a function of particle mobility diameter showing no systematic size dependence. The average effective densities are also listed in g cm− 3. The solid line represents the average effective density of pure ammonium sulfate particles.
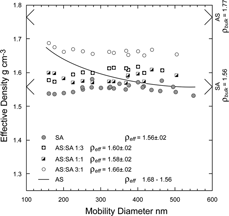
TABLE 5 Effective densities and dynamic shape factors for SA and SA/AS mixed particles
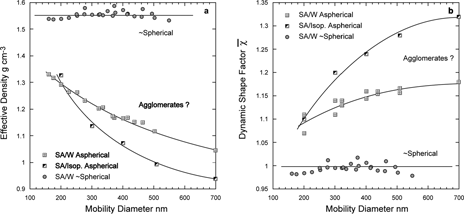
The other data points in and entries in provide the results of measurements on internally mixed AS:SA particles. Also shown in is a line representing the average measured effective density of pure ammonium sulfate particles presented in the section above. Unlike ammonium sulfate, SA and AS:SA particles show no systematic size dependence. The observed effective densities of the internally mixed particles can be compared with the calculated densities of the binary mixtures assuming a simple additive rule is applicable. The same binary mixture densities were used to calculate corresponding DSFs. All measured effective densities, line shapes, and derived DSFs suggest that the AS:SA particles are slightly more spherical than pure ammonium sulfate particles and that the dependence of the DSF on size is diminished.
As in the case of NaCl it is possible to produce SA particles of more than one type. Under drying conditions similar to those that tend to produce NaCl agglomerates we observe succinic acid particles with significantly lower effective densities and higher DSF. Similar results are observed when SA particles are produced from a solution of isopropyl alcohol instead of water. and show the derived effective densities and shape factors for all types of SA particles we observed.
FIG. 22 Size dependence of the derived effective densities (a) and DSFs (b) plotted as a function of succinic acid particle mobility diameter. Three distinct types of succinic acid particles are evident: Nearly spherical, and two types of aspherical particles generated from water, SA/W, and isopropyl alcohol, SA/IP.
Since we do not have microscopic evidence for this system, it is not possible to definitively conclude that these larger DSF particles are agglomerates. However, the derived DSFs are similar to those derived for the compact agglomerates of PSL spheres. The larger shape factors for succinic acid particles generated from isopropyl alcohol solution as compared with those from water are consistent with the lower solubility of SA in IP that in turn would produces smaller primary particles and hence higher agglomerate for the same size particle.
It might also be possible to explain the data shown in by considering the fact that macroscopic succinic acid crystals grown from water have plate like shapes and those grown from isopropyl alcohol are needle shaped (CitationDavey and Garside 2000; CitationVan der Voort 1991). The derived DSF shown in are consistent with these particle shapes as well.
For lauric acid we present results only for 300 nm mobility diameter particles, which we summarize in . The observed effective density of pure LA particles was in perfect agreement with the bulk density suggesting that like succinic acid, in small LA particles are nearly spherical. When mixed with ammonium sulfate at a ratio of 20:80 (AS:LA) the effective density is only slightly more than 10% lower than what would be calculated on the basis of a simple additive rule. However, the effective density of the ammonium sulfate-rich, 80:20 mixture is 25% lower than the calculated density. On the basis of these data alone it is not possible to distinguish between internally mixed AS:LA particles with a density of 1.2 g cm− 3 that are nearly spherical and aspherical particles whose density is 1.59 g cm− 3 and a shape factor of 1.17.
TABLE 6 Effective densities and dynamic shape factors for LA and AS/LA mixed particles
To conclude this section, the very limited data on organic particles suggest that they tend to be nearly spherical unless agglomerated. When neither shape nor density are known the limited information available from the two measured diameters, d va and d b , does not provide the means to distinguish between a nearly spherical particle of low density and an aspherical particle of high density, making the interpretation of effective density in terms of mixing ratios unreliable.
CONCLUSION
We presented a study of the effect of asphericity on two quantifiable particle diameters, d va and d b , and on the relationship between them. We used the model system of the PSL spheres agglomerates, for which shape, density and volume equivalent diameters are all known, to develop a fundamental understanding of the information content in these measured quantities. Next we applied the SPLAT/DMA system to irregularly shaped particles, but with known density. And we concluded the study with measurements of particles of unknown shape and density.
Using the DMA to measure DSFs of PSL spheres agglomerates in the transition regime proved that alignment effects in the DMA electric field play an important role. Consequently the measured DSFs for the doublets and triplets were found to depend on particle size, because of the sensitivity of charge separation to particle size and the voltage in the DMA. Our observations were found to be excellent agreement with other studies.
Taking advantage of the DMA capacity to isolate one of the agglomerates at a time made it possible for the first time measure the DSFs of PSL spheres agglomerates in the free molecular regime. We find perfect agreement between the predicted DSFs for the doublets and those measured here. Applying the same formulation, which was developed for straight chains, to compact triplets and quadruplets is somewhat less successful. Aided by microscopy the DSFs for higher compact agglomerates were measured as well. A comparison between χ t,θ and χ v reveals that alignment and pressure can play an important role in determining the observed d va and d b . For the larger compact agglomerates these effects seem to diminish and χ v ≅ χ t,θ.
The high sizing resolution of the SPLAT/DMA system (CitationZelenyuk et al. 2005) has made it possible to establish an unambiguous relationship between asphericity and the line shape of the d va distribution, with the general rule being that asphericity causes broadening of the d va distribution. For spheres agglomerates this phenomenon correlates with an orientation dependent DSF.
For systems with known density but unknown shape,—unknown volume equivalent diameter, it is not possible to extract a DSF from the measured d
va
and d
b
alone. Here we apply a simplifying approximation in which we set χ
v
= χ
t,θ = , making it possible to derive a DSF. Using the data for the PSL spheres agglomerates we showed empirically, that
is approximately equal to the average of χ
t,θ and χ
v
. It is important to keep in mind that the average DSFs, while well defined and clearly related to particle shape can be affected by alignment in the DMA. We presented an application of this approach to ammonium sulfate, sodium chloride, succinic acid, and lauric acid particles. Based on what we now know about the shape of particles composed of ammonium sulfate, sodium chloride, and the organics there is a good reason to expect that alignment, in the DMA, should not play a significant role for these systems. This would be true of course as long as no agglomeration takes place.
For the smaller ammonium sulfate particles we find = 1.03, in agreement with mildly aspherical particles. However, our data indicate a slight but systematic increase of the DSF with particle size.
Three types of NaCl particles were generated and characterized: (1) Nearly spherical particles with shape factor of ∼ 1.02; (2) Cubes, with size dependent DSFs increasing from 1.065 to 1.17 as particle size increases from 160 nm to 900 nm. Cubic particles with lower than 1.08 shape factors had rounded edges and cubic particles with higher shape factors were associated with larger fraction of rectangular prisms; (3) Agglomerates of NaCl, with DSFs of 1.3 to 1.4, which were generated by coagulation.
SA and LA particles were found to be nearly spherical, although here as well, under some preparations SA particles with higher DSFs were produced. These were tentatively explained by the formation of agglomerates.
The density and shape of internally mixed particles are unknown. Based on the line width we speculate that AS:SA particles are nearly spherical and the densities of the internally mixed SA/AS particles are reasonably close to the average density of the two compounds. In contrast the effective density of particles with 80:20 (AS:LA) composition was found to be significantly lower than that predicted on the basis of a simple average. Here it is not possible to distinguish between nearly spherical particles with lower density or dense particles but with a shape factor of 1.17.
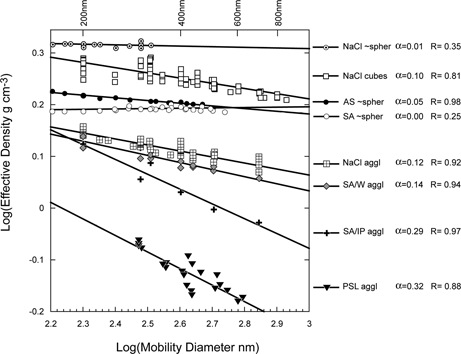
and summarize the entire dataset; the first figure accentuates the differences between the systems, while the second illustrates their similarities. is a plot of log(ρ eff ) vs. log(d b ). In this representation the data segregate by slopes into three types: (1) nearly spherical particles with slopes < 0.05 that include AS, SA, SA:AS mixtures, and nearly spherical NaCl; (2) NaCl cubes, NaCl agglomerates, and SA/W agglomerates with slopes of 0.10 to 0.14; (3) Agglomerates of PSL spheres and SA/IP agglomerates with a slope of ∼ 0.3.
FIG. 23 A plot of log(ρ eff ) versus log(d b ) summarizes nearly all the data presented in this paper. The data segregate into three groups: nearly spherical particles, AS, SA, and NaCl (the SA/AS mixed particles, not shown, fit into this group) with a slope < 0.05; NaCl cubes, NaCl agglomerates and aspherical SA particles generated from water with slopes between 0.1 and 0.14; and PSL agglomerates and aspherical SA particles generated from isopropyl alcohol with slopes ∼ 0.3.
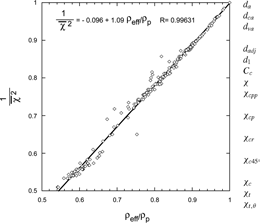
puts the entire dataset on the same footing in a plot of 1/χ2 vs. the normalized effective density (ρ eff /ρ p ). In this presentation the entire dataset can be fit with a straight line that approaches 1 when the DSF approaches 1. The simple straight line formula given in the and in Equation (Equation11) provides convenient means for calculating an approximate DSF from the ratio d va /d b for this range.
NOMENCLATURE
| λ | = |
mean free path of gas molecules |
| Kn | = |
Knudsen number = 2λ/d |
| d | = |
particle diameter |
| d ve | = |
volume equivalent diameter |
| d b | = |
electrical mobility diameter |
| d a | = |
aerodynamic diameter in any flow regime |
| d ca | = |
continuum regime aerodynamic diameter |
| d va | = |
vacuum aerodynamic diameter or free-molecular regime aerodynamic diameter |
| d adj | = |
adjusted sphere diameter |
| d 1 | = |
primary particle diameter |
| C c | = |
Cunningham slip correction factor |
| χ | = |
dynamic shape factor in any flow regime |
| χ cpp | = |
dynamic shape factor in the continuum regime for perpendicular orientation |
| χ cp | = |
dynamic shape factor in the continuum regime for parallel orientation |
| χ cr | = |
dynamic shape factor in the continuum regime for random orientation |
| χ c45ˆ | = |
dynamic shape factor in the continuum regime for 45ˆ orientation |
| χ c | = |
dynamic shape factor in continuum regime |
| χ t | = |
dynamic shape factor in transition regime |
| χ t,θ | = |
operationally defined dynamic shape factor as derived from the DMA operated in the transition regime, at 298 K and 1 Atm and a flow dependent voltage that orients particles at angle θ. |
| χ v | = |
dynamic shape factor in vacuum or free molecular regime |
| ρ0 | = |
standard density (1 g cm− 3) |
| ρ eff | = |
effective or “apparent” density |
| δ | = |
parameter related to the fraction of internal void spaces |
Acknowledgments
We thank Dr. Alexander Laskin for his help with the Scanning Electron Microscope.
This research was performed in the Environmental Molecular Sciences Laboratory, a national scientific user facility sponsored by the Department of Energy's Office of Biological and Environmental Research at Pacific Northwest National Laboratory (PNNL). PNNL is operated by the US Department of Energy by Battelle Memorial Institute under contract No. DE-AC06-76RL0 1830.
This work was supported by the U.S. Department of Energy Office of Basic Energy Sciences, Chemical Sciences Division.
Notes
a Calculated using ψ = d adj /d 1 = 1.262 and χ c45ˆ = 1.08 (CitationKousaka et al. 1996).
b Calculated using ψ = d adj /d 1 = 1.21 and χ cp = 1.025 (CitationCheng et al. 1988).
c Appears to be between 45ˆ and randomly oriented.
a Calculated using ψ = d adj /d 1 = 1.456 and χ c45ˆ = 1.115 (CitationKousaka et al. 1996).
b Calculated using ψ = d adj /d 1 = 1.41 and χ cp = 1.087 (average of CitationCheng et al. 1988; CitationHorvath 1974).
c Appears to be between 45ˆ and randomly oriented.
c, b Average of CitationCheng et al. (1988) and CitationHorvath (1974).
d Calculated from equation χ = χ c C c (d ve )/C c (d adj ) and following the equation from CitationHappel and Brenner (1965) 1/χ vr = (1/3χ vp + 23χ vpp ).
e Observed in this study.
a Calculated using Equation (Equation10) and the densities listed in the table.
b Calculated assuming simple average.
c The reported values for the density of SA vary between 1.55 and 1.57 g cm− 3. Here we use 1.56 g cm− 3.
d Tentatively assigned to agglomerates.
a Calculated using Equation (Equation10) and the densities listed in the table.
b Calculated assuming simple average.
REFERENCES
- Allen , M. D. and Raabe , O. G. 1982 . Re-Evaluation of Millikan Oil Drop Data for the Motion of Small Particles in Air . J. Aerosol Sci. , 13 ( 6 ) : 537 – 547 . [CSA]
- Allen , M. D. and Raabe , O. G. 1985a . Slip Correction Measurements of Spherical Solid Aerosol-Particles in an Improved Millikan Apparatus . Aerosol Sci. Technol. , 4 ( 3 ) : 269 – 286 . [CSA]
- Allen , M. D. and Raabe , O. G. 1985b . Slip Correction Measurements for Aerosol Particles of Doublet and Triangular Triplet Aggregates of Spheres . J. Aerosol Sci , 16 ( 1 ) : 57 – 67 . [CROSSREF] [CSA]
- Baron , P. A. and Willeke , K. 2001 . “ Gas and Particle Motion ” . In Aerosol Measurement: Principles, Techniques, and Applications , Edited by: Baron , P. A. and Willeke , K. pp. 61 – 97 . New York : Wiley .
- Buzorius , G. , Zelenyuk , A. , Brechtel , F. and Imre , D. 2002 . Simultaneous Determination of Individual Ambient Particle Size, Hygroscopicity and Composition . Geophys. Res. Lett. , 29 ( 20 ) : 1974 [CROSSREF] [CSA]
- Chan , P. and Dahneke , B. 1981 . Free-Molecule Drag on Straight Chains of Uniform Spheres . J. Appl. Phy. , 52 ( 5 ) : 3106 – 3110 . [CROSSREF] [CSA]
- Chen , B. T. , Irwin , R. , Cheng , Y. S. , Hoover , M. D. and Yeh , H. C. 1993 . Aerodynamic Behavior of Fiber-Like and Disk-Like Particles in a Millikan Cell Apparatus . J. Aerosol Sci. , 24 ( 2 ) : 181 – 195 . [CROSSREF] [CSA]
- Cheng , Y. S. 1991 . Drag Forces on Nonspherical Aerosol-Particles . Chem. Eng. Comm. , 108 : 201 – 223 . [CSA]
- Cheng , Y. S. , Allen , M. D. , Gallegos , D. P. , Yeh , H.-C. and Peterson , K. 1988 . Drag Force and Slip correction of Aggregate Aerosols . Aerosol Sci. Technol. , 8 ( 3 ) : 199 – 214 . [CSA]
- Cheng , Y. S. , Chen , B. T. , Yeh , H.-C. , Marshall , I. A. , Mitchell , J. P. and Griffiths , W. D. 1993 . Behavior of Compact Nonspherical Particles in the TSI Aerodynamic Particle Sizer Model APS33B: Ultra-Stokesian Drag Forces . Aerosol Sci. Technol. , 19 ( 3 ) : 255 – 267 . [CSA]
- Dahneke , B. 1982 . Viscous Resistance of Straight-Chain Aggregates of Uniform Spheres . Aerosol Sci. Technol. , 1 ( 2 ) : 179 – 185 . [CSA]
- Dahneke , B. E. 1973a . Slip Correction Factors for Nonspherical Bodies-I: Introduction and Continuum Flow . J. Aerosol Sci. , 4 : 139 – 145 . [CROSSREF] [CSA]
- Dahneke , B. E. 1973b . Slip Correction Factors for Nonspherical Bodies—II: Free Molecule Flow . J. Aerosol Sci. , 4 : 147 – 161 . [CROSSREF] [CSA]
- Dahneke , B. E. 1973c . Slip Correction Factors for Nonspherical Bodies—III: The Form of the General Law . J. Aerosol Sci. , 4 : 163 – 170 . [CROSSREF] [CSA]
- Davey , R. J. and Garside , J. 2000 . From Molecules to Crystallizer: An Introduction to Crystallization , Oxford University Press .
- DeCarlo , P. F. , Slowik , J. G. , Worsnop , D. R. , Davidovits , P. and Jimenez , J. L. 2004 . Particle Morphology and Density Characterization by Combined Mobility and Aerodynamic Diameter Measurements. Part 1: Theory . Aerosol Sci. Technol. , 38 : 1185 – 1205 . [CROSSREF] [CSA]
- Dick , W. D. , Ziemann , P. J. , Huang , P.-F. and McMurry , P. H. 1998 . Optical Shape Fraction Measurements of Submicrometre Laboratory and Atmospheric Aerosols . Meas. Sci. Technol. , 9 : 183 – 196 . [CROSSREF] [CSA]
- Hansson , H. C. and Ahlberg , M. S. 1985 . Dynamic Shape Factors of Sphere Aggregates in an Electric-Field and their Dependence on the Knudsen Number . J. Aerosol Sci. , 16 ( 1 ) : 69 – 79 . [CROSSREF] [CSA]
- Happel , J. and Brenner , H. 1965 . Low Reynolds Number Hydrodynamics , pp. 182 – 207 . Englewood Cliffs, NJ : Prentice Hall .
- Hinds , W. C. 1999 . Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles , New York : Wiley .
- Hochrainer , D. and Hänel , G. 1975 . Der Dynamische Formfaktor Nicht-Kugelförmiger Teilchen als Funktion des Luftdrucks . J. Aerosol Sci. , 6 : 97 – 103 . [CROSSREF] [CSA]
- Horvath , H. 1974 . The Sedimentation Behavior of Non-Spherical Particles . Staub Reinhaltung Reinhaltung der Luft (in English) , 34 : 197 – 202 . [CSA]
- Horvath , H. 1979 . Method for the Determination of Dynamic Shape Factors of Sphere Aggregates by Measuring the Sedimentation Velocity in a Capacitor . J. Aerosol Sci. , 10 ( 3 ) : 309 – 315 . [CROSSREF] [CSA]
- Huffman , J. A. , Jayne , J. T. , Drewnick , F. , Aiken , A. C. , Onasch , T. , Worsnop , D. R. and Jimenez , J. L. 2005 . Design, Modeling, Optimization, and Experimental Tests of a Particle Beam Width Probe for the Aerodyne Aerosol Mass Spectrometer , Aerosol Science and Technology . Submitted to
- Jimenez , J. L. , Bahreini , R. , Cocker , D. R. , Zhuang , H. , Varutbangkul , V. , Flagan , R. C. , Seinfeld , J. H. , O'Dowd , C. D. and Hoffmann , T. 2003 . New Particle Formation from Photooxidation of Diiodomethane (CH2I2) . J. Geophys. Res.—Atmos. , 108 ( D10 ) : 4318 [CROSSREF] [CSA]
- Kasper , G. and Berner , A. 1978 . Generator for production of Extreme Monodisperse Sodium Chloride Aerosols . Staub Reinhaltung der Luft , 38 ( 5 ) : 183 – 186 . [CSA]
- Katrib , Y. , Martin , S. T. , Rudich , Y. , Davidovits , P. , Jayne , J. T. and Worsnop , D. R. 2005 . Density Changes of Aerosol Particles as a Result of Chemical Reaction . Atmos. Chem. Phys. , 5 : 275 – 291 . [CSA]
- Kousaka , Y. , Endo , Y. , Ichitsubo , H. and Alonso , M. 1996 . Orientation-Specific Dynamic Shape Factors for Doublets and Triplets of Spheres in the Transition Regime . Aerosol Sci. Technol. , 24 ( 1 ) : 36 – 44 . [CSA]
- Krämer , L. , Pöschl , U. and Niessner , R. 2000 . Microstructural Rearrangement of Sodium Chloride Condensation Aerosol Particles on Interaction with Water Vapor . J. Aerosol Sci. , 31 ( 6 ) : 673 – 685 . [CROSSREF] [CSA]
- Kreidenweis , S. M. , Koehler , K. , DeMott , P. , Prenni , A. J. , Carrico , C. and Ervens , B . 2005 . Water Activity and Activation Diameters from Hygroscopicity data—Part I: Theory and Application to Inorganic Salts . Atmos. Chem. Phys. Discuss. , 5 : 287 – 323 . [CSA]
- Laskin , A. , Cowin , J. P. and Jedema , M. J. 2005 . Off-Line Analysis of Individual Environmental Particles using Modern Methods of Electron Microscopy and X-ray Microanalysis . Submitted to J. Electr. Spectr. , [CSA]
- Li , J. , Posfai , M. , Hobbs , P. V. and Buseck , P. R. 2003 . Individual Aerosol Particles from Biomass Burning in Southern Africa: 2. Compositions and Aging of Inorganic Particles . J. Geophys. Res. , 108 ( D13 ) : 8484 doi:10.1029/2002JD002310[CROSSREF] [CSA]
- Mikhailov , E. , Vlasenko , S. , Niessner , R. and Pöschl , U. 2004 . Interaction of Aerosol Particles Composed of Protein and Salts with Water Vapor: Hygroscopic Growth and Microstructural Rearrangement . Atmos. Chem. Phys. , 4 : 323 – 350 . [CSA]
- Murphy , D. M. , Cziczo , D. J. , Hudson , P. K. , Schein , M. E. and Thomson , D. S. 2004 . Particle Density Inferred from Simultaneous Optical and Aerodynamic Diameters Sorted by Composition . J. Aerosol Sci. , 35 ( 1 ) : 135 – 139 . [CROSSREF] [CSA]
- Perry , R. J. , Hunt , A. J. and Huffman , D. R. 1978 . Experimental Determinations of Mueller Scattering Matrices for Nonspherical Particles . Appl. Optics , 17 ( 17 ) : 2700 – 2710 . [CSA]
- Rader , D. J. 1990 . Momentum Slip Correction Factor for Small Particles in 9 Common Gases . J. Aerosol Sci. , 21 ( 2 ) : 161 – 168 . [CROSSREF] [CSA]
- Scheibel , H. G. and Porstendörfer , J. 1983 . Generation of Monodisperse Ag Aerosol and NaCl Aerosol with Particle Diameters between 2 nm and 300 nm . J. Aerosol Sci. , 14 ( 2 ) : 113 [CROSSREF] [CSA]
- Scheuch , G. and Heyder , J. 1986 . Diffusional Losses of Nonspherical Particles in tubes . J. Aerosol Sci. , 17 ( 3 ) : 432 – 435 . [CROSSREF] [CSA]
- Schreiner , J. , Schild , U. , Voigt , C. and Mauersberger , K. 1999 . Focusing of Aerosols into a Particle Beam at Pressures from 10 to 150 Torr . Aerosol Sci. Technol. , 31 ( 5 ) : 373 – 382 . [CROSSREF] [CSA]
- Slowik , J. G. , Stainken , K. , Davidovits , P. , Williams , L. R. , Jayne , J. T. , Kolb , C. E. , Worsnop , D. R. , Rudich , Y. , DeCarlo , P. F. and Jimenez , J. L. 2004 . Particle Morphology and Density Characterization by Combined Mobility and Aerodynamic Diameter Measurements. Part 2: Application to Combustion Generated Soot Aerosols as a Function of Fuel Equivalence Ratio . Aerosol Sci. Technol. , 38 : 1206 – 1222 . [CROSSREF] [CSA]
- Stöber , W. and Flachsbart , H. 1969 . Size-Separating Precipitation of Aerosols in a Spinning Spiral Duct . Environ. Sci. Technol. , 3 ( 12 ) : 1280 – 1296 . [CROSSREF] [CSA]
- Van , d er and Voort , E. 1991 . The Morphology of Succinic Acid Crystals: The Role of Solvent Interaction . J. Crystal Growth , 110 ( 4 ) : 662 – 668 . [CROSSREF] [CSA]
- Zelenyuk , A. and Imre , D. 2005 . Single Particle Laser Ablation Time-of-Flight Mass Spectrometer: An Introduction to SPLAT . Aerosol Sci. Technol. , 39 ( 6 ) : 554 – 568 . [CSA]
- Zelenyuk , A. , Cai , Y. , Chieffo , L. and Imre , D. 2005 . High Precision Density Measurements of Single Particles: The Density of Metastable Phases . Aerosol Sci. Technol. , 39 ( 10 ) : 972 – 986 . [CROSSREF] [CSA]
- Zhang , X. F. , Smith , K. A. , Worsnop , D. R. , Jimenez , J. , Jayne , J. T. and Kolb , C. E. 2002 . A Numerical Characterization of Particle Beam Collimation by an Aerodynamic Lens—Nozzle System: Part I. An Individual Lens or Nozzle . Aerosol Sci. Technol. , 36 ( 5 ) : 617 – 631 . [CROSSREF] [CSA]