An intensive sampling campaign was performed in Fresno, CA during December 2003 measuring fine particulate matter including both the semi-volatile and nonvolatile fractions of the aerosol. Both the newly developed R&P FDMS Monitor and a PC-BOSS have been shown to measure total PM 2.5 concentrations including semi-volatile nitrate and organic material. Good agreement was observed between the PC-BOSS and the R&P FDMS Monitor in this study with linear regression analysis resulting in a zero-intercept slope of 1.00 ± 0.02 and an R 2 = 0.93. Several real-time measuring systems including the R&P Differential TEOM, the Met One BAMS, and a GRIMM Monitor were also employed and comparisons of total PM 2.5 mass were made with the R&P FDMS Monitor. Agreement among these various monitors was generally good. However, differences were sometimes seen. Reasons for observed differences in the real-time mass measurement systems are explained by the composition and complexity of the measured aerosol, most importantly the composition of semi-volatile material. A newly automated ion chromatographic system developed by Dionex was also field tested and compared to both R&P 8400N Nitrate and integrated PC-BOSS inorganic species measurements. Sulfate and nitrate determined by the Dionex and PC-BOSS systems agreed. However, nitrate measured by the 8400N was low during fog events compared to the other two systems.
INTRODUCTION
Exposure to fine particulate matter (PM2.5, particles with an aerodynamic diameter less than 2.5 μ m) has been implicated as a contributor to adverse human health effects including increases in cardiovascular and pulmonary disease, which leads to elevated human mortality and morbidity (CitationPope 2000; CitationSchwartz 1996; CitationU.S. EPA 2002). PM2.5 has also been associated with visibility degradation in urban (CitationWatson 2002; CitationEatough et al. 2003) and pristine environments (CitationWatson 2002; CitationEatough et al. 1996) and contributes to changes in the global radiative balance (CitationChung and Seinfeld 2002; CitationConant et al. 2003). The exacerbation of observed health problems is believed to be associated more closely with exposure to fine particles, especially those generated by combustion, than coarse particles. Adverse human health effects have been observed at concentrations substantially below the U.S. PM10 national ambient air quality standards (NAAQS). As a result, in 1997 the U.S. Environmental Protection Agency (EPA) promulgated revised standards for PM, which established new annual and 24-hour PM2.5 NAAQS. The PM2.5 Federal Reference Method (FRM), based on the collection of PM2.5 on a single filter, is used as the indicator for PM2.5 mass measurement compliance in monitoring networks (Musick 1999; CitationSchaefer et al. 1997). This recognition of fine and coarse particles as different classes of PM was an advancement in the understanding and control of PM (CitationChow 1995; CitationWilson and Shuh 1997). Ambient PM2.5 is not a single pollutant but is composed of both stable and semi-volatile species. Stable species include trace metals (including toxic, crustal, and transition metals), elemental carbon, and sulfate. Some species such as ammonium nitrate and lower molecular weight organic species can be semi-volatile in nature, existing in dynamic equilibrium between the gas and particle phases.
Single filter methods, such as the PM2.5 FRM, can accurately measure stable species such as sulfate, and trace and crustal elements (Musick 1999) but cannot accurately determine semi-volatile fine particulate species such as ammonium nitrate and semi-volatile organic material (CitationEatough et al. 2003; CitationHering and Cass 1995). Identification of the component(s) of fine particles responsible for the epidemiologically identified health effects would significantly aid implementation of the new PM2.5 standard. New research is focusing on the improved characterization of urban PM2.5.
Several integrated samplers have been developed that accurately determine nonvolatile material and semi-volatile material (SVM) concentrations including a modified speciation sampler (CitationCarter et al. 2002) and the Particle Concentrator-Brigham Young University Organic Sampling System, PC-BOSS, (CitationTang et al. 1994; CitationSioutas et al. 1994; CitationDing et al. 2002a; CitationModey et al. 2001, Citation2002; CitationDing et al. 2002b; CitationLewtas et al. 2001). Although these samplers can accurately speciate PM2.5, including SVM, there are several drawbacks. Integrated samplers are very labor and cost intensive. Collection of filter media and in-lab analysis are time consuming and expensive, resulting in data interpretation weeks and months from the time of collection. The potential for sample contamination is increased with collection, transport, and laboratory analysis. Furthermore, 1 h time resolved data is often not possible with integrated samplers which inhibits the ability to temporally resolve short term changes in pollution levels that often occur in urban environments.
To overcome these problems, the development of real-time or near real-time instruments has been attempted. One of the most universally used real-time PM2.5 measurement techniques is the Tapered Element Oscillating Microbalance (TEOM) developed by Rupprecht & Patashnick Co., Inc. Under some conditions the TEOM does not accurately determine total PM2.5 mass because the particle collection filter is heated to 30–50°C to remove particle bound water which also results in loss of SVM. The Real-Time Total Ambient Mass Sampler (RAMS) and more recently the Rupprecht & Patashnick Filter Dynamic Measuring System (FDMS), have been developed to measure total PM2.5 mass, including SVM. Real-time instruments have several advantages including, reliability, cost effectiveness, ease of sampling, and reduction in labor requirements. One of the main advantages of real-time instruments is the ability to temporally resolve short term episodes of PM2.5 that occur in urban environments. One-hour real-time data has also been shown to increase the performance (i.e., reduce uncertainty) of source apportionment techniques to determine sources, both primary and secondary, of urban PM (CitationGrover et al. 2006).
The application of these samplers to the study of atmospheric chemistry in urban environments has shown that a substantial fraction of the fine particulate matter (PM) in these environments is semi-volatile organic and nitrate material (SVM) (CitationEatough et al. 2001; CitationLong et al. 2002, Citation2003). Furthermore, these studies have shown that the majority of the semi-volatile organic material (SVOM) is secondary (CitationEatough et al. 2003). Because a significant portion of PM2.5 has been shown to be semi-volatile and SVM may be important in cardiovascular human health effects (CitationPope et al. 2000, Citation2004), the development of instruments to accurately determine all components of urban PM2.5 is important.
An intensive research campaign was conducted in Fresno, CA to determine most of the major components of ambient PM2.5 including both the nonvolatile and semi-volatile organic and inorganic fractions of the urban aerosol. Inter-comparisons were made between several real-time measurement systems and one integrated method.
EXPERIMENTAL
Sampling Site
The sampling site was located in the parking area of the Veterans Hall Federal Post 509 adjacent to the Fresno, CA EPA Supersite (CitationWatson et al. 2000) operated by the California Air Resource Board (CARB). This site is centrally located in the major Fresno metropolitan area and approximately 0.8 km east of California highway 41. Fresno is located in the central San Joaquin valley which is home to an estimated 3.2 million residents. Fresno is an urban area consisting of approximately 850,000 residents according to U.S. Census Bureau estimates in 2003 (http://www.census.gov; http://www.census.gov). It is located on the valley floor approximately 100 meters above sea level and is surrounded by the Sierra Nevada mountain range on the east and coastal mountains to the west. These geographical conditions abet the development of high urban pollution aerosol concentrations which often occur in the Fresno area.
During the winter months, Fresno is often impacted by strong inversion layers resulting in time periods of high PM2.5 concentrations as well as other pollutants (CitationWatson and Chow 2002). Mobile emission sources of particulate pollution as well as wood combustion from wood burning stoves contribute to PM2.5 concentrations during the winter months. High humidity often results in persistent low altitude fog during inversion episodes resulting in a highly water saturated aerosol.
Sampling Methods
Real-Time PM 2.5 Samplers
The various samplers used to monitor PM2.5 included samplers to determine mass and chemical components. Each of the samplers used in this study is detailed in the following sections.
R&P TEOM Monitor
Hourly data from two TEOMs were obtained during the study including a BYU TEOM operating at 30°C and the CARB TEOM operating at 50°C (CitationPatashnick and Rupprecht 1991). Semi-volatile PM is not retained on a conventional TX40 filter at these elevated temperatures, which is required to remove particle-bound water (CitationMignacca and Stubbs 1999). With the elevated TEOM filter temperature, this technique measures only non-volatile PM.
R&P FDMS 8500 Unit
This system is designed to measure total PM2.5 mass including both the non-volatile and semi-volatile fractions using TEOM technology. Ambient air is sampled constantly and passes through a Sample Equilibration System (SES) diffusion Nafion dryer to remove particle bound water. The main flow is subsequently split into a base flow and a reference flow every six minutes using a switching valve. The base flow is sampled directly through a TX40 filter on a TEOM microbalance providing a direct measurement of PM deposited on the filter. After six minutes, the flow is directed to a reference flow in which the air stream is sampled through a 47 mm quartz purge filter, maintained at 4°C, to remove particles in the sampled aerosol. The quartz purge filter is maintained at 4°C to minimize SVM loss from the purge filter and to provide an integrated sample that can be used for subsequent chemical analysis. The reference flow is then directed to the TX40 filter in the TEOM mass sensor unit providing a direct mass measurement of particle-free aerosol. The reference flow mass measurement is used to adjust the mass concentration of the base flow measurement. If particles collected on the TX40 filter during the base flow contain SVM, this SVM will be lost from the TX40 filter over the measurement period following volatilization dynamics. The 6-min particle-free reference flow allows sufficient time for the rate of evaporative losses of SVM to be measured, and results in a measurement of the amount of SVM present in the aerosol. For example, if a negative mass is measured during the reference flow cycle, due to loss of SVM from the TX40 filter, this mass is added to the mass measurement made during the initial base flow (particle-laden) measurement to obtain a measurement of total PM2.5 concentration. Two FDMS TEOMs were used in this study. In each unit the TEOM TX40 filter was maintained at 30°C.
R&P Differential TEOM
The Differential TEOM is similar in method to the R&P FDMS 8500 unit and was developed primarily as a research instrument. An electrostatic precipitator (CitationMeyer et al. 2002; CitationYi et al. 2004) is used in place of the chilled filter (R&P FDMS) to remove particles during the 6 minute reference purge cycle. Similar to the FDMS, during the purge cycle SVM is lost from the TX40 collection filter and measured as a negative mass. This measured negative mass is than added back on to the mass measured during the collection cycle to determine total PM2.5 concentrations. Two Differential TEOMs were employed in this study.
GRIMM Model 1100 Monitor
This unit uses a semiconductor laser as a light source to monitor light scattering of single particles. An internal volume controlled pump is used to sample the ambient aerosol at a rate of 1.2 L/min. This pump also is used to generate a clean sheath air which is filtered and subsequently passed through a sheath air regulator to the optical chamber. The sheath air is used: (1) to prevent dust contamination in the laser-optic assembly and (2) as a reference zero test during the auto-calibration procedure. As single particles pass through the laser beam in the optical chamber, light scattering occurs and is culminated by a mirror located approximately 90 degrees from the laser source and subsequently measured by a recipient diode. The signal of the diode is recorded with a multi-channel size classifier. A pulse height analyzer then classifies the transmitted signal in each channel which is sent to the data storage card for analysis. Conversion from the measured particle number to volume and volume to mass distribution is done using protocols developed by GRIMM Technologies, Inc.
The conventional GRIMM monitor measures particles at ambient temperature. A collocated GRIMM monitor was also applied in this study which used an inlet equipped with a heater operated at 80°C, resulting in a temperature of 50°C in the laser measured aerosol, for comparison with heated TEOM mass data.
Met One Instruments Beta Attenuation Mass (BAM) Monitor
In the BAM, particles are deposited on a continuous glass fiber type tape. A C14 beta source and a beta detector are used to measure beta ray attenuation following the deposition of aerosol PM on the glass-fiber filter tape. Prior to sample collection, baseline beta attenuation values are obtained. Subsequently, beta attenuation is re-measured following the collection of PM on the filter tape to determine mass concentrations of PM2.5. An automated advanced microprocessor system is used to obtain semi-continuous measurements by advancing the filter tape following each sampling period and drift is avoided because the baseline beta attenuation is measured before each sampling period (Chung et al. 2001; Jaklevic et al. 1981).
Dionex GP-IC: Fine Particulate Ammonium, Sulfate, and Nitrate
The development of instrumentation for the measurement of atmospheric inorganic particulate composition has been recently reviewed (CitationDasgupta and Poruthoor 2002). The instrument used in this study is similar to that previously described and has recently been commercialized by Dionex Corporation. The air sample is first passed through a cyclone with a 50% cutpoint at 2.5 μ m flowing at 5 L/min and then proceeds through a parallel plate wet denuder using 0.5 mM hydrogen peroxide as the scrubber liquid; this removes soluble gases, notably sulfur dioxide, nitric acid, and ammonia (CitationBoring et al. 2002). Air exiting the denuder enters the annular channel of a concentric nozzle, deionized water is pumped into the center tube using a peristalic pump. The liquid generates a spray that attaches to the aerosol particles. The flow is ultimately drawn out through a 0.5 μ m pore size PTFE filter. Liquid droplets coalesce and fall below. The liquid is aspirated by a peristalic pump and sent to a Dionex TAC-ULP preconcentration column of an ion chromatograph (IC) for anion analysis and a Dionex TCC-ULP preconcentration column for cation analysis. Anion analysis is performed using an IonPac AG11-HC guard column and an IonPac AS11-AC column with 15 minute chromatographic cycles largely following protocols described previously (CitationDasgupta and Poruthoor 2002; CitationAl-Horr et al. 2003). The system can collect particles down to 100 nm aerodynamic diameter with high efficiency. This technology has been field tested in Philadelphia, PA in the summer of 2001 and in Tampa, FL in the summer of 2002. The recently commercialized version of this instrument, the Dionex GP-IC, was field tested for the first time during this study.
R&P Series 8400N Nitrate Monitor
In this method, the aerosol is sampled through a 2.5 μ m cyclone inlet, passed through a charcoal honeycomb denuder to remove interfering nitric acid, humidified in a Nafion® tube to increase collection efficiency, and collected on a nickel-chrome impaction strip. Following collection, the sample is flash vaporized and nitrate concentrations are measured by a NOx pulse analyzer (CitationStolzenburg and Hering 2000).
R&P Series 8400S Sulfate Monitor
This system operates under a similar flash vaporization technology to that used in the R&P Nitrate monitor. The aerosol is drawn first through a 2.5 μ m cyclone and then passes through an activated carbon denuder to remove interfering gases. A Nafion® humidifying system is used to humidify the particle-laden aerosol to increase collection efficiency of PM on the impactor. Following collection, flash vaporization of the aerosol particles with temperature exceeding 600°C occurs producing SO2 which is subsequently measured using a SO2-pulsed fluorescence sensor. Due to problems associated with this instrument, data were only obtained during the initial 10 days of the sampling period.
R&P Series 5400 Ambient Carbon Particulate Monitor
This instrument performs a thermal-CO2 analysis to determine hourly carbon concentrations present in a sample collected on an impactor. During the analysis phase the collected sample is first heated to 375°C to determine OC and then to 750°C to determine total carbon (TC) concentrations. EC is determined by the difference between the TC and the OC measurements. The low temperature evolved carbon was assumed to be organic material. A conversion factor of 1.6 was used to convert carbon to organic material which is typical of an aged urban aerosol (CitationTurpin and Lim 2001). Carbon concentrations using the R&P 5400 were obtained by both an instrument operated by Brigham Young University and one operated by the EPA Fresno Supersite.
Sunset Lab Carbon Monitor
This instrument is a semi-continuous thermal/optical transmission (TOT) method for the measurement of particulate carbon. A 2.5 μ m sharp-cut cyclone inlet (R&P) is used with a total flow of 16 L/min. Eight L/min is directed to the carbon instrument and the remaining 8 L/min is removed as a bypass flow. An in-line parallel plate charcoal impregnated filter denuder, similar to that used in the RAMS (CitationEatough et al. 1999), is used to remove gas phase organic compounds in the aerosol which eliminates positive artifacts. The air stream is sampled on a 12.3 mm diameter quartz filter for a designated time period, normally 45 minutes followed by a 15 min analysis to yield an estimate of an hour average. Sample collection is then interrupted and the sample analyzed, using a (TOT) method similar to the NIOSH Method 5040. Initially OC concentrations are determine by heating the filter in a pure helium atmosphere to temperatures of 250, 500, 650, and 850°C. A 98% helium 2% oxygen atmosphere is used in the second stage and heated to temperatures of 650, 750, and 850°C to determine EC concentrations. Pyrolyzed carbon is corrected for based on the laser transmission (CitationBirch and Cary 1996). Carbon thermally evolved from the filter is converted to CO2 in a manganese dioxide catalyst and detected by a non-dispersive infrared detector (NDIR). Each sample analysis is followed by a calibration step.
Integrated PC-BOSS Determination of PM 2.5 Composition and Mass
The combination of technology used in the High-Volume Brigham Young University Organic Sampling System (BIG BOSS) and the Harvard particle concentrator has resulted in the Particle Concentrator-Brigham Young University Organic Sampling System (PC-BOSS) (CitationTang et al. 1994; CitationSioutas et al. 1994; CitationDing et al. 2002a, Citation2002b; CitationLewtas et al. 2001). The configuration and operation of the PC-BOSS that was used in this study has been previously described (CitationLong et al. 2003).
Samples for the chemical characterization of PM2.5 in the minor flow following a particle concentrator and a BOSS diffusion denuder are collected in a filter pack containing a pre-fired 47 mm quartz filter (Pallflex) followed by a 47 mm carbon impregnated glass fiber (CIG) filter to determine fine particulate sulfate, and carbonaceous material and nitrate, including SVM. A second parallel filter pack containing a 47 mm Teflon (Whatman) filter followed by a 47 mm Nylon (Gelman, Nylasorb) filter is used to determine PM2.5 filter-retained (non-volatile) mass, sulfate and nitrate, plus any semi-volatile nitrate lost from the particles during sample collection. A side flow filter pack, located prior to the particle concentrator, containing a 47 mm polycarbonate (Corning nuclepore, 0.4 μ m pore size) filter followed by a 47 mm CIG collects particles (excluding SVM lost during sampling) and gas phase organic material after the 2.5 μ m inlet cut. These data are compared to data from the minor flow filters to determine the particle concentrator efficiency. The filters can also be used to determine elemental content by PIXE.
Sample Collection
Sampling was conducted in Fresno, CA from December 1–23, 2003. One-hr averaged data were obtained from all instruments listed above excluding the PC-BOSS and the Dionex GP-IC system. Several days were forecasted, in which high concentrations of PM were expected, during the study for the collection of PC-BOSS data. Subsequently, 3-h PC-BOSS data were obtained on 4 days during the sampling campaign (15, 17, 18, and 22). The Dionex GP-IC provided 15 min data resolution which was then averaged to provide 1-h averaged data for comparison with the other instruments used in the study. Several of the instruments including the R&P 8400N Nitrate and 8400S Sulfate Monitors, the Met One BAMs, the Sunset Carbon Monitor and an R&P 5400 C Monitor were operated by the EPA Fresno Supersite. Particle separation for all continuous mass measurements, excluding the Dionex GP-IC, was done with an R&P16.67 L/min PM10 inlet followed by an R&P 2.5 μ m Sharp Cut Cyclone (SCC) at ambient temperature. The Dionex GP-IC used a Teflon coated aluminum (URG-2000-30EN, University Research Glassware) 2.5 μ m SCC at ambient temperature following protocols previously described (CitationAl-Horr et al. 2003). Quality assurance flow checks were performed weekly throughout the study using a calibrated mass flow controller.
Statistical Treatment of Data
Comparisons between monitoring techniques were performed by linear and orthogonal regression. For linear regression, both a zero-intercept and slope-calculated intercept analysis were performed. Linear regression analysis assumes that the variability in the X component is zero. To overcome this constraint, slopes were also calculated using orthogonal regression which symmetrically fits the slope of the data so that variability in both the X and Y components is accounted for. A bias corrected precision (σ) was calculated as:
RESULTS
PC-BOSS Integrated Constructed Mass Versus Real-Time Total Mass Measurements
Total PM2.5 mass concentrations from the PC-BOSS can be obtained as the sum of: sulfate and nitrate (assumed to be present as the ammonium salts), nonvolatile and SVOM, employing an organic carbon to organic material conversion factor of 1.6, typical of an aged urban aerosol (CitationTurpin and Lim 2001), and EC. Comparisons between constructed mass obtained by the PC-BOSS and R&P FDMS and R&P Differential TEOM Monitors were made. Three PC-BOSS 3-h samples were not included in the comparisons due to incomplete analysis of the organic material, resulting in 29 comparisons between constructed integrated mass and real-time mass measurements. Real-time concentration data were averaged over the PC-BOSS sampling time periods for comparison.
Excellent agreement was observed between the PC-BOSS and the R&P FDMS Monitor, as shown in . Linear regression analysis of the R&P FDMS (x) and the PC-BOSS (y) data resulted in a zero-intercept linear regression slope = 1.00 ± 0.02 with an R2 = 0.93 and an intercept calculated slope of 0.88 ± 0.04 with an R2 = 0.95 and an intercept of 6.7 ± 4.3 μ g/m3. An orthogonal regression resulted in a slope of 0.87± 0.04 with an intercept of 7.0 ± 1.9. The calculated uncertainty in the comparison resulted in σ = ±3.6 μ g/m3 or ±7.3%, which is consistent with the expected uncertainty in the PC-BOSS measurement.
TABLE 1 Regression analysis results of the R&P FDMS (X) compared to other real-time mass measurement instruments
Linear regression analysis was also performed on a comparison between the average PM2.5 mass concentrations obtained with the collocated R&P Differential TEOMs (x) and the PC-BOSS constructed mass data (y). A zero-intercept linear regression slope of 1.28 ± 0.03 with an R2 = 0.88 was observed. An intercept calculated slope of 1.11 ± 0.07 resulted in an R2 = 0.90 and a intercept of 7.5 ± 6.1 μ g/m3. An orthogonal regression resulted in a slope of 1.18 ± 0.07 and an intercept of 5.0 ± 3.2. The uncertainty in the comparison was σ = ±9.2 μ g/m3 (± 21.4%).
A comparison between the R&P FDMS and the R&P Differential TEOM Monitor, , reveals good agreement at lower concentrations, and deviation at higher concentrations is obtained. These high concentration values were consistently observed at peak episodes throughout the study and a plausible explanation for the difference between the PC-BOSS and R&P FDMS compared to the R&P Differential TEOM Monitor is explained in a subsequent section of this article.
R&P FDMS Versus FRM
Every sixth day, 24-hr FRM samples were collected at the Fresno Supersite. R&P FDMS concentrations were averaged over each 24-hr period and compared to concentrations obtained by the FRM. Generally, the FRM underestimates PM2.5 concentrations due to the loss of SVM not retained by the single filter sampler (CitationGrover et al. 2005). However during this sampling campaign, good agreement was observed between the R&P FDMS and the FRM. Regression statistics are given in .
The agreement between the R&P FDMS and the FRM can be explained. Lower PM2.5 concentrations were observed during December 11, 23, and 29 with little or no SVM present in the aerosol. These three sampling days were also impacted by intermittent rain resulting in the observed low concentrations of PM2.5. The FRM sample obtained on December 5 occurred during the strong persistent inversion, accompanied by high humidity and high PM2.5 concentrations, that was observed at the beginning of the sampling period. During this time period, the SVM was dominated by ammonium nitrate. Previous studies have indicated semi-volatile ammonium nitrate is often not lost from the FRM Teflon filter under winter temperature conditions and high humidity (CitationLong et al. 2003). SVM on December 17 was not, however, dominated by ammonium nitrate and approximately 88% of the SVM was SVOM. However during the evening period, when the aerosol had high concentrations of SVM, high humidity (∼ 90% RH) and cold temperatures (∼ 7°C) were also observed. Reasonably, SVM could be stabilized by cold and humid conditions as were observed on December 17.
Real-Time Mass Comparisons
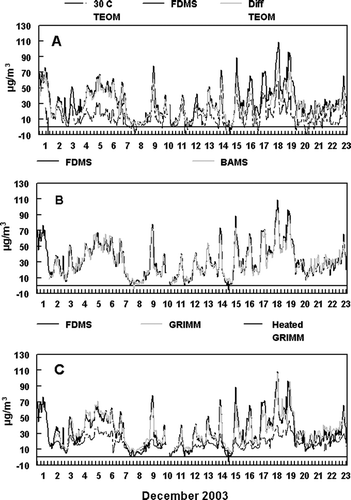
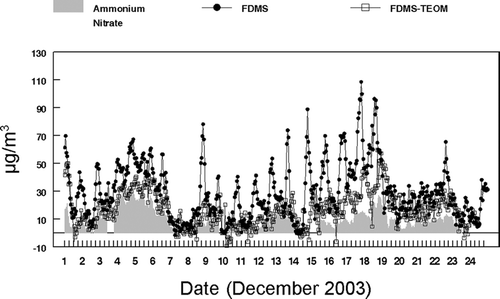
Hourly averaged mass measurements from the monitoring instruments were compared: two R&P FDMS Monitors, two R&P Differential TEOM Monitors, an R&P TEOM Monitor, a Met One BAMS, a conventional GRIMM Monitor and a GRIMM Monitor equipped with a heated inlet. Data recovery for each of the instruments was; greater than 99% for the R&P FDMS, 86% for the Differential TEOMs, 99% for the conventional 50°C and 30°C TEOM, 98% for the BAMS, 98% for the GRIMM, and 95% for the GRIMM with a heated inlet. Incomplete data recovery was mostly the result of two major power outages that occurred at the sampling site during the study. Comparisons between instruments were only made when both instruments were operating and the number of data points are indicated in . The R&P 8500 FDMS Monitor is used as a benchmark for comparison because this monitor has been shown to measure total PM2.5 concentrations including both the nonvolatile and semi-volatile fractions (CitationGrover et al. 2005) and was in agreement with the PC-BOSS data. As shown in , a diurnal pattern was observed with concentrations increasing during the evening rush hour period and reaching maxima during the nighttime hours. This increase in PM2.5 mass concentration is believed to be associated with a decrease in meteorological boundary layer as the temperature decreased during the winter nights throughout the study period together with contributions from evening rush hour and wood smoke emissions (CitationWatson and Chow 2002). Generally low daytime concentrations were observed throughout the study. An exception to this pattern was seen December 4–6 when a persistent inversion with fog was present.
Precision of Collocated R&P FDMS and R&P Differential TEOM Monitors
Precision measurements were only made for the R&P FDMS Monitors during the December 1–13 period. After this time period, one of the FDMS monitors was not operating properly for the remainder of the study period due to the malfunction of one of the computer boards in the system. As shown in , good agreement was observed between the two FDMS monitors with a bias of only −1.2 μ g/m3 and σ = ±2.0 μ g/m3 or ± 6.6%.
Precision measurements for the R&P Differential TEOM monitors were also only made during the December 1–13 time period. Starting on December 14, operating conditions on one of the R&P Differential TEOM monitors was changed for the rest of the study period. Good agreement was also observed between the collocated R&P Differential TEOMs as shown in . A bias of 2.2 μ g/m3 between the two instruments was observed with σ = ±2.3 μ g/m3 or ± 8.5%.
R&P FDMS Versus R&P TEOM
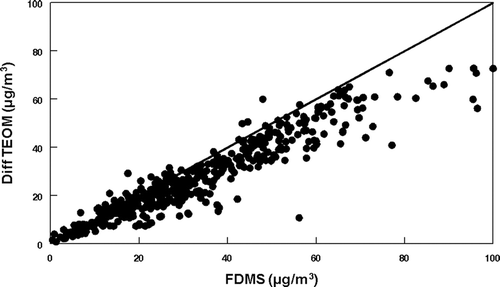
Due to the loss of SVM from the heated inlet of the TEOM Monitor (CitationGrover et al. 2005; CitationLong et al. 2002, Citation2003), TEOM measured mass concentrations were normally below or equal to FDMS measured mass concentrations as shown in . Regression analysis, shown in , indicates the magnitude of SVM loss during the study period. A linear regressoion zero-intercept slope of 0.46 ± 0.01 was obtained with R2 = 0.67. The intercept calculated slope was 0.50 ± 0.01 with R2 = 0.68 and an intercept of −1.7 ± 6.9 μ g/m3. Orthogonal regression statistics are shown in . A sigma value of ± 15.0 μ g/m3 or ±67% was obtained for the comparison.
Two TEOMs operating at 50°C and 30°C respectively were employed during the study. The TEOM operating at 50°C often measured lower concentrations of PM2.5 throughout the study period. This indicates that more of the SVM is lost from the collection filter at the higher operating temperature. Regression statistics for the TEOMs operating at different temperatures are given in .
R&P FDMS Versus R&P Differential TEOM
During the majority of the sampling period good agreement was observed between the FDMS and the Differential TEOM measurements. Some exceptions were observed at peak concentration time periods on December 8, 13, 14, 15, 17, and 18 as shown in . Linear regression analysis, for the entire sampling period, resulted in a zero-intercept slope of 0.81 ± 0.01 with an R2 = 0.89 and n = 465. An intercept calculated slope linear regression gave a slope = 0.78 ± 0.01 with R2 = 0.90 and an intercept of 1.5 ± 5.4 μ g/m3. An orthogonal regression resulted in a slope of 0.82 ± 0.01 and an intercept of 0.3 ± 0.5 μ g/m3. The bias between the two measurement systems was 5.7 μ g/m3 with σ = ± 6.4 μ g/m3 or ± 21.5%.
Focusing on time periods in which the observed peak exceptions did not occur (December 1–7 and December 10–13), a linear regression analysis resulted in a zero-intercept linear slope of 0.90 ± 0.01 and R2 = 0.97 and the intercept calculated slope was 0.90 ± 0.01 with an R2 = 0.97 and an intercept of −0.1 ± 2.9 μ g/m3. An orthogonal regression gave a slope of 0.91 ± 0.02 with an intercept of −0.4 ± 0.4 μ g/m3. The observed bias excluding peak exceptions was 3.3 μ g/m3 with σ = ± 3.4 μ g/m3 or ±11.4%.
R&P FDMS Versus Met One BAMS
Good agreement was observed during the sampling period with some scatter occurring at higher mass concentrations as shown in . A linear regression analysis zero-intercept slope of 0.94 ± 0.01 with an R2 = 0.84 was obtained. A slope of 0.85 ± 0.02 with an R2 = 0.85 and an intercept of 3.9 ± 7.9 μ g/m3 was observed for an intercept calculated linear regression. Orthogonal regression gave a slope of 0.91 ± 0.02 with an intercept of 1.9 ± 0.6 μ g/m3. Although the comparison between the two instruments was somewhat noisy as indicated by the lower R2 value, little bias was observed (bias = 0.7 μ g/m3) with σ = ± 0.6 μ g/m3 or ±2.0%.
R&P FDMS Versus Non-Heated and Heated GRIMM 1100 Monitor
As shown in , good agreement was observed between the conventional GRIMM Monitor and the R&P FDMS Monitor with few exceptions. During peak concentration periods from December 8–17 the GRIMM mass concentrations were substantially lower than the R&P FDMS mass, and were also lower than the concurrent BAMS measurement. However, at other peak concentration time periods the GRIMM Monitor measurement was equal to that obtained by the R&P FDMS Monitor. The GRIMM Monitor measurement was also noticeably higher during an initial inversion period on December 3–6 and some rainy periods throughout the study as seen on December 8–9 and again on December 21–22. The inversion period was accompanied by high humidity and low level fog as well as high nitrate concentrations. Therefore, the overestimated mass measurement by the GRIMM Monitor at these time periods may be the result of water uptake due to the hydroscopic nature of urban PM2.5 especially with high nitrate concentrations (CitationGrover et al. 2004).
Linear regression analysis of the R&P FDMS (x) and the conventional GRIMM Monitor (y) resulted in a zero-intercept calculated slope of 0.97 ± 0.01, R2 = 0.80 with n = 496. The intercept calculated slope was 0.80 ± 0.02 with an R2 = 0.85 and an intercept of 7.4 ± 6.9 μ g/m3. An orthogonal regression resulted in a slope of 0.86 ± 0.02 with an intercept of 5.5 ± 0.6 μ g/m3. The uncertainty in the comparison was σ = ± 5.7 μ g/m3 or ±17.7%.
The PM2.5 concentration measurement by the heated GRIMM Monitor was substantially lower than the R&P FDMS measurement and the conventional GRIMM measurement throughout the study. This is mainly due to the loss of SVM by the heated inlet of the modified GRIMM Monitor. PM2.5 mass from the heated-inlet GRIMM Monitor more closely resembled those obtained by the conventional TEOM monitor. Regression statistics are given in .
Particulate Carbon Measurements
Diurnal patterns in carbon concentrations tended to track PM2.5 mass concentrations as measured by the R&P FDMS. Generally good agreement between the two semi-continuous carbon monitors was observed for TC at low concentrations and significant deviations were observed at higher concentrations with regression statistics given in . During the inversion period observed at the beginning of the study (December 1–7), good agreement was observed between the two carbon monitors. During the later part of the study, when a diurnal pattern with high nighttime concentrations was observed, the Sunset monitor concentration was often greater than the R&P 5400 with very low correlations (R2 = 0.22). Previously, the R&P 5400 Carbon Monitor has been shown to underestimate SVOM (CitationAnderson et al. 2002) especially at higher concentrations as were observed in this period of the study. Furthermore, the aerosol during the initial inversion period was dominated by ammonium nitrate with low particulate carbon concentrations. During the later part of the study, when deviations were observed, the aerosol had much higher carbon concentrations as well as significant concentrations of semi-volatile carbon.
TABLE 2 Results of regression analysis for fine particulate chemical species
Where PC-BOSS data were available, comparisons were made between PC-BOSS 3-h integrated total carbon concentrations with averaged carbon concentrations determined by the respective semi-continuous monitors and regression statistics are given in . Linear regression zero-intercept slopes of 0.67 ± 0.04 and 0.80 ± 0.03 were determined for the R&P 5400 and the Sunset, respectively.
Inorganic Constituent Measurements
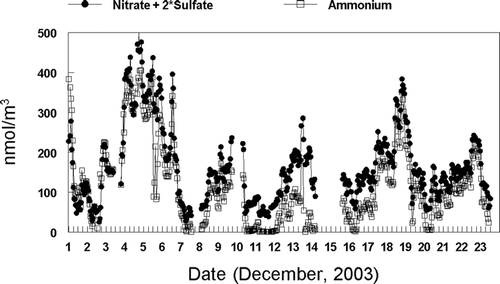
Hourly averaged concentrations of particulate sulfate, nitrate, and ammonium ion determined by the Dionex GP-IC and the R&P systems during the study period are shown in . Throughout the study, concentrations of nitrate were higher than sulfate concentrations with the averaged concentrations, for the more complete GP-IC data set, of 8.09 μ g/m3 and 1.51 μ g/m3, respectively. Fine particulate ammonium ion concentrations tended to track fine particulate nitrate concentrations through the study.
FIG. 3 Real-Time Concentrations of Nitrate, Sulfate, and Ammonium Ions Determined by the Dionex GP-IC System. R&P Nitrate Concentrations and R&P Sulfate Concentrations are Included. Also Shown are Data from the Sunset Carbon Monitor and the R&P 5400 Carbon Monitor. Note that X-Axis Concentrations are Different for Each Graph.
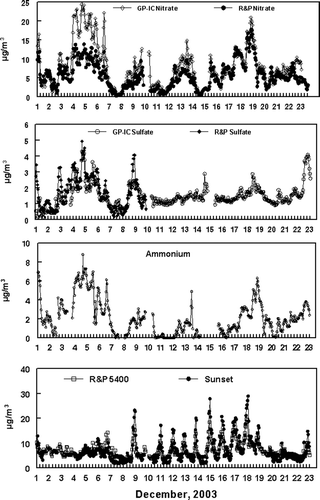
The sum of the concentrations of 3-h averaged nitrate collected on the PC-BOSS Teflon filter and the lost semi-volatile nitrate collected on the PC-BOSS backup nylon filter were compared to nitrate concentrations (averaged from 12–15 min. nitrate samples) determined by the GP-IC system. As shown in , good agreement was observed between the 29 paired nitrate measurements on the two systems with a zero-intercept calculated slope close to unity and a bias of only −0.5 μ g/m3. The uncertainty in the comparison was σ = ±1.3 μ g/m3 or ± 13.7%. We conclude that total nitrate concentrations, including semi-volatile nitrate, were measured by the GP-IC system under the range of conditions observed in this study with an acceptable precision.
Major differences were observed between the R&P 8400N and the GP-IC nitrate data as shown in . The most significant differences were observed during a persistent inversion at the beginning of the sampling period, which was associated with ground level fog and high humidity. Incomplete flash volatilization by the R&P Nitrate Monitor under conditions of high humidity (CitationLong and McClenny 2006) may explain the differences observed between the two monitoring techniques. Unfortunately, PC-BOSS data were not obtained during this time period of the sampling campaign.
A comparison was made between sulfate collected on the Teflon filter of the PC-BOSS and sulfate concentrations obtained by the GP-IC system. A resulting bias of 0.3 μ g/m3 was observed. Two statistical outliers were removed for regression analysis resulting in 27 data pairs. Although generally low concentrations of sulfate were observed throughout the sampling period, shows good agreement was observed between the two measurement systems, with a precision of σ = ±0.3 μ g/m3 or ±11.0%.
Sulfate data from the R&P 8400S were only available for the initial 10 days of the sampling period as shown in . The R&P Sulfate Monitor and the GP-IC system were in good agreement with an uncertainty of ± 0.4 μ g/m3 which is comparable to the estimated uncertainty of semi-continuous monitoring data of ± 0.3 μ g/m3. Linear regression statistics show low correlations but give an intercept near zero and a slope close to unity.
An increased understanding of atmospheric chemical processes can be obtained by the simultaneous determination of atmospheric cations and anions. An acid neutral aerosol would be expected to exhibit equal equivalents of the major anions (sulfate + nitrate) and the ammonium ion. During the initial portion of the sampling period (December 1–7), an acid neutral aerosol was observed when a persistent inversion occurred. The equivalents of anions were sometimes greater than that of the ammonium ion during the later portion of the study. Because the Fresno aerosol has excess ammonia and is normally neutral, most likely other cations besides the ammonium ion were present in the aerosol that were not measured during these time periods. This is illustrated in . During the later portion of the study, concentrations of fine particulate mass measured by the R&P FDMS monitor exhibited increased concentrations during the late evening and nighttime hours associated with the formation of nocturnal inversion layers. A corresponding pattern was also observed for carbon measured by the R&P 5400 Carbon Monitor. The same pattern however is not observed with nitrate concentrations, which typically exhibited a diurnal pattern with increased concentrations during the daytime hours, shown in . This diurnal trend is in accordance with daytime photochemistry that occurs, resulting in the formation of nitrate during time periods of high photochemical activity. Concentration data for nitrite and chloride ions were also routinely determined during the study using the Dionex GP-IC. Nitrite and chloride ion concentrations were typically low and approximately 2–5% of the nitrate ion concentrations observed during the study.
R&P FDMS and Constructed Real-Time Mass
A time-resolved sum of the major species of PM2.5 determined by various instruments resulted in a semi-continuous constructed mass shown in . Nitrate and sulfate concentrations were determined by the Dionex GP-IC and represented as ammonium nitrate and ammonium sulfate, respectively. Particulate carbon concentrations, including OC and EC, were measured using the Sunset Carbon Monitor. GP-IC inorganic and Sunset carbon data were used instead of the respective R&P nitrate and carbon monitors, because these instruments had better correlation with PC-BOSS data obtained during the study. OC concentrations were converted to organic material concentrations using a factor of 1.6 (CitationTurpin et al. 2001). Constructed mass data were compared to total PM2.5 concentration data obtained by the R&P FDMS as shown in . The difference between constructed mass and FDMS measured mass concentrations can be explained by the amount of SVOM which is measured by the FDMS but not by the Sunset carbon monitor. During the initial inversion period of the study (December 1–7), when SVM was dominated by ammonium nitrate, constructed mass approached those measured by the R&P FDMS Monitor. During the later part of the study, (December 9–23) high concentrations of organic material were observed during nighttime inversion periods. SVM during these time periods was not dominated by ammonium nitrate and had significant amounts of SVOM. Constructed mass concentrations more closely resembled non-volatile mass concentrations measured by the TEOM monitor because the SVOM was not measured by the Sunset Carbon Monitor or the TEOM monitor.
FIG. 5 (A) R&P FDMS versus Constructed Mass. Constructed Mass Consists of Dionex GP-IC Nitrate and Sulfate Data represented as the Ammonium Salts, and Sunset Carbon Data (B) X-Y plot of FDMS measured mass vs. constructed mass with error bars indicating estimates in the propagation of error in the constructed mass measurement.
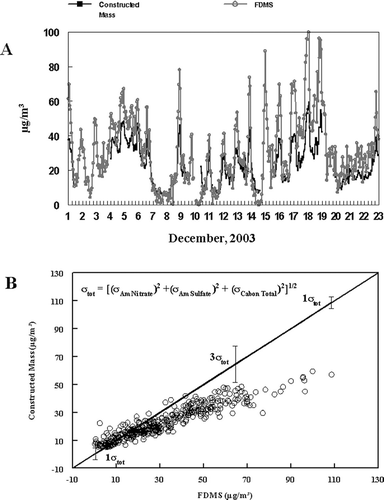
An estimate of the propagation of error for the various chemical species measurements was calculated as shown as σtot. The uncertainty in each chemical species measurement was estimated by comparison with the PC-BOSS with the resulting σ values given in . As shown, the propagation of the various instrument measurement error, does not entirely account for the difference seen between the constructed mass and the FDMS measurement. At time periods mentioned previously, when high concentrations of SVOM was present in the aerosol, the difference between constructed mass and FDMS mass is often greater than 3 σtot, .
SVM Comparison
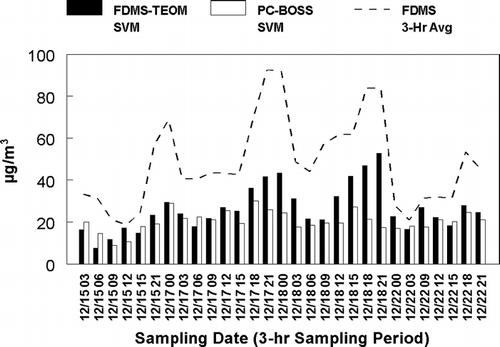
Less of the SVM material was observed to be lost from the respective semi-continuous monitors than the TEOM monitor. A test of the difference between the amount of SVM lost was made by comparing the amount of SVM lost from the heated filter of the TEOM monitor and that collected by the PC-BOSS (including both SVOM and semi-volatile nitrate). Three-h averaged concentrations were determined for the FDMS and TEOM monitors for comparison with the PC-BOSS sampling periods. Three-h averaged SVM concentrations lost from the TEOM monitor were calculated as the FDMS concentration minus the TEOM concentration. During the majority of periods when PC-BOSS data were collected, the amount of SVM lost from the TEOM monitor was 15–18 μ g/m3 greater than the amount lost from the Teflon (nitrate) and Quartz (organic material) filters of the PC-BOSS, as shown in . Including the entire study period, an average of 6.0 μ g/m3 more SVM was lost from the TEOM monitor than the respective PC-BOSS filters for all time periods when PC-BOSS data were available.
Discussion
Meteorological conditions resulted in a persistent inversion that occurred during the initial week of the study period (December 1–7) causing high PM2.5 concentrations. This inversion was accompanied by high RH (%) and persistent ground level fog. During this initial inversion period relatively good agreement was observed for the R&P FDMS, the R&P Differential TEOM, the GRIMM Monitor, and the Met-One BAMS with the exceptions of the higher GRIMM measurement seen at some time periods during the inversion due to the presence of water in the aerosol as previously mentioned (CitationGrover et al. 2004). Following this initial persistent inversion, a typical diurnal pattern with high nighttime concentrations developed and good agreement was observed between the measurement systems during daytime non-peak concentration periods. However, significant differences were observed during nighttime peak concentrations during the later part of the study.
The differences observed at peak concentrations between the various measurement systems in the later part of the study (December 8–19) warrants some discussion. A comparison was made between each of the real-time mass measurement systems, excluding the conventional TEOM monitor and the heated GRIMM Monitor. The R&P FDMS at the maximum 1-h peak concentration for each day during December 8–19 frequently gave higher concentrations than were observed between the other continuous measurement techniques. On December 8, 13, and 14 the R&P FDMS exhibited over 5 μ g/m3 higher concentrations than all the other measurement systems. On all other days during the later period of the study, one or more of the other measurement techniques was comparable to the R&P FDMS. The difference at peak concentrations between the R&P FDMS and the Met-One BAMs, the conventional GRIMM, and the R&P Differential TEOM for all peak values between December 8–19 was 6.0 μ g/m3, 9.1 μ g/m3, and 16.1 μ g/m3, respectively.
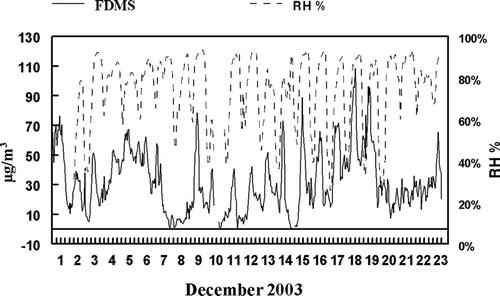
The high degree of variability between the measurement systems at the peak concentrations is most likely associated with the complexity of the aerosol being measured. Meteorological conditions resulted in consistent nighttime inversion layers and high PM2.5 concentrations. These diurnal peak concentrations were normally associated with dense low altitude fog resulting in a high water content associated with the PM2.5. This high water content in the aerosol may play a role in the variance seen between the different measurement techniques. The RH (%) and R&P FDMS concentrations are shown throughout the study in . The differences seen between measurement systems at the initial inversion period and at peak concentrations during the later part of the study are associated with time periods of high RH (%). However, both the R&P FDMS Monitor and the R&P Differential TEOM Monitor use Nafion® dryers to remove particle bound water prior to measurement and therefore, would not be expected to be affected by aerosol water content. The FDMS monitor measures the RH (%) downstream of the Nafion dryers and an error code is triggered when the dew point of the aerosol exceeds 2°C.
Another factor that may be associated with the differences between the measurement systems is the composition of the SVM in the aerosol. An estimation of the amount of 1-hr averaged SVM concentrations in the aerosol can be made by subtracting the hourly TEOM from the hourly FDMS mass measurements as shown in as FDMS-TEOM. Previous studies have shown that the sum of ammonium nitrate concentrations and SVOM concentrations accounts for the difference seen between the FDMS and TEOM measurement (CitationGrover et al. 2005) or the amount of SVM present in the aerosol. Also displayed in are the 1-h averaged concentrations of nitrate measured by the Dionex GP-IC represented as ammonium nitrate. During the initial portion of the sampling period when good agreement was observed between the FDMS and the other measurement systems, the SVM was dominated by ammonium nitrate. When peak exceptions were present, SVM is not dominated by ammonium nitrate but contains a large fraction of SVOM. Difference in composition of the SVM present in the aerosol may explain the differences in mass measurement seen between the FDMS and the other measurement systems at the peak concentration periods during the later period of the study. This explanation also accounts for the difference seen between PC-BOSS integrated constructed mass measurements and the Differential TEOM mass measurements because PC-BOSS samples were only obtained on days in which SVOM was a large fraction of the total SVM.
CONCLUSIONS
During the time periods when collocated R&P FDMS or collocated Differential TEOM monitors were operating properly, excellent agreement was observed. The resulting precision is well within the expected precision of the monitoring techniques.
Excluding peak concentration time periods during the later part of the study, relatively good agreement was observed between the R&P FDMS, the R&P Differential TEOM, the Met One BAMs and the conventional GRIMM 1100. The conventional GRIMM monitor, at some high humidity time periods, may measure higher mass concentrations due to the inclusion of water in the measured mass. PM2.5 mass acquired by the R&P TEOM and the GRIMM monitor with a heated inlet were substantially lower than the other measurement techniques throughout the majority of the study due to the loss of SVM.
Good agreement was observed between the PC-BOSS constructed mass measurement and the R&P FDMS averaged measurement for the four days of 3-h PC-BOSS samples obtained. All four PC-BOSS sampling days occurred when significant differences were observed between the real-time measurement techniques. Also, good agreement was observed between the inorganic species concentrations obtained by the PC-BOSS and the Dionex GP-IC system. PM2.5 nitrate, including the semi-volatile nitrate, was measured by the GP-IC system. The GP-IC proved to be a robust instrument with the advantage of the semi-continuous measurement of the major fine particulate ammonium ion and anion concentrations. Lower nitrate concentrations were measured with the R&P 8400N Nitrate Monitor under conditions of high humidity.
The observed peak concentration variance between the different real-time measurement techniques may be associated with the complexity of the aerosol being measured, including water content and SVM composition. However, a definitive explanation is not known at this time. Further investigation of the effect of SVM composition, especially SVOM, on real-time mass measurement is needed.
The real-time mass concentration instruments used during the study were all very robust, requiring little or no attention from the operator during the study period. The combination of real-time particulate mass with chemical species measurement is useful in gaining a better understanding of atmospheric aerosols.
Appreciation is expressed for the assistance of the California Air Resource Board in Fresno CA and EPA Fresno Supersite. Special thanks is given to Scott Scheller for his assistance during the study and Bill Roe with GRIMM technologies for his help. The U.S. Environmental Protection Agency (EPA) funded and collaborated in the research described under grant 3C-R044-NAEX to Brigham Young University. This study was also jointly funded as part of the U.S. EPA Supersite program under STAR Grant R831086. The views expressed in this paper are those of the authors and do not reflect the views or policies of the United States government or EPA. Mention of trade names and commercial products does not constitute endorsement.
Notes
a Slopes are given for (1) linear regression zero intercept, (2) linear regression calculated intercept, and (3) orthogonal regression.
a Slopes are given for (1) zero intercept, (2) calculated intercept, and (3) orthogonal regression.
b Two Statistical Outliers were not included in the analysis.
REFERENCES
- Al-Horr , R. , Samantha , G. and Dasgupta , P. K. 2003 . A Continuous Analyzer for Soluble Anionic Constituents and Ammonium in Atmospheric Particulate Matter . Environ. Sci. Technol. , 37 : 5711 – 5720 . [INFOTRIEVE] [CSA]
- Al-Horr , R. , Later , D. W. , Grover , B. D. , Eatough , N. L. , Watson , J. , Chow , J. and Eatough , D. J. A New Gas-Particle Ion Chromatographic System for the Continuous Monitoring of Soluble Gases and Ionic Constituents of Particulate Matter . Proceedings of A&WMA 97th Annual Conference . June 2004 . Paper #763
- Anderson , R. A. , Martello , D. V. , Rohar , P. C. , Strazisar , B. R. , Tmilia , J. P. , Waldner , K. , White , C. M. , Modey , W. K. , Mangelson , N. F. and Eatough , D. J. 2002 . Sources and Composition of PM2.5 at the National Energy Technology Laboratory in Pittsburgh during July and August 2000 . Energy and Fuels. , 16 : 261 – 269 . [CROSSREF] [CSA]
- Birch , M. E. and Cary , R. A. 1996 . Elemental Carbon-Based Method for Occupational Monitoring of Particulate Diesel Exhaust: Methodology and Exposure Issues . Analyst , 121 : 1183 – 1190 . [INFOTRIEVE] [CROSSREF] [CSA]
- Boring , C. B. , Al-Horr , R. , Genfa , Z. , Dasgupta , P. K. , Martin , M. W. and Smith , W. F. 2002 . Field Measurements of Acid Gases and Soluble Anions in Atmospheric Particulate Matter using a Parallel Plate Wet Denuder and Alternating Filter-Based Automated Analysis System . Anal. Chem. , 74 : 1256 – 1268 . [INFOTRIEVE] [CROSSREF] [CSA]
- Carter , C. , Long , R. W. , Eatough , N. L. and Eatough , D. J. Comparison of Speciation Sampler and PC-BOSS PM2.5 Organic Material Results Obtained on the Wasatch Front During the Winter of 2001–2002 . Pro. AWMA Annual Conf. 2002 . pp. 767
- Chow , J. C. 1995 . Measurement Methods to Determine Compliance with Ambient Air Quality Standards for Suspended Particles . J. Air & Waste Manage. Assoc. , 45 : 320 – 382 . [CSA]
- Chung , S. H. and Seinfeld , J. H. 2002 . Global Distribution and Climate Forcing of Carbonaceous Aerosols . J. Geophysical Research , 107 : 4407 [CROSSREF] [CSA]
- Conant , W. C. , Seinfeld , J. H. , Wang , J. , Carmichael , G. R. , Tang , Y. , Uno , I. , Flatau , P. J. , Markowicz , K. M. and Quinn , P. K. 2003 . A Model for the Radiative Forcing During ACE-Asia Derived from CIRPAS Twin Otter and R/V Ronald H. Brown Data and Comparison with Observation . J. Geophysical Research , 108 : 8661 [CROSSREF] [CSA]
- Dasgupta , P. K. and Poruthoor , S. K. 2002 . Comprehensive Analytical Chemistry, , 37th ed. , Edited by: Pawliszyn , J. 161 – 276 . Amsterdam : Elsevier Science B.V. .
- Ding , Y. , Pang , Y. , Eatough , D. J. , Eatough , N. L. and Tanner , R. L. 2002a . High-Volume Diffusion Denuder Samplers for the Routine Monitoring of Fine Particulate Matter: I. Design and Optimization of the PC-BOSS . Aerosol Sci. Technol. , 36 : 369 – 382 . [CROSSREF] [CSA]
- Ding , Y. , Pang , Y. , Eatough , D. J. , Eatough , N. L. and Tanner , R. L. 2002b . High-Volume Diffusion Denuder Samplers for the Routine Monitoring of Fine Particulate Matter: II. Field Evaluation of the PC-BOSS . Aerosol Sci. Technol. , 36 : 383 – 396 . [CROSSREF] [CSA]
- Eatough , D. J. , Eatough , D. A. , Lewis , L. and Lewis , E. A. 1996 . Fine Particulate Chemical Composition and Light Extinction at Canyonlands National Park using Organic Particulate Material Concentrations Obtained with a Multisystem, Multichannel Diffusion Denuder Sampler . J. Geophysical Research , 101 : 19514 – 19531 . [CSA]
- Eatough , D. J. , Obeidi , F. , Pang , Y. , Ding , Y. , Eatough , N. L. and Wilson , W. E. 1999 . Integrated and real-time diffusion denuder samplers for PM2.5 based on BOSS, PC and TEOM technology . Atmos. Environ. , 33 : 2835 – 2844 . [CROSSREF] [CSA]
- Eatough , D. J. , Eatough , N. L. , Obeidi , F. , Pang , Y. , Modey , W. and Long , R. 2001 . Continuous determination of PM2.5 mass, Including Semi-Volatile Species . Aerosol Sci. Technol. , 34 : 1 – 8 . [CSA]
- Eatough , D. J. , Long , R. W. , Modey , W. K. and Eatough , N. L. 2003 . Semi-Volatile Secondary Organic Aerosol in Urban Atmospheres: Meeting a Measurement Challenge . Atmos. Environ. , 37 : 1277 – 1292 . [CROSSREF] [CSA]
- Grover , B. D. , Carter , C. B. , Kleinman , M. A. , Richards , J. S. , Eatough , N. L. and Eatough , D. J. 2006 . Monitoring and Source Apportionment of Fine Particulate Matter at Lindon, Utah . Aerosol Sci. Technol. , In press[CSA]
- Grover , B. D. , Kleinman , M. A. , Eatough , N. L. , Eatough , D. J. , Hopke , P. K. , Long , R. W. , Wilson , W. E. , Meyer , M. B. and Ambs , J. L. 2005 . Measurement of Total PM2.5 Mass (Nonvolatile plus Semi-Volatile) with the FDMS TEOM Monitor . J. Geophysical Research , 110 : D07S03 [CROSSREF] [CSA]
- Grover , B. D. , Eatough , N. L. , Hopke , P. K. and Eatough , D. J. 2004 . Measurement of Fine Particulate Mass, Including the Semi-Volatile Constituents, with a GRIMM Monitor . Proceedings of A&WMA 97th Annual Conference . June 2004 . Paper #51
- Godleski , J. J. , Verrier , R. L. , Koutrakis , P. and Catalano , P. 2000 . Mechanisms of morbidity and mortality from exposure to ambient air particles , Health Effects Institute . research report no. 91
- Hering , S. and Cass , G. 1995 . The Magnitude of Bias in the Measurement of PM2.5 Arising from Volatilization of Particulate Nitrate from Teflon Filters . J. Air & Waste Manage. Assoc. , 49 : 725 – 733 . [CSA]
- Lewis , C. W. , Norris , G. A. , Conner , T. L. and Henry , R. C. 2003 . Source Apportionment of Phoenix PM2.5 Aerosol with the Unmix Receptor Model . J. Air & Waste Manage. Assoc. , 53 : 325 – 338 . [CSA]
- Lewtas , J. , Booth , D. , Pang , Y. , Reimer , S. , Eatough , D. J. and Gundel , L. 2001 . Comparison of sampling methods for semi-volatile organic carbon (SVOC) associated with PM2.5 . Aerosol Sci. Technol. , 34 : 9 – 22 . [CROSSREF] [CSA]
- Long , R. W. , Smith , R. , Smith , S. , Eatough , N. L. , Mangelson and Eatough , D. J. 2002a . Sources of Fine Particulate Material along the Wasatch Front . Energy and Fuels. , 16 : 282 – 293 . [CROSSREF] [CSA]
- Long , R. W. , Eatough , N. L. , Mangelson , N. F. , Thompson , W. , Fiet , K. , Smith , S. , Smith , R. and Eatough , D. J. 2003 . The Measurement of PM2.5, Including Semi-Volatile Components, in the EMPACT Program: Results from the Salt Lake City Study and Implications for Public Awareness, Health Effects, and Control Strategies . Atmos. Environ. , 37 : 4407 – 4417 . [CROSSREF] [CSA]
- Long , R. W. and McClenny , W. A. 2006 . Laboratory and Field Evaluation of Instrumentation for the Semi-Continuous Determination of Particulate Nitrate (and other Water Soluble Components . J. Air & Waste Manage. Assoc. , In press[CSA]
- Meyer , M. B. , Patashnick , H. and Ambs , J. L. Ongoing development of a continuous reference standard particulate matter mass monitor for ambient air . paper presented at the Symposium on Air Quality Measurement Methods and Technology . San Francisco, CA.
- Mignacca , D. and Stubbs , K. 1999 . Effects of Equilibration temperature on PM10 Concentrations from the TEOM Method in the Lower Fraser Valley . J. Air & Waste Manage. Assoc. , 49 : 1250 – 1254 . [CSA]
- Modey , W. K. , Pang , Y. , Eatough , N. L. and Eatough , D. J. 2001 . Fine Particulate (PM2.5) Composition in Atlanta, USA: Assessment of the Particle Concentrator Brigham Young University Organic Sampling System, PC-BOSS, During the EPA Supersite Study . Atmos. Environ. , 35 : 6493 – 6502 . [CROSSREF] [CSA]
- Modey , W. K. and Eatough , D. J. 2003 . Trends in Composition at the Department of Energy OST NETL PM2.5 Characterization Site in Pitsburgh, Pennsylvania, USA . Adv. Environ. Res. , 7 : 859 – 869 . [CROSSREF] [CSA]
- Musick , D. 2000 . A Summary of the Ambient Air Program for PM2.5 . Environ. Manager. , : 17 – 20 . [CSA]
- Patashnick , H. and Rupprecht , E. G. 1991 . Continuous PM10 measurements using the tapered element oscillating microbalance . J. of Air & Waste Manage. Assoc. , 41 : 1079 – 1083 . [CSA]
- Pope , C. A. III . 2000 . Epidemiology of fine particulate air pollution and human health; Biological mechanisms and who's at risk? . Environ. Health Perspect. , 108 ( Sup 4 ) : 713 – 723 . [INFOTRIEVE] [CSA]
- Pope , C. A. III , Hansen , M. L. , Long , R. W. , Nielson , K. R. , Eatough , N. L. , Wilson , W. E. and Eatough , D. J. 2004 . Particulate Air Pollution, HRV, and Inflammation . Environ. Health Perspect. , 112 : 339 – 345 . [INFOTRIEVE] [CSA]
- Schaefer , G. , Hamilton , W. and Mathai , C. V. 1997 . Implementing the revised NAAQS and the FACA subcommittee for ozone, particulate matter and regional haze . Environ. Manager , : 22 – 28 . [CSA]
- Seinfeld , J. H. and Pandis , S. N. 1996 . Atmospheric Chemistry and Physics , 23 – 38 . New York : John Wiley & Sons .
- Sioutas , C. , Koutrakis , P. and Burton , R. M. 1994 . Development and Evaluation of a Low Cutpoint Virtual Impactor . Aerosol Sci. Technol. , 21 : 223 – 235 . [CSA]
- Schwartz , J. , Dockery , D. W. and Neas , L. M. 1996 . Is Daily Mortality Associated Specifically with Fine Particles? . J. Air & Waste Manage. Assoc. , 46 : 927 – 939 . [CSA]
- Stolzenburg , M. R. and Hering , S. V. 2000 . Method for the Automated Measurement of Fine Particulate Nitrate in the Atmosphere . Environ. Sci. Technol. , 34 : 907 – 914 . [CROSSREF] [CSA]
- Tang , H. , Lewis , E. A. , Eatough , D. J. , Burton , R. M. and Farber , R. J. 1994 . Determination of the Particle Size Distribution and Chemical Composition of Semi-Volatile Organic Compounds in Atmospheric Fine Particles . Atmos. Environ. , 28 : 939 – 947 . [CROSSREF] [CSA]
- Turpin , B. J. and Lim , H. J. 2001 . Species Contributions to PM2.5 Mass Concentrations: Revisiting Common Assumptions for Estimating Organic Mass . Aerosol Sci. Technol. , 35 : 602 – 610 . [CSA]
- U.S. Environmental Protection Agency . 2002 . Air Quality Criteria for Particulate Matter , Research Triangle Park, NC : Environmental Protection Agency . EPA/600/P-99/002aC
- Annual Estimates of Population for Incorporated Places over 100,000. http://eire.census.gov/popest/data/cities/tables/SUB-EST2003-01.pdf Accessed July 8, 2004
- Watson , J. G. , Chow , J. C. , Bowen , J. L. , Lowenthal , D. H. , Hering , S. , Ouchida , P. and Oslund , W. 2000 . Air quality measurements from the Fresno Supersite . J. Air & Waste Manage. Assoc. , 50 ( 8 ) : 1321 – 1334 . [CSA]
- Watson , J. G. and Chow , J. C. 2002 . A Wintertime PM2.5 Episode at the Fresno, CA, Supersite . Atmos. Environ. , 36 ( 3 ) : 465 – 475 . [CROSSREF] [CSA]
- Watson , J. G. 2002 . Visibility: Science and Regulation . J. Air & Waste Manage. Assoc. , 52 : 628 – 713 . [CSA]
- Wilson , W. E. and Suh , H. H. 1997 . Fine Particles and Coarse Particles: Concentration Relationships Relevant to Epidemiologic Studies . J. Air & Waste Manage. Assoc. , 47 : 1238 – 1249 . [CSA]
- Yi , S. M. , Ambs , J. L. , Patashnick , H. , Rupprecht , G. and Hopke , P. K. 2004 . Evaluation of a Prototype Electrostatic Precipitator (ESP) for a Differential TEOM System . Aerosol Sci. Technol. , 38 : 46 – 51 . [CROSSREF] [CSA]