PM2.5 combustion emissions from small engines (string trimmer and chainsaw) using gasoline containing biogenic ethanol were collected and analyzed for their 14 C content. The sampling methodology was designed to minimize potential bias from organic artifact effects. The 14 C in the PM2.5 emissions was found to be drastically smaller (approximately a factor of 40) than the 14 C amounts measured in the fuels. This suggests that the current method of using 14 C measurements on ambient aerosol to estimate the contribution from fossil fuel combustion will be little affected by increased use of ethanol-containing gasoline.
INTRODUCTION
Radiocarbon (14C) is present in living and recently living material at an approximate concentration of one 14C atom per 1012 ordinary carbon atoms (12C + 13C). This equilibrium amount is a result of the gain of 14C from its steady production by cosmic rays in the atmosphere—some of which in the form of 14CO2 is taken up by the biosphere through photosynthesis—versus the loss of 14C from its radioactive decay (5730 y half-life). However 14C is absent from fossil fuels, having radioactively decayed to unmeasurably small amounts because of the ancient age of fossil carbon. This dichotomy is the basis of inferring the fraction of fossil carbon in an ambient aerosol sample by comparing its 14C content to that of living material, a methodology that has become increasingly popular in recent years (CitationKlinedinst and Currie 1999; CitationLemire et al. 2002; CitationTanner et al. 2004; CitationSzidat et al. 2004a, Citation2004b; CitationEndo et al. 2004; CitationLewis et al. 2004, Citation2006; CitationBench and Herckes 2004; CitationWard et al. 2006; CitationSzidat et al. 2006).
When using this methodology to estimate the contribution of mobile source emissions to ambient aerosol it is assumed that there is no 14C in the fuels used by the vehicles. However this assumption may be invalid for those situations in which the fuel is either gasohol (gasoline with approximately 10% vol. ethanol, usually from corn fermentation) or biodiesel (diesel with approximately 20% vegetable oils). For both political and petroleum conservation reasons, such biogenic additives are expected to become increasingly utilized in the United States. Depending on the particle- versus gas-phase distribution of the combustion products of the 14C-containing compounds in the fuel, the 14C percentage in the particle emissions could be larger or smaller than in the fuel.
Previous work (CitationBuchholz et al. 2002a, Citation2002b; CitationCheng et al. 2002) has used radiocarbon methodology to infer the origin of the carbon in emissions from a diesel engine fueled with diesel blended with several oxygenates, including biogenic ethanol. The principal focus of the work was in gaining a better understanding of the mechanism by which oxygenate addition reduces particulate emissions. The 14C measurements showed that the ethanol carbon is only about half as likely to form particulate matter compared to carbon from the diesel portion of the fuel. However the low solubility and cetane number of ethanol is a barrier to its routine use as a diesel oxygenate (CitationBuchholz et al. 2002a).
In contrast the present work is a first step in exploring the implications for 14C in ambient aerosol from the expected increasing usage of common commercially available biofuels. A laboratory study was conducted in which PM2.5 emissions samples from small engines using conventional gasoline and ethanol-containing gasoline were collected and analyzed for their 14C content. The objective was to compare any 14C found in PM2.5 emissions from ethanol-containing gasoline with the 14C amount in the fuel, using PM2.5 emissions from conventional gasoline (14C = 0) as the control. The present work also develops a methodology to account for sampling artifacts that can distort measurements of 14C in emissions. The methodology is directly applicable to the more important case of the measurement of 14C in PM2.5 emissions from motor vehicles, which is not part of this study.
EXPERIMENTAL
Emissions Measurements
Engine Descriptions
Measurements were performed on two used, handheld, 2-stroke small engines—a 1999 Echo model SRM-2601 string trimmer and a 1999 Husqvarna model 55 chainsaw (CitationSnow and Crews 2004). Both were operated “as is” with little if any maintenance performed beforehand.
Fuel and Oil Types
Three fuels were used: (1) regular, unleaded summer gasoline (UNL); (2) reformulated gasoline containing ethanol (RFG); and (3) regular, winter gasohol (GHL). The first two were specialty fuels purchased in June 2003 (Haltermann Products Inc., Channelview, TX) with specifications intended to be representative of the national average for these two fuel types. The third was acquired during December 2004 near Detroit, MI from a gasohol-labeled service station pump. A “low-smoke” mineral oil, Pro-Mix #54001, purchased at a local retailer, was blended 1:50 with the fuels for use in the 2-stroke engines.
Sampling
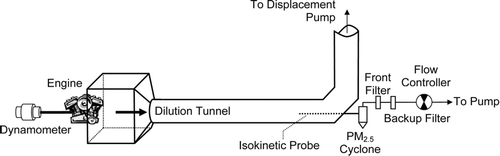
shows schematically the arrangement used to collect filter samples of PM2.5 from the small engines' exhaust emissions. Emissions measurements were performed at the EPA Small Engine Dynamometer Facility, Research Triangle Park, NC. Because a complete technical description of the facility and its sampling procedures have been given elsewhere (CitationSnow and Crews 2004), only a brief description of the sampling measurements is given here. Exhaust emissions from each engine were directed into a dilution tunnel along with laboratory air at a constant total flow rate of 13.0 m3 min−1 and then vented to the outside. An isokinetic probe extracted exhaust samples from the tunnel at a flow rate of 16.7 L min−1, which passed through a PM2.5 cyclone (URG, Chapel Hill, NC) to either a single 47-mm dia. filter or two filters in series (front and backup filter) held in a filter pack. The backup filter was used to correct for organic artifact, as described below. For the string trimmer tests two parallel filter systems were used concurrently (quartz-behind-teflon and quartz only). The teflon filters were Teflo type (Pall Life Sciences, Ann Arbor, MI). The quartz filters were type 2500QAOT (Pallflex Products Corp., Putnam, CT), pre-baked for 4 h at 500°C. For the chainsaw tests only a single quartz-behind-quartz system was used. The two alternative filter configurations reflect a current difference of opinion on which is the better method for estimating organic sampling artifact from measurements made on a backup filter (CitationTurpin et al. 2000). Analytical measurements were performed only on the quartz filters.
Each engine was coupled to a dynamometer that provided a prescribed engine-loading over the test runs. Composite Two-Mode (C2M) duty cycles (which employ 90% full throttle and 10% idle times) were used for testing—four consecutive 10-min cycles for the string trimmer and either two or three consecutive 6-min cycles for the chainsaw. These test cycles were designed to emulate the SAE J1088 Certification duty cycle (CitationGabele 2000). For the string trimmer the UNL and RFG fuels were paired, while for the chainsaw the UNL and GHL fuels were used. For each engine test duplicate sample collections were performed with UNL, followed by duplicate sample collections with one of the ethanol-containing fuels (RFG or GHL). Laboratory air background runs were performed with the engines not running. Because the two engine tests were conducted six months apart (April and December 2004) background samples were collected on both occasions. For the April tests duplicate 60-min background sample collections were performed, while in the December tests one 75-min background sample was collected.
In contrast to previous work (CitationBuchholz et al. 2002a) no gaseous emissions samples were collected for 14C analysis in the present study. While this might have served to generally confirm—by 14C mass balance considerations—the measurements made only on the particulate emissions it would be a less accurate approach, since it would have involved a difference measurement between quantities of nearly identical size (the 14C contents of the fuel and the gaseous emissions) each with their own uncertainties.
Sample Analyses
The NIOSH 5040 method of thermo-optical analysis (CitationBirch and Cary 1996) was used by Sunset Laboratory (Hillsborough, NC) to measure organic carbon (OC) and elemental carbon (EC) on 1.4 cm2 punches from the quartz filters. None of the samples showed any presence of carbonate, and the EC component was generally below the detection limit, so the total carbon on a filter was virtually all OC.
Gas Chromatography/Flame Ionization Detection/Mass Spectrometry (GC/FID/MS) methodology was used to identify and quantify C2–C12 compounds in liquid samples of the fuels, as described previously (CitationHarley et al. 2001). Ethanol was the only identified compound that was not a pure hydrocarbon, and presumably was the only source of 14C in the fuel. Because the FID per carbon response for ethanol is less than that for pure hydrocarbons, the measured response for ethanol was increased by the factor 2.0/1.5 (CitationScanlon and Willis 1985; CitationJorgensen et al. 1990).
The quartz filters and fuel samples were analyzed for 14C by Accelerator Mass Spectrometry (AMS) at the National Ocean Sciences AMS Facility (Woods Hole, MA). Each filter sample was submitted as a 41-mm dia. circle punched from within the aerosol deposit area, in order to decrease the filter blank, and analyzed for 14C as previously described (CitationTanner et al. 2004). Fuel samples were analyzed by an adaptation of a previously described method (CitationMcNichol et al. 2000). In the adaptation the liquid sample was transferred in a capillary tube and immersed in a dry-ice/acetone bath prior to evacuation. Additionally, we tested that ethanol was not preferentially removed from the sample during evacuation by quantifying the amount of ethanol in a separate sample both before and after the evacuation step. All 14C measurement results were expressed in the usual way in terms of “percent Modern Carbon (pMC),” which is 100 times the 14C/12C ratio for the sample normalized to the same ratio for a standard that is equivalent to wood grown in 1890.
RESULTS AND DISCUSSION
14C in Gasoline and Gasohol
The pMC of each of the three fuels was determined by two independent methods: (1) indirectly from the GC-measured ethanol content of the fuel, assuming the ethanol is biogenic, and (2) directly from 14C analysis of the fuel. For the former, first the ethanol fractional abundance (ppbC/ppbC) was calculated from the ratio of the (corrected) ethanol chromatographic peak to the sum of all the peaks (identified or not) in the chromatogram. Unidentified peaks constituted only a few percent of the sum. shows a portion of the chromatogram near the expected location of the ethanol peak for both UNL and RFG fuels. As expected, ethanol is clearly present in RFG, but absent in UNL. The chromatogram for GHL was similar to that of RFG, showing a clear presence of ethanol. The measured ethanol abundances for the fuels are shown in the second column of .
FIG. 2 Chromatograms for conventional gasoline (UNL) and ethanol-containing reformulated gasoline (RFG)
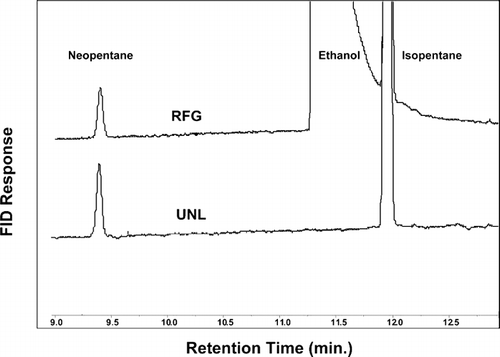
TABLE 1 Ethanol abundance and pMC for three fuels. Measurement uncertainties represent one standard deviation
The inferred pMC for a fuel is the product of its ethanol abundance and the pMC of the ethanol. Because of mid-twentieth-century atmospheric nuclear weapons testing the pMC of Northern Hemisphere atmospheric CO2—and consequently the pMC of contemporaneous biogenic material—has ranged from 100% (pre-1950) to 190% (1964), declining since then to its current value of about 106% (CitationLevin and Kromer 2004). Although the ages of the ethanol fuel additives were unknown, the ethanol was presumed to be of recent origin, and thus the ethanol pMC was taken to be 106%. With this assumption the inferred pMCs are shown in the third column of . The average pMC for the ethanol-containing fuels is 8.25%.
The rightmost column of shows the pMCs from direct 14C measurements on the liquid samples. The average pMC for the ethanol-containing fuels is 7.95% and virtually zero for the regular gasoline, both of which are in good agreement with the inferred results. The direct 14C measurement is the more definitive approach, but its good agreement with the GC-based approach suggests that the latter is a satisfactory surrogate for determining fuel pMC when 14C measurements are unavailable.
14C in Combustion Emissions
suggests that the pMC of the engine particle emissions will be relatively small, and thus considerable care is needed in performing accurate measurements of the emissions. Two effects can distort the filter measurements of engine emissions: (1) laboratory background (carbon aerosol present in the lab air in addition to the test engine emissions), and (2) organic sampling artifact (positive and/or negative). In their review of prior work CitationTurpin et al. (2000) have concluded that during sampling, positive artifact (adsorption of gaseous organic species by a filter) is expected to dominate negative artifact (loss of semi-volatile organic compounds—SVOC—from the filter). In the derivations below we make the simplifying assumption that only positive artifact may be occurring. We make the additional simplifying assumption that any adsorbed gas is in equilibrium between the front and backup filters, so that the adsorbed amount is identical for the two filters. The combined impact of background and positive artifact are calculated separately for carbon mass and its associated pMC in the two following sections.
Carbon Mass Correction
Define the following quantities:
gives the four measured carbon masses and the resulting corrected particulate carbon emission mass for each of the eight experimental runs (duplicate runs for both engines using conventional unleaded gasoline (UNL) and ethanol-containing reformulated gasoline (RFG) or gasohol (GHL)). A comparison of M1 and PE for each run in shows that the combined effects of background and positive artifact leads to a quite small correction for carbon mass. However the real importance of the derivation is that it lays the foundation for the consideration of these effects in calculating the corrected pMC, the main focus of this article, as given in the following section.
TABLE 2 Particulate carbon mass measurements (μg cm−2) with one-standard-deviation uncertainties. M1 and PE (Equation (Equation9)) are the uncorrected and corrected results, respectively
Percent Modern Carbon correction
In analogy with Equation (Equation1) define p1 = measured pMC on front filter (engine on).
Similarly define p2, p3, and p4 in analogy with Equations (2)–(4), with each p i measured on the same quartz filter as was M i . The analog of Equation (Equation5) is then
lists all the pMC measurements, and is the analog of where the carbon mass measurements were given. The pPE column of (the artifact- and background-corrected pMC) gives the principal results of this study, as calculated from Equation (Equation11) using the measurements in both and . Comparing each pPE with its uncorrected measurement p1 shows that the correction is significant, decreasing pMC by about one-half. The uncertainty in each pPE was calculated from the uncertainties in the associated Mi and pi measurements by standard propagation of errors, as in the case of PE in .
TABLE 3 Percent Modern Carbon measurements (%) with one-standard-deviation uncertainties. p1 and pPE (Equation (Equation12)) are the uncorrected and corrected results, respectively
The average pMC for the UNL runs is [0.57 ± 0.42 (sd)]%, compared with 0% anticipated for purely fossil fuel. Surprisingly, the average pMC for the ethanol-containing runs (RFG and GHL) is [0.22 ± 0.12 (sd)]%, statistically equivalent to the UNL average, and consistent with 0% (2 sd). The conclusion is that 14C in particulate carbon emissions from ethanol-containing fuels is drastically smaller than in the fuel itself, with a reduction factor on the order of 40 (8%/0.2%).
Recall that the quantitative aspects of this conclusion rest both on a particular model of organic artifact correction and on sampling configurations for implementing the model that were different for the two engines tested. The latter may at least partially account for pPE being larger on average for the chainsaw versus the string trimmer tests.
In the Appendix it is shown that the model used to correct the mass and pMC measurements can be generalized to include negative as well as positive organic artifact effects. The derivation shows that the corrected mass result given by Equation (Equation9) is actually an underestimate when negative artifact is allowed to occur, although this is of less interest than the effect of negative artifact on pMC. Remarkably, the original pMC result given by Equation (Equation12) is unchanged in spite of the generalization. Thus the results for pPE in are more robust than might be suggested by the somewhat restrictive assumptions that originally led to Equation (Equation12). Whatever the adequacy of the model and its implementation however it is important to appreciate that even the uncorrected pMC for the RFG and GHL test runs showed large reductions in comparison to the pMC of the fuels.
Implication for Mobile Source Emissions
Because the emissions of gasoline/gasohol-fueled motor vehicles make a far greater contribution to ambient aerosol than those of small engines it is interesting to consider the extent to which the (2-stroke) small-engine pMC findings are applicable to motor vehicles. The small-engine results however cannot be immediately generalized to gasohol-fueled motor vehicles. This is because 2-stroke engine emissions are known to contain a significant component of unburned lubricating oil from the gasoline-oil blend of the fuel. Because the oil is expected to be devoid of 14C the presence of oil in the emissions in effect dilutes any 14C in particles from gasohol combustion.
An estimate of the dilution effect can be obtained by measuring the particulate mass emission rate as a function of the oil to gasoline percentage (OGP) of the fuel, extrapolating the emission rates to 0% OGP, and comparing that emission rate to the emission rate for the OGP at which the pMC measurements were performed (2%). Then the 0% OGP emission rate and the difference of the 2% and 0% OGP emission rates are estimates of the gasoline combustion and unburned oil contributions, respectively, to the 2% OGP emission rate. shows data from a chainsaw test that were collected during the earlier comprehensive small-engine testing program conducted at the EPA facility (CitationSnow and Crews 2004). The data are well-represented by a parabolic relationship: Emissions Rate = 0.3933 + 0.2302 (OGP)2, r2 = 0.9978. This implies a mass ratio (at 2% OGP) for unburned oil to gasoline combustion particulate matter of (1.29 − 0.39)/0.39 = 2.31, corresponding to a dilution factor of 3.31. Consequently the average pMC for only the gasohol particulate emissions would be increased by this dilution factor to [0.73 ± 0.40 (sd)] %, a substantial increase, but still more than an order of magnitude smaller than the pMC of the fuel (7.95%). Thus it seems likely that particulate emissions with very small pMC will also be the case for the combustion of gasohol in other spark-ignition engines (in particular, motor vehicles) for which unburned oil is a smaller component of the emissions.
TABLE 4 Chainsaw mass emission rates for varying oil to gasoline fuel blends
CONCLUSIONS
This work has shown that 14C levels in PM2.5 emitted by the combustion of ethanol-containing fuel in 2-stroke small engines are drastically smaller (approximately a factor of 40) than what is present in the fuel. It should not be surprising that the combustion of ethanol, as a low molecular weight compound with a “built-in” oxidant, is more likely to lead to gaseous products—CO2 in particular—than particle emissions. The earlier findings (CitationBuchholz et al. 2002a) for compression-ignition combustion of ethanol-containing diesel fuel in a diesel engine showed such an effect, although to a much lesser extent than for the present spark-ignition small-engine results. While the extension of this small-engine result to other gasohol-fueled spark-ignition engines is complicated by the presence of substantial unburned oil in the combustion emissions of 2-stroke engines, the consideration given to the oil issue suggests the likelihood of a very small pMC for the particulate emissions from gasohol-fueled motor vehicles as well.
APPENDIX
Equations (9) and (12) assume the complete absence of negative organic artifact—volatilization during subsequent sampling of previously collected carbon mass on a filter. As shown below this assumption can be relaxed, resulting in equations with only one simple change from before.
The degree of loss of organic material by volatilization will depend on the volatility of the material. For example, filter-adsorbed organic gases may be more susceptible to volatilization than filter-collected carbonaceous particulate matter. Thus to be as general as possible, let the quantities v, w, x, y, and z be the fraction of carbon remaining on a filter after any volatilization for PE, PB, VE, VB, and BL, respectively. The latter five quantities, defined prior to equation (Equation1), are now re-interpreted to be the carbon masses prior to any volatilization loss. Then Equations (5)–(8) become
As before, the greater interest is in calculating the corrected pMC, now when negative as well as positive organic artifact is allowed to occur. The starting point is Equation (Equation10), but modified by inserting the previously defined v, w, x, y, and z volatilization residual fractions as coefficients of each of the five terms in the equation. As before, three more analogous equations can be written, mirroring Equations (6′)–(8′), just as the modified Equation (Equation10) mirrors Equation (5′). Remarkably, when the equations are solved for the corrected pMC of the engine emissions particulate carbon (pPE), the result is identical to Equation (Equation12). That is, there is no difference whether or not negative organic artifact is allowed to occur, and there are no additional parameters that need to be estimated. In this sense the corrected pMC result is more robust than the corrected carbon mass result, since the latter requires knowledge of the volatilization residual fraction v.
Acknowledgments
The United States Environmental Protection Agency through its Office of Research and Development managed and funded the research described here under Contract No. 68-D-00-269 to Bevilacqua-Knight, Inc. and Purchase Order No. 3D-5079-NTHX to Woods Hole Oceanographic Institution. It has been subjected to Agency review and approved for publication. We thank Jerry Faircloth, Mike Pleasant, Kevin Hicks, Jason Mills, and Richard Snow for their assistance in collecting the small engine samples. We also thank Li Xu and Dana Gerlach for their technical expertise and assistance with 14C analysis of the fuel and filter samples.
Notes
a Average of two background measurements, adjusted for run duration.
b One background measurement, adjusted for run duration.
a Datum from Run 1.
b Datum from Run 2.
c Datum from Run 5.
1 > 99% organic carbon (OC).
REFERENCES
- Bench , G. and Herckes , P. 2004 . Measurements of Contemporary and Fossil Carbon Contents of PM2.5 Aerosols: Results from Turtleback Dome, Yosemite National Park . Environ. Sci. Technol. , 38 ( 10 ) : 2424 – 2427 . [INFOTRIEVE] [CSA]
- Birch , M. E. and Cary , R. A. 1996 . Elemental Carbon-based Method for Monitoring Occupational Exposures to Particulate Diesel Exhaust . Aerosol Sci. Technol. , 25 : 221 – 241 . [CSA]
- Buchholz , B. A. , Cheng , A. S. and Dibble , R. W. 2002a . Isotopic Tracing of Bio-Derived Carbon from Ethanol-in-Diesel Blends in the Emissions of a Diesel Engine. SAE Technical Paper No. 2002-01-1704.[CSA]
- Buchholz , B. A. , Cheng , A. S. , Dibble , R. W. , Mueller , C. J. and Martin , G. C. 2002b . Isotopic Tracing of Fuel Component Carbon in the Emissions from Diesel Engines. SAE Technical Paper No. 2002-01-1942.[CSA]
- Cheng , A. S. , Dibble , R. W. and Buchholz , B. A. 2002 . The Effects of Oxygenates on Diesel Engine Particulate Matter. SAE Technical Paper No. 2002-01-1705.[CSA]
- Endo , M. , Yamamoto , N. , Yoshinaga , J. , Yanagisawa , Y. , Endo , O. , Goto , S. , Yoneda , M. , Shibata , Y. and Morita , M. 2004 . 14C Measurement for Size-Fractionated Airborne Particulate Matters . Atmos. Environ. , 38 ( 36 ) : 6263 – 6267 . [CROSSREF] [CSA]
- Gabele , P. A. 2000 . Characterization of Emissions from Handheld Two-Stroke Engines. Paper No. AS3A, Air and Waste Management Association Annual Meeting, Salt Lake City, UT.[CSA]
- Harley , R. A. , McKeen , S. A. , Pearson , J. , Rodgers , M. O. and Lonneman , W. A. 2001 . Analysis of Motor Vehicle Emissions during the Nashville/Middle Tennessee Ozone Study . J. Geophys. Res. , 106 ( D4 ) : 3559 – 3567 . [CROSSREF] [CSA]
- Jorgensen , A. D. , Picel , K. C. and Stamoudis , V. C. 1990 . Prediction of Gas Chromatography Flame Ionization Detector Response Factors from Molecular Structure . Anal. Chem. , 62 : 683 – 689 . [CROSSREF] [CSA]
- Klinedinst , D. B. and Currie , L. A. 1999 . Direct Quantification of PM2.5 Fossil and Biomass Carbon within the Northern Front Range Air Quality Study's Domain . Environ. Sci. Technol. , 33 ( 23 ) : 4146 – 4154 . [CROSSREF] [CSA]
- Lemire , K. R. , Allen , D. T. , Klouda , G. A. and Lewis , C. W. 2002 . Fine Particulate Matter Source Attribution for Southeast Texas using 14C/13C Ratios . J. Geophys. Res. , 107 ( D22 ) : 4613 1029/2002JD002339.[CROSSREF] [CSA]
- Levin , I. and Kromer , B. 2004 . The Tropospheric 14CO2 Level in Mid-Latitudes of the Northern Hemisphere . Radiocarbon , 46 ( 3 ) : 1261 – 1272 . [CSA]
- Lewis , C. W. , Klouda , G. A. and Ellenson , W. D. 2004 . Radiocarbon Measurement of the Biogenic Contribution to Summertime PM-2.5 Ambient Aerosol in Nashville, TN . Atmos. Environ. , 38 ( 35 ) : 6053 – 6061 . [CROSSREF] [CSA]
- Lewis , C. W. and Stiles , D. C. 2006 . Radiocarbon Content of PM2.5 Ambient Aerosol in Tampa, FL . Aerosol Sci. Technol. , 40 ( 3 ) : 189 – 196 . [CROSSREF] [CSA]
- McNichol , A. P. , Ertel , J. R. and Eglinton , T. I. 2000 . The Radiocarbon Content of Individual Lignin-Derived Phenols: Technique and Initial Results . Radiocarbon , 42 ( 2 ) : 219 – 227 . [CSA]
- Scanlon , J. T. and Willis , D. E. 1985 . Calculation of Flame Ionization Detector Relative Response Factors Using the Effective Carbon Number Concept . J. Chromatogr. Sci. , 23 : 333 – 340 . [CSA]
- Snow , R. F. and Crews , W. 2004 . Characterization of Emissions from Small, Hand-Held, In-Use 2-Cycle Engines. EPA Report No. EPA/600/X-04/191, U.S. Environmental Protection Agency, Washington, D.C., October 2004.[CSA]
- Szidat , S. , Jenk , T. M. , Gaggeler , H. W. , Synal , H.-A. , Fisseha , R. , Baltensperger , U. , Kalberer , M. , Samburova , V. , Reimann , S. , Kasper-Giebl , A. and Hajdas , I. 2004a . Radiocarbon (14C)-deduced Biogenic and Anthropogenic Contributions to Organic Carbon (OC) of Urban Aerosols from Zurich, Switzerland . Atmos. Environ. , 38 : 4035 – 4044 . [CROSSREF] [CSA]
- Szidat , S. , Jenk , T. M. , Gaggeler , H. W. , Synal , H.-A. , Fisseha , R. , Baltensperger , U. , Kalberer , M. , Samburova , V. , Wacker , l. , Sauer , M. , Schwikowski , M. and Hajdas , I. 2004b . Source Apportionment of Aerosols by C-14 Measurements in Different Carbonaceous Particle Fractions . Radiocarbon , 46 ( 1 ) : 475 – 484 . [CSA]
- Szidat , S. , Jenk , T. M. , Synal , H.-A. , Kalberer , M. , Wacker , L. , Hajdas , I. , Kasper-Giebl , A. and Baltensperger , U. 2006 . Contributions of Fossil Fuel, Biomass-burning, and Biogenic Emissions to Carbonaceous Aerosols in Zurich as Traced by 14C . J. Geophys. Res. , 111 : D07206 doi:10.1029/2005JD006590.[CROSSREF] [CSA]
- Tanner , R. L. , Parkhurst , W. J. and McNichol , A. P. 2004 . Fossil Sources of Ambient Aerosol Carbon Based on 14C Measurements . Aerosol Sci. Technol. , 38 ( S1 ) : 133 – 139 . [CROSSREF] [CSA]
- Turpin , B. J. , Saxena , P. and Andrews , E. 2000 . Measuring and Simulating Particulate Organics in the Atmosphere: Problems and Prospects . Atmos. Environ. , 34 ( 18 ) : 2983 – 3013 . [CROSSREF] [CSA]
- Ward , T. J. , Rinehart , L. R. and Lange , T. 2006 . The 2003/2004 Libby, Montana PM2.5 Source Apportionment Research Study . Aerosol Sci. Technol. , 40 ( 3 ) : 166 – 177 . [CROSSREF] [CSA]