Because of increasing incidence of virus-containing aerosols, ozone was potentially considered to be a promising method to inactivate airborne viruses. In this investigation, bacteriophages MS2, phi X174, phi 6, and T7 are under evaluation. The effects of ozone concentration, contact time, different capsid architecture of virus and relative humidity (RH) on inactivating airborne viruses by ozone were evaluated in a laboratory test chamber. It was observed that the survival fraction of airborne virus decreased exponentially with increasing ozone dose. Airborne viruses required ozone doses of 0.34 to 1.98 and 0.80 to 4.19 min-mg/m 3 for 90% and 99% inactivation, respectively. For all four tested, the ozone dose for 99% inactivation was 2 times higher than that for 90% inactivation. At airborne phase with a short contact time, viruses with more complex capsid architectures were observed to be less susceptible to ozone inactivation than those with simple ones. For all tested viruses at the same inactivation, the required ozone concentration at 85% RH was lower than that at 55% RH, possibly because the generation of more radicals from ozone reacting with water vapor at the higher RH. In summary, it was concluded that ozone is highly effective for the inactivation of airborne virus.
INTRODUCTION
Viruses are obligating parasites that cannot multiply or propagate outside specific host cells. Viruses can be transmitted by various routes, including vector and vehicle transmission. The vehicle transmission pathways include respiratory transmission by droplets and aerosols. To reduce infection risk from virus-containing aerosols, there are many control techniques including filtration (CitationDemers 2001), ultraviolet germicidal irradiation (UVGI) (CitationJensen 1964; CitationTseng and Li 2005a), and ozone (CitationDemik and Degroot 1977; CitationKekez and Sattar 1997) have been extensively researched. Among these methods, ozone is known in decreasing the viral load (CitationBerrington and Pedler 1998) and used as a potent oxidizing agent in food and other industries (CitationKim et al. 1999).
Up to now, the primary mechanism by which ozone inactivates virus is not well understood. Ozone may react with virus either by direct reaction with molecular ozone or by indirect reaction with the radical species formed when ozone decomposes (CitationKim et al. 1999). It was generally accepted that the inactivation is achieved mostly by the attack of molecular ozone instead of free radicals (CitationZhou and Smith 2001). Ozone is known to attack unsaturated bonds, and form aldehydes, ketones or carbonyl compounds (CitationLanglais et al. 1991). Additionally, ozone could react with amino acids, proteins, protein functional groups and nucleic acids very rapidly (CitationLanglais et al. 1991). Therefore, viruses may be inactivated by ozone acting on the protein structure of a virus capsid, or nucleic acids of virus.
For viruses, early research on ozone applications focused mainly in water (CitationLazarova et al. 1998; CitationShin and Sobsey 2003; CitationThurston-Enriquez et al. 2005; CitationTyrrell et al. 1995). Recent studies report that relatively low ozone concentration (less than 1 mg/liter) and short contact time (1 min) are sufficient to inactivate 99% virus, such as rotaviruses, parvoviruses, feline calicivirus, and hepatitis A virus (CitationShin and Sobsey 2003; CitationThurston-Enriquez et al. 2005). These previous studies reveal that the susceptibility of viruses is highly related to ozone concentrations, pH value, water temperature, residence time, mixing degree, and organic compounds.
Regarding ozone for inactivation of airborne microorganisms, our previous study demonstrated that ozone can be used for inactivating 1–3 logs of airborne bacteria (Escherichia coli) and yeast (CitationLi and Wang 2005). However, there were no ozone-inactivation effects observed for spore types of Bacillus subtilis and Penicillium citrinum at a very high ozone concentration of 20 ppm. Our study revealed that the microorganism susceptibility to ozone is highly depended on microorganism species. In addition, it was also observed that microorganism susceptibility to ozone is significantly higher when relative humid (RH) increased.
Up to now, only limited data has been available on the inactivation of airborne viruses by ozone (CitationDemik and Degroot 1977; CitationKekez and Sattar 1997). At a long exposure time of 30 min, the use of ozone could inactivate 3 logs of airborne phi X174 phage at an ozone concentration of 0.04 ppm (CitationDemik and Degroot 1977). In addition, phi X174 was observed to retain biological activity of extracted DNA after 30 min. Therefore, ozone may primarily inactivate the protein capsid, and then the naked nucleic acid may be secondarily inactivated (CitationDemik and Degroot 1977; CitationKim et al. 1980). In virus inactivation, ozone concentration, contact time, and the type of viral capsid protein are suggested to play critical roles.
In our current study, ozone for inactivation of virus-containing aerosols was evaluated in a laboratory test chamber. In general, virus-containing aerosols less than 2 μ m in size should have higher infectivity than those of the virus itself (CitationCouch et al. 1965). Therefore, virus-containing aerosols were generated by Collison three-jet nebulizer to range from 0.5 μ m to 3.0 μ m. Regarding the virus target, bacteriophages were used as a model because it is safe and easy to handle. The bacteriophages used in this study have been used as indicators of poliovirus, enterovirus, enveloped viruses, and Human Immunodeficiency Virus (CitationDileo et al. 1993; CitationLytle et al. 1991; CitationMaillard et al. 1994). Moreover, bacteriophages can grow to higher titers that could constitute a more sensitive assay. According to the types of the nucleic acids, viruses could be divided into four groups including single-stranded RNA (ssRNA), single-stranded DNA (ssDNA), double-stranded RNA (dsRNA), and double-stranded DNA (dsDNA). Viruses with different architecture of capsid protein were also assessed, because ozone may cause capsid protein damage. In this current study, MS2 (ssRNA), phi X174 (ssDNA), phi 6 (dsRNA), and T7 (dsDNA) phages are composed of 180, 60, 120, and 415 molecules of the capsid protein, respectively. The effects of ozone concentration, contact time, nucleic acid type of virus, architecture of capsid protein, and RH on virus survival were under evaluation.
MATERIALS AND METHODS
Test Viruses
As shown in , the evaluated viruses were four different bacteriophages: ssRNA (MS2, ATCC 15597-B1), ssDNA (phi X174, ATCC 13706-B1), dsRNA (phi 6 with envelope lipid, ATCC 21781-B1), and dsDNA (T7, ATCC 11303-B1). The host bacteria were Escherichia coli F-amp (ATCC 15597) for MS2, Escherichia coli CN-13 (ATCC 13706) for phi X174, Escherichia coli 11303 (ATCC 11303) for T7, as well as Pseudomonas syringae (ATCC 21781) for phi 6. Because ozone mainly caused capsid protein damage, viruses with different architecture of capsid protein were also considered. MS2, phi X174, phi 6, and T7 phages are composed of 180, 60, 120, and 415 molecules of the capsid protein, respectively. Moreover, plaque assay and phage cultivation methods were followed from the ATCC product information sheet. A high titer stock of bacteriophages (109–1010 PFU/ml, where PFU is Plaque Forming Units) was prepared via plate lysis and elution. To allow the phage to attach the host, the bacteriophages were mixed with their own respective host. First, 5 ml of top agar was added to a sterile tube of infected cells. The medium for MS2, phi X174, T7, and phi 6 phage cultivation include Luria-Bertani Agar (Difco Laboratories, 244520), Nutrient Agar (Difco Laboratories, 213000) with 0.5% NaCl, Trypticase Soy Agar (Difco Laboratories, 236950), and NBY Agar (containing Nutrient Broth, Yeast extract, K2HPO4, KH2PO4, and MgSO4·7H2O), respectively. Then, the contents of the tube were mixed by gentle tapping for 5 sec and poured onto the center of a labeled agar plate. Finally, the plate was incubated for 24 h either at 37°C for coliphages or at 26°C for phi 6. After cultivation, 5 ml SM buffer (containing NaCl, MgSO4 · 7H2O, Tris, and gelatin) was pipetted onto a plate that showed confluent lysis. Then, the plate was slowly rocked for 40 min and the buffer was transferred to a tube for centrifugation at 4,000 × g for 10 min. After the supernatant was removed, the remaining phage stock was kept at −80°C. From our preliminary results (data not shown), virus infectivity could be maintained for 24 h at 4°C. Before ozone experiments, the virus titers were determined by plaque assay, and the virus suspension was stored at 4°C within 24 h.
TABLE 1 Characteristics of bacteriophages used in this study
Aerosol Test System
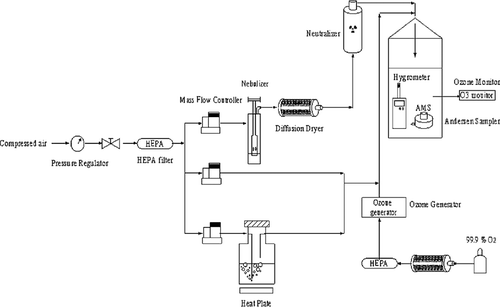
Aerosol Generation Unit
In our current study, a Collison three-jet nebulizer (BGI Inc., Waltham, MA) was used to nebulize the bacteriophage stock in deionized water at 3 L/min with dry, filtered, compressed laboratory air, then passed though a Kr-85 particle-charge neutralizer (model 3077, TSI). The aerosolized suspension was then diluted with filtered, compressed air. The stock solutions of bacteriophages MS2, phi X174, and T7 were diluted in sterile, deionized water for nebulization. For phi 6 phage, the stock solution was diluted in sterile, deionized water containing 0.03% Tween 80 to preserve infectivity (CitationThompson and Yates 1999). In all of the experiments, the phage concentrations in the nebulizer were ranged from 2 × 108 to 7 × 108 PFU/ml.
RH Regulation Unit
A humidified gas stream was generated by passing pure compressed air through a humidity saturator. The water vapor contents (i.e., RH) in the gas stream was adjusted by changing the flow rate ratio of humidified gas stream to dry gas stream, and finally measured using a hygrometer (Testo, Sekunden-Hygrometer 601) placed in the sampling chamber. For evaluating the effect of RH, the humidified gas stream was heated by adding a dry gas stream to reach the medial (RH 55%) or humid condition (85%) at 25–28°C.
Ozone Exposure Unit
As shown in , the exposure chamber was approximately 23 liters in volume (ID 14 cm and height 38 cm). The ozone was generated from an ozone generator (OZ1PCS-V/SW, Ozotech Inc., Yreka, CA) with pure oxygen at 3 L/min. Ozone levels were measured by an ozone analyzer (model 401, Advanced Pollution Instruments, San Diego, CA) with a detection limit of 1.0 ppb. The gas flow rates were 45 L/min and 60 L/min to obtain contact time at 18.4 sec and 13.8 sec, respectively. The gaseous ozone was continuously generated through a Teflon tube into the chamber at a flowrate of 3 L/min. In addition, the virus-containing aerosols were also generated continuously into this chamber from a Collison three-jet nebulizer at a flowrate of 3 L/min. The ozone generator voltage was adjusted to produce the appropriate ozone/oxygen ratio for attaining the target ozone concentrations in the range of 0.1–10 ppm. Experiments were done at least in triplicate for each set of conditions with different ozone concentrations (0.1–10 ppm), contact time (13.8 sec and 18.4 sec) RH (55% and 85%), and test virus (MS2, phi X174, T7, and phi 6). The test system was located in a chemical hood so that the exhausted gas was vented outside.
Virus Aerosol Sampling
From our previous investigation (CitationTseng and Li 2005b), an aerodynamic particle sizer (APS, Model 3310A, TSI, Inc., St. Paul, MN) was used to measure the real-time number concentration and size distribution of the virus-containing aerosols in the test chamber. By using the Andersen 6-STG sampler, the measured geometric mean aerodynamic diameter of MS2, phi X174, T7, and phi 6 was found to be 1.23 μ m, 1.25 μ m, 1.24 μ m, and 1.25 μ m, respectively, with a geometric standard deviation of 1.5 (data not shown). In addition, more than 95% of virus-containing aerosols were found to be less than 2.1 μ m in diameter. In the current study, an Andersen one-stage viable impactor (Andersen Samplers, Inc., Atlanta, GA) was used to collect virus-containing aerosols before and after ozone treatment. This stage has four hundred 0.25-mm holes and has a sampling flow rate of 28.3 L/min (corresponding to a velocity of 24 m/s) when 20 ml LB (Luria-Bertani) broth is used with 3% gelatin plates. The measured and theoretical cut-point diameters of this stage are 0.57 μ m and 0.65 μ m, respectively (CitationNevalainen et al. 1993). The samples of each virus aerosol were taken without and with ozone exposure. To collect a sufficient concentration of virus (at least 30 plaques), sampling times ranged from 30 sec to 1 min without ozone exposure, and ranged from 1 min to 5 min with ozone exposure. The lower limit of 30 plaques is necessary to obtain sufficient statistical power for comparison purposes (CitationLembke et al. 1981; CitationThorne et al. 1992). After sampling, the plate with collection medium from the impactor was placed in an incubator at 37°C for 10 min. All of the viral samples were immediately subjected to plaque assay for coliphages at 37°C and for phi 6 at 26°C. Then, PFU per cubic meter (PFU/m3) was calculated based on plaque numbers, dilution ratio, plated volume, sampling time, and sampling flowrate.
Survival Fraction of Viruses vs. Ozone Exposure
The ozone dose to which an airborne virus exposed was defined as the product of ozone concentration on the virus and the contact time (Ct). The survival fraction (SF, unitless) is a ratio that represents the virus concentration after ozone exposure, and defined as
Statistics
The parameter exponential log of the survival fraction versus ozone concentration and ozone dose for each experiment was used to perform regression analysis on the data for each virus. R2 values were obtained by regression analysis. Generation of regression curves and prediction of the ozone concentration required for 90% and 99% viral reduction were accomplished by including data points from all experiments for each virus. Comparisons of survival fraction among the viruses were performed using a t-test to evaluate statistically significant differences.
RESULTS AND DISCUSSION
In this study, airborne ozone was evaluated for inactivation of airborne viruses. The effect of ozone dose and RH was evaluated for four different bacteriophages selected to represent all types of virus. In our experimental chamber, the virus infectivity in the aerosolized suspension and aerosol phase (at 55% and 85% RH) could be maintained up to 90 min with a coefficient of concentration variation less than 25% (CitationTseng and Li 2005b). Therefore, the natural decay rates of the aerosolized suspension were found to be insignificant.
At 55% RH, to obtain 90% virus inactivation at the contact time of 13.8 sec (as shown in ), phi 6, phi X174, MS2, and T7 required ozone of 1.16 ppm, 1.87 ppm, 3.43 ppm, and 5.20 ppm, respectively. For 99% virus inactivation, phi 6, phi X174, MS2, and T7 required ozone of 2.50 ppm, 3.84 ppm, 6.63 ppm, and 10.33 ppm, respectively. There were two times in which ozone concentration differences between 90% and 99% inactivation for all types of viruses. Moreover, it is indicated that the ozone concentration for both 90% and 99% inactivation of MS2 and T7 is approximately 2–4 times higher than those of phi X174 and phi6 (p < 0.05). For 90% virus inactivation at the contact time of 18.4 sec, phi 6, phi X174, MS2, and T7 required ozone of 0.64 ppm, 0.85 ppm, 1.45 ppm, and 2.32 ppm, respectively. For 99% virus inactivation at 18.4 sec, phi 6, phi X174, MS2, and T7 required ozone of 1.43 ppm, 1.90 ppm, 2.90 ppm, and 5.12 ppm, respectively. It was observed that the required ozone concentration at 85% RH was 1.2–1.7 times lower than those found at 55% RH at the same 90% and 99% inactivation.
FIG. 2 Survival fraction of airborne MS2, phi X174, phi 6 and T7 exposed to different ozone concentrations at RH 55% and 85%. Error bars represent one standard deviation of the mean of at least three trials
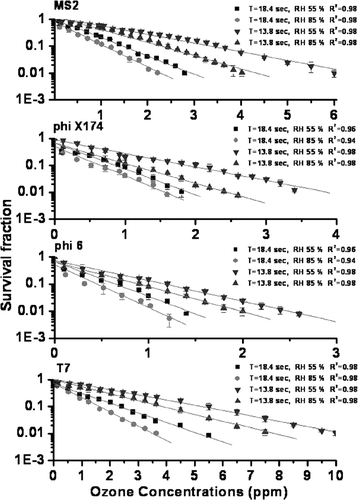
In comparison with our previous study (CitationLi and Wang 2005), Escherichia coli and yeast required ozone concentration of 8.7 ppm and 19 ppm for 90% inactivation, respectively. In this study, the required ozone concentrations for cell type bacteria and fungi were 4–13 times and 8–30 times higher than those of the tested four viruses, respectively. Moreover, there was no inactivation for spore types of Bacillus subtilis and Penicillium citrinum at a very high ozone level of 20 ppm. Therefore, it was demonstrated that virus susceptibility to ozone is much higher than those of bacterial and fungal aerosols. The higher susceptibility of airborne bacteriophage indicated that ozone reacts more readily with capsid proteins than with lipids of cell membrane (CitationKomanapalli and Lau 1998). The differences in the phage capsid protein and bacterial cell membrane may result in the different microorganism susceptibility to ozone (CitationMudd et al. 1969; CitationPryor et al. 1991).
From our findings, it was indicated that the survival fraction of all four viruses decreased exponentially with increasing ozone dose (as shown in ). At 55% RH, to obtain 90% viral inactivation, phi 6, phi X174, MS2, and T7 required ozone dose of 0.47, 0.72, 1.28, and 1.98 min-mg/m3, respectively. For 99% viral inactivation, phi 6, phi X174, MS2, and T7 required ozone dose of 1.05, 1.58, 2.60, and 4.19 min-mg/m3, respectively. The results obviously indicated that T7 and MS2 phages are more resistant to ozone than those of phi X174 and phi 6 phages (p < 0.05). Furthermore, our results of ozone doses for phi X174 were similar to those evaluated at a long exposure time of 30 min (CitationDemik and Degroot 1977). Our findings demonstrated that the germicidal effects of ozone for airborne virus inactivation depended on ozone dose, and were not individually influenced by ozone concentration or the contact time. From our previous investigation of airborne E. coli and yeast, it is revealed that survival fraction did not decline exponentially with ozone dose increase (CitationLi and Wang 2005). These differences could be due to the different inactivation mechanism of ozone between viruses, and cell type bacteria and fungi. Currently, US EPA recommended that using Ct values, (ozone dose), as an indicator for viral inactivation in water by ozone (CitationUS EPA Guidance Manual, 1989). The Ct values of 0.15–0.30 min-mg/L for 2–4 logs viral inactivation by ozone at 25°C are suggested. Compared with airborne viruses, viruses in water were more difficult to inactivate.
FIG. 3 Survival fraction of airborne MS2, phi X174, phi 6, and T7 exposed to different ozone doses at RH 55% and 85%. Error bars represent one standard deviation of the mean of at least three trials
Based on the exponential decay model, the virus susceptibility factors, K (expressed in m3/mg-min), were found to vary widely. It was observed that the K factors of phi X174 and phi 6 phages (3.12–5.94) were higher than those of T7 and MS2 phages (1.25–2.54). This may be related to the fact that virus with a more complex capsid but without envelope lipids could provide protection from ozone inactivation. For the four types of viruses, K values at 85% RH (1.54–5.94) were higher than those (1.25–4.25) at 55% RH. A higher ozone dose is required to inactivate virus at lower RH (p < 0.05). This could be related to generation of more radicals from ozone reacted with more water vapor at higher RH, which agreed with those from the previous studies (CitationFoarde et al. 1997; CitationLi and Wang 2003).
In the current study, the order of resistance to ozone was phi 6 < phi X174 < MS2 < T7. In comparison with simple capsid architecture (phi X174), more complex virus capsid (MS2 and T7) was observed to be less susceptible to ozone (p < 0.05). Virus capsid would be degraded into protein subunits by ozonation (CitationKim et al. 1980), as well as more complex virus capsid could provide protection from ozone inactivation. From the previous investigations (CitationDemik and Degroot 1977; CitationShin and Sobsey 2003), it was indicated that ozone could act on both nucleic acids and virus capsid to inactivate virus. At a low ozone concentration (0.04 ppm) and long contact time (30 min), airborne phi X174 phage was observed to keep biological activity of extracted DNA (CitationDemik and Degroot 1977). Therefore, it is suggested that ozone may mainly alter the capsid protein, and inactivate the naked nucleic acid later. In our study, the ozone contact time is very short (13.8 and 18.4 sec), therefore, ozone may mainly cause capsid protein damage rather than damage the nucleic acid. In our findings, phi 6 was found to be the most sensitive virus to ozone, however, phi 6 has higher molecules of the capsid protein than phi X174. This may be related to the higher sensitivities of the enveloped viruses to physical and chemical challenges than those of the naked viruses. (CitationWoolwine and Gerberding 1995). Moreover, it is recommended that only one strand of the nucleic acid is damaged during inactivation, and the undamaged strand might then serve as a template for repair by host enzymes in the host cell repair mechanisms (Thurston-Enriquez et al. 2003). This may cause T7 to be less susceptible to ozone. Consequently, viral components such as capsid protein, nucleic acid, and lipid content would be oxidized by ozone. Further studies are needed to determine which component is the primary target of ozone at different ozone concentrations and contact time.
Up to now, bacteriophages of the male-specific group are used as indicators of the efficiency of wastewater disinfection (CitationHavelaar et al. 1991). MS2 with high resistance has commonly served as an indicator of viral inactivation (CitationShin and Sobsey 2003). In our current study, T7 with more complex capsid architecture is less susceptible to ozone than MS2. Therefore, MS2 could not be a suitable indicator for virus inactivation by ozone in air. In summary, our current findings demonstrated that the survival fraction of viruses decreased exponentially with increasing ozone dose. In the airborne phase with a short contact time, virus susceptibility to ozone could be mainly related to the type of virus capsid architecture.
CONCLUSION
The effects of ozone concentrations, contact time, and RH for virus inactivation were evaluated in a laboratory test chamber. Our findings demonstrated that the survival fraction of airborne viruses decreased exponentially with increasing ozone dose. Virus required ozone doses of 0.34–1.98 and 0.80–4.19 min-mg/m3for 90% and 99% inactivation, respectively. In the airborne phase with a short contact time, virus susceptibility to ozone could be related to the type of virus capsid architecture, and with or without envelope. A more complex virus capsid could provide protection from ozone inactivation, as well as the enveloped viruses which were found to have higher susceptibility to ozone. Regarding the RH effects, the susceptibility for viruses was higher at 85% RH than that at 55% RH. This might be related to the generation of more radicals from ozone which reacted with more water vapor at higher RH.
Acknowledgments
This work was supported by grant NSC 93-2621-Z-002-003- from the National Science Council, Republic of China. Chun-Chieh Tseng was supported by a graduate scholarship from the same grant during part of this research effort.
REFERENCES
- Benbough , J. E. 1971 . Some Factors Affecting the Survival of Airborne Vruses . J. Gen. Virol. , 10 : 209 – 220 . [INFOTRIEVE] [CSA]
- Berrington , A. W. and Pedler , S. J. 1998 . Investigation of Gaseous Ozone for MRSA Decontamination of Hospital Side-Rooms . J. Hosp. infect. , 40 : 61 – 65 . [INFOTRIEVE] [CROSSREF] [CSA]
- Buckland , F. E. and Tyrell , D. A. J. 1962 . Loss of Infectivity on Drying Various Viruses . Nature , 195 : 1063 – 1064 . [INFOTRIEVE] [CSA]
- Couch , R. B. , Gerone , P. J. , Cate , T. R. , Griffith , W. R. , Alling , D. W. and Knight , V. 1965 . Preparation and Properties of a Small-Particle Aerosol of Coxsackie A21 . Proc. Soc. Exp. Biol. Med. , 118 : 818 – 822 . [INFOTRIEVE] [CSA]
- Demers , R. R. 2001 . Bacterial/viral filtration—Let the breather beware . Chest , 120 : 1377 – 1389 . [INFOTRIEVE] [CROSSREF] [CSA]
- Demik , G. and Degroot , I. 1977 . Mechanisms of Inactivation of Bacteriophage Psi-X174 and Its DNA in Aerosols by Ozone and Ozonized Cyclohexene . J. Hygiene. , 78 : 199 – 211 . [CSA]
- Dileo , A. J. , Vacante , D. A. and Deane , E. F. 1993 . Size-Exclusion Removal of Model Mammalian Viruses Using a Unique Membrane System. 1. Membrane Qualification . Biologicals. , 21 : 275 – 286 . [INFOTRIEVE] [CROSSREF] [CSA]
- Foarde , K. K. , VanOsdell , D. W. and Steiber , R. S. 1997 . Investigation of Gas-Phase Ozone as a Potential Biocide . Appl. Occup. Environ. Hyg. , 12 : 535 – 542 . [CSA]
- Havelaar , A. H. , Butler , M. , Farrah , S. R. , Jofre , J. , Marques , E. , Ketratanakul , A. , Martins , M. T. , Ohgaki , S. , Sobsey , M. D. and Zaiss , U. 1991 . Bacteriophages as Model Viruses in Water-Quality Control . Water Res. , 25 : 529 – 545 . [CROSSREF] [CSA]
- Ijaz , M. K. , Karim , Y. G. , Sattar , S. A. and Johnson-Lussenburg , C. M. 1987 . Development of Methods to Study the Survival of Airborne Viruses . J. Virol. Methods. , 18 : 87 – 106 . [INFOTRIEVE] [CROSSREF] [CSA]
- Jensen , M. M. 1964 . Inactivation of Airborne Viruses by Ultraviolet Irradiation . Appl. Microbiol. , 12 : 418 – 420 . [INFOTRIEVE] [CSA]
- Kekez , M. M. and Sattar , S. A. 1997 . A New Ozone-Based Method for Virus Inactivation: Preliminary Study . Phys. Md. Biol. , 42 : 2027 – 2039 . [CROSSREF] [CSA]
- Komanapalli , I. R. and Lau , B. H. S. 1998 . Inactivation of Bacteriophage λ, Escherichia coli, and Candida albicans by ozone . Appl. Microbiol. Biotechnol. , 49 : 766 – 769 . [INFOTRIEVE] [CROSSREF] [CSA]
- Kim , C. K. , Gentile , D. M. and Sproul , O. J. 1980 . Mechanism of Ozone Inactivation of Bacteriophage-F2 . Appl. Eviron. Vcrobiol. , 39 : 210 – 218 . [CSA]
- Kim , J. G. , Yousef , A. E. and Dave , S. 1999 . Application of Ozone for Enhancing the Microbiological Safety and Quality of Foods: A review . J. Food Prot. , 62 : 1071 – 1087 . [INFOTRIEVE] [CSA]
- Kowalski , W. J. and Bahnfleth , W. 1998 . Airborne Respiratory Diseases and Mechanical Systems for Control of Microbes . HPAC Eng. , 70 : 34 – 48 . [CSA]
- Langlais , B. , Reckhow , D. A. and Brink , D. R. 1991 . Ozone in Water Treatment, Applications and Engineering , Chelsea, MI : Lewis Publishers, Inc. .
- Lazarova , V. , Janex , M. L. , Fiksdal , L. , Oberg , C. , Barcina , I. and Pommepuy , M. 1998 . Advanced Wastewater Disinfection Technologies: Short and Long Term Efficiency . Wat. Sci. Tech. , 38 : 109 – 117 . [CROSSREF] [CSA]
- Lembke , L. L. , Kniseley , R. N. , Van Mostrand , R. C. and Hale , M. D. 1981 . Precision of the All-Glass Impinger and the Andersen Microbial Impactor for Air Sampling in Solid-Waste Handling Facilities . Appl. Environ. Microbiol. , 42 : 222 – 225 . [INFOTRIEVE] [CSA]
- Li , C. S. and Wang , Y. C. 2003 . Surface Germicidal Effects of Ozone for Microorganisms . AIHA Journal. , 64 : 533 – 537 . [INFOTRIEVE] [CROSSREF] [CSA]
- Li , C. S. and Wang , Y. C. 2005 . Inactivation Effects of Airborne Ozone for Bioaerosols . J. Environ. Heal. , submitted.[CSA]
- Lytle , C. D. , Budacz , A. P. , Keville , E. , Miller , S. A. and Prodouz , K. N. 1991 . Differential Inactivation of Surrogate Viruses with Merocyanine-540 . Photochem. Photobiol. , 54 : 489 – 493 . [INFOTRIEVE] [CSA]
- Maillard , J. Y. , Beggs , T. S. , Day , M. J. , Hudson , R. A. and Russell , A. D. 1994 . Effect of Biocides on MS2-Coliphage and K-Coliphage . Appl. Environ. Microbiol. , 60 : 2205 – 2206 . [INFOTRIEVE] [CSA]
- Mudd , J. B. , Leavitt , R. , Ongun , A. and McManus , T. T. 1969 . Reaction of Ozone with Amino Acids and Poteins . Atmos. Environ. , 3 : 669 – 682 . [INFOTRIEVE] [CROSSREF] [CSA]
- Nevalainen , A. , Willeke , K. , Liebhaber , F. , Pastuszka , J. , Burge , H. and Henningson , E. 1993 . “ Bioaerosol Sampling ” . In Aerosol Measurement: Principles, Techniques, and Applications , Edited by: Willeke , K. and Baron , P. A. 471 – 492 . New York : Van Nostrand Reinhold .
- Pryor , W. A. , Das , B. and Church , D. F. 1991 . The Ozonation of Unsaturated Fatty Acids: Aldehydes and Hydrogen Peroxide as Products and Possible Mediators of Ozone Toxicity . Chem. Res. Toxicol. , 4 : 341 – 348 . [INFOTRIEVE] [CROSSREF] [CSA]
- Shin , G. A. and Sobsey , M. D. 2003 . Reduction of Norwalk Virus, Poliovirus 1, and Bacteriophage MS2 by Ozone Disinfection of Water . Appl. Environ. Microbiol. , 69 : 3975 – 3978 . [INFOTRIEVE] [CROSSREF] [CSA]
- Thompson , S. S. and Yates , M. V. 1999 . Bacteriophage Inactivation at the Air-Water-Solid interface in Dynamic Batch Systems . Appl. Environ. Microbiol. , 65 : 1186 – 1190 . [INFOTRIEVE] [CSA]
- Thorne , P. S. , Kiekhaefer , M. S. , Whitten , P. and Donham , K. J. 1992 . Comparison of Bioaerosol Sampling Methods in Barns Housing Swine . Appl. Environ. Microbiol. , 58 : 2543 – 2551 . [INFOTRIEVE] [CSA]
- Thurston-Enriquez , J. A. , Haas , C. N. , Jacangelo , J. and Gerba , C. P. 2005 . Inactivation of Enteric Adenovirus and Feline Calicivirus by Ozone . Wat. Res. , 39 : 3650 – 3656 . [CROSSREF] [CSA]
- Tseng , C. C. and Li , C. S. 2005a . Inactivation of Virus-Containing Aerosols by Ultraviolet Germicidal Irradiation . Aerosol Sci. Technol. , 39 : 1136 – 1142 . [CROSSREF] [CSA]
- Tseng , C. C. and Li , C. S. 2005b . Collection Efficiencies of Aerosol Samplers for Virus-Containing Aerosols . J. Aerosol. Sci. , 36 : 593 – 607 . [CROSSREF] [CSA]
- Tyrrell , S. A. , Rippey , S. R. and Watkins , W. D. 1995 . Inactivation of Bacterial and Viral Indicators in Secondary Sewage Effluents, Using Chlorine and Ozone . Wat. Res. , 29 : 2483 – 2490 . [CROSSREF] [CSA]
- USEPA . 1989 . Guidance Manual for Compliance with the Filtration and Disinfection Requirements for Public Water Systems Using Surface Water Sources , Washington, DC : Office of Water, US Environmental Protection Agency .
- Woolwine , J. D. and Gerberding , J. L. 1995 . Effect of Testing Method on Apparent Activities of Antiviral Disinfectants and Antiseptics . Antimicrob. Agents Chemother. , 39 : 921 – 923 . [INFOTRIEVE] [CSA]
- Zhou , H. and Smith , D. W. 2001 . Advanced Technologies in Water and Wastewater Treatment . Can. J. Civ. Eng. , 28 : 49 – 66 . [CROSSREF] [CSA]